Ecosystem productivity has a stronger influence than soil age on surface soil carbon storage across global biomes
Ecosystem productivity has a stronger influence than soil age on surface soil carbon storage across global biomes"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Interactions between soil organic matter and minerals largely govern the carbon sequestration capacity of soils. Yet, variations in the proportions of free light (unprotected) and
mineral-associated (protected) carbon as soil develops in contrasting ecosystems are poorly constrained. Here, we studied 16 long-term chronosequences from six continents and found that the
ecosystem type is more important than soil age (centuries to millennia) in explaining the proportion of unprotected and mineral-associated carbon fractions in surface soils across global
biomes. Soil carbon pools in highly productive tropical and temperate forests were dominated by the unprotected carbon fraction and were highly vulnerable to reductions in ecosystem
productivity and warming. Conversely, soil carbon in low productivity, drier and colder ecosystems was dominated by mineral-protected carbon, and was less responsive to warming. Our findings
emphasize the importance of conserving ecosystem productivity to protect carbon stored in surface soils. SIMILAR CONTENT BEING VIEWED BY OTHERS LAND-USE INDUCED SOIL CARBON STABILIZATION AT
THE EXPENSE OF ROCK DERIVED NUTRIENTS: INSIGHTS FROM PRISTINE ANDEAN SOILS Article Open access 20 March 2023 GLOBAL STOCKS AND CAPACITY OF MINERAL-ASSOCIATED SOIL ORGANIC CARBON Article
Open access 01 July 2022 VULNERABILITY OF MINERAL-ASSOCIATED SOIL ORGANIC CARBON TO CLIMATE ACROSS GLOBAL DRYLANDS Article 30 July 2024 INTRODUCTION Surface soil carbon (C) in the form of
organic matter supports essential ecosystem services such as climate regulation, plant production, nutrient cycling and water storage and purification1,2,3,4,5. Global data show that soil C
content is larger in cold and mesic than in warm and xeric ecosystems6. Moreover, we know that soil C often accumulates during the first thousands of years of ecosystem development7, and
declines during ecosystem retrogression over millennia8. Soil C gains and losses are driven by intertwined changes in primary productivity and stabilization and destabilization processes,
which in turn are in part governed by the interaction of soil organic matter with minerals1,9. Specifically, the long-term preservation of soil C is mediated by occlusion within aggregates
and by the formation of organo-mineral associations10,11, which limit the accessibility of microorganisms and their extracellular enzymes to the organic substrates and increasing energy
requirements for respiration9,12,13,14. Thus, the mineral-associated C fraction of soil organic matter may be less vulnerable to microbial decomposition, and consequently to
warming-induced-increases of microbial respiration, than the particulate and free light C fractions, which are largely unprotected by minerals15,16,17. The mineral control of soil C
accumulation and loss has been highlighted in recent studies across large temporal11,18,19 and spatial gradients20,21. For example, studies conducted on single chronosequences in Hawaii and
California have found positive relationships between soil C and mineral reactivity, and suggested that weathering first increases and subsequently reduces the potential of soil to stabilize
C9,19. This pattern is particularly evident in soils where short-range order and non-crystalline minerals form in relatively large amounts22,23,24. Despite these important advances, we still
lack a comprehensive understanding of how, why and to what extent the mineral protection of C (assessed by the proportion of soil C in mineral-associated vs. free fractions) changes as soil
develops over centuries to millennia across different ecosystems. Such fundamental knowledge is crucial to improve our understanding of soil C storage and turnover over large spatiotemporal
scales, reduce uncertainties in the representation of soil processes in land C models, and ultimately shape land management strategies to foster the multiple ecosystem services of soil
organic matter, including climate regulation. Most studies to date on this topic have been based on single chronosequences9,19,25,26. Here, we advance on this by exploring the major drivers
of soil organic C fractionation as soil develops across 16 globally distributed soil chronosequences from contrasting ecosystems. The objective of this work was to determine how and why the
amount and the proportion of surface (0–10 cm) soil C stored in different organic matter fractions changes during soil development across biomes. These chronosequences cover a range of
parent material (volcanic material, sedimentary rocks, and sand dunes) and ecosystems (from deserts to tropical forests and croplands; Supplementary Fig. 1 and Supplementary Data 1)27.
Within each soil chronosequence, we selected four to six stages ranging from hundreds to millions of years sharing similar parent material and climate. We applied a density-based, soil
organic matter fractionation to separate and quantify soil organic C content in three distinct fractions based on their association with minerals: free light fraction (not protected by
minerals); occluded light fraction (protected by occlusion within aggregates); and mineral-associated fraction (protected by sorption to minerals)28. Using this information, we calculated
the proportion of organic C stored in each fraction in relation to the total organic C content (proportion of organic C fraction i = organic C fraction i content × 100/sum of all organic C
fraction contents). We focused on the surface soil (top 10 cm, after removing any litter/plant debris on the soil surface) because it is the most biologically active layer and the most
exposed to environmental factors, as well as because the surface soil C is especially relevant to support soil functioning and multiple environmental services1,29. This sampling depth is
also consistent with previous studies on soil C storage across spatiotemporal scales19,21,30. Additional details on methods are in Appendix S1: Section S1. RESULTS AND DISCUSSION CHANGES IN
C FRACTIONS DURING SOIL DEVELOPMENT Total C stocks in the surface soil were generally largest in grasslands and forests, specifically in temperate and tropical regions (Supplementary Fig.
2). Moreover, soil C stocks in these ecosystems generally tracked a hump-shaped relationship with soil age, with the largest stocks occurring at intermediate stages of soil development
(Supplementary Fig. 2). This relationship agrees with results from single-chronosequence studies on soils relatively dominated by short-range order and non-crystalline minerals9,18,19,22,23.
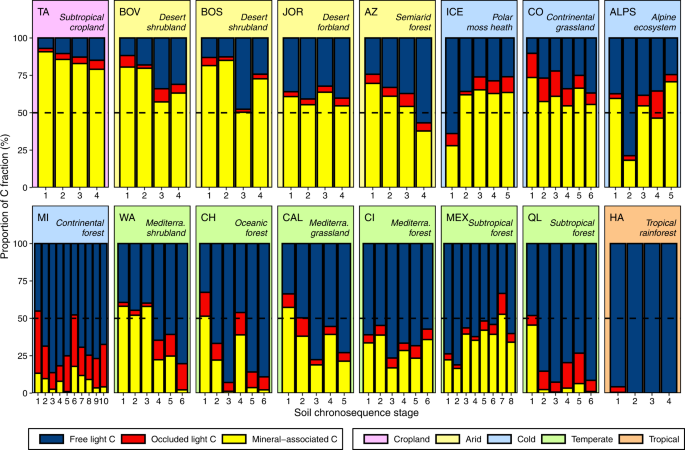
We further show that the free light and mineral-associated fractions consistently dominated the soil C stocks across chronosequences (Fig. 1 and Supplementary Fig. 3), while the occluded
light fraction only represented a minor part (less than 10% on average across chronosequence sites). Because of this, we focused on the free light and mineral-associated C fractions for
downstream analyses. We also focused our discussion mainly on the proportion of soil C stored in each fraction rather than changes in stocks, because the quantification of the latter would
require no changes in soil bulk density through time31, and this condition was not met in our chronosequences. Our data revealed that while the mineral protection of soil C differed vastly
across ecosystems, it changed to a much lesser extent during soil development within each chronosequence (Fig. 1). In fact, these results were confirmed by linear mixed-effects modeling (see
Methods for details), which revealed that ecosystem productivity significantly alters the proportion of organic C in the free light (_p_ = 0.002) and mineral-associated (_p_ = 0.005)
fractions across chronosequences but soil age class (i.e., thousands of years, hundreds of thousand years and millions of years) does not (_p_ > 0.05; Supplementary Fig. 4). In
particular, warm and wet tropical and temperate (e.g., subtropical and Mediterranean) ecosystems (often forests) had a consistently larger proportion of free light relative to
mineral-associated C fraction irrespective of soil age (7/8 chronosequences, 35/45 plots; Fig. 1 and Supplementary Fig. 3). These tropical and temperate ecosystems also had relatively larger
contents of soil organic C (Supplementary Figs. 2, 3 and 5). In contrast, in arid and cold ecosystems with less primary production (e.g., desert shrublands), most organic C was stored in
the mineral-associated fraction (7/8 chronosequences and 29/42 plots; Fig. 1 and Supplementary Figs. 2, 3 and 5). The chronosequences from Arizona (AZ) and Hawaii (HA) illustrate the point
that mineral protection of surface soil organic C varies more between than within ecosystems as soil develops from centuries to millennia. Both forest ecosystems are on volcanic substrates
and developed over the same period (3–4 million years), yet the semiarid ecosystem from AZ is, on average, dominated by the mineral-associated C fraction (56%), while the tropical ecosystem
from HA is permanently dominated by the free light fraction (average proportion of mineral-associated C <2%; Fig. 1 and Supplementary Data 1). Similarly, cold grasslands from Colorado
(CO), which developed over millennia on sedimentary substrates, were dominated by the mineral-associated C fraction (61%), while comparable, but warmer, ecosystems from California (CAL) were
dominated by the free light fraction (35% of mineral-associated C on average; Fig. 1 and Supplementary Data 1). The C distribution found in two soil chronosequences contrasted with the cold
and arid vs. warm and wet ecosystem pattern regarding the dominance of C stored in the mineral-associated vs. free light organic matter fractions. In particular, soil C in the
chronosequence located in a highly productive continental forest in North America (MI, 10 plots) was predominantly stored in free light organic matter, rather than in the mineral-associated
organic matter fraction as was found in the other cold ecosystems (Fig. 1 and Supplementary Fig. 3). Conversely, soil C contents of a subtropical soil chronosequence cropped with tea for the
last 100 years in an area naturally dominated by broad-leaved evergreen forests in Taiwan (TA, 4 plots)32 were much smaller (Supplementary Fig. 2) than those found in other productive and
warm ecosystems (e.g., Queensland, QL, Mexico, MEX, and Hawaii, HA; Supplementary Fig. 2), and were also dominated by mineral-associated rather than free light organic matter fractions (Fig.
1 and Supplementary Fig. 3). Overall, these local contingencies lend support to the global pattern reported here, supporting the importance of free light organic matter as a dominant C pool
in the soil of undisturbed forest ecosystems. In addition, these results suggest that land use conversion of broad-leaved evergreen forest to agriculture in the TA chronosequence altered
the millennial balance of C contents, resulting in relevant losses of unprotected C fractions from the soil. The proportion of surface soil C stored in free light organic matter was
positively correlated with microbial respiration rates, whereas the opposite was found for the proportion of mineral-associated C (Fig. 2). An additional experiment across a subset of eight
chronosequences that represented all the major biomes examined here revealed a positive relationship between the proportion of C stored in the free organic matter fraction and the
temperature sensitivity of soil respiration (i.e., evaluated with the _Q_10 coefficient as the increase in soil respiration with a temperature increase of 10 °C; Fig. 2). Together, these
findings suggest that surface soil C dominated by the free C fraction, mainly associated with temperate and tropical ecosystems in our chronosequence network, is relatively more vulnerable
to microbial decomposition and dependent on temperature increases. This is consistent with recent observations demonstrating a surprisingly high temperature sensitivity of soil C in tropical
forests that may result in substantial soil C losses with warming and an important positive feedback to climate change33. Our results also show that, although C contents and stocks in arid
and cold grasslands and shrublands are lower than those in the tropical and temperate forests evaluated, they are predominantly protected by minerals, and therefore relatively more stable
against microbial decomposition and elevated temperatures at a millennial scale34,35. BIOTIC AND ABIOTIC DRIVERS OF C STORAGE IN DIFFERENT FRACTIONS DURING SOIL DEVELOPMENT Given the
important reported changes in the proportion of C associated with different fractions across ecosystems, we conducted structural equation modeling (SEM, Fig. 3) to investigate the major
environmental drivers of the mineral protection of surface soil C. The spatial autocorrelation in our database was accounted by including the geographical distance across all plots in our
model design (space) (see Methods and Supplementary Table 1 for details on this and other variables). We found that contemporary levels of ecosystem productivity (as measured with the
Normalized Difference Vegetation Index (NDVI)) and soil fine texture (% of clay and silt) were the main factors explaining the vast differences in the proportion of free light and
mineral-associated C fractions across ecosystems (Fig. 3). Our analysis showed that higher precipitation, higher temperature and forest vegetation resulted in greater levels of ecosystem
productivity (Fig. 3). Soil C in highly productive ecosystems was predominantly stored in the free light organic matter fraction (Fig. 3 and Supplementary Figs. 2–6; but see TA in Fig. 1).
In contrast, soil C in less productive environments was mainly stored in the mineral-associated organic matter fraction, which was significantly and positively associated with fine texture
content, and indirectly associated with soil age (Fig. 3 and Supplementary Figs. 5 and 6). LIMITATIONS AND IMPLICATIONS While our findings contribute toward a more comprehensive
understanding of the changes and drivers of soil C fractions during soil development across a wide range of chronosequences, it is important to note that they cannot be extrapolated directly
to infer changes in soil C storage globally9,19,25,26. Further, the implications of our study can only be extended to the surface soil layer (0–10 cm) and not to total C stocks of the whole
soil profile9. Our data from globally distributed chronosequences indicate that mineral protection of surface soil C changes relatively little with soil development, but varies considerably
across terrestrial ecosystems. Soil age alone, however, may not be an accurate proxy for the progression of mineral weathering, which strongly depends on parent material, and even though we
used well-established soil chronosequences, the occurrence of regressive processes (i.e., erosion) cannot be discarded without specific mineralogical data. Broad climatic and ecosystem
drivers of soil organic C storage may also mask site-specific changes attributable to soil geochemistry36. In fact, it is noteworthy that previous single-chronosequence studies indicated
that C stabilization in the surface soil of dry ecosystems, and in deeper soil layers of moist ecosystems, is largely driven by soil mineralogy and weathering9,19. Weathering controls the
formation and availability of reactive mineral surfaces that can stabilize C through sorption or formation of aggregates19. As such, the amount of C stored in deeper soils layers in
temperate and tropical systems and associated with minerals might also be less vulnerable to microbial decomposition as soil ages prior to ecosystem retrogression. While our approach enabled
comparison of factors driving soil C contents across soil surface and across different ecosystems, it did not allow us to estimate how soil depth interacted with soil age to influence soil
C stabilization, which remains a future challenge. CONCLUSIONS In summary, our work demonstrates that mineral protection of surface soil C varies considerably across ecosystem types, but not
that much as soil develops from centuries to millennia in a wide range of environmental contexts. We found that soil C in warm and wet forest ecosystems is predominantly stored in free
organic matter fractions as soil develops, and that these soils are highly vulnerable to reductions in ecosystem productivity and warming temperatures. In contrast, soil C in cold and arid
ecosystems is mainly stored in mineral-associated organic matter fractions during soil development, and is mainly driven by soil texture and far less vulnerable to warming. Taken together,
this research advances our understanding on the dynamics of soil C stability and temperature dependence during soil development. METHODS LONG-TERM CHRONOSEQUENCES We used a globally
distributed set of 16 long-term soil chronosequences located in nine countries from six continents, each consisting of four to ten sites representing an increasing temporal stage during
ecosystem development. The chronosequences cover a wide range of parent material (volcanic material, sedimentary rocks, and sand dunes), climates (tropical, temperate, continental, polar,
and arid), vegetation (grasslands, shrublands, forests, and croplands), and age gradient (from hundreds to millions of years). Further information of the study sites was provided in27,37 and
summarized here in Supplementary Fig. 1 and Supplementary Data 1. Climatic type was obtained from the Koppen classification (http://koeppen-geiger.vu-wien.ac.at/present.htm)38; here we used
the term warm in a broad sense for equatorial, arid and warm temperate climates, and cold for boreal and polar climates sensu the Koppen classification38. Mean annual temperature and
precipitation was determined from the WorldClim database (https://www.worldclim.org). VEGETATION SURVEY For each site, we set up a 50 × 50 m plot representative for the spatial heterogeneity
of the ecosystem. Total plant cover was determined from data collected in three parallel transects of 50 m, spaced 25 m apart, using the line-intercept method27,39. We used the NDVI as our
proxy for plant primary productivity (NPP), referred to as ecosystem productivity throughout the text. This index provides a global measure of the “greenness” of vegetation across Earth’s
landscapes for a given composite period, and has been widely used to quantify NPP in large-scale studies40,41,42. Data were obtained from the Moderate Resolution Imaging Spectroradiometer
(MODIS) aboard NASA’s Terra satellites (http://neo.sci.gsfc.nasa.gov/), which provides data 23 times per year with a pixel size of 250 m × 250 m. We calculated the mean value of NDVI index
from monthly data (2008–2017). SOIL SAMPLING We collected five soil core samples under the dominant vegetation type of each plot (a total of 435 soil samples) with an open-tube sampler to
avoid compaction. Each soil sample consisted of a composite of five cores taken from a depth of 0 to 10 cm (topsoil; excluding litter/plant debris from the soil surface). This sampling
depth, which is consistent with previous works on soil C storage across spatiotemporal scales19,21,30, was suitable since a number of the study sites (e.g., youngest sites in volcanic
chronosequences) are very shallow, which does not allow deeper sampling and a comparative analysis of deeper layers across all chronosequences. The topsoil is the most biologically active
layer29, has the largest concentration of organic C along the soil profile and is particularly important in a context of climate and land use change1,30,43. Bulk density was calculated as
the dry mass of the soil sample divided by its volume in the field. Prior to further analysis, the soil samples were passed through a 2-mm sieve. A portion of each soil sample was air-dried
and used for chemical analysis and soil organic matter fractionation, whereas another portion was stored at −20 °C and used for biological analysis27. Each soil analysis was conducted in the
same laboratory. Fine texture (% clay + silt) was determined on air-dried samples by sieving and sedimentation44. SOIL ORGANIC C ANALYSIS AND FRACTIONATION We analyzed the 435 soil samples
for total organic C content by colorimetry after oxidation with K2Cr2O7 and H2SO4 at 150 °C for 30 min45 at the Biology and Geology Department of Rey Juan Carlos University (Móstoles,
Spain). One of the composite soil samples of each study site was fractionated to isolate soil organic C pools characterized by different mechanisms of stability and protection from
decomposition. We used the well-established density-based fractionation scheme developed by ref. 28 with slight modifications46,47. This scheme is widely used in soil organic matter
research15,48 and is intended to isolate three soil organic matter fractions directly connected to conceptual mechanisms of stabilization: a free light fraction organic matter, located
between soil aggregates and accessible to microbial decomposers; an occluded, intra-aggregate light fraction organic matter, physically protected by occlusion within soil aggregates and
disconnection from decomposers; and a mineral-associated organic matter fraction, chemically protected from decomposition by sorption to mineral surfaces and, consequently, with longer
turnover times than free and intra-aggregate fractions. Briefly, we added 40 ml of NaI at a density of 1.85 g ml−1 to 4 g of soil in a 50-ml centrifuge tube. The centrifuge tube was rotated
at 1 revolution s−1 for 30 s in an overhead shaker. After centrifugation at 2500 × _g_ for 30 min, the floating (free) light fraction was separated from the heavy fraction by suction and
filtration through a glass fiber filter and washed with deionized water. The NaI solution was added to the heavy fraction in the centrifuge tube, and the mixture was sonicated at an energy
input of 1500 J g−1 (calibrated calorimetrically49,50,51). The floating light (occluded) fraction was separated from the heavy (mineral-associated) fraction by centrifugation at 2500 × _g_
for 60 min, suction, and filtration through a glass fiber filter, and washed with deionized water. We used a density of 1.85 g ml−1 and a dispersion energy of 1500 J g−1 for consistency with
previous studies46,52,53,54. The three isolated fractions were oven-dried at 60 °C, weighed, ground with a ball mill and analyzed for organic C concentration by dry combustion and gas
chromatography using a Thermo Scientific Flash 2000 CN analyzer (Thermo Fisher Scientific, MA). In the case of the mineral-associated organic matter fraction, the organic C concentration was
determined after acid fumigation to remove carbonates55. Soil organic matter fractionation and C concentration analysis of the isolated fractions were performed at the Institute of
Agricultural Sciences of the Spanish National Research Council (CSIC, Madrid, Spain). We found a high correlation between the sum of free light, occluded light and mineral-associated C and
the total organic C content analyzed on whole soil samples colorimetrically as described above (_r_ = 0.987, _p_ < 0.001, _n_ = 87). The median (interquartile range) C recovery (sum of C
content recovered in free light, occluded light and mineral-associated C fractions relative to the total organic C content) was 89.4 (34.5)%. We found no significant differences in C
recovery based on parent material nor between high and low productive ecosystems (Kruskal-Wallis rank sum tests, _p_ = 0.669 and 0.368, respectively). We estimated the stocks of total
organic C and its free light, occluded light and mineral-associated fractions (kg C m−2) as the product of sampling depth, C content (g C kg−1 soil) and bulk density (Supplementary Fig. 2).
Bulk density was calculated as the dry weight of the soil sample collected with the core cylinder divided by its volume, assuming negligible coarse fraction (>2 mm) contents. The
quantification of changes in soil C stocks to a fixed depth over time, however, requires that soil bulk density does not change through time. This is because the same depths at different
times correspond to equivalent soil mass and layers only if bulk density remains constant31. Since this condition was not met in our chronosequences, we focused our analyses mainly on the
content and proportion of soil organic C fractions (proportion of organic C fraction i = organic C fraction i content × 100/sum of all organic C fraction contents) rather than the stocks. To
address whether our results were contingent on the fractionation method used (density-based), we repeated the analysis on a subset of samples across contrasting chronosequences (_n_ = 24)
using a size fractionation method, which is also widely used in soil organic matter research15,18,56. In particular, we used an automated wet sieving system to separate particulate organic
matter (>53 µm) from mineral-associated soil organic matter (<53 µm) after dispersion with sodium hexametaphosphate. The free light C separated by density was highly correlated with
the particulate organic C separated by size (_r_ = 0.975, _p_ < 0.001), and the same was found for the mineral-associated C fraction (_r_ = 0.687, _p_ < 0.001) (Supplementary Fig. 7).
This cross-validation supports previous findings suggesting that the light fraction separated by density is similar to the particulate organic matter fraction separated by size15,18. In
agreement with previous results47, the choice of heavy liquid may affect the proportion of occluded and mineral-associated organic C. In particular, compared to NaI solutions, less viscous
liquids such as sodium polytungstate solutions may result in greater intra-aggregate and smaller mineral-associated organic C concentrations, because of a greater effectiveness of the
ultrasonic disruption treatment to break up aggregates and the release of intra-aggregate C, which in turn decreases the amount of C recovered in the mineral-associated C pool. Also in
agreement with previous observations47, this bias may be expected to be systematic and operate in the same direction across soils, and thus not to affect our interpretation and discussion on
the evolution and drivers of soil C fractions across ecosystems. BIOLOGICAL SOIL ANALYSIS We measured soil basal heterotrophic respiration to examine soil biological activity across
chronosequences. This analysis was conducted on a composite soil sample per plot by quantifying the total CO2 released over 16 days from 1 g of bulk soil incubated in the dark at 28 °C and
50% water holding capacity in 20-mL glass vials, after a 1-week-long pre-incubation period. These incubation conditions were within the ranges found in the literature to measure soil basal
respiration57. In addition to these determinations, we measured the potential response of soil heterotrophic respiration to temperature (_Q_10) to assess the vulnerability of soil C losses
to warming. The temperature sensitivity of the soil microbial respiration (_Q_10) was determined on a composite soil sample per soil age (location) from a subset of eight chronosequences
selected to cover a wide range of global environmental conditions in terms of climate, vegetation types and soil age. The _Q_10 coefficient represents the increase in soil respiration as
temperature increases by 10 °C, and is commonly used to assess warming effects on soil C losses and to model soil C dynamics58,59,60. Soil respiration rates were measured after short-term
(10 h) incubations in triplicate at three increasing temperatures (5, 15 and 25 °C) in 96 deep well microplates61, using the MicroResp technique62. We then calculated the _β_ and _R_0
coefficients for the exponential relationship between heterotrophic soil respiration rate (_R_S, in μg CO2−C g−1 h−1) and temperature (_T_, in °C): _R_s = _R_0 × exp(_β_ × _T_); and used _β_
to compute _Q_10 using the equation _Q_10 = exp(10 × _β_). The MicroResp technique has been successfully used in previous studies for high-throughput quantification of _Q_10 values61.
Higher soil respiration rates and _Q_10 values were interpreted as higher soil C vulnerability to microbial decomposition and increases in temperature. We used short incubations to prevent
microbial acclimation to the assay temperature used in the laboratory63,64,65. We acknowledge that C respired during incubations may also represent chemically labile old C that is physically
protected in the field but is freed during soil sampling and sieving in the lab66. DATA ANALYSIS The effects of ecosystem productivity, soil age (binned in three arbitrary categories:
thousands of years, hundreds of thousand years and millions of years) and their interaction were analyzed by linear mixed-effects models, with an intercept structure in the random term to
account for the lack of independence among soils from the same chronosequence. We excluded a subtropical chronosequence in an area naturally dominated by broad-leaved evergreen forests in
Taiwan (TA) from this analysis, because its natural productivity was altered being cropped with tea for the last 100 years. Normality and homoscedasticity were examined visually using
residual plots. The relationships between soil respiration and temperature sensitivity of soil respiration and the relative abundance of free light and mineral-associated C fractions were
also assessed with linear mixed-effects modeling, including net primary productivity (evaluated with the NDVI index) and fine texture (percentage of clay and silt) as covariates. P values
for fixed effects were computed via the Satterthwaite approximation. We used SEM67 to examine the complex (direct and indirect) effects of climate, vegetation, soil age, soil properties on
the proportion of free light and mineral-associated soil organic C. For soil age, we used a standardized composite variable created from three complementary metrics: a quantitative index of
soil age (years; log10 + 1-transformed), a semi-quantitative index of age range (i.e., thousands of years, hundreds of thousand years and millions of years) and a qualitative soil age index
(standardized soil age range from 0 to 1 calculated for each chronosequence following approaches used in previous works68). We first created an a priori model that incorporated all potential
relationships between variables based on prior ecological knowledge (Supplementary Fig. 8). We included a geographical variable (space) created from the coordinates and distances between
sites to account for spatial dependencies and information redundancy. The statistical analysis and plots were performed using IBM SPSS v. 2669, R70 and the R packages ggeffects71,
ggnewscale72, ggstar73, ggtext74, lme475, lmerTest76, patchwork77, readxl78, rgeos79, rnaturalearth80, tidyverse81. REPORTING SUMMARY Further information on research design is available in
the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The data associated with this study are publicly available in Figshare
(https://doi.org/10.6084/m9.figshare.12619466). REFERENCES * Jackson, R. B. et al. The ecology of soil carbon: pools, vulnerabilities, and biotic and abiotic controls. _Annu. Rev. Ecol.
Evol. Syst._ 48, 419–445 (2017). Article Google Scholar * Paul, E. A. The nature and dynamics of soil organic matter: plant inputs, microbial transformations, and organic matter
stabilization. _Soil. Biol. Biochem._ 98, 109–126 (2016). Article CAS Google Scholar * Amundson, R. et al. Soil and human security in the 21st century. _Science_ 348, 1261071–1261071
(2015). Article Google Scholar * Batjes, N. H. Harmonized soil property values for broad-scale modelling (WISE30sec) with estimates of global soil carbon stocks. _Geoderma_ 269, 61–68
(2016). Article CAS Google Scholar * Crowther, T. W. et al. The global soil community and its influence on biogeochemistry. _Science_ 365, eaav0550 (2019). Article CAS Google Scholar *
Hengl, T. et al. SoilGrids250m: global gridded soil information based on machine learning. _PLoS ONE_ 12, e0169748 (2017). Article Google Scholar * Schlesinger, W. H. Evidence from
chronosequence studies for a low carbon-storage potential of soils. _Nature_ 348, 232–234 (1990). Article CAS Google Scholar * Peltzer, D. A. et al. Understanding ecosystem retrogression.
_Ecol. Monogr._ 80, 509–529 (2010). Article Google Scholar * Torn, M. S., Trumbore, S. E., Chadwick, O. A., Vitousek, P. M. & Hendricks, D. M. Mineral control of soil organic carbon
storage and turnover. _Nature_ 389, 170–173 (1997). Article CAS Google Scholar * Kleber, M. et al. Mineral–organic associations: formation, properties, and relevance in soil environments.
in _Advances_ in _Agronomy_ (ed. Sparks, D. L.) 130 1–140 (Academic Press, 2015). * Kleber, M. et al. Dynamic interactions at the mineral–organic matter interface. _Nat. Rev. Earth
Environ_. https://doi.org/10.1038/s43017-021-00162-y (2021). * Schmidt, M. W. I. et al. Persistence of soil organic matter as an ecosystem property. _Nature_ 478, 49–56 (2011). Article CAS
Google Scholar * Lehmann, J. & Kleber, M. The contentious nature of soil organic matter. _Nature_ 528, 60–68 (2015). Article CAS Google Scholar * Hemingway, J. D. et al. Mineral
protection regulates long-term global preservation of natural organic carbon. _Nature_ 570, 228–231 (2019). Article CAS Google Scholar * Lavallee, J. M., Soong, J. L. & Cotrufo, M. F.
Conceptualizing soil organic matter into particulate and mineral-associated forms to address global change in the 21st century. _Glob. Chang. Biol._ 26, 261–273 (2020). Article Google
Scholar * Liu, X. J. A., Frey, S. D., Melillo, J. M. & DeAngelis, K. M. Physical protection regulates microbial thermal responses to chronic soil warming. _Soil. Biol. Biochem._ 159,
108298 (2021). Article CAS Google Scholar * Lugato, E., Lavallee, J. M., Haddix, M. L., Panagos, P. & Cotrufo, M. F. Different climate sensitivity of particulate and
mineral-associated soil organic matter. _Nat. Geosci_. https://doi.org/10.1038/s41561-021-00744-x (2021). * Mikutta, R. et al. Microbial and abiotic controls on mineral-associated organic
matter in soil profiles along an ecosystem gradient. _Sci. Rep._ 9, 10294 (2019). Article Google Scholar * Doetterl, S. et al. Links among warming, carbon and microbial dynamics mediated
by soil mineral weathering. _Nat. Geosci._ 11, 589–593 (2018). Article CAS Google Scholar * Cotrufo, M. F., Ranalli, M. G., Haddix, M. L., Six, J. & Lugato, E. Soil carbon storage
informed by particulate and mineral-associated organic matter. _Nat. Geosci._ 12, 989–994 (2019). Article CAS Google Scholar * Doetterl, S. et al. Soil carbon storage controlled by
interactions between geochemistry and climate. _Nat. Geosci._ 8, 780–783 (2015). Article CAS Google Scholar * Masiello, C. A., Chadwick, O. A., Southon, J., Torn, M. S. & Harden, J.
W. Weathering controls on mechanisms of carbon storage in grassland soils. _Glob. Biogeochem. Cycles_ 18, 1–9 (2004). Article Google Scholar * Lawrence, C. R., Harden, J. W., Xu, X.,
Schulz, M. S. & Trumbore, S. E. Long-term controls on soil organic carbon with depth and time: a case study from the Cowlitz River Chronosequence, WA USA. _Geoderma_ 247–248, 73–87
(2015). Article Google Scholar * Rasmussen, C. et al. Controls on soil organic carbon partitioning and stabilization in the california sierra nevada. _Soil. Syst._ 2, 1–18 (2018). Article
Google Scholar * Baisden, W. T. et al. A multiisotope C and N modeling analysis of soil organic matter turnover and transport as a function of soil depth in a California annual grassland
soil chronosequence. _Glob. Biogeochem. Cycles_ 16, 82-1-82–26 (2002). Article Google Scholar * Baisden, W. T., Amundson, R., Cook, A. C. & Brenner, D. L. Turnover and storage of C and
N in five density fractions from California annual grassland surface soils. _Glob. Biogeochem. Cycles_ 16, 64-1-64–16 (2002). Article Google Scholar * Delgado-Baquerizo, M. et al. Changes
in belowground biodiversity during ecosystem development. _Proc. Natl. Acad. Sci. USA_ 116, 6891–6896 (2019). Article CAS Google Scholar * Golchin, A., Oades, J. M., Skjemstad, J. O.
& Clarke, P. Soil structure and carbon cycling. _Aust. J. Soil. Res._ 32, 1043–1063 (1994). Article Google Scholar * Rey, A., Pegoraro, E. & Jarvis, P. G. Carbon mineralization
rates at different soil depths across a network of European forest sites (FORCAST). _Eur. J. Soil. Sci._ 59, 1049–1062 (2008). Article CAS Google Scholar * Crowther, T. W. et al.
Quantifying global soil carbon losses in response to warming. _Nature_ 540, 104–108 (2016). Article CAS Google Scholar * Ellert, B. H., Janzen, H. H., VandenBygaart, A. J. & Bremer,
E. Measuring change in soil organic carbon storage. in _Soil Sampling and Methods of Analysis_ (eds. Carter, M. R. & Gregorich, E. G.) 25–38 (CRC Press, 2007). * Tsai, H., Huang, W. S.,
Hseu, Z. Y. & Chen, Z. S. A river terrace soil chronosequence of the Pakua tableland in central Taiwan. _Soil. Sci._ 171, 167–179 (2006). Article CAS Google Scholar * Nottingham, A.
T., Meir, P., Velasquez, E. & Turner, B. L. Soil carbon loss by experimental warming in a tropical forest. _Nature_ 584, 234–237 (2020). Article CAS Google Scholar * Giardina, C. P.
& Ryan, M. G. Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. _Nature_ 404, 858–861 (2000). Article CAS Google Scholar * Qin, S. et
al. Temperature sensitivity of SOM decomposition governed by aggregate protection and microbial communities. _Sci. Adv._ 5, eaau1218 (2019). Article CAS Google Scholar * Nave, L. E. et
al. Patterns and predictors of soil organic carbon storage across a continental-scale network. _Biogeochemistry_ https://doi.org/10.1007/s10533-020-00745-9 (2021). * Delgado-Baquerizo, M. et
al. The influence of soil age on ecosystem structure and function across biomes. _Nat. Commun._ 11, 4721 (2020). Article CAS Google Scholar * Kottek, M., Grieser, J., Beck, C., Rudolf,
B. & Rubel, F. World map of the Köppen-Geiger climate classification updated. _Meteorol. Z._ 15, 259–263 (2006). Article Google Scholar * Maestre, F. T. et al. Plant species richness
and ecosystem multifunctionality in global drylands. _Science_ 335, 214–218 (2012). Article CAS Google Scholar * Paruelo, J. M., Epstein, H. E., Lauenroth, W. K. & Burke, I. C. ANPP
estimates from NDVI for the Central Grassland Region of the United States. _Ecology_ 78, 953 (1997). Article Google Scholar * Running, S. W. Estimating terrestrial primary productivity by
combining remote sensing and ecosystem simulation. in _Remote Sensing of_ _Biosphere Functioning_ (eds. Hobbs, R. J. & Mooney, H. A.) 65–86 (Springer, 1990). * Rafique, R., Zhao, F., De
Jong, R., Zeng, N. & Asrar, G. R. Global and regional variability and change in terrestrial ecosystems net primary production and NDVI: a model-data comparison. _Remote Sens_. 8, 177
(2016). * Jobbágy, E. G. & Jackson, R. B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. _Ecol. Appl._ 10, 423–436 (2000). Article Google
Scholar * Kettler, T. A., Doran, J. W. & Gilbert, T. L. Simplified method for soil particle-size determination to accompany soil-quality analyses. _Soil. Sci. Soc. Am. J._ 65, 849–852
(2001). Article CAS Google Scholar * Baillie, I. C., Anderson, J. M. & Ingram, J. S. I. _Tropical Soil Biology and Fertility. A Handbook of Methods_ 78 (C.A.B. International, 1990). *
Sohi, S. P. et al. A procedure for isolating soil organic matter fractions suitable for modeling. _Soil. Sci. Soc. Am. J._ 65, 1121–1128 (2001). Article CAS Google Scholar * Plaza, C.,
Giannetta, B., Benavente, I., Vischetti, C. & Zaccone, C. Density-based fractionation of soil organic matter: effects of heavy liquid and heavy fraction washing. _Sci. Rep_. 9, 10146
(2019). * Gosling, P., Parsons, N. & Bending, G. D. What are the primary factors controlling the light fraction and particulate soil organic matter content of agricultural soils? _Biol.
Fertil. Soils_ 49, 1001–1014 (2013). Article Google Scholar * North, P. F. Towards an absolute measurement of soil structural stability using ultrasound. _J. Soil. Sci._ 27, 451–459
(1976). Article Google Scholar * Oorts, K., Vanlauwe, B., Recous, S. & Merckx, R. Redistribution of particulate organic matter during ultrasonic dispersion of highly weathered soils.
_Eur. J. Soil. Sci._ 56, 77–91 (2005). Article CAS Google Scholar * Roscoe, R., Buurman, P. & Velthorst, E. J. Disruption of soil aggregates by varied amounts of ultrasonic energy in
fractionation of organic matter of a clay Latosol: carbon, nitrogen and δ13C distribution in particle-size fractions. _Eur. J. Soil. Sci._ 51, 445–454 (2000). Article Google Scholar * Six,
J., Elliott, E. T., Paustian, K. & Doran, J. W. Aggregation and soil organic matter accumulation in cultivated and native grassland soils. _Soil. Sci. Soc. Am. J._ 62, 1367–1377 (1998).
Article CAS Google Scholar * Six, J., Elliott, E. T. & Paustian, K. Aggregate and soil organic matter dynamics under conventional and no-tillage systems. _Soil. Sci. Soc. Am. J._ 63,
1350–1358 (1999). Article CAS Google Scholar * Six, J., Paustian, K., Elliott, E. T. & Combrink, C. Soil structure and organic matter: I. Distribution of aggregate-size classes and
aggregate-associated carbon. _Soil. Sci. Soc. Am. J._ 64, 681–689 (2000). Article CAS Google Scholar * Harris, D., Horwáth, W. R. & Van Kessel, C. Acid fumigation of soils to remove
carbonates prior to total organic carbon or carbon-13 isotopic analysis. _Soil. Sci. Soc. Am. J._ 65, 1853–1856 (2001). Article CAS Google Scholar * Sokol, N. W. & Bradford, M. A.
Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. _Nat. Geosci._ 12, 46–53 (2019). Article CAS Google Scholar * Creamer, R. E. et al.
Measuring basal soil respiration across Europe: do incubation temperature and incubation period matter? _Ecol. Indic._ 36, 409–418 (2014). Article Google Scholar * Melillo, J. M. et al.
Long-term pattern and magnitude of soil carbon feedback to the climate system in a warming world. _Science_ 358, 101–105 (2017). Article CAS Google Scholar * Carey, J. C. et al.
Temperature response of soil respiration largely unaltered with experimental warming. _Proc. Natl. Acad. Sci. USA_ 113, 13797–13802 (2016). Article CAS Google Scholar * Meyer, N., Welp,
G. & Amelung, W. The temperature sensitivity (Q10) of soil respiration: controlling factors and spatial prediction at regional scale based on environmental soil classes. _Glob.
Biogeochem. Cycles_ 32, 306–323 (2018). Article CAS Google Scholar * Dacal, M., Bradford, M. A., Plaza, C., Maestre, F. T. & García-Palacios, P. Soil microbial respiration adapts to
ambient temperature in global drylands. _Nat. Ecol. Evol._ 3, 232–238 (2019). Article Google Scholar * Campbell, C. D., Chapman, S. J., Cameron, C. M., Davidson, M. S. & Potts, J. M. A
rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole
soil. _Appl. Environ. Microbiol._ 69, 3593–3599 (2003). Article CAS Google Scholar * Bradford, M. A., Watts, B. W. & Davies, C. A. Thermal adaptation of heterotrophic soil respiration
in laboratory microcosms. _Glob. Chang. Biol._ 16, 1576–1588 (2010). Article Google Scholar * Bradford, M. A. et al. Cross-biome patterns in soil microbial respiration predictable from
evolutionary theory on thermal adaptation. _Nat. Ecol. Evol._ 3, 223–231 (2019). Article Google Scholar * Tucker, C. L., Bell, J., Pendall, E. & Ogle, K. Does declining carbon-use
efficiency explain thermal acclimation of soil respiration with warming? _Glob. Chang. Biol._ 19, 252–263 (2013). Article Google Scholar * Ewing, S. A., Sanderman, J., Baisden, W. T.,
Wang, Y. & Amundson, R. Role of large-scale soil structure in organic carbon turnover: Evidence from California grassland soils. _J. Geophys. Res. Biogeosci._ 111, G03012 (2006). *
Grace, J. B. _Structural Equation Modeling and Natural Systems_ (Cambridge University Press, 2006). * Laliberté, E. et al. Soil fertility shapes belowground food webs across a regional
climate gradient. _Ecol. Lett._ 20, 1273–1284 (2017). Article Google Scholar * IBM Corp. _IBM SPSS Statistics for Macbook_ (IBM Corp, 2017). * R Core Team. _R: A Language and Environment
for Statistical Computing_ (R Core Team, 2021). * Lüdecke, D. ggeffects: tidy data frames of marginal effects from regression models. _J. Open. Source Softw._ 3, 772 (2018). Article Google
Scholar * Campitelli, E. ggnewscale: multiple fill and colour scales in ‘ggplot2’. (2022). * Xu, S. ggstar: multiple geometric shape point layer for ‘ggplot2’. (2021). * Wilke, C. O.
ggtext: improved text rendering support for ‘ggplot2’. R package version 0.1.1. (2020). * Bates, D., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting linear mixed-effects models using
lme4. _J. Stat. Softw._ 67, 1–48 (2015). Article Google Scholar * Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest Package: tests in linear mixed effects models. _J.
Stat. Softw._ 82, 1–26 (2017). Article Google Scholar * Pedersen, T. L. patchwork: the Composer of Plots. R package version 1.1.1. https://CRAN.R-project.org/package=patchwork. (2020). *
Wickham, H. & Brian, J. readxl: Read Excel Files (Version 1.3.1). R package version 1.3.1. https://cran.r-project.or (2019). * Bivand, R. & Rundel, C. rgeos: Interface to Geometry
Engine - Open Source (‘GEOS’). (2021). * South, A. rnaturalearth: world map data from Natural Earth. R package version 0.1. 0. The R Foundation.
https://CRAN.R-project.org/package=rnaturalearth (2017). * Wickham, H. et al. Welcome to the Tidyverse. _J. Open. Source Softw._ 4, 1686 (2019). Article Google Scholar Download references
ACKNOWLEDGEMENTS M.D.-B. and P.G.-P. were supported by Ramón y Cajal grants from the Spanish Ministry of Science and Innovation (RYC2018-025483-I and RYC2018-024766-I). This project received
funding from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement 702057 and the Spanish State Plan for Scientific and
Technical Research and Innovation (2013–2016), award ref. AGL201675762-R (AEI/FEDER, UE). M.D.-B. acknowledges support from the Spanish Ministry of Science and Innovation for the I+D+i
project PID2020-115813RA-I00 funded by MCIN/AEI/10.13039/501100011033. M.D.-B. is also supported by a project of the Fondo Europeo de Desarrollo Regional (FEDER) and the Consejería de
Transformación Económica, Industria, Conocimiento y Universidades of the Junta de Andalucía (FEDER Andalucía 2014–2020 Objetivo temático “01—Refuerzo de la investigación, el desarrollo
tecnológico y la innovación”) associated with the research project P20_00879 (ANDABIOMA). C.P. acknowledges support from the EU H2020 research and innovation programme under grant agreement
No 101000224. F.B. acknowledges support from CSIC i-LINK + 2018 (LINKA20069), PID2020-114942RB-I00 funded by MCIN/AEI/10.13039/501100011033 and Fundación Séneca from Murcia Province
(19896/GERM/15). We thank the researchers involved in the CLIMIFUN project for collection of field data and soil samples. We would like to thank Fernando Maestre, Victoria Ochoa and Beatriz
Gozalo for their help with lab analyses, Lynn Riedel, Julie Larson, Katy Waechter and Drs. David Buckner and Brian Anacker for their help with soil sampling in the chronosequence from
Colorado, and to the City of Boulder Open Space and Mountain Parks for allowing us to conduct these collections. We also thank Melissa Martin for revising the English in this manuscript. We
also thank Dr. Santiago Soliveres (University of Alicante, Spain) and Dr. Miguel Berdugo (Pompeu Fabra University, Spain) for helpful comments on an earlier version of the manuscript. AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Instituto de Ciencias Agrarias (ICA), CSIC, Serrano 115 bis, 28006, Madrid, Spain César Plaza & Pablo García-Palacios * Department of Life and
Environmental Sciences and Sierra Nevada Research Institute, University of California Merced, Merced, CA, 95343, USA Asmeret Asefaw Berhe * Departamento de Sistemas Físicos, Químicos y
Naturales, Universidad Pablo de Olavide, 41013, Sevilla, Spain Jesús Barquero * CEBAS-CSIC, Department of Soil and Water Conservation, Campus Universitario de Espinardo, 30100, Murcia, Spain
Felipe Bastida * Department of Earth and Environmental Sciences, Michael Smith Building, The University of Manchester, Oxford Road, Manchester, M13 9PT, UK G. Kenny Png & Richard D.
Bardgett * Asian School of the Environment, Nanyang Technological University, 50 Nanyang Avenue, Singapore, 639798, Singapore G. Kenny Png * Departamento de Biogeografía y Cambio Global,
Museo Nacional de Ciencias Naturales (MNCN), CSIC, Serrano 115 bis, 28006, Madrid, Spain Ana Rey * Laboratorio de Biodiversidad y Funcionamiento Ecosistémico, Instituto de Recursos Naturales
y Agrobiología de Sevilla (IRNAS), CSIC, Av. Reina Mercedes 10, E-41012, Sevilla, Spain Manuel Delgado-Baquerizo * Unidad Asociada CSIC-UPO (BioFun), Universidad Pablo de Olavide, 41013,
Sevilla, Spain Manuel Delgado-Baquerizo Authors * César Plaza View author publications You can also search for this author inPubMed Google Scholar * Pablo García-Palacios View author
publications You can also search for this author inPubMed Google Scholar * Asmeret Asefaw Berhe View author publications You can also search for this author inPubMed Google Scholar * Jesús
Barquero View author publications You can also search for this author inPubMed Google Scholar * Felipe Bastida View author publications You can also search for this author inPubMed Google
Scholar * G. Kenny Png View author publications You can also search for this author inPubMed Google Scholar * Ana Rey View author publications You can also search for this author inPubMed
Google Scholar * Richard D. Bardgett View author publications You can also search for this author inPubMed Google Scholar * Manuel Delgado-Baquerizo View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS M.D.-B., C.P. and P.G.-P. designed the study presented in the manuscript. M.D.-B. devised the chronosequence survey and
coordinated all the research activities. C.P., P.G.-P., J.B., F.B., G.K.P., A.R. and M.D.-B. conducted laboratory research. M.D.-B., P.G.-P. and C.P. performed data analysis. C.P., P.G.-P.,
A.A.B., J.B., F.B., G.K.P., A.R., R.D.B. and M.D.-B. substantially discussed the results. C.P., M.D.-B. and P.G.-P. wrote the initial draft of the manuscript, and A.A.B., J.B., F.B., G.K.P.,
A.R. and R.D.B. significantly contributed to editing. CORRESPONDING AUTHORS Correspondence to César Plaza or Manuel Delgado-Baquerizo. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications Earth & Environment_ thanks Wenjuan Huang and the other, anonymous, reviewer(s) for their contribution
to the peer review of this work. Primary Handling Editors: Erika Buscardo and Clare Davis. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1
REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Plaza, C., García-Palacios, P., Berhe, A.A. _et al._ Ecosystem productivity has a stronger influence than soil age on surface soil carbon storage across global biomes.
_Commun Earth Environ_ 3, 233 (2022). https://doi.org/10.1038/s43247-022-00567-7 Download citation * Received: 25 November 2021 * Accepted: 28 September 2022 * Published: 07 October 2022 *
DOI: https://doi.org/10.1038/s43247-022-00567-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
What we’ve learnt from building africa’s biggest genome libraryThe human genome was first sequenced in 2003 by multiple research centres across the world. The breakthrough was hailed ...
Microfluidic electronic paper | Nature PhotonicsPigmented inks in a microfluidic structure provide a new approach for fabricating bright, colourful electronic paper wit...
Targeted modulation of immune cells and tissues using engineered biomaterialsABSTRACT Therapies modulating the immune system offer the prospect of treating a wide range of conditions including infe...
Jalisco officials say this indigenous leader died in a car crash. His community isn't buying itThe Indigenous Wixárika community in northern Jalisco is raising doubts about the official explanation that a vehicle ac...
Over 500 chiapas residents flee cartel violence into guatemalaHundreds of Mexican families fleeing cartel violence in Mexico’s southern state of Chiapas have sought refuge across the...
Latests News
Ecosystem productivity has a stronger influence than soil age on surface soil carbon storage across global biomesABSTRACT Interactions between soil organic matter and minerals largely govern the carbon sequestration capacity of soils...
Pfizer-biontech covid-19 vaccine gets full fda approvalMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
Javascript support required...
Livestock farmers reminded of new export forms from december - farmers weekly© Savo Ilic/Adobe Stock Livestock farmers will need to join a farm assurance scheme or ask a vet to vouch for the health...
Tesla semi truck event live - how to watch elon musk unveiling new...UPDATE TWO Some people are stuggling to access the live stream for the Tesla Semit truck unveiling. Once entering their...