Fatty acid synthase, a novel poor prognostic factor for acute lymphoblastic leukemia which can be targeted by ginger extract
Fatty acid synthase, a novel poor prognostic factor for acute lymphoblastic leukemia which can be targeted by ginger extract"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Altered metabolism of fatty acid synthesis is considered a hallmark characteristic of several malignancies, including acute lymphoblastic leukemia (ALL). To evaluate the impact of
fatty acid synthase (FASN) on drug resistant ALL, bone marrow samples were collected from 65 pediatric ALLs, including 40 de novo and 25 relapsed patients. 22 non-cancer individuals were
chosen as controls. Quantitative RT-PCR showed increased expression levels of _FASN_ in drug resistant patients compared with the therapy responders. Single and combined treatment of
malignant cells were analyzed using Annexin-V/PI double staining and MTT assays. Incubation of resistant primary cells with ginger showed simultaneous increased apoptosis rates and reduced
_FASN_ expression levels. Furthermore, docking studies demonstrated high affinity bindings between ginger derivatives and FASN thioesterase and ketosynthase domains, compared with their
known inhibitors, fenofibrate and morin, respectively. Finally, combined treatment of in-house multidrug resistant T-ALL subline with ginger and dexamethasone induced drug sensitivity and
down regulation of _FASN_ expression, accordingly. To the best of our knowledge, this is the first study that introduces _FASN_ upregulation as a poor prognostic factor for drug resistant
childhood ALL. Moreover, it was revealed that FASN inhibition may be applied by ginger phytochemicals and overcome dexamethasone resistance, subsequently. SIMILAR CONTENT BEING VIEWED BY
OTHERS STEAROYL-COA DESATURASE INHIBITION IS TOXIC TO ACUTE MYELOID LEUKEMIA DISPLAYING HIGH LEVELS OF THE DE NOVO FATTY ACID BIOSYNTHESIS AND DESATURATION Article Open access 26 August 2024
FUNCTIONAL CHARACTERIZATION OF THE PI3K/AKT/MTOR SIGNALING PATHWAY FOR TARGETED THERAPY IN B-PRECURSOR ACUTE LYMPHOBLASTIC LEUKEMIA Article Open access 06 July 2022 TARGETING ONCOGENIC
ACTIVATION OF FLT3/SREBP/FASN PROMOTES THE THERAPEUTIC EFFECT OF QUIZARTINIB INVOLVING DISRUPTION OF MITOCHONDRIAL PHOSPHOLIPIDS Article Open access 22 April 2025 INTRODUCTION Acute
lymphoblastic leukemia (ALL) is the most common type of hematological malignancy in children1,2. Despite the enormous advances in modern medicine and development of innovative therapeutic
strategies, disease relapse remains a leading cause of cancer-related morbidity and mortality in children3. Metabolic rearrangements are vital to satisfy the different requirements of cancer
cells during tumorigenesis4. Elevated de novo fatty acid biosynthesis is a hallmark adaptation in many cancers that supply signaling molecules and basic elements for lipid biosynthesis5.
While most normal cells supply their fatty acids from dietary sources, cancer cells reactivate de novo fatty acid synthesis6. Fatty acid synthase (FASN) is a multifunctional protein
containing six enzymatic domains that catalyzes the biosynthesis of palmitate5. Elevated expression of _FASN_ is found to be associated with poor prognosis and higher risk of recurrence in a
number of human cancers. Indeed, _FASN_ overexpression has been shown to contribute to multidrug resistance (MDR). Multi-drug resistance is one of the major obstacles to the successful
treatment of various types of cancer, particularly childhood ALL5,7,8. Glucocorticoids (GCs) such as prednisone and dexamethasone (DEX) are indispensable drugs for childhood ALL treatment9.
Early response to glucocorticoids is a positive prognostic indicator and glucocorticoid resistance has been associated with an increased risk of relapse and poor clinical response10,11.
Glucocorticoids regulate _FASN_ expression and subsequently affect lipogenesis12. Therefore, FASN knock down or inhibition of its activity is recognized as an attractive therapeutic
approach. Moreover, FASN can be considered as a target in combinational therapy. However, early generation of FASN inhibitors including cerulenin, orlistat and C75 have limitations such as
chemical instability, low bioavailability and undesirable side effects like body weight loss, that restrict their clinical development5,13. The aim of the current study was to evaluate
_FASN_ expression levels in children with ALL and those who were resistant to chemotherapy. Furthermore, we examined the effect of ginger extract (_Zingiber officinale_) on _FASN_ expression
levels in leukemic cell lines and patients primary cells. Recent studies revealed that some ginger components can reduce FASN expression. Therefore, combined treatment was performed with
ginger and dexamethasone together on the CCRF-CEM/MVCD resistant subline. In the final step, molecular docking was recruited to determine the best ginger phytochemicals which could interfere
with FASN activity through binding its first and last catalytic domains. To the best of our knowledge, this is the first study to introduce _FASN_ as a poor prognostic marker for pediatric
ALL patients. In addition, we evaluated the cytotoxicity of ginger extract and its capacity to down-regulate _FASN_ expression in ALL relapsed patients. Finally, we demonstrated that ginger
phytochemicals may inhibit FASN activity and ginger may induce susceptibility to dexamethasone in the ALL resistant subline, and decrease _FASN_ expression levels, accordingly. RESULTS
PATIENTS CHARACTERISTICS The clinical characteristics of the ALL patients are summarized in Table 1b. Among the 65 patients, 40 cases were newly diagnosed, and 25 patients were relapsed
cases. 22 non-cancer bone marrow specimens were used as the control group. Controls were age/gender-matched children (12 (54.5%) males and 10 (45.5%) females < 12 years of age) who were
administered to the hospital with thrombocytopenia. However, no evidence of cancer was found in their bone marrow aspirates. Regarding their response to one year chemotherapy, the newly
diagnosed patients were divided into 9 MRD+ and 31 MRD− patients followed by PCR-SSCP analyses. RELATIVE EXPRESSION LEVELS OF _FASN_ IN DE NOVO PATIENTS In order to characterize the
expression pattern of _FASN_ in children with ALL, quantitative reverse transcriptase polymerase chain reaction was used to determine the expression levels of this gene in the bone marrow
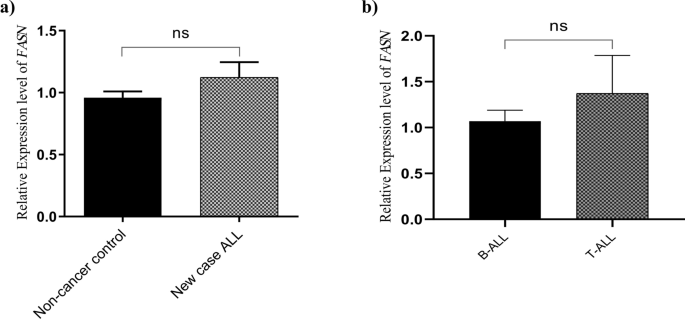
mononuclear cell samples of 40 children with newly diagnosed ALL and 22 non-cancer control cases. Results indicated no significant difference in the expression levels of _FASN_ in de novo
patients compared to the control group [1.123 ± 0.1228 vs. 0.9596 ± 0.05020 vs. mean ± SEM, _P_ = 0.3432] (Fig. 1a). To determine whether there was any significant difference between two
subtypes of ALL, the mRNA expression levels of _FASN_ was analyzed in 36 B-ALL and 4 T-ALL samples. As shown in Fig. 1b, there was no significant difference in _FASN_ expression levels in
these two groups [1.069 ± 0.1197 vs. 1.372 ± 0.4133, mean ± SEM, _P_ = 0.3884]. _FASN_ EXPRESSION LEVELS IN DRUG SENSITIVE VS. RESISTANT PATIENTS The relative gene expression levels of
_FASN_ in MRD+ and MRD− patients are presented in Fig. 2a. A significantly higher mRNA expression level of _FASN_ was determined in MRD+ patients compared with the MRD− patients [1.841 ±
0.3311, n = 9 vs. 0.9242 ± 0.1134, n = 31, _P_ = 0.0021]. Moreover, ROC curve analysis introduced the mRNA _FASN_ level as a prognostic biomarker which may distinguish MRD+ from MRD− ALL
patients. The total area under the curve (AUC) was 0.82, confirming the ability and accuracy of this measurement to classify the innate drug resistant patients from the sensitive group (95%
CI 0.675–0.978, _P_ = 0.0039) (Fig. 2a,b). In order to determine the association between _FASN_ and adaptive drug resistance, the expression levels of _FASN_ was measured in 25 relapsed
patients. It was revealed that _FASN_ was significantly upregulated in ALL relapsed group compared with the MRD− patients (1.169 ± 0.15 vs. 0.7669 ± 0.09448, mean ± SEM, _P_ = 0.0180).
Moreover, ROC curve analysis revealed that _FASN_ expression levels could discriminate between the relapsed and MRD− patients (AUC = 0.7023, 95% CI 0.545–0.891, _P_ = 0.0187) (Fig. 2c,d).
EFFECT OF GINGER EXTRACT ON _FASN_ EXPRESSION IN PRIMARY ALL CELLS The anti-leukemic effect of ginger extract was previously introduced by our group. Moreover, it was shown that this effect
was not attributed to the expression levels of ABC transporters14. In order to identify the possible mechanism through which ginger could conquer ALL multidrug resistance in patients primary
cells, fresh samples were collected from 7 children with relapsed ALL and 1 non-cancer control, treated with 167 μg/ml ginger extract for 48 h, and analyzed for any post-treatment
alteration of the _FASN_ expression levels. Cell death was measured using Annexin V/PI double staining and flow cytometry analysis. Results supported our previous data considering the
significantly increased cell death in ginger treated patient samples compared with the untreated cells [39.11 ± 9.089% vs. 18.80 ± 7.433%, mean ± SEM, _P_ = 0.0023]. In addition normal
mononuclear cells (MNCs) were not significantly sensitive to proliferation inhibition of ginger extract (Fig. 3a). On the other hand, RT-PCR showed that _FASN_ expression in relapsed
patients was decreased upon cells exposure to ginger in comparison with the untreated samples (0.5894 ± 0.08593, mean ± SEM, _P_ = 0.0031) but in normal MNCs were not significantly decreased
(Fig. 3b). EFFECT OF GINGER EXTRACT ON CCRF-CEM AND DEXAMETHASONE RESISTANT CCRF-CEM/MVCD SUBLINE The cytotoxic effect of ginger extract on the in house multidrug resistant CCRF-CEM/MVCD
subline was previously defined14, and RT-PCR showed overexpression of _FASN_ in this cell line compared with normal MNCs and its parental cell (_P_ = 0.0119 and _P_ = 0.0241, respectively)
(Fig. 4a). Considering our previous data regarding CCRF-CEM/MVCD, among diverse examined chemotherapy drugs, dexamethasone showed the highest half maximal concentration (IC50) for inhibiting
cell growth (Table 2). To investigate the possible impact of ginger in generating CCRF-CEM/MVCD sensitivity to dexamethasone, cells were treated with ginger extract, alone and in
combination with dexamethasone. MTT assay was performed in addition to RT-PCR in order to determine the expression levels of _FASN_. Results showed that the cytotoxic effect of
dexamethasone/ginger extract was significantly more than that of the dexamethasone alone. In other words, cell viability was reduced down to 15.4 ± 0.821% in the presence of a combination of
1,000 μM dexamethasone with 167 μg/ml ginger, compared with dexamethasone alone (56.794 ± 0.808%, _P_ = 0.0008) (Fig. 4b). Subsequently, RT-PCR demonstrated decrease in _FASN_ expression
followed by combination therapy compared with single drug treatment [1.791 ± 0.043 vs. 3.2 ± 0.210 (mean ± SEM; n = 2), P = 0.0225] (Fig. 4c). Interestingly, _FASN_ expression level was
increased in response to incubation with dexamethasone alone (_P_ = 0.0116). DOCKING RESULTS OF THE THIOESTERASE (TE) DOMAIN The molecular surfaces of the FASN TE and KS domains were
illustrated using in silico studies (Fig S1). The related interacting residues and binding energy of each docked ligand to the active site of the TE domain were calculated and demonstrated
in Table 3. As shown, among diverse ginger phytochemicals (Fig S2), gingerenone family molecules had the highest affinity to the substrate binding site of the TE domain. Interestingly, the
binding energies of these molecules were as low as fenofibrate, an experimentally-proved TE inhibitor15, and their binding energies were markedly lower than orlistat; which is another known
TE inhibitor. The interactions of fenofibrate and other gingerenones with the TE domain are illustrated in Fig. 5 and Fig. S3. As shown, gingerenone C covers both, the interface cavity and
specificity channel, generating the highest affinity towards the TE domain (see Fig. S4 for more details). Other gingerenone family members, not only lie on the specificity channel and block
the substrate binding site, but also extend to the catalytic site and form several hydrogen bonds with important residues for catalytic activity such as Ser2308 and Tyr234316, through which
the enzyme could be suppressed. DOCKING RESULTS OF THE KETOSYNTHASE (KS) DOMAIN Binding energies of docked ligands to the active-site cavity of the KS domain and interacting residues of
each KS-ligand complex are shown in Table 4. In the present study, none of our ligands displayed a significant affinity to the distal substrate binding site. By contrast, several ginger
compounds, including quercetin and gingerenone family molecules, showed high affinity to the active-site cavity. Through the occupation of the cavity volume, these ligands may block
accessibility to the active site residues Cys161, His293 and His331 and inhibit FASN activity. Morin, the well-known KS domain inhibitor, shares similar molecular scaffold and binding modes
to the KS domain with quercetin. However, none of them show direct interaction with the main KS active site residues, implying that their inhibitory function might be due to the blocking of
the substrate entry (Fig. 6, Fig. S5). In contrast, the gingerenone family molecules, may not only block the substrate entry, but also enter deep inside the cavity and form several hydrogen
bonds with some of the main active site residues such as His293 and His331. (Fig. 6, Figs. S5, S6). Moreover, gingerenones form pi stacking interactions with His293 (data not shown).
Cerulenin and C75, the two other docked known inhibitors, showed lower affinity to the KS domain in comparison to the majority of docked ginger phytochemicals. DISCUSSION Despite remarkable
advances in the treatment strategies, drug resistance is still a major cause of chemotherapy failure leading to relapse in pediatric acute lymphoblastic leukemia17. Multiple studies
suggested that metabolic rearrangements, newly recognized as prominent features of cancer18, are associated with the development of drug resistance in cancer cells19. Changes in lipid
metabolism, in particular increased synthesis of fatty acids, are recognized as one of the key hallmarks in several cancer cells. Besides, _FASN_ overexpression has shown to be associated
with poor prognosis and resistance to chemotherapy20. In the current study, the expression profile of _FASN_ was determined in children with ALL. Although our findings showed no increase in
_FASN_ mRNA levels in de novo ALL patients compared with the control group, _FASN_ showed significant upregulation in positive MRD patients known as drug resistant group compared with the
drug sensitive or MRD− group. These data supported the hypothesis that _FASN_ up-regulation contributes to poor response to chemotherapy. We also examined the potential prognostic value of
_FASN_ dysregulation in both intrinsic and adaptive drug resistance using ROC curve analysis. Results showed AUCs of 0.82 and 0.7 in discriminating MRD+ from MRD− (_P_ = 0.0039) new case
samples with one year follow up, and relapsed patients from MRD− individuals (_P_ = 0.0187), respectively. Accordingly, it can be hypothesized that _FASN_ might be served as a potential
prognostic biomarker in pediatric ALL. The increased expression levels of _FASN_ in a relapsed patient, compared with the expression levels of this gene at the time of diagnosis (0.075 vs.
1.14, respectively) was another interesting support for this hypothesis (data not shown). Investigating larger populations of ALL paired samples in prospective cohort studies may help
intensify the validity of these results. An increasing number of studies have examined the anti-cancer activity of ginger and its bioactive compounds in drug resistant cancer cells21. Our
group previously reported the anti-leukemic effect of ginger extract. On the other hand, It was shown that fresh normal peripheral MNCs were not significantly sensitive to proliferation
inhibition induced by 50% inhibition concentration of the ginger extract14. Furthermore, it was shown that this effect was not ascribed to the expression levels of ABC transporters. To
identify the possible targets through which ginger may exert its cytotoxic effect on drug-resistant cells, the expression profile of the _FASN_ was analyzed after treating the relapsed ALL
patients primary cells with ginger extract. Results revealed that cell death was significantly increased in ginger treated samples compared with the untreated cells. On top of that, _FASN_
expression was decreased upon cells exposure to ginger compared with the untreated samples. Our results shared a number of similarities with Impheng et al. findings which demonstrated that
[6]-gingerol, one of the derivatives of ginger, reduces de novo fatty acid synthesis, resulting in mitochondrial dysfunction and induction of cell death in HepG2 cells22. Considering the
possible contribution of FASN in drug resistance, and the negative impact of glucocorticoids on _FASN_ expression levels in B-ALL cell lines12, we further investigated the effect of
dexamethasone on the sensitive and resistant T-leukemic cells, using RT-PCR followed by MTT assays. The rationale for selecting T cells in these examinations was the aggressive behavior of
this phenotype and the low survival rate of T-ALL patients compared with those with B-ALL23. Results showed that resistance to dexamethasone was associated with failure to _FASN_
downregulation (Fig. 4b). Considering the cytotoxicity of ginger extract on resistant patient primary cells, combination treatments were designed to determine whether ginger extract may
overcome resistance to dexamethasone. Intriguingly, results showed that ginger/dexamethasone combined therapy was associated with decreased expression levels of _FASN_ and cell growth
inhibition (Fig. 4b,c). These data may open up new avenues for improved combination therapies against leukemia drug resistance. In cancer cells dysregulation of de novo FA synthesis and
upregulation of enzymes involved in this pathway occur largely at the transcriptional levels through the activation of sterol regulatory element-binding proteins (SREBPs)24. The activity of
SREBP is regulated by mTORC1, one of the crucial downstream effector of AKT25. In both B-cell and T-cell ALL primary bone marrow samples, AKT hyperactivation has been observed11. Similar to
this data was our comparison between the transcripts levels of _FASN_ in B-ALL and T-ALL patients which revealed no marked difference between these two groups. Cancer cells are extremely
dependent on de novo lipogenesis for proliferation and survival. Therefore, FASN inhibitors seem to play promising role in cancer treatment. It is shown that FASN inhibitors can induce tumor
cell apoptosis and sensitize breast cancer cells to chemotherapies7. However, off-target activities and detrimental systemic side effects of such components have prevented their clinical
development. On the other hand, anticancer activity of the plant components is currently undergoing preclinical evaluation. Therefore, in the next step of this research, molecular docking
was used to determine which one of the ginger phytochemicals can interfere with FASN activity. From the six different catalytic domains of FASN, KS (β-ketoacyl synthase) and TE
(thioesterase), known as the first and the last catalytic domains of this enzyme, were chosen26. Subsequently, we retrieved twenty ginger phytochemicals from published literatures and
investigated their inhibitory interactions with FASN. Since FASN thioesterase domain is involved in palmitate synthesis termination and also in maintaining of the length of fatty acid chain,
it is particularly a promising target to inhibit the enzyme activity (TE dom). Orlistat and fenofibrate are the FASN inhibitors which can prevent tumor growth and induce malignant cell
death through blocking the TE domain15. In order to predict the inhibitory effects of ginger phytochemicals on FASN activity, they were docked with the crystal structure of TE domain and
their binding energies were compared to those of orlistat and fenofibrate, in order to prioritize these ligands. Docking results revealed the binding energies of fenofibrate and orlistat to
be − 7.4 and − 6.7 kcal/mol, respectively. Of all docked ginger phytochemicals, gingernone family molecules showed the highest binding affinity to FAS-TE domain (C form: − 7.5 kcal/mol, B
form: − 7.4 kcal/mol and A form: − 7.1 kcal/mol) even higher than orlistat which is a US FDA-approved and marketed drug for management of obesity, acting through FAS-TE inhibition26 (Fig. 5,
Fig. S3). The first catalytic domain of FASN is KS27. Concerning the KS domain, several inhibitors such as morin, cerulenin and C75 have been reported28. The binding free energy of the 20
selected compounds against FAS-KS domain showed the highest binding affinity to the KS in quercetin (− 8.9 kcal/mol), which was even more than morin inhibitor (− 8.5), followed by
gingernones (B form: − 7.7 kcal/mol, A form: − 7.7 kcal/mol and C form: − 7.5 kcal/mol) and Isogingernone B (− 7.5 kcal/mol) (Fig. 6), showing much higher binding affinities than the known
KS inhibitors, cerulenin and C75 (− 6.5 kcal/mol). Interestingly, it can be noted that gingernones elicit the greater inhibitory effects on both TE and KS domains among all tested ginger
compounds. Collectively, these results suggest that ginger may suppress FASN activity and overcome drug resistance through its gingernones. Additional cell-based studies and test tube
experiments including dual luciferase (Firefly-Renila) reporter assays are required to confirm the molecular docking data. In conclusion, our findings emphasize the significance of fatty
acid synthesis as a potential target for leukemia treatment. Moreover, ginger constituents are introduced as promising agents able to effectively overcome drug resistance by possibly
reducing FASN expression level and inhibiting its activity. Followed by additional FASN overexpression and knockdown studies confirming the causative role of ginger ingredients in FASN
downregulation and drug sensitivity, this laboratory investigation could be taken into consideration for the design of animal model studies followed by clinical trials to evaluate the effect
of combined treatment of ginger constituents and chemotherapeutic drugs in multidrug resistant leukemia. MATERIALS AND METHODS IN VITRO STUDIES PATIENTS AND CONTROL SAMPLES 65 children with
ALL and 22 non-cancer controls were included in the present study. Individuals were referred to Sayed-ol-Shohada Hospital, Isfahan, Iran in 2014–2017 for bone marrow evaluation. The project
was performed in accordance with the Declaration of Helsinki and permitted by the Ethics Committee of the University of Isfahan (agreement number 94/31540). All Samples of children with ALL
and non-cancer controls were collected with full written informed parents’ consents in compliance with the ethical protocols and standards of Sayed-ol-Shohada Hospital. Two to five
milliliters of bone marrow heparinized sample was collected from cALL patients and controls and sent on ice to the Cellular and Molecular Biology laboratory of University of Isfahan.
Mononuclear cells (MNCs) were isolated by sedimentation on lymphoprep density gradients (Axis Shailed Diagnostics Ltd., Oslo, Norway), according to the manufacturer recommended protocol.
HERBAL MATERIAL AND CHEMICALS Extract of ginger dried root (batch number ZSKY20140123) was purchased from Shaanxi Zhengsheng Kangyuan Bio-medical Co., Ltd (Shaanxi, China). Detailed
information about this extract is summarized in Table 1a. Dexamethasone was bought from caspiantamin (Rasht, Iran). Dimethyl-sulfoxide (DMSO) was obtained from Cinnagen (Tehran, Iran).
Roswell Park Memorial Institute-1640 (RPMI1640), fetal bovine serum (FBS), and penicillin streptomycin (Pen Strep) were purchased from Bioidea (Tehran, Iran). l-Glutamine was from Gibco (Sao
Paulo, Brazil) and phosphate buffered saline (PBS) was bought from Sigma-Aldrich (Munich, Germany). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was obtained from
Atocel (Graz, Austria). FITC Annexin-V apoptosis detection kit with PI was purchased from BioLegend (London, United Kingdom). TRIzol reagent was from Invitrogen (California, CA) and
Ficoll–Hypaque was bought from Inno-train (Kronberg, Germany). CELL LINES AND PATIENT PRIMARY CELLS CCRF-CEM (derived from a 4-year-old girl with T-ALL) human cell lines was purchased from
Pasteur Institute (Tehran, Iran). Multidrug resistant CCRF-CEM/MVCD subline was generated in-house. Briefly, CCRF-CEM cells sequentially exposed to stepwise concentrations of Methotrexate
(MTX) from 5 nM to 1.2 μM. In order to allow cells reaching regular growth rate, cells were kept in the same concentration of MTX for two or three passages. After full growth recovery, the
concentration of MTX was increased by twofold each time. Finally, it was revealed that CCRF-CEM/MVCD subline had developed cross-resistance to a number of other chemotherapy drugs including
dexamethasone. For further experiments, parental and resistant cell lines were cultured in RPMI1640 containing 10% (v/v) heat-inactivated FBS and 100 μg/ml streptomycin and 1% (v/v) 100
IU/ml penicillin. Freshly collected patient samples were grown in RPMI-1640 supplemented with 20% FBS and 1% l-glutamine. CELL TREATMENT Cell lines were seeded in 96-well cell culture plates
at a density of 15 × 104 cells per well. Cells were suspended in 100 μl supplemented media and treated with 50 μl of freshly made ginger extract (167 μg/ml) for 72 h. Combination treatment
was carried out by the addition of 25 μl ginger extract to the same volumes of dexamethasone (1,000 μM), followed by the same incubation time. MTT assay was initiated by the addition of MTT
dye to each well. After 3 h incubation at 37 °C, in order to dissolve the formazan crystals in each well, the supernatant was removed and replaced with 100 μl of DMSO. The absorbance was
measured at a wavelength of 492 nm using a Stat Fax-2100 microtiter plate reader (Palm City, FL). Cell viability ratio was evaluated as mentioned before14. FLOW CYTOMETRY ANALYSIS
Mononuclear cells isolated from relapsed patients samples and normal MNCs were seeded at a density of 25 × 104 cells per well and treated with 167 μg/ml ginger extract for 48 h. At the end
of the treatment period, cells were harvested, washed with PBS supplemented by 0.5% FBS, resuspended in 100 μl of cold 1 × Annexin-V-binding buffer after centrifugation, and incubated with 5
μl of FITC conjugated Annexin-V and 10 μl of PI at room temperature for 15 min. The quantitative analysis of cell death was conducted by BD FACSCalibur Flow Cytometer (London, UK). Data was
acquired and analyzed using Cell Quest Pro (BD Biosciences, San Jose, CA) and FlowJo softwares (Tree Star Inc., Ashland, OR). RNA EXTRACTION AND CDNA SYNTHESIS In accordance with the
manufacturer’s protocol, total RNA isolation was performed from treated cell lines as well as MNCs of patient and control samples using TRIzol reagent. Extracted RNA was transformed to cDNA
in accordance with the instructions provided by PrimeScript RT reagent kit (Takara, Japan) utilizing random hexamers and oligo dT primers. The obtained cDNA was preserved at − 20 °C for
further analyses. REAL-TIME PCR ANALYSIS Gene expression assessment was conducted utilizing ExiLENT SYBR Green master mix and Chromo4 system (Bio-Rad, Foster City, CA), according to the
manufacturer’s instructions. Data normalization was carried out utilizing glyceraldehyde-3-phosphate dehydrogenase (_GAPDH_) as the internal control gene. qRT-PCR was carried out in
duplicate according to the following cycling conditions: 5 min pre-incubation at 95 °C followed by 95 °C denaturation for 20 s, 60 °C annealing for 30 s, and 72 °C product expansion for 30
s. All relative expression levels were evaluated and reported using the 2−ΔΔCt method. The forward and reverse primers sequences for _FASN_ were CCGCTTCCGAGATTCCATCCTACGC and
GGATGGCAGTCAGGCTCACAAACG; and for _GAPDH _were GCCCCAGCAAGAGCACAAGAGGAAGA and CATGGCAACTGTGAGGAGGGGAGATT, respectively. RESPONSE TO CHEMOTHERAPY ALL patients were treated based on the
Australian and New Zealand Children's Cancer Study Group ALL study (https://www.anzctr.org.au/trial_view.aspx?ID=1568). For evaluation of the treatment response, at the end of the first
year, the presence of minimal residual disease (MRD) in new patients was assessed utilizing PCR-SSCP (Polymerase chain reaction coupled single-strand conformation polymorphism) analyses for
T-cell receptor gamma (TcRγ) and immunoglobulin heavy chain (IgH) gene rearrangements. MRD provides evidence for the presence of post-therapeutic leukemia cells within the bone marrow and
more rarely in peripheral blood circulation. Persistent monoclonality, referred to as MRD+, was considered as drug resistance and non-response chemo-treatment. MRD− individuals were
appointed as drug sensitive patients. STATISTICAL ANALYSIS SPSS23.0 and GraphPad Prism8.0.2 softwares were used to analyze the data of each experiment statistically. The Kolmogorov–Smirnov
normality (KS test) and Shapiro–Wilk tests were applied to evaluate the normality of data distribution. Kruskal–Wallis and Mann–Whitney two tailed tests were carried out to compare the
difference of continuous variables between two groups. The statistical significance of differences between two sets of data were estimated using unpaired nonparametric _t_ test. Receiver
operating characteristic (ROC) curves and the area under the ROC (AUC) were depicted, using GraphPad Prism, to evaluate the specificity and sensitivity of _FASN_ as a prognostic biomarker
for ALL patients. The greater the area under the curve, the more accurate the test. All data were expressed as mean ± standard error of mean (SEM). Data were results of 2 to 3 independent
experiments which were performed in triplicates for cell line analyses, and in duplicates for patient samples. _P_ < 0.05 was considered significant, statistically. IN SILICO STUDIES In
order to determine the inhibitory impact of ginger phytochemicals on hFASN through in silico studies, various biologically active ginger compounds were selected from literatures29,30,31,32.
Two domains of the enzyme were selected to perform docking simulation study; the ketosynthase (KS) and thioesterase (TE) domains. These domains are particularly important in targeting and
inhibiting the FASN activity since the KS domain initiates the fatty acid synthesis cycle27 and the TE domain terminates the cycle by hydrolyzing the thioester bond, which results in
releasing the 16-carbon fatty acid, palmitate33. To determine and prioritize the significance of the docking results of the chosen phytochemicals, some known inhibitors of these two domains
were selected and their binding affinities were determined as well26. DOCKING SIMULATION Methods for selection and preparation of ligands and receptors were described in the supplementary
methods. All molecular docking simulations were performed using AutoDock Vina 1.1.234 on a windows platform. Compounds and the protein preparations as well as results analyses were done
using UCSF Chimera 1.13.135, AutoDockTools 1.5.636 and LIGPLOT + 2.137. AutoDock Vina was used to perform semi-flexible docking simulations in which proteins were considered as rigid, while
ligands were allowed to be completely rotatable. Since the majority of our ligands were conformationally-flexible with large numbers of rotatable bonds, exhaustiveness was set to 24 for each
docking simulation38. Other parameters were set to default. Grid boxes were defined for each domain using AutoDockTools. For TE domain, chain B of the pdb file (ID: 2PX6) was used.
Subsequently, the Grid box was adjusted around the catalytic triad (Ser2308, Asp2338, His2481), specificity channel and interface cavity, all of which are important for substrate binding as
described by John et al.16. For KS domain, chain A of the pdb file (ID: 3HHD) was utilized and since it was comprised of KS-MAT didomain, the KS domain was isolated by removing the sequence
from Pro410 to Pro824 as described by Pappenberger et al.39. The Grid box was then adjusted around the active site residues (Cys161, His293, His331) which are deep inside the active-site
cavity and the distal substrate binding sites40. Docking simulation was done for each ligand and results were compared and analyzed. CHANGE HISTORY * _ 25 NOVEMBER 2020 An amendment to this
paper has been published and can be accessed via a link at the top of the paper. _ REFERENCES * Bhojwani, D., Yang, J. J. & Pui, C.-H. Biology of childhood acute lymphoblastic leukemia.
_Pediatr. Clin._ 62, 47–60 (2015). Google Scholar * Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. _Nat. Rev. Cancer_ 18, 471–484 (2018). Article CAS PubMed
PubMed Central Google Scholar * Iacobucci, I. & Mullighan, C. G. Genetic basis of acute lymphoblastic leukemia. _J. Clin. Oncol._ 35, 975 (2017). Article CAS PubMed PubMed Central
Google Scholar * Boroughs, L. K. & DeBerardinis, R. J. Metabolic pathways promoting cancer cell survival and growth. _Nat. Cell Biol._ 17, 351–359 (2015). Article CAS PubMed PubMed
Central Google Scholar * Buckley, D. _et al._ Fatty acid synthase–modern tumor cell biology insights into a classical oncology target. _Pharmacol. Ther._ 177, 23–31 (2017). Article CAS
PubMed Google Scholar * Chen, M. & Huang, J. The expanded role of fatty acid metabolism in cancer: new aspects and targets. _Precis. Clin. Med._ 2, 183–191 (2019). Article PubMed
PubMed Central Google Scholar * Liu, H., Liu, Y. & Zhang, J.-T. A new mechanism of drug resistance in breast cancer cells: fatty acid synthase overexpression-mediated palmitate
overproduction. _Mol. Cancer Ther._ 7, 263–270 (2008). Article PubMed Google Scholar * Wu, X., Qin, L., Fako, V. & Zhang, J.-T. Molecular mechanisms of fatty acid synthase
(FASN)-mediated resistance to anti-cancer treatments. _Adv. Biol. Regul._ 54, 214–221 (2014). Article CAS PubMed Google Scholar * Bhadri, V. A., Trahair, T. N. & Lock, R. B.
Glucocorticoid resistance in paediatric acute lymphoblastic leukaemia. _J. Paediatr. Child Health_ 48, 634–640 (2012). Article PubMed Google Scholar * Goossens, S. & Van Vlierberghe,
P. Overcoming steroid resistance in T cell acute lymphoblastic leukemia. _PLoS Med._ 13, e1002208 (2016). Article PubMed PubMed Central Google Scholar * Hall, C. P., Reynolds, C. P.
& Kang, M. H. Modulation of glucocorticoid resistance in pediatric T-cell acute lymphoblastic leukemia by increasing BIM expression with the PI3K/mTOR inhibitor BEZ235. _Clin. Cancer
Res._ 22, 621–632 (2016). Article CAS PubMed Google Scholar * Dehghan-Nayeri, N., Goudarzi Pour, K. & Eshghi, P. Over expression of the fatty acid synthase is a strong predictor of
poor prognosis and contributes to glucocorticoid resistance in B-cell acute lymphoblastic leukemia. _World Cancer Res. J._ 3, e746 (2016). Google Scholar * Jones, S. F. & Infante, J. R.
Molecular pathways: fatty acid synthase. _Clin. Cancer Res._ 21, 5434–5438 (2015). Article CAS PubMed Google Scholar * Babasheikhali, S. R., Rahgozar, S. & Mohammadi, M. Ginger
extract has anti-leukemia and anti-drug resistant effects on malignant cells. _J. Cancer Res. Clin. Oncol._ 145, 1987–1998 (2019). Article Google Scholar * You, B. J. _et al._ Fenofibrate
induces human hepatoma Hep3B cells apoptosis and necroptosis through inhibition of thioesterase domain of fatty acid synthase. _Sci. Rep._ 9, 3306. https://doi.org/10.1038/s41598-019-39778-y
(2019). Article ADS CAS PubMed PubMed Central Google Scholar * John, A., Vetrivel, U., Subramanian, K. & Deepa, P. R. Comparative docking of dual conformations in human fatty acid
synthase thioesterase domain reveals potential binding cavity for virtual screening of ligands. _J. Biomol. Struct. Dyn._ 35, 1350–1366. https://doi.org/10.1080/07391102.2016.1184183
(2017). Article CAS PubMed Google Scholar * Ghodousi, E. S. & Rahgozar, S. MicroRNA-326 and microRNA-200c: two novel biomarkers for diagnosis and prognosis of pediatric acute
lymphoblastic leukemia. _J. Cell. Biochem._ 119, 6024–6032 (2018). Article CAS PubMed Google Scholar * Montellier, E. & Gaucher, J. Targeting the interplay between metabolism and
epigenetics in cancer. _Curr. Opin. Oncol._ 31, 92–99 (2019). Article CAS PubMed Google Scholar * Morandi, A. & Indraccolo, S. Linking metabolic reprogramming to therapy resistance
in cancer. _Biochim. Biophys. Acta (BBA) Rev. Cancer_ 1868, 1–6 (2017). Article CAS Google Scholar * Kuo, C.-Y. & Ann, D. K. When fats commit crimes: fatty acid metabolism, cancer
stemness and therapeutic resistance. _Cancer Commun._ 38, 47 (2018). Article Google Scholar * de Lima, R. M. T. _et al._ Protective and therapeutic potential of ginger (_Zingiber
officinale_) extract and [6]-gingerol in cancer: a comprehensive review. _Phytother. Res._ 32, 1885–1907 (2018). Article PubMed Google Scholar * Impheng, H. _et al._ [6]-Gingerol inhibits
de novo fatty acid synthesis and carnitine palmitoyltransferase-1 activity which triggers apoptosis in HepG2. _Am. J. Cancer Res._ 5, 1319 (2015). PubMed PubMed Central Google Scholar *
Tasian, S. K. & Hunger, S. P. Genomic characterization of paediatric acute lymphoblastic leukaemia: an opportunity for precision medicine therapeutics. _Br. J. Haematol._ 176, 867–882
(2017). Article CAS PubMed Google Scholar * Koundouros, N. & Poulogiannis, G. Reprogramming of fatty acid metabolism in cancer. _Br. J. Cancer_ 122, 4–22 (2019). Article PubMed
PubMed Central Google Scholar * Chen, Y. & Li, P. Fatty acid metabolism and cancer development. _Sci. Bull._ 61, 1473–1479 (2016). Article CAS Google Scholar * Angeles, T. S. &
Hudkins, R. L. Recent advances in targeting the fatty acid biosynthetic pathway using fatty acid synthase inhibitors. _Expert Opin. Drug Discov._ 11, 1187–1199 (2016). Article CAS PubMed
Google Scholar * Nisthul, A. A., Retnakumari, A. P., Shabana, A., Anto, R. J. & Sadasivan, C. In silico screening for identification of fatty acid synthase inhibitors and evaluation of
their antiproliferative activity using human cancer cell lines. _J. Recept. Signal Transduct. Res._ 38, 335–341. https://doi.org/10.1080/10799893.2018.1511730 (2018). Article CAS Google
Scholar * Wang, J., Hudson, R. & Sintim, H. O. Inhibitors of fatty acid synthesis in prokaryotes and eukaryotes as anti-infective, anticancer and anti-obesity drugs. _Future Med. Chem._
4, 1113–1151 (2012). Article PubMed Google Scholar * Ali, B. H., Blunden, G., Tanira, M. O. & Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger
(_Zingiber officinale_ Roscoe): a review of recent research. _Food Chem. Toxicol._ 46, 409–420. https://doi.org/10.1016/j.fct.2007.09.085 (2008). Article CAS PubMed Google Scholar *
Choi, J. G., Kim, S. Y., Jeong, M. & Oh, M. S. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. _Pharmacol. Ther._ 182, 56–69.
https://doi.org/10.1016/j.pharmthera.2017.08.010 (2018). Article CAS PubMed Google Scholar * Mahomoodally, M. F. _et al._ Ginger and its active compounds in cancer therapy: from folk
uses to nano-therapeutic applications. _Semin. Cancer Biol._ https://doi.org/10.1016/j.semcancer.2019.08.009 (2019). Article PubMed Google Scholar * Mao, Q. Q., Xu, X. Y., Cao, S. Y.
& Gan, R. Y. Bioactive compounds and bioactivities of ginger (_Zingiber_ _officinale_ Roscoe). _Foods_ https://doi.org/10.3390/foods8060185 (2019). Article PubMed PubMed Central
Google Scholar * Panman, W. _et al._ Computational screening of fatty acid synthase inhibitors against thioesterase domain. _J. Biomol. Struct. Dyn._ 36, 4114–4125.
https://doi.org/10.1080/07391102.2017.1408496 (2018). Article CAS PubMed Google Scholar * Trott, O. & Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a
new scoring function, efficient optimization, and multithreading. _J. Comput. Chem._ 31, 455–461. https://doi.org/10.1002/jcc.21334 (2010). Article CAS PubMed PubMed Central Google
Scholar * Pettersen, E. F. _et al._ UCSF Chimera—a visualization system for exploratory research and analysis. _J. Comput. Chem._ 25, 1605–1612. https://doi.org/10.1002/jcc.20084 (2004).
Article CAS PubMed Google Scholar * Morris, G. M. _et al._ AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. _J. Comput. Chem._ 30, 2785–2791 (2009).
Article CAS PubMed PubMed Central Google Scholar * Laskowski, R. A. & Swindells, M. B. LigPlot+: Multiple ligand− protein interaction diagrams for drug discovery. _J. Chem. Inf.
Model._ 51, 2778–2786 (2011). Article CAS PubMed Google Scholar * Forli, S. _et al._ Computational protein-ligand docking and virtual drug screening with the AutoDock suite. _Nat.
Protoc._ 11, 905–919. https://doi.org/10.1038/nprot.2016.051 (2016). Article CAS PubMed PubMed Central Google Scholar * Pappenberger, G. _et al._ Structure of the human fatty acid
synthase KS-MAT didomain as a framework for inhibitor design. _J. Mol. Biol._ 397, 508–519. https://doi.org/10.1016/j.jmb.2010.01.066 (2010). Article CAS PubMed Google Scholar * Soulère,
L., Alix, P. M., Croze, M. L. & Soulage, C. O. Identification of novel antilipogenic agents targeting fatty acid biosynthesis through structure-based virtual screening. _Chem. Biol.
Drug Des._ 92, 1366–1372. https://doi.org/10.1111/cbdd.13202 (2018). Article CAS PubMed Google Scholar Download references AUTHOR INFORMATION Author notes * These authors contributed
equally: Somayeh Babasheikhali Rahimi, Arman Safavi, and Elaheh Sadat Ghodousi. AUTHORS AND AFFILIATIONS * Department of Cell and Molecular Biology and Microbiology, Faculty of Biological
Science and Technology, University of Isfahan, 81746-73441, Isfahan, Iran Maryam Ghaeidamini Harouni, Soheila Rahgozar, Somayeh Rahimi Babasheikhali, Arman Safavi & Elaheh Sadat Ghodousi
Authors * Maryam Ghaeidamini Harouni View author publications You can also search for this author inPubMed Google Scholar * Soheila Rahgozar View author publications You can also search for
this author inPubMed Google Scholar * Somayeh Rahimi Babasheikhali View author publications You can also search for this author inPubMed Google Scholar * Arman Safavi View author
publications You can also search for this author inPubMed Google Scholar * Elaheh Sadat Ghodousi View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS M.G.H. and S.R.B. conceived the ideas and performed the experiments. M.G.H. and E.S.G. wrote the article. A.S. and E.S.G. did and wrote in silico studies. S.R. conceived the
ideas, designed the study, wrote and reviewed the article. CORRESPONDING AUTHOR Correspondence to Soheila Rahgozar. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Ghaeidamini Harouni, M., Rahgozar, S., Rahimi Babasheikhali, S. _et al._ Fatty acid synthase, a novel poor prognostic factor for acute lymphoblastic leukemia
which can be targeted by ginger extract. _Sci Rep_ 10, 14072 (2020). https://doi.org/10.1038/s41598-020-70839-9 Download citation * Received: 09 April 2020 * Accepted: 27 July 2020 *
Published: 21 August 2020 * DOI: https://doi.org/10.1038/s41598-020-70839-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
First Cyber Security Policy Going To Be Implement In State - Jamshedpur NewsBY: INEXTLIVE | Updated Date: Sun, 01 Sep 2013 12:55:00 (IST) बढ़ रहे हैं CYBER CRIME INCIDENTS अब साइबर क्राइम काफी बढ़...
Gillmor Gang on Streaming TechnologiesTechCrunch Desktop LogoTechCrunch Mobile LogoLatestStartupsVentureAppleSecurityAIAppsEventsPodcastsNewslettersSearchSubm...
Missing jogger found safe and well in France: she left with a friendThey say the jogger - identified as Chloé - is around 1.56m tall and of slim build with short brown hair and blond highl...
Molecule-based nonlinear optical switch with highly tunable on-off temperature using a dual solid solution approach | Nature CommunicationsDownload PDF Article Open access Published: 02 June 2020 Molecule-based nonlinear optical switch with highly tunable on-...
Telehandler test: bobcat tl 38. 70 hf agri - farmers weeklyOur test machine was the biggest in Bobcat’s line-up of ag-spec TL telehandlers, offering a lift capacity of 3.8t and a ...
Latests News
Fatty acid synthase, a novel poor prognostic factor for acute lymphoblastic leukemia which can be targeted by ginger extractABSTRACT Altered metabolism of fatty acid synthesis is considered a hallmark characteristic of several malignancies, inc...
Missing jogger found safe and well in France: she left with a friendThey say the jogger - identified as Chloé - is around 1.56m tall and of slim build with short brown hair and blond highl...
Molecule-based nonlinear optical switch with highly tunable on-off temperature using a dual solid solution approach | Nature CommunicationsDownload PDF Article Open access Published: 02 June 2020 Molecule-based nonlinear optical switch with highly tunable on-...
Telehandler test: bobcat tl 38. 70 hf agri - farmers weeklyOur test machine was the biggest in Bobcat’s line-up of ag-spec TL telehandlers, offering a lift capacity of 3.8t and a ...
Portrait | arts education | season 1Portrait Clip: 5/23/1991 | 1m 54sVideo has Closed Captions | CC After graduating from MCAD, Heart Warrior Chosa traveled...