New insights into the electrochemical behavior of acid orange 7: convergent paired electrochemical synthesis of new aminonaphthol derivatives
New insights into the electrochemical behavior of acid orange 7: convergent paired electrochemical synthesis of new aminonaphthol derivatives"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Electrochemical behavior of acid orange 7 has been exhaustively studied in aqueous solutions with different pH values, using cyclic voltammetry and constant current coulometry. This
study has provided new insights into the mechanistic details, pH dependence and intermediate structure of both electrochemical oxidation and reduction of acid orange 7. Surprisingly, the
results indicate that a same redox couple (1-iminonaphthalen-2(1H)-one/1-aminonaphthalen-2-ol) is formed from both oxidation and reduction of acid orange 7. Also, an additional purpose of
this work is electrochemical synthesis of three new derivatives of 1-amino-4-(phenylsulfonyl)naphthalen-2-ol (3a–3c) under constant current electrolysis via electrochemical oxidation (and
reduction) of acid orange 7 in the presence of arylsulfinic acids as nucleophiles. The results indicate that the electrogenerated 1-iminonaphthalen-2(1 H)-one participates in Michael
addition reaction with arylsulfinic acids to form the 1-amino-3-(phenylsulfonyl)naphthalen-2-ol derivatives. The synthesis was carried out in an undivided cell equipped with carbon rods as
an anode and cathode. SIMILAR CONTENT BEING VIEWED BY OTHERS GREEN ELECTROCHEMICAL METHOD FOR THE SYNTHESIS OF NITRO AND AZO DERIVATIVES BASED ON MEFENAMIC ACID Article Open access 20
January 2022 A NEW TYPE OF CONVERGENT PAIRED ELECTROCHEMICAL SYNTHESIS OF SULFONAMIDES UNDER GREEN AND CATALYST-FREE CONDITIONS Article Open access 16 October 2023 ELECTROCHEMICAL GENERATION
OF PHENOTHIAZIN-5-IUM. A SUSTAINABLE STRATEGY FOR THE SYNTHESIS OF NEW BIS(PHENYLSULFONYL)-10_H_-PHENOTHIAZINE DERIVATIVES Article Open access 21 February 2024 INTRODUCTION 2-Naphthol
orange (acid orange 7), C16H11N2NaO4S, is a mono-azo water-soluble dye that extensively used for dyeing paper, leather and textiles1,2. The structure of acid orange 7 involves a hydroxyl
group in the ortho-position to the azo group. This resulted an azo-hydrazone tautomerism, and the formation of two tautomers, which each show an acid−base equilibrium3,4,5,6,7,8,9,10,11,12.
Despite the number of articles dealing with acid−base properties of the acid orange 7, this topic is not yet well known, and only one pKa, (pKa = 11.4) is reported6. On the other hand, azo
dyes have been widely used for developing and testing theories of color and constitution, tautomerism, indicator action, and acid-base equilibria5. Therefore, detailed mechanistic
information is important in understanding of the stability and in identifying of the intermediates structure resulting from the oxidative or reductive decomposition of dye. Consequently,
detailed mechanistic information is particularly attractive from the point of view of environmental pollution because of residual dye and the commercial applications13,14. Additionally,
green/sustainable synthesis is much more important than conventional synthetic methods. The concept and significance of green sustainable chemistry (GSC), has been recognized throughout the
world, and nowadays new processes cannot be developed without consideration of GSC. In recent years, much attention has been paid to electroorganic synthesis as a typical environmentally
friendly process15,16,17,18,19,20,21,22,23. This method contains the simultaneous incidence of both oxidation (at the anode) and reduction (at the cathode). In conventional electroorganic
synthesis, the synthesis of the desired products is done either by the anodic or by the cathodic reaction and so; the reaction product at the counter electrode is undesirable. The
simultaneous use of both oxidation and reduction reactions to synthesis of a product is the dream of an organic electrochemist and is a wonderful strategy24,25,26,27,28. At ideal conditions,
a 200% current efficiency is achievable for paired electrosynthesis when both anodic and cathodic reactions to provide the similar product (convergent strategy)15. Furthermore, sulfone
compounds and naphthalene derivatives are found in antibiotic drugs such as nafacillin and 4,4-diaminodiphenylsulfone (dapsone) and antifungal drugs such as naftifine, tolnaftate and
terbinafine29,30,31. These compounds have an effective inhibitory effect against the bacteria30,31 and antimicrobials effect against wide range of human pathogens29. Based on these advances,
we anticipate that naphthalene derivatives containing sulfone groups reveals such properties. The results discussed above prompted us to investigate the electrochemical oxidation and
reduction of acid orange 7 in aqueous solutions with different pH values to achieve the following goals: (i) new insights into the electrochemical oxidation and reduction of acid orange 7,
(ii) definitive detection of intermediates formed during the oxidative and reductive degradation of acid orange 7, and (iii) convergent paired electrochemical synthesis of new
1-amino-2-naphthol derivatives by constant current electrolysis of acid orange 7 in the presence of arylsulfinic acids as nucleophiles. RESULTS AND DISCUSSION ELECTROCHEMICAL STUDY OF ACID
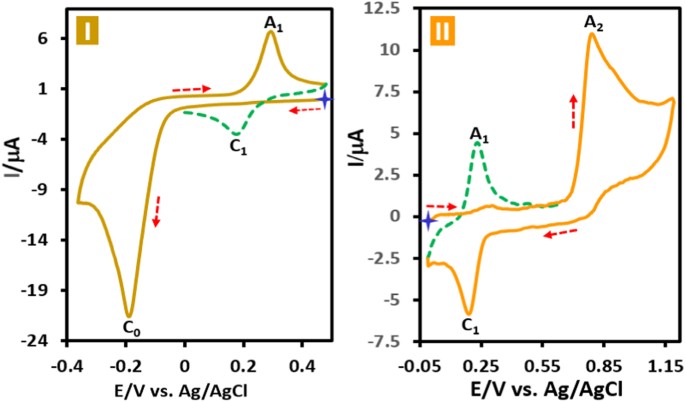
ORANGE 7 Cyclic voltammograms of acid orange 7 (AO7) in aqueous phosphate buffer solution (_c_ = 0.2 M, pH = 2.0) in two different potential regions (+0.5 to −0.4 and 0.0 to +1.2 V vs.
Ag/AgCl) is shown in Fig. 1. When the electrode potential was scanned from +0.5 V versus Ag/AgCl to a sufficiently negative voltage (−0.4 V versus Ag/AgCl), the cyclic voltammogram exhibits
a large cathodic peak (C0) at −0.19 with an anodic peak (A1) at +0.30 V versus Ag/AgCl at 50 mV s−1 (Fig. 1I). Under these conditions, in the second cycle a new cathodic peak (C1), which is
the counterpart of anodic peak (A1) appears with an _E_p value of +0.18 V versus Ag/AgCl. Moreover, when the electrode potential was scanned from 0.0 V vs. Ag/AgCl to the positive potentials
(+1.2 V vs. Ag/AgCl), the voltammogram shows an anodic peak (A2) at +0.80 V vs. Ag/AgCl with a cathodic peak (C1) (Fig. 1II). As in the previous experiment, a quasi-reversible couple,
A1/C1, appears in the second cycle of the voltammogram. A very important point in this study is the presence of the same redox couple in both cyclic voltammograms. This confirms the
generation of the same intermediates from both oxidation and reduction of AO7. When the potential scan rate increases from 250 to 8000 mV s−1, the cyclic voltammograms of AO7 in anodic
region (Fig. S1) show the following changes: (1) the appearance of peak C2, which is the counterpart of peak A2. (2) The increase of the anodic and cathodic peak current ratios
(_I_pA2/_I_pA1) and (_I_pC2/_I_pCA1) and (3) the decrease of peak current function for anodic peak A2 (_I_pA2/v1/2). Increase in the potential scan rate causes a decrease in the CV
time-scale and therefore a decrease in the progress of the following chemical reaction. These data confirm the occurrence of a following chemical reaction and generation of a
quasi-reversible system after oxidation of AO732. In addition, the effect of potential scan rate, _v_, (250 to 8000 mV s−1) on the cyclic voltammetric response of AO7 in cathodic region
which confirms the Irreversibility of the reduction process corresponding to peak C0 (Fig. S2). The oxidative and reductive controlled-potential coulometry of AO7 was performed by applying
potentials +0.90 and −0.20 V vs. Ag/AgCl, respectively. The solutions after coulometry are shown in Supporting Information (Fig. S3). The monitoring of the electrolysis progress was carried
out by cyclic voltammetry (Fig. 2). This Figure show that, in both experiments, proportional to the advancement of coulometry, parallel to the decrease in the current of peaks A2 (Fig. 2I)
and C0 (Fig. 2II), the peaks A1 and C1, increases. In these conditions, the number of transferred electrons in oxidative controlled potential coulometry was obtained 2.9 electrons pre each
AO7 molecule. On the other hand, the number of transferred electrons in reductive controlled potential coulometry was obtained 4.1 electrons pre each AO7 molecule. An important point on the
oxidation behaviour of AO7 can be seen in Fig. 2Ia. This Figure (and also Fig. 1II) represents a typical behaviour of an _ECE_ pathway in the kinetic region32. However, the comparison of
_I_pA2 at the start of coulometry with _I_pC1 at the end of coulometry shows that, _I_pC1 at the end of coulometry is equal to _I_pA2 at the start of coulometry . It should be noted that, in
an _ECE_ mechanism, the peak current ratio of the starting compound to the product (_I_st/_I_pr) in the kinetic region is {(_n__1_ + _n__2__)/n__2_}3/2. Where, _n_1 and _n_2 are the number
of electrons involved in the oxidation of starting compound and product, respectively32. This discrepancy implying that oxidation pathway of AO7, is not an _ECE_ and confirms the reaction
pathway presented in Fig. 3, for oxidative behaviour of AO7. Contrary to Fig. 2I,II shows that, _I_pC0 at the start of coulometry, , is more than 2.5 times than that of _I_pA1 at the end of
coulometry which is near to theoretical value of 2.8 when n1 = n233. Diagnostic criteria of cyclic voltammetry accompanied by previously published data on oxidation of
AO734,35,36,37,38,39,40,41,42,43 allow us to propose the mechanism presented in Fig. 3 for the electrochemical oxidation and reduction of AO7. In oxidation pathway, generation of AO7OX is
followed by the addition of H2O and formation of AO7OH. At the final step, AO7OH degraded into 4-nitrosobenzenesulfonate (4NB) and 1-iminonaphthalen-2(1 _H_)-one (INO). It should be noted
that, according to the Fig. 3, the number of transferred electron in oxidative pathway is two electrons, while, the obtained number of transferred electron (from controlled potential
electrolysis) was 2.9 electrons. This discrepancy can be related to partially oxidation of 4NB19. In the reduction pathway, the first two-electron reduction converts AO7 to AO7R. In the next
step, AO7R via an irreversible two-electron degradation process converts to 4-aminobenzenesulfonate (4AB) and 1-aminonaphthalen-2-ol (ANO). According to the above data, the cathodic peak C0
corresponds to reduction of AO7 to its reduced form (AO7R). The anodic peak A2 corresponds to the two-electron oxidation of AO7 to its oxidized form (AO7OX). Obviously, the cathodic peak C2
corresponds to the reduction of AO7OX into AO7 and the redox couple A1/C1, are related to the oxidation of ANO to INO and reduction of INO to ANO within a quasi-reversible two-electron,
two-proton process. ADSORPTION STUDY Fig. S4 shows the normalized oxidative cyclic voltammograms of AO7 (the data of Fig. S4, obtained from Fig. S1). The normalization was performed by
dividing the current by the square root of the potential scan rate (_I_/v1/2). According to the proposed pathway for the electrochemical oxidation of AO7, the increasing of normalized A2
peak current (_I_pA2/v1/2), was unexpected. One possibility for such inconsistency is adsorption of AO7 on the electrode surface. To confirm this finding, the plot of log _I_PA2 vs. log _v_
at pH values 2.4, 7.2 and 10.3 is shown in Fig S5. It was reported that when the slope log _I_PA2 vs. log _v_ is 0.5, the electrochemical reaction is a diffusion controlled process, while
when the slope increases to 1, the electrochemical reaction occurs via an adsorption-controlled process32. It is clear that, in all pHs, the slope is more than 0.5 and increases with
increasing pH from 0.58 to 0.74. These values are greater than 0.5 for the diffusion controlled process and are less than one, which is theoretical value for the adsorption-controlled
electrode process. Therefore, it is clear that the electrochemical oxidation of AO7 at glassy carbon electrode in aqueous media is adsorption/diffusion process. These results show that the
interaction between anionic forms of AO7 and the electrode surface is stronger than neutral form. THE EFFECT OF PH The electrochemical behavior of AO7 has been studied in different pH
values. The oxidative and reductive cyclic voltammograms of AO7 in aqueous solution with various pHs are shown in Figs S6 and S7, respectively. As seen in Fig. S6, with increasing pH, _E_pA2
shifts to negative values. This confirms the participation of proton(s) in the oxidation of AO7. The variation of EpA2 with pH is given by: where _m_ is the number of protons involved in
the reaction, is the anodic peak potential at pH = 0.0 and _R, T_, and _F_ have their usual meaning. The _E_pA2–pH diagram comprise three linear segments with different equations and slopes
at pH values 7.4 and 11.4 (Fig. 4I). This diagram indicates that in the aqueous solutions, AO7 is in different reduced and oxidized forms, that their relative amounts are dependent on the pH
and electrode potential. At pHs lower than 7.4, the _E_pA2 value shifts by −29 mV/pH indicating that the redox reaction is two-electron/one-proton process involving the oxidation protonated
AO7 (HAO7) to protonated AO7OX (Fig. 5, Eq. 1)44,45,46. Moreover, at pH range 7.4–11.4, the _E_pA2 value shifts by −61 mV/pH. In this range of pH, the redox reaction is
two-electron/two-proton process involving the oxidation of HAO7 to AO7OX (Fig. 5, Eq. 2). Finally, at pHs >11.4, the _E_pA2 value is independent of pH, showing that the redox reaction
involves a two-electron process without participation of any proton including the oxidation of AO7 anion (AO7−) to AO7OX (Fig. 5, Eq. 3). The important point of Fig. 5 is the absence of
neutral AO7, which can be related to the high tendency of AO7 to keep the proton on the nitrogen atoms due to the intramolecular hydrogen bonding (Fig. S8). In addition, the calculated pKa
for HAO7OX/AO7OX and AO7/AO7− equilibria which is also shown in Fig. 5, Eqs 4 and 5, are: 7.4 and 11.4, respectively. The reductive cyclic voltammograms of AO7 in aqueous solution with
different pHs are shown in Fig. S7. As seen, with increasing pH, peak C0 shifts to negative potentials, indicating the participation of proton(s) in the electrode process. The _E_PC0–pH
diagram is shown in Fig. 4II. It has two linear segments at pH 7.7. At pH values lower than 7.7, _E_pC0 value shifts by −90 mV/pH indicating the redox reaction is two-electron/three-proton
process involving the reduction of HAO7 to H2AO7R (Fig. 5, Eq. 6). However, at pHs >7.7, the situation is a little complicated, as the _E_pC0 value shifts by −40 mV/pH. It probably
including unrecognizable reductions HAO7 to HAO7R (two-electron/two-proton process) and HAO7 to AO7R (two-electron/one-proton process) (Fig. 5, Eqs. 7 and 8). The cyclic voltammograms
regarding A1/C1 peaks at different pHs and its _E_1/2–pH diagram are shown in Fig. S9. The _E_1/2 values were calculated as the average of the anodic and cathodic peak potentials (_E_pA1 +
_E_pC1)/2. The _E_1/2–pH diagram displays a simple linear dependence of the _E_1/2 on pH, with the slope of −85 mV/pH, indicating that the redox reaction is two-electron/three-proton process
involving the oxidation of HANO to INO in the forward scan and reduction of INO to HANO in the reverse scan (Fig. 5, Eq. 9). The extrapolation of this line to pH = 0.0 provides the _E_1/2 =
+0.60 V versus Ag/AgCl for redox couple A1/C1. ELECTROCHEMICAL STUDY OF AO7 IN THE PRESENCE OF ARYLSULFINIC ACIDS Because of two reasons: a) confirmation of the proposed mechanism in Fig. 3
and b) electrochemical synthesis of some new organic compounds, in this part, the electrochemical behavior of AO7 in the presence of 4-toluenesulfinic acid (1A) was studied and compared
with that of AO7 in the absence of 1A (Fig. 6). The cyclic voltammogram of AO7 in aqueous phosphate buffer (_c_ = 0.2 M, pH = 2.0), in the presence of 1A is shown in Fig. 6b. Comparison of
the voltammogram with that of AO7 in the absence of 1A (Fig. 6a), shows two important differences: (a) the appearance of a new redox couple (peaks A3 and C3) at _E_1/2 = 0.39 V vs Ag/AgCl.
(b) The disappearance of the cathodic peak C1 in the reverse scan. Under these conditions, the peak current ratio, _I_pC1/_I_pA1, depends on the both potential scan rate and 1A
concentration, so that, _I_pC1/_I_pA1 increases with increasing scan rate and decreasing 1A concentration. In Fig. 6, curve c is the voltammogram of 1A in the same conditions and in the
absence of AO7 that does not show any peak in the working potential range. In addition, cyclic voltammogram d, is belong to the isolated product from the electrolysis of AO7 in the presence
of 1A. In this part, the same results were obtained for the other two arylsulfinic acids (1B and 1C). These results and the spectroscopic data of the isolated electrolysis product all point
out to compound 3A (final product), which would have been formed according to the pathway shown in Fig. 7. The first step in the synthesis of 3A is the generation of INO. This compound is
directly generated from oxidative cleavage of AO7 (Fig. 3), and/or from the two-electron oxidation of ANO (Fig. 7). In the next step, INO would serve as a Michael acceptor in a reaction with
1A to form the final product 3A. The oxidation of 3A did not occur during the electrolysis due to the insolubility of 3A in electrolysis solution (aqueous phosphate buffer). Based on Fig.
7, the anodic and cathodic peaks A3 and C3 pertain to the oxidation of 3A to 4A and vice versa. According to the proposed mechanism in Fig. 3, we have designed a paired electrochemical
strategy for the synthesis of the sulfone derivatives 3A–3C. The paired electrochemical synthesis of 3A–3C has been successfully performed in a one-pot process at the current density of 0.32
mA/cm2, in an undivided cell equipped with carbon rods as cathode and anode. The electrolysis was terminated when the cathodic peak that corresponds to the reduction of AO7 (C0) disappears.
This peak disappears after consumption of 4.0 F/mol electricity. Under these conditions, the monitoring of the electrolysis progress was carried out by cyclic voltammetry and shown in Fig.
8. This Figure shows that, proportional to the advancement of coulometry, _I_pC0, _I_pA1 and _I_pA2 decreases while _I_pA3 and _I_pC3 increases. The variation of _I_pC0 vs. charge consumed
is also shown in Fig. 8 (inset). This curve show that _I_pC0 decrease exponentially with advancing coulometry (_I_pC0 = 10.15 e−0.03_Q_). The total amount of charge passed for terminating
the reaction was determined from the extrapolation to the X-axis. The calculated charge passed confirms consumption of about 4e− per molecule of AO7. In addition, the UV–visible spectra of
AO7 in the presence of 4-toluenesulfinic acid (1A) were collected during a constant current coulometry in the same conditions as before (Fig. S11). Under these conditions, the absorption
spectrum of AO7 consists of three absorption bands at 312, 410 and 488 nm. Our data show as the coulometry is carried out, the height of all three peaks decrease and a new peak appears at
352 nm and grows in intensity. INO is an asymmetric Michael acceptor and can be attacked by 1A via 1,4 (site A) or 1,6 (site B) Michael addition reaction to yield two types of products (3A
and 3A’) (Fig. 9). However, the recorded NMR spectrum shows a singlet peak at 8.04 ppm belongs to aromatic proton. This result confirms that the 1,4-Michael addition is more probable
reaction and compound 3A is the final product of the electrochemical oxidation of AO7 in the presence of 1A. In order to increase the yield of 3A–3C, some affecting factors must be
optimized. Therefore, the effects of two of the most important factors, applied current density and charge were investigated by setting all parameters to be constant and optimizing one each
time. The effect of charge passed was studied in the range of 1 to 6 F mol−1, while the other parameters are as follows: temperature = 298 K, current density = 0.32 mA/cm2, electrode surface
= 31.2 cm2, AO7 = 0.1 mmol and 1A = 0.1 mmol are kept constant. As is shown in Fig. S10a, the maximum product yield appears in 4.1 F mol−1 charge consumed. The product yield decreases with
increasing charge passed from 4.1 F mol−1 probably due to the occurrence of side reaction(s) such as over-oxidation. Furthermore, the effect of applied current density on product yield was
studied in the range 0.16–1.6 mA cm−2, while the other parameters (temperature = 298 K, charge consumed = 40 C, electrode surface = 31.2 cm2, AO7 = 0.1 mmol, and of 1A = 0.1 mmol) are kept
constant. The results show that, with increasing the current density from 0.32 mA cm−2, the product yield decreases (Fig. S10b). The product yield decreasing in current densities greater
than 0.32 mA cm−2, can be related to some side reactions such as oxidation of solvent, nucleophile or over-oxidation of 3A and/or INO. To evaluate the usefulness of the pair strategy in the
synthesis of 3A–3C, electrochemical synthesis of 3A was performed in a divided cell in both oxidative and reductive conditions. Our data confirms that in a divided cell (in both cases)
(unpaired condition), (a) the yield of 3A is lower and (b) the charge consumption is greater than that of in undivided cell. CONCLUSIONS This work provides new insights into the
electrochemical behavior of AO7 in aqueous solutions in both oxidative and reductive regions and shows that both oxidation and reduction of AO7 leading to the formation of a redox couple
(ANO/INO) (Fig. 3). In addition, the pH dependence of AO7 and other intermediates was studied in order to understand the predominant species, oxidation and reduction pathways and adsorption
study. For example, our data shows that, the interaction between anionic forms of AO7 and the electrode surface is stronger than neutral form. Furthermore, in this work, the electrochemical
oxidation/reduction of AO7 has been investigated in the presence of arylsulfinic acids (1A–1C) as nucleophiles, in acidic solutions. Our data display that the intermediate (INO) is attacked
by the nucleophile, 1A–1C, to give the final product 3A–3C (Fig. 7). Clean synthesis, technical feasibility (using galvanostatic method and simple cell), use of electricity instead of
oxidative or reductive reagents, one-step process, work in room temperature and pressure and use of aqueous solution instead of organic solvents, are the advantages of this method. MATERIALS
AND METHODS APPARATUS AND REAGENTS Cyclic voltammetry, controlled-potential coulometry and preparative electrolysis were performed using an Autolab model PGSTAT 30 and a Behpazho
potentiostat/galvanostat. The working and counter electrode used in macro-scale electrolysis and coulometry was an assembly of four ordinary soft carbon rods (6 mm diameter and 4 cm length).
Working electrode used in the cyclic voltammetry experiments was a glassy carbon disc (1.8 mm diameter) and a platinum rod was used as a counter electrode. The electrosynthesis were
performed under constant-current condition in an undivided cell. The glassy carbon electrode was polished using alumina slurry (from Iran Alumina Co.). More details are described in our
previous paper47. Acid orange 7, arylsulfinic acids and phosphate salts were obtained from commercial sources. These chemicals were used without further purification. ELECTROORGANIC
SYNTHESIS OF 3A–3C An aqueous phosphate buffer solution (70 ml, _c_ = 0.2 M, pH 2.0) containing AO7 (0.25 mmol) and arylsulfinic acid (1A–1C) (0.25 mmol) was electrolyzed in an undivided
cell under constant current conditions (current density = 0.32 mA cm−2) for 2 h 45 min. At the end of electrolysis, the cell was placed in a refrigerator overnight. The precipitated solid
was collected by filtration and washed several times with water. After recrystallization in ethyl ether, the products were characterized by IR, 1H NMR, 13C NMR and mass spectroscopy.
1-AMINO-3-TOSYLNAPHTHALEN-2-OL (C17H15NO3S) (3A) mp: 163–164 °C; isolated yield 65%. 1H NMR (400 MHz, DMSO-_d_6): _δ_ = 2.32 (s, 3 H, methyl), 6.22 (s, ~1 H, NH, this peak disappeared upon
addition of D2O), 7.36 (m, 4 H, _J_ = 8 Hz, aromatic), 7.71 (d, 2 H, _J_ = 8.4, aromatic), 8.04 (s, 1 H, aromatic), 8.11 (m, 1 H, aromatic), 8.29 (m, 1 H, aromatic), 9.86 (s, ~1 H, OH, this
peak disappeared upon addition of D2O); 13C NMR (100 MHz, DMSO-_d_6): _δ_ = 21.3, 119.5, 120.5, 122.7, 123.1, 123.9, 124.8, 125.0, 125.8, 126.9, 130.2, 136.7, 137.7, 140.4, 143.7; IR (KBr):
3384, 2926, 1704, 1622, 1354, 1266, 1200, 1140, 1083, 951, 755, 668, 571, 529 cm−1; MS (EI, 70 eV): m/z (relative intensity %): 313 (M+, 31), 270 (6), 158 (100), 139 (11), 130 (65), 91 (24),
77 (15), 65(18). 1-AMINO-3-(PHENYLSULFONYL)NAPHTHALEN-2-OL (C16H13NO3S) (3B) mp: 209–210 °C; isolated yield 60%. 1H NMR (400 MHz, DMSO-_d_6): _δ_ = 6.27 (s, ~1 H, NH, this peak disappeared
upon addition of D2O), 7.38 (dd, 2 H, _J_ = 3.2 and 10.0 Hz, aromatic), 7.57 (m, 3 H, aromatic), 7.85 (dd, 2 H, _J_ = 2.0 and 6.8 Hz, aromatic), 8.09 (s, 1 H, aromatic), 8.18 (dd, 1 H, _J_ =
3.2 and 10.0 Hz, aromatic), 8.33 (dd, 1 H, _J_ = 3.2 and 10.0 Hz, aromatic), 9.91 (s, ~1 H, OH, this peak disappeared upon addition of D2O); 13C NMR (100 MHz, DMSO-_d_6): _δ_ = 118.8,
120.7, 122.7, 123.2, 123.9, 124.7, 125.1, 125.8, 126.8, 129.8, 133.1, 136.8, 138.2, 143.4; IR (KBr): 3473, 3379, 3073, 3025, 1739, 1616, 1358, 1286, 1207, 1136, 1082, 952, 784, 740, 557
cm−1; MS (EI, 70 eV): m/z (relative intensity %): 299 (M+, 21), 257 (7), 160 (16), 159 (55), 158 (100), 130 (58), 103 (16), 77(44), 51(24), 43 (74).
1-AMINO-3-((4-CHLOROPHENYL)SULFONYL)NAPHTHALEN-2-OL (C16H12CLNO3S) (3C) mp: 164–165 °C; isolated yield 62%. 1H NMR (400 MHz, DMSO-_d_6): _δ_ = 6.33 (s, ~1 H, NH, this peak disappeared upon
addition of D2O), 7.38 (dd, 2 H, _J_ = 3.2 and 9.6 Hz, aromatic), 7.62 (d, 2 H, _J_ = 8.8 Hz, aromatic), 7.85 (d, 2 H, _J_ = 8.8 Hz, aromatic), 8.06 (d, 1 H, _J_ = 2.8 Hz, aromatic), 8.19
(m, 1 H, aromatic), 8.28 (m, 1 H, aromatic), 9.92 (s, ~1 H, OH, this peak disappeared upon addition of D2O); 13C NMR (100 MHz, DMSO-_d_6): _δ_ = 118.2, 120.6, 122.6, 123.1, 123.7, 124.3,
124.9, 125.0, 126.2, 128.8, 129.9, 136.7, 138.2, 142.0; IR (KBr): 3362, 3286, 3068, 1630, 1309, 1284, 1174, 1144, 1085, 906, 824, 781, 752, 648, 580 cm−1; MS (EI, 70 eV): m/z (relative
intensity %): 333 (M+, 24), 174 (12), 158 (100), 130 (58), 111 (21), 103 (18), 77 (17), 75 (28), 50 (18). ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Momeni, S. and Nematollahi, D. New
insights into the electrochemical behavior of acid orange 7: Convergent paired electrochemical synthesis of new aminonaphthol derivatives. _Sci. Rep._ 7, 41963; doi: 10.1038/srep41963
(2017). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES * Silva, J. P. et al.
Adsorption of acid orange 7 dye in aqueous solutions by spent brewery grains. _Sep. Purif. Technol._ 40, 309–315 (2004). Article CAS Google Scholar * Zollinger, H. _Color Chemistry:
Syntheses, Properties, and Applications of Organic Dyes and Pigments_. (John Wiley & Sons, 2003). * Hihara, T., Okada, Y. & Morita, Z. Azo-hydrazone tautomerism of phenylazonaphthol
sulfonates and their analysis using the semiempirical molecular orbital PM5 method. _Dyes Pigm_. 59, 25–41 (2003). Article CAS Google Scholar * Özen, A. S., Doruker, P. & Aviyente, V.
Effect of cooperative hydrogen bonding in azo-hydrazone tautomerism of azo dyes. _J. Phys. Chem. A_ 111, 13506–13514 (2007). Article CAS PubMed Google Scholar * Gordon, P. F. &
Gregory, P. _Organic Chemistry in Colour_ (Springer Science & Business Media, 2012). * Oakes, J. & Gratton, P. Kinetic investigations of azo dye oxidation in aqueous media. _J. Chem.
Soc. Perk. T_. 2, 1857–1864 (1998). Article Google Scholar * Oakes, J., Gratton, P., Clark, R. & Wilkes, I. Kinetic investigation of the oxidation of substituted arylazonaphthol dyes
by hydrogen peroxide in alkaline solution. _J. Chem. Soc. Perk. T_. 2, 2569–2576 (1998). Article Google Scholar * Hihara, T., Okada, Y. & Morita, Z. Reactivity of phenylazonaphthol
sulfonates, their estimation by semiempirical molecular orbital PM5 method, and the relation between their reactivity and azo-hydrazone tautomerism. _Dyes Pigm_. 59, 201–222 (2003). Article
CAS Google Scholar * Allen, R. L. _Colour Chemistry_ (Springer Science & Business Media, 2013). * Christie, R. _Colour Chemistry_ (Royal Society of Chemistry, 2014). * Thomas, F.
& Boto, K. _The Chemistry of the Hydrazo, Azo and Azoxy Groups_. 443–493 (Wiley-VCH, Weinheim, 1975). * Ember, E., Rothbart, S., Puchta, R. & van Eldik, R. Metal ion-catalyzed
oxidative degradation of orange II by H2O2. High catalytic activity of simple manganese salts. _New J. Chem._ 33, 34–49 (2009). Article CAS Google Scholar * Robinson, T., McMullan, G.,
Marchant, R. & Nigam, P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. _Bioresource Technol._ 77, 247–255
(2001). Article CAS Google Scholar * Waring, D. R. & Hallas, G. _The Chemistry and Application of Dyes_ (Springer Science & Business Media, 2013). * Fuchigami, T., Atobe, M. &
Inagi, S. _Fundamentals and Applications of Organic Electrochemistry: Synthesis, Materials, Devices_ (John Wiley & Sons, 2014). * Varmaghani, F., Nematollahi, D., Mallakpour, S. &
Esmaili, R. Electrochemical oxidation of 4-substituted urazoles in the presence of arylsulfinic acids: an efficient method for the synthesis of new sulfonamide derivatives. _Green Chem._ 14,
963–967 (2012). Article CAS Google Scholar * Salehzadeh, H., Nematollahi, D. & Hesari, H. An efficient electrochemical method for the atom economical synthesis of some benzoxazole
derivatives. _Green Chem._ 15, 2441–2446 (2013). Article CAS Google Scholar * Nematollahi, D., Hosseiny Davarani, S. S. & Mirahmadpour, P. A green approach for the electroorganic
synthesis of new dihydroxyphenyl-indolin-2-one derivatives. _ACS Sustainable Chem. Eng._ 2, 579–583 (2014). Article CAS Google Scholar * Khazalpour, S. & Nematollahi, D.
Electrochemical and chemical synthesis of different types of sulfonamide derivatives of _N,N_-dimethyl-1,4-benzenediamine using 4-nitroso-_Nα_-dimethylaniline. _Green Chem._ 17, 3508–3514
(2015). Article CAS Google Scholar * Maleki, A., Nematollahi, D., Rasouli, F. & Zeinodini-Meimand, A. Electrode instead of catalyst and enzyme. A greener protocol for the synthesis of
new 2-hydroxyacetamide derivatives containing a γ-lactone ring. _Green Chem._ 18, 672–675 (2016). Article CAS Google Scholar * Nematollahi, D. & Rafiee, M. Diversity in
electrochemical oxidation of dihydroxybenzoic acids in the presence of acetylacetone. A green method for synthesis of new benzofuran derivatives. _Green Chem._ 7, 638–644 (2005). Article
CAS Google Scholar * Salahifar, E., Nematollahi, D., Bayat, M., Mahyari, A. & Amiri Rudbari, H. Regioselective green electrochemical approach to the synthesis of nitroacetaminophen
derivatives. _Org. Lett._ 17, 4666–4669 (2015). Article CAS PubMed Google Scholar * Paddon, C. A., Pritchard, G. J., Thiemann, T. & Marken, F. Paired electrosynthesis: micro-flow
cell processes with and without added electrolyte. _Electrochem. Commun._ 4, 825–831 (2002). Article CAS Google Scholar * Amatore, C., Lund, H. & Baizer, M. _Organic Electrochemistry_
(Marcel Dekker, New York, 1991). * Hammerich, O. & Lund, H. _Organic Electrochemistry_ (CRC Press, 2000). * Nematollahi, D. & Varmaghani, F. Paired electrochemical synthesis of new
organosulfone derivatives. _Electrochim. Acta_ 53, 3350–3355 (2008) Article CAS Google Scholar * Habibi, D., Pakravan, N. & Nematollahi, D. The green and convergent paired Diels–Alder
electro-synthetic reaction of 1,4-hydroquinone with 1,2-bis (bromomethyl) benzene. _Electrochem. Commun._ 49, 65–69 (2014). Article CAS Google Scholar * Sharafi-Kolkeshvandi, M.,
Nematollahi, D. & Nikpour, F. A Green CC bond formation reaction between _N,N_′-diphenylbenzene-1, 4-diamine and Michael donors: A convergent paired strategy. _J. Electrochem. Soc._ 163,
G75–G78 (2016). Article CAS Google Scholar * Beale, Jr. J. M. & Block, J. (eds) _Wilson and Gisvold’s Textbook of Organic Medicinal and Pharmaceutical Chemistry_. 12th ed.
(Lippincott Williams and Wilkins, Philadelphia, 2011). * Nematollahi, D., Baniardalan, M., Khazalpour, S. & Pajohi-Alamoti, M. R. Product diversity by changing the electrode potential.
_Synthesis, kinetic evaluation and antibacterial activity of arylsulfonyl-4,4′-biphenol and bis-arylsulfonyl-4, 4′-biphenol derivatives. Electrochim. Acta_ 191, 98–105 (2016). CAS Google
Scholar * Khazalpour, S., Nematollahi, D. & Pajohi-Alamoti, M. R. A green approach for the synthesis of bis (substituted sulfabenzamide) _para_-benzoquinone based on the reaction of
sulfabenzamide with electrochemically generated _para_-benzoquinone and its antibacterial evaluation. _New J. Chem._ 39, 6734–6737 (2015). Article CAS Google Scholar * Bard, A. J. &
Faulkner, L. R. _Electrochemical Methods: Fundamentals and Applications_ (Wiley, 2000). * Nicholson, R. S. & Shain, I. Theory of stationary electrode polarography. _Single scan and
cyclic methods applied to reversible, irreversible, and kinetic systems. Anal. Chem_. 36, 706–723 (1964). CAS Google Scholar * Abbott, L. C., Batchelor, S. N., Smith, J. R. L. & Moore,
J. N. Reductive reaction mechanisms of the azo dye orange II in aqueous solution and in cellulose: from radical intermediates to products. _J. Phys. Chem. A_ 113, 6091–6103 (2009). Article
CAS PubMed Google Scholar * Mu, Y., Rabaey, K., Rozendal, R. A., Yuan, Z. & Keller, J. Decolorization of azo dyes in bioelectrochemical systems. _Environ. Sci. Technol._ 43,
5137–5143 (2009). Article ADS CAS PubMed Google Scholar * Zhang, S. J., Yu, H. Q. & Li, Q. R. Radiolytic degradation of acid orange 7: A mechanistic study. _Chemosphere_ 61,
1003–1011 (2005). Article ADS CAS PubMed Google Scholar * Lopez, C. et al. Mechanism of enzymatic degradation of the azo dye orange II determined by _ex situ_1H nuclear magnetic
resonance and electrospray ionization-ion trap mass spectrometry. _Anal. Biochem._ 335, 135–149 (2004). Article CAS PubMed Google Scholar * Ozcan, A., Oturan, M. A., Oturan, N. &
Şahin, Y. Removal of acid orange 7 from water by electrochemically generated Fenton’s reagent. _J. Hazard. Mater._ 163, 1213–1220 (2009). Article CAS PubMed Google Scholar * Hammami, S.,
Bellakhal, N., Oturan, N., Oturan, M. A. & Dachraoui, M. Degradation of acid orange 7 by electrochemically generated OH radicals in acidic aqueous medium using a boron-doped diamond or
platinum anode: A mechanistic study. _Chemosphere_ 73, 678–684 (2008).; Article ADS CAS Google Scholar * Wu, J., Zhang, H. & Qiu, J. Degradation of acid orange 7 in aqueous solution
by a novel electro/Fe 2+/peroxydisulfate process. _J. Hazard. Mater._ 215, 138–145 (2012). Article ADS CAS PubMed Google Scholar * Zhao, H. Z., Sun, Y., Xu, L. N. & Ni, J. R.
Removal of acid orange 7 in simulated wastewater using a three-dimensional electrode reactor: Removal mechanisms and dye degradation pathway. _Chemosphere_ 78, 46–51 (2010). Article ADS
CAS PubMed Google Scholar * Zheng, J., Gao, Z., He, H., Yang, S. & Sun, C. Efficient degradation of acid orange 7 in aqueous solution by iron ore tailing Fenton-like process.
_Chemosphere_ 150, 40–48 (2016). Article ADS CAS PubMed Google Scholar * Cai, C., Zhang, Z. & Zhang, H. Electro-assisted heterogeneous activation of persulfate by Fe/SBA-15 for the
degradation of orange II. _J. Hazard. Mater._ 313, 209–218 (2016). Article ADS CAS PubMed Google Scholar * Baymaky, M. S. & Zuman, P. Equilibria of formation and dehydration of the
carbinolamine intermediate in the reaction of benzaldehyde with hydrazine. _Tetrahedron_ 63, 5450–5454 (2007). Article CAS Google Scholar * Baymak, M. S., Celik, H., Ludvik, J., Lund H.
& Zuman P. Diprotonated hydrazones and oximes as reactive intermediates in electrochemical reductions. _Tetrahedron Lett._ 45, 5113–5115 (2004). Article CAS Google Scholar * Shine, H.
J., Zmuda, H. & Park, K. H. Mechanism of the benzidine rearrangement. Kinetic isotope effects and transition states. _Evidence for concerted rearrangement. J. Am. Chem. Soc._ 103,
955–956 (1981). Article CAS Google Scholar * Nematollahi, D., Dehdashtian, S. & Niazi, A. Electrochemical oxidation of some dihydroxybenzene derivatives in the presence of indole. _J.
Electroanal. Chem._ 616, 79–86 (2008). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We acknowledge the Bu-Ali Sina University Research Council and Center of Excellence
in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) for their support of this work. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Faculty of Chemistry,
Bu-Ali Sina University, Hamedan, Zip Code 65178-38683, Iran Shima Momeni & Davood Nematollahi Authors * Shima Momeni View author publications You can also search for this author inPubMed
Google Scholar * Davood Nematollahi View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.M. and D.N. conceived and designed the study. S.M.
did the experiments. S.M. and D.N. wrote the manuscript. D.N. directed the research. CORRESPONDING AUTHOR Correspondence to Davood Nematollahi. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPORTING INFORMATION (PDF 1981 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons
Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the
credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of
this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Momeni, S., Nematollahi, D. New insights into the
electrochemical behavior of acid orange 7: Convergent paired electrochemical synthesis of new aminonaphthol derivatives. _Sci Rep_ 7, 41963 (2017). https://doi.org/10.1038/srep41963 Download
citation * Received: 24 August 2016 * Accepted: 29 December 2016 * Published: 06 February 2017 * DOI: https://doi.org/10.1038/srep41963 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative
Trending News
What we’ve learnt from building africa’s biggest genome libraryThe human genome was first sequenced in 2003 by multiple research centres across the world. The breakthrough was hailed ...
At an average of 1,200 passengers per day, maya train numbers far from eventual targetWill the Maya Train railroad eventually become a popular mode of travel between beach resorts, colonial cities and archa...
Targeted modulation of immune cells and tissues using engineered biomaterialsABSTRACT Therapies modulating the immune system offer the prospect of treating a wide range of conditions including infe...
Jalisco officials say this indigenous leader died in a car crash. His community isn't buying itThe Indigenous Wixárika community in northern Jalisco is raising doubts about the official explanation that a vehicle ac...
Over 500 chiapas residents flee cartel violence into guatemalaHundreds of Mexican families fleeing cartel violence in Mexico’s southern state of Chiapas have sought refuge across the...
Latests News
New insights into the electrochemical behavior of acid orange 7: convergent paired electrochemical synthesis of new aminonaphthol derivativesABSTRACT Electrochemical behavior of acid orange 7 has been exhaustively studied in aqueous solutions with different pH ...
Properties: see what you can buy in orne in rural france for €40,000 and €1. 123mEXPLORE ORNE'S SCENIC ROUTES, TRADITIONAL SAUSAGES, AND SURPRISINGLY AFFORDABLE PROPERTY MARKET Dept 61 Orne Prefe...
Irrigation intensification impacts sustainability of streamflow in the western united statesABSTRACT Quantifying the interconnected impacts of climate change and irrigation on surface water flows is critical for ...
Ecosystem productivity has a stronger influence than soil age on surface soil carbon storage across global biomesABSTRACT Interactions between soil organic matter and minerals largely govern the carbon sequestration capacity of soils...
Pfizer-biontech covid-19 vaccine gets full fda approvalMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...