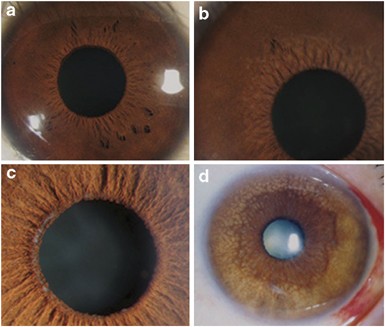
Aqueous flare and cells in fuchs syndrome
Aqueous flare and cells in fuchs syndrome"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT PURPOSE To quantitatively evaluate aqueous flare and cells in patients with Fuchs syndrome. METHODS The medical records of 40 patients (47 eyes) diagnosed with Fuchs syndrome
between February 2006 and January 2007 at the Uveitis Study Center of Sun Yat-sen University were retrospectively reviewed. Aqueous flare and cells were clinically evaluated and quantified
with laser flare-cell meter. Statistical analysis was performed to investigate the relationship between flare values and cell counts, and clinical parameters including patients' age,
sex, duration of disease, best-corrected visual acuity, keratic precipitate, iris depigmentation, intraocular pressure, and posterior subcapsular lens opacities. RESULTS Aqueous flare values
(photon counts/ms) were significantly higher in Fuchs syndrome (9.40±5.85) than in normal controls (5.77±1.89, _P_=0.000). Aqueous cell counts (cells/0.5 mm3) were also significantly higher
in Fuchs syndrome (5.09±4.84) than in normal controls (1.14±1.03, _P_=0.000). The flare values were positively correlated with the cell counts (_r_=0.331, _P_=0.001). Both flare values and
cell counts were higher in eyes with keratic precipitates scored 2+ or 3+ as compared to those with a 1+ score. Higher flare values and cell counts were also observed in eyes with a 2+ or 3+
iris depigmentation score as compared to those with a 1+ score. No difference was found between flare values and cell counts and other parameters. CONCLUSION Breakdown of blood-aqueous
barriers and increased cell counts are present in the affected eyes in patients with Fuchs syndrome. These changes are positively associated with the degree of keratic precipitates and iris
depigmentation. SIMILAR CONTENT BEING VIEWED BY OTHERS AQUEOUS HUMOR ANALYSES OF DIABETIC MACULAR EDEMA PATIENTS WITH SUBRETINAL FLUID Article Open access 25 October 2021 UNRAVELING THE
EFFECTS OF SERIAL INTRAVITREAL AFLIBERCEPT INJECTIONS ON THE OCULAR SURFACE OF PATIENTS WITH GLAUCOMA AND RETINAL COMORBIDITY Article Open access 15 April 2025 AN OBJECTIVE METHOD OF
DIAGNOSING HYDROXYCHLOROQUINE MACULOPATHY Article 14 September 2020 INTRODUCTION The term Fuchs syndrome, usually referred to Fuchs heterochromic iridocyclitis, is used for a specific
uveitis entity usually manifesting as a mild unilateral anterior uveitis with stellate or medium-sized keratic precipitates (KP) and varying degrees of iris depigmentation or
heterochromia.1, 2, 3, 4, 5 It occurs mostly in the third or fourth decades of adults and affects both sexes equally. Complicated cataract and secondary glaucoma are common in these
patients. With careful slit-lamp examination, signs of intraocular inflammation including KP, flare and cells in the anterior chamber, iris depigmentation or heterochromia are detectable in
most affected eyes. Alteration of the blood-aqueous barrier (BAB) in patients with Fuchs syndrome has been demonstrated by iris fluorescein angiography.6 Our recent study using laser
flare-cell meter (LFCM) analysis has confirmed that aqueous flare values and cell counts are increased in the affected eyes with Fuchs syndrome.7 However, it is not yet known which clinical
parameters, such as patients’ age, sex, laterality, best-corrected visual acuity (BCVA), duration of disease, KP, iris depigmentation, intraocular pressure, and posterior subcapsular lens
opacities are associated with these changes. In the present study, we quantitatively evaluated the breakdown of the BAB and the changes in the cells in the anterior chamber of the affected
eyes in a consecutive series of patients with Fuchs syndrome and evaluated the relationship between the LFCM results and clinical parameters. METHODS Forty patients with Fuchs syndrome
referred to the Uveitis Study Center of the Sun Yat-sen University were included in this study. The diagnosis of Fuchs syndrome was based on clinical biomicroscopic findings including
chronic anterior uveitis with low inflammatory activity, characteristic stellate or medium-sized KP diffusely or centrally distributed on the corneal endothelium, diffuse iris depigmentation
or heterochromia, and lack of posterior synechiae.7, 8, 9 Idiopathic chronic anterior uveitis, Posner–Schlossman syndrome, and other conditions that may cause impairment of the BAB
(diabetes mellitus, pseudoexfoliation, occlusion of retinal vessels, ocular trauma, intraocular tumors, and so on) were excluded from this study through careful examination. In cases of
diagnostic doubt, we performed HLA-B27 typing in suspected patients to rule out HLA-B27-associated anterior uveitis. Serum angiotensin-converting enzyme assay and chest X-ray were performed
in suspected patients to rule out sarcoidosis. A detailed history, including age, laterality, complaints, the BCVA, and associated systemic diseases, was taken from each patient. All
patients were examined by the same uveitis specialist. The examinations included the BCVA, slit-lamp biomicroscopy for bilateral anterior segment analysis of the eye, gonioscopy, and
ophthalmoscopy through a dilated pupil. The severity of anterior chamber activity was clinically evaluated using the grading system proposed by the Standardization of Uveitis Nomenclature
(SUN) Working Group.10 The degree of KP was defined as (0, 1+, 2+, 3+, and 4+) according to the number of KP, as described by La Hey _et al_9 and Hogan _et al_11. Iris depigmentation was
identified as 0, 1+, 2+, and 3+ based on the colour and depigmentation of the iris (Table 1; Figures 1 and 2). Intraocular pressure was measured using the noncontact tonometry method. Most
patients received nonsteroid anti-inflammatory eye drops topically when anterior chamber reaction was present. Few patients received corticosteroid eye drops for a short time because of the
presence of overt anterior chamber cells (1+). None of the tested patients received topical or systemic steroids treatment at least for 5 days before examination by LFCM. The quantitative
measurement of aqueous flare and cells was performed by an experienced technician who did not know the clinical results. All data were acquired using standardized data sheets that were
designed before the study was started. LFCM was performed on 47 eyes of 40 cases using the FC-2000 LFCM (version 1.0) (Kowa Company Ltd, Tokyo, Japan) to quantitatively evaluate aqueous
flare and cells. This examination was also concurrently performed on 47 eyes of 36 normal individuals as age-matched controls. Three individual measurements were averaged and those with
artifacts were discarded. The results of flare values and cell counts were expressed as photon counts/ms (pc/ms) and cells/0.5 mm3, respectively. The results were given as mean±SD and were
analysed using the software of SPSS 11.0. The statistical methods used in this study included the _t_-test, nonparametric correlation analysis (Bivariate Kendall method), and one-way ANOVA
analysis (_Post hoc_ multiple comparisons). We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this
research. The study and data accumulation were carried out with approval from the Institutional Review Board (IRB) of Zhongshan Ophthalmic Center. All patients and healthy volunteers were
informed about the nature of the noninvasive examinations of LFCM and consented to this examination. RESULTS A total of 47 eyes with Fuchs syndrome from 40 patients (15 males, 25 females,
mean age 33.8±10.1 years, range 16–72 years) and 47 normal control eyes of 36 healthy individuals (13 males, 23 females, mean age 34.8±12.1 years, range 17–66 years) were enrolled in this
study. The duration of disease (mainly according to the patients’ complaints) varied from 17 days to 20 years, with a median of 3 years. The BCVA of Fuchs eyes ranged from 0.1 (20/200) to
1.5 (20/13.3), with a mean BCVA of 0.87±0.44 (20/23±20/45). Clinically, the anterior chamber reaction was mild in these patients with Fuchs syndrome. Flare (+) was observed in 72.3% (34 of
47) of affected eyes. More than half of eyes (53.2%, 25 of 47) had no detectable cells in the anterior chamber. Aqueous cells (1+) and (0.5+) were observed in 29.8% (14 of 47) and 17.0% (8
of 47) of eyes. There were 7, 17, 14, and 9 eyes graded as KP (0+), (1+), (2+), and (3+), respectively. Among the eyes with KP, more than half showed a diffuse distribution (65%) and
stellate morphology (52.5). Iris depigmentation was graded as (1+), (2+), and (3+) in 19, 20 and, 8 eyes, respectively. Posterior subcapsular lens opacity was present in less than 30% of
eyes. LFCM FLARE VALUES AND CELL COUNTS IN PATIENTS AND CONTROLS The mean flare values in 40 patients and 36 controls were 9.40±5.85 and 5.77±1.89 pc/ms. The number of anterior chamber cells
in 40 patients and 36 controls was 5.09±4.84 cells/0.5 mm3 and 1.14±1.03 cells/0.5 mm3, respectively. There was a significant difference between patients and controls with regard to flare
values (_t_=4.041, _P_=0.000) and cell counts (_t_=6.465, _P_=0.000) in the anterior chamber. LFCM FLARE VALUES _VS_ CELL COUNTS The LFCM results showed a good association between flare
values and cell counts. There was a significant correlation between these two parameters (Kendall’s correlation coefficient, _r_=0.331, _P_=0.001; Figure 3). LFCM FLARE VALUES AND CELL
COUNTS _VS_ SLIT-LAMP GRADINGS Figure 4 shows the relation between the LFCM results and slit-lamp gradings of anterior inflammation in patients with Fuchs syndrome. Flare values ranged from
1.9 to 34.9 pc/ms and slit-lamp gradings varied from (0+) to (1+). There was a good correlation between the LFCM results and slit-lamp readings in flare measurements (Kendall’s correlation
coefficient, _r_=0.402, _P_=0.001). Higher cell counts were also noted in the eyes with higher gradings by slit-lamp analysis (Kendall’s correlation coefficient, _r_=0.756, _P_=0.000). LFCM
FLARE VALUES AND CELL COUNTS _VS_ DEGREE OF KP LFCM flare values and cell counts _vs_ degree of KP in Fuchs syndrome are shown in Table 2. There was a good correlation between the results of
flare values and slit-lamp gradings of KP (Kendall’s correlation coefficient, _r_=0.381, _P_=0.001). There was also a good correlation between the results of LFCM cell counts and slit-lamp
gradings of KP (Kendall’s correlation coefficient, _r_=0.696, _P_=0.000). These results suggest that increased flare values and cell counts are present in eyes with higher slit-lamp gradings
of KP. LFCM FLARE VALUES AND CELL COUNTS _VS_ DEGREE OF IRIS DEPIGMENTATION Flare values and cell counts _vs_ degree of iris depigmentation in Fuchs syndrome are shown in Table 3. There was
a good correlation between the results of flare values and slit-lamp gradings of iris depigmentation (Kendall’s correlation coefficient, _r_=0.441, _P_=0.000). A similar result was also
observed between cell counts and slit-lamp gradings of iris depigmentation (Kendall’s correlation coefficient, _r_=0.482, _P_=0.000). LFCM FLARE VALUES AND CELL COUNTS _VS_ OTHER CLINICAL
PARAMETERS With respect to the distribution of KP, we noted significantly higher flare values and cell counts in eyes with diffusely distributed KP compared to those with centrally
distributed KP (13.26 _vs_ 6.45 pc/ms, _P_=0.048; 7.08 _vs_ 2.50 cells/0.5 mm3, _P_=0.037). However, there were no differences either between patients with diffusely distributed KP and those
with inferiorly distributed KP or between patients with centrally distributed KP and those with inferiorly distributed KP concerning flare values and cell counts. No differences were
observed between flare values, cell counts, and other parameters, including patients’ age, sex, laterality, BCVA, duration of disease, morphology of KP, intraocular pressure, and posterior
subcapsular lens opacities. DISCUSSION In this study, we used LFCM to quantitatively evaluate the breakdown of the BAB and cell reaction in the anterior chamber in patients with Fuchs
syndrome. Our study showed that patients with Fuchs syndrome display significantly higher flare values and cell counts than normal controls. Further analysis showed that these changes were
positively associated with the degree of KP and iris depigmentation. However, these changes were not linked with other clinical parameters, such as patients’ age, sex, laterality, BCVA,
duration of disease, morphology of KP, intraocular pressure, or posterior subcapsular lens opacities. The Kowa LFCM has been proven to be a reliable tool in quantifying aqueous flare and
cells _in vivo_ by measuring light scattering from a low-power He–Ne beam. The accuracy and reproducibility of the method have been shown in several studies.12, 13, 14, 15, 16, 17, 18, 19,
20, 21, 22, 23 Using LFCM, we recently showed breakdown of the BAB and presence of anterior chamber cells in Chinese patients with Fuchs syndrome.7 The present study confirmed our previous
observation in another group of Chinese patients with Fuchs syndrome using the same technique. These results suggest that breakdown of the BAB and increased cell counts are a prominent
feature of this disease. In the present study, the LFCM results showed a good correlation with a clinical grading system. These results, on the one hand, indicate that careful slit-lamp
examination provides a simple and rapid assessment for the anterior reaction. On the other hand, the LFCM may provide quantitative information for the evaluation of the anterior
inflammation. Our results also showed that the flare values were positively associated with the cell counts. These results show that breakdown of BAB in this disease can directly be
attributed to the degree of inflammation in the anterior chamber. To clarify which clinical parameters could influence the results of LFCM, we divided patients into subgroups according to a
number of factors including patients’ age, sex, duration of disease, BCVA, KP, iris depigmentation, intraocular pressure, and posterior subcapsular lens opacities. Our results showed that
the flare values and cell counts were associated with the degree of KP. As the presence of KP is one of the important indexes of inflammation in the anterior chamber, it is not difficult to
understand the positive link between them. We further analysed the association between morphology and distribution of KP and LFCM results. Our results showed no relationship between the
morphology of KP and the LFCM results. Interestingly, we found a strong association of LFCM results with diffusely distributed KP. This result may be explained by the fact that the diffusely
distributed KP are usually seen in eyes with KP score 2+ and 3+ (19 of 26 eyes). Another parameter that was associated with flare values and cell counts was the degree of iris
depigmentation. Since iris depigmentation has been considered as a sequence of long-lasting or chronic anterior inflammation in Fuchs syndrome, it is obvious that this sign may represent, to
a certain degree, the activity of the anterior chamber reaction. These findings support the theory that the chronic anterior chamber inflammation is responsible for the iris
depigmentation.24 It is interesting to note that the flare values and cell counts were not associated with other clinical parameters, for instance, patients’ age, sex, laterality, BCVA,
intraocular pressure, and posterior subcapsular lens opacities. Unexpectedly, the breakdown of BAB indirectly revealed by LFCM was not found to be associated with the disease duration. This
result could be misinterpreted by the uncertainty of the disease duration described by patients themselves. More importantly, this result indicates that the breakdown of BAB in these
patients is mainly caused by the active anterior inflammation. Interestingly, our results show that the LFCM readings are not associated with the presence of posterior subcapsular lens
opacity. We suppose this relationship would have existed if chronic inflammation were a sole factor contributing to posterior subcapsular lens opacity. Actually we failed to find this
relationship in this study. Therefore, other factors, such as prolonged use of topical steroids eye drops before referring to us, might also contribute to the development of posterior
subcapsular lens opacity. In conclusion, our study characterized the anterior chamber reaction in patients with Fuchs syndrome using LFCM. The results showed a concurrent presence of
elevated flare values and cell counts. There was a strong association between LFCM results and the degree of KP and iris depigmentation, both mostly indicative of the inflammation in the
anterior chamber. The combined results suggest that the low-grade inflammation in the anterior chamber is responsible for the BAB breakdown observed in patients with Fuchs syndrome.
REFERENCES * Loewenfeld IE, Thompson S . Fuchs heterochromic cyclitis: a critical review of the literature. I. Clinical characteristics of the syndrome. _Surv Ophthalmol_ 1973; 17: 394–457.
CAS PubMed Google Scholar * Jones NP . Fuchs heterochromic uveitis: a reappraisal of the clinical spectrum. _Eye_ 1991; 5: 649–661. Article Google Scholar * Jones NP . Fuchs
heterochromic cyclitis: an update. _Surv Ophthalmol_ 1993; 37: 253–272. Article CAS Google Scholar * Fearnley IR, Rosenthal AR . Fuchs heterochromic iridocyclitis revisited. _Acta
Ophthalmol Scand_ 1995; 73: 166–170. Article CAS Google Scholar * Mohamed Q, Zamir E . Update on Fuchs’ uveitis syndrome. _Curr Opin Ophthalmol_ 2005; 16: 356–363. Article Google Scholar
* Norrsell K, Holmer AK, Jacobson H . Aqueous flare in patients with monocular iris atrophy and uveitis. A laser flare and iris angiography study. _Acta Ophthalmol Scand_ 1998; 76:
405–412. Article CAS Google Scholar * Yang P, Fang W, Jin H, Li B, Chen X, Kijlstra A . Clinical features of Chinese patients with Fuchs syndrome. _Ophthalmology_ 2006; 113: 473–480.
Article Google Scholar * Kimura SJ, Hogan MJ, Thygeson P . Fuchs syndrome of heterochromic cyclitis. _Arch Ophthalmol_ 1955; 54: 179–186. Article CAS Google Scholar * La Hey E, Baarsma
GS, De Vries J, Kijlstra A . Clinical analysis of Fuchs heterochromic cyclitis. _Doc Ophthalmol_ 1991; 78: 225–235. Article CAS Google Scholar * The Standardization of Uveitis
Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. _Am J Ophthalmol_ 2005; 118: 338–342. Google Scholar * Hogan MJ, Kimura SJ, Thygeson P
. Signs and symptoms of uvietis. I Anterior uveitis. II classification of the posterior manifestations of uveitis. _Am J Ophthalmol_ 1959; 47: 155–176. Article CAS Google Scholar * Ohara
K, Okubo A, Miyazawa A, Miyamoto T, Sasaki H, Oshima F . Aqueous flare and cell measurement using laser in endogenous uveitis patients. _Jpn J Ophthalmol_ 1989; 33: 265–270. CAS PubMed
Google Scholar * Oshika T, Nishi M, Mochizuki M, Nakamura M, Kawashima H, Iwase K _et al_. Quantitative assessment of aqueous flare and cells in uveitis. _Jpn J Ophthalmol_ 1989; 33:
279–287. CAS PubMed Google Scholar * Yoshitomi T, Wong SA, Daher E, Sears LM . Aqueous flare measurement with laser flare-cell meter. _Jpn J Ophthalmol_ 1990; 34: 57–62. CAS PubMed
Google Scholar * Sawa M . Clinical application of laser flare-cell meter. _Jpn J Ophthalmol_ 1990; 34: 346–363. CAS PubMed Google Scholar * Guex-Crosier Y, Pittet N, Herbort PC .
Evaluation of laser flare-cell photometry in the appraisal and management of intraocular inflammation in uveitis. _Ophthalmology_ 1994; 101: 728–735. Article CAS Google Scholar *
Guex-Crosier Y, Pittet N, Herbort PC . Sensitivity of laser flare photometry to monitor inflammation in uveitis of the posterior segment. _Ophthalmology_ 1995; 102: 613–621. Article CAS
Google Scholar * Saari MK, Guillen-Monterrubio MO, Hartikainen J, Hamalainen MM, Taskinen K . Measurement of protein concentration of aqueous humour _in vivo_: correlation between laser
flare measurements and chemical protein determination. _Acta Ophthalmol Scand_ 1997; 75: 63–66. Article CAS Google Scholar * Kuchle M, Nguyen NX, Horn F, Naumann GOH . Quantitative
assessment of aqueous flare and aqueous “cells” in pseudo-exfoliation syndrome. _Acta Ophthalmol_ 1992; 70: 201–208. Article CAS Google Scholar * Kuchle M, Nguyen NX, Naumann GOH .
Quantitative assessment of the blood-aqueous barrier in human eyes with malignant or benign uveal tumors. _Am J Ophthalmol_ 1994; 117: 521–528. Article CAS Google Scholar * Kuchle M,
Nguyen NX, Martus P, Freissler K, Schalnus R . Aqueous flare in retinitis pigmentosa. _Graefe's Arch Clin Exp Ophthalmol_ 1998; 236: 426–433. Article CAS Google Scholar * Jandrasits
K, Krepler K, Wedrich A . Aqueous flare and macular edema in eyes with diabetic retinopathy. _Ophthalmologica_ 2003; 217: 49–52. Article Google Scholar * Nguyen NX, Kuchle M, Naumann GOH .
Quantification of blood-aqueous barrier breakdown after phaco-emulsification in Fuchs’ heterochromic uveitis. _Ophthalmologica_ 2005; 219: 21–25. Article Google Scholar * Bonfioli AA,
Curi LLA, Orefice F . Fuchs’ heterochromic cyclitis Semin. _Ophthalmol_ 2005; 20: 143–146. Google Scholar Download references ACKNOWLEDGEMENTS This study was supported in part by the Fund
for Project of Science and Technology of Guangdong province (2005B60302009), Key Project of Natural Science Foundation (30630064), National supporting project of P.R. China, and ‘5010’
Clinical Project of Sun Yat-sen University. AUTHOR INFORMATION Author notes * W Fang and H Zhou: Both these authors contributed equally to the study. AUTHORS AND AFFILIATIONS * Zhongshan
Ophthalmic Center, Sun Yat-sen University, Guangzhou, P.R., China W Fang, H Zhou, P Yang, X Huang & L Wang * Uveitis Study Center and International Uveitis Study Laboratory of Sun
Yat-sen University, Guangzhou, P.R., China W Fang, H Zhou, P Yang, X Huang, L Wang & A Kijlstra, * State Key Laboratory of Ophthalmology of Sun Yat-sen University, Guangzhou, P.R., China
W Fang, H Zhou, P Yang, X Huang & L Wang * Department of Ophthalmology, Eye Research Institute Maastricht, University Hospital Maastricht, Maastricht, The Netherlands A Kijlstra,
Authors * W Fang View author publications You can also search for this author inPubMed Google Scholar * H Zhou View author publications You can also search for this author inPubMed Google
Scholar * P Yang View author publications You can also search for this author inPubMed Google Scholar * X Huang View author publications You can also search for this author inPubMed Google
Scholar * L Wang View author publications You can also search for this author inPubMed Google Scholar * A Kijlstra, View author publications You can also search for this author inPubMed
Google Scholar CORRESPONDING AUTHOR Correspondence to P Yang. ADDITIONAL INFORMATION None of the authors has a proprietary or financial interest in any product mentioned. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Fang, W., Zhou, H., Yang, P. _et al._ Aqueous flare and cells in Fuchs syndrome. _Eye_ 23, 79–84 (2009).
https://doi.org/10.1038/sj.eye.6702991 Download citation * Received: 08 March 2007 * Accepted: 02 September 2007 * Published: 26 October 2007 * Issue Date: January 2009 * DOI:
https://doi.org/10.1038/sj.eye.6702991 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * laser flare-cell meter * Fuchs syndrome * uveitis
Trending News
Canadian police begin arresting protesters, removing trucks in downtown ottawaOTTAWA — Hundreds of Canadian police officers launched a methodical operation Friday to clear the streets of truckers an...
R. Kelly's motion to get out of jail because of the coronavirus denied by judgeR. Kelly can't get out of jail because of the coronavirus pandemic, a judge has decided.Kelly, the disgraced R&B star wh...
“Long Corbyn” haunts LabourWe can all agree that Prime Minister Boris Johnson is having a bad time. His own MPs, the Tory press, and even, on occas...
Initiative aims to up hispanic college-graduation ratesWith the help of $21 million in grants from the W.K. Kellogg Foundation and the Houston Endowment Inc., 13 communities a...
'ajlt’ showrunner says writers were 'split' on charlotte's bomb cyclone parenting decision (exclusive)_WARNING: THIS POST CONTAINS SPOILERS FROM THURSDAY'S EPISODE OF _AND JUST LIKE THAT... Charlotte York might just ...
Latests News
Aqueous flare and cells in fuchs syndromeABSTRACT PURPOSE To quantitatively evaluate aqueous flare and cells in patients with Fuchs syndrome. METHODS The medical...
Jai Sena Movie: Showtimes, Review, Songs, Trailer, Posters, News & Videos | eTimesNetflix CEO Ted Sarandos calls Shah Rukh Khan his favorite Indian celebrity: “We hit it off immediately” - Deets insideK...
Soft-hearted fish | Nature Reviews GeneticsAccess through your institution Buy or subscribe The authors identified the mutant by screening a mutagenized zebrafish ...
Unscrambling the egg–sperm divideKey to the production of gametes is the time at which the germ cells they are derived from enter meiosis. In mice, meios...
2 Youths Wounded in Confrontation : Thousand Oaks: Westlake High athletes are caught in gunfire stemming from student rivalry. It follows after-schoolTwo Westlake High football players were wounded Thursday during a shooting on a Thousand Oaks street corner, as animosit...