Delta-radiomics features for the prediction of patient outcomes in non–small cell lung cancer
Delta-radiomics features for the prediction of patient outcomes in non–small cell lung cancer"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Radiomics is the use of quantitative imaging features extracted from medical images to characterize tumor pathology or heterogeneity. Features measured at pretreatment have
successfully predicted patient outcomes in numerous cancer sites. This project was designed to determine whether radiomics features measured from non–small cell lung cancer (NSCLC) change
during therapy and whether those features (delta-radiomics features) can improve prognostic models. Features were calculated from pretreatment and weekly intra-treatment computed tomography
images for 107 patients with stage III NSCLC. Pretreatment images were used to determine feature-specific image preprocessing. Linear mixed-effects models were used to identify features that
changed significantly with dose-fraction. Multivariate models were built for overall survival, distant metastases, and local recurrence using only clinical factors, clinical factors and
pretreatment radiomics features, and clinical factors, pretreatment radiomics features, and delta-radiomics features. All of the radiomics features changed significantly during radiation
therapy. For overall survival and distant metastases, pretreatment compactness improved the c-index. For local recurrence, pretreatment imaging features were not prognostic, while
texture-strength measured at the end of treatment significantly stratified high- and low-risk patients. These results suggest radiomics features change due to radiation therapy and their
values at the end of treatment may be indicators of tumor response. SIMILAR CONTENT BEING VIEWED BY OTHERS IDENTIFICATION OF CT RADIOMIC FEATURES ROBUST TO ACQUISITION AND SEGMENTATION
VARIATIONS FOR IMPROVED PREDICTION OF RADIOTHERAPY-TREATED LUNG CANCER PATIENT RECURRENCE Article Open access 19 April 2024 MRI-BASED DELTA-RADIOMIC FEATURES FOR PREDICTION OF LOCAL CONTROL
IN LIVER LESIONS TREATED WITH STEREOTACTIC BODY RADIATION THERAPY Article Open access 03 November 2022 DEVELOPMENT OF A ROBUST RADIOMIC BIOMARKER OF PROGRESSION-FREE SURVIVAL IN ADVANCED
NON-SMALL CELL LUNG CANCER PATIENTS TREATED WITH FIRST-LINE IMMUNOTHERAPY Article Open access 15 June 2022 INTRODUCTION Lung cancer is responsible for the largest number of cancer deaths in
both men and women in the United States1. Over 85% of lung cancer cases are non-small cell lung cancers (NSCLC)1, 2. Predicting a particular NSCLC patient’s response to treatment, even
compared to patients in the same disease stage group, is extremely difficult. A model that could effectively identify patients whose tumors are not responding to treatment would be
beneficial and could be used to recommend patients for adjuvant chemotherapy or a radiation boost. Radiomics is the extraction of quantitative imaging features from medical images. These
quantitative values can be used to develop models for cancer diagnosis, patient prognosis, or relative tumor heterogeneity that can then guide clinical decisions3, 4. This process is similar
to the current application of tumor stage or genetic information derived from tumor biopsy specimens for clinical decision making. Radiomics has the combined advantages of being highly
patient-specific and non-invasive. Additionally, unlike biopsy specimens, radiomics allows for sampling the heterogeneity over the entire tumor. In recent years, numerous studies have
examined the potential clinical utility of radiomics features calculated from computed tomography (CT) images of NSCLC. These studies have identified features that are linked to tumor
histology5, 6, tumor stage7, patient overall survival8,9,10,11,12,13,14,15, and genetic mutations16,17,18. Changes in radiomics features, called delta-radiomics features, have also been
studied for their prognostic potential in cancer. Delta-radiomics features have been successful in predicting the response of colorectal cancer liver metastases19 and metastatic renal cell
cancer20 to chemotherapies. Delta-radiomics features have also been used to identify patients with esophageal cancer who would develop radiation pneumonitis during treatment21.
Delta-radiomics features calculated from PET images were shown to be predictive of overall survival for NSCLC patients22. To our knowledge, however, no published reports have investigated
the possibility of using delta-radiomics features calculated from CT images for determining prognosis in NSCLC patients. Currently, NSCLC tumor response is analyzed with the Response
Evaluation Criteria in Solid Tumors (RECIST) guidelines23,24,25. These guidelines depend on changes in tumor size to evaluate tumor response. Tumor size is widely known to be correlated with
survival and probability for distant metastases in NSCLC. However, it does not reflect changes in tumor heterogeneity or genetic profiles, both of which may be more indicative of individual
tumor biology. By sampling the entire tumor and analyzing changes in the spatial variations in intensity, delta-radiomics features may fill this gap and provide better patient-specific
outcome predictions. The main objective of this work was to determine whether therapy-induced changes in radiomics features, called delta-radiomics features, can improve models for
predicting patient outcome when used in conjunction with clinical factors and radiomics features measured prior to treatment. METHODS PATIENT DATA For this study, we retrospectively reviewed
the images and medical records for 137 NSCLC patients with a waiver of informed consent from the Institutional Review Board (IRB) at the University of Texas MD Anderson Cancer center. These
patients were selected because they had been enrolled on an IRB approved clinical trial at the University of Texas MD Anderson Cancer Center where they were imaged weekly with a
four-dimensional CT (4DCT) during their treatment26. Informed consent had been obtained from each study participant. All methods for the trial were performed in accordance with the
University of Texas MD Anderson Cancer Center IRB guidelines and regulations and all experimental protocols were approved by the same IRB. Inclusion criteria for the trial were
pathologically proven advanced NSCLC, Karnofsky performance status (KPS) ≥70 or ECOG score 0–1, forced expiratory volume ≥1 liter, and age between 18 and 85 years26. Exclusion criteria
included small cell histology, prior radiotherapy to the planned radiation therapy fields, pregnancy, or oxygen dependence due to pre-existing lung disease26. The patients were treated with
radiation therapy and concurrent chemotherapy. They had been randomized to receive treatment with either photons or protons to 66 or 74 Gy. Full trial details are available online26. In this
retrospective analysis, treatment modality was not considered for classification purposes. For this retrospective analysis, the medical records of these patients were reviewed to determine
their clinical factors: sex, age, smoking status, pack years, tumor histology, overall disease stage, T stage, N stage, KPS, and total prescribed radiation dose (Table 1). The primary
endpoints for this retrospective analysis of the trial data were overall survival, freedom from distant metastases, and local-regional control. LANDMARK ANALYSIS Survival studies using
measures of response that are calculated at multiple time points, such as the radiomics features measured at weekly intervals in this study, require a landmark time point to be used for
calculating the time until the endpoint is reached27, 28. Otherwise a bias can be introduced by responders since they must have already survived to the time of treatment to be classified as
responders27, 28. In this study, patients were classified as high or low risk using multivariate models that included clinical factors (recorded at the time of entrance to the study),
pre-treatment radiomics features (measured from the treatment planning images), and delta-radiomics features (measured from the different weekly images through treatment). Because a variable
number of days occurred for each patient between when they entered the trial and when their pre-treatment images were acquired and between their pre-treatment images and last weekly images,
a landmark time point was required to measure survival. For this study, endpoints were defined from a landmark time point of 90 days from the day the patient was entered on the clinical
trial until one of the endpoints of death, presence of distant metastases, or local-regional failure was met. Patients not reaching the endpoint were censored at their last follow-up date.
The landmark point was calculated by determining the total number of days from entering the trial to end of treatment for each patient. The maximum interval (by which all measures of
response had been determined) was 83 days, which was rounded to 90 days for simplicity. This ensures that the time until the endpoint is reached or the patient is censored is uniformly
measured across all patients and not biased by the number of days they have already survived to reach the end of treatment. IMAGING PARAMETERS The pretreatment and weekly 4DCTs were acquired
on either a GE Discovery ST or GE LightSpeed RT16 (GE Medical, Waukesha, WI) with a peak tube voltage of 120 kVp, tube current of 100 or 200 mA, and rotation times of 0.5 or 0.8 second.
Axial images were reconstructed in a 512 × 512 matrix at an in-plane resolution of 0.98 mm and image thickness of 2.5 mm. These acquisition and reconstruction parameters are our
institutional standards for CT imaging. For this study, the features were calculated from the end-of-exhale phase images for each scan. This phase was selected because it was considered the
most stable and has been used in other radiomics studies15, 29, 30. The three-dimensional gross tumor volume contoured from the treatment plan was used as the region of interest (ROI) for
feature extraction. The gross tumor volume contour from the treatment plan was deformably registered to each subsequent weekly 4DCT scan using clinical software developed in-house31,32,33.
During this step, only the contour is deformed, the images themselves are not changed. All contours were visually inspected by the same person to ensure consistency and modified if necessary
to remove any non-tumor regions. Furthermore, to ensure that normal lung and bone were excluded from the final ROI used for feature calculation, a thresholding step was also applied to each
image with a lower threshold of −100 Hounsfield Units (HU) and upper threshold of 200 HU. EXCLUSION CRITERIA Patients were excluded from this dataset for any of the following reasons: (i) a
small ROI volume (<5 cm3) (n = 18); (ii) imaging using a different protocol, such as breath hold instead of 4DCT (n = 9); or (iii) occurrence of an endpoint, e.g. death, before the
landmark point used for calculating survival times (n = 3). After exclusion, 107 patients were included in the final data analysis. FEATURE EXTRACTION AND SELECTION Feature calculation was
performed using the IBEX software34, 35. This software allowed for customization of feature parameters and image preprocessing. Extracted features included shape features (n = 16), intensity
histogram features (n = 11), co-occurrence matrix (COM) features (n = 22)36, 37, neighborhood gray-tone difference matrix (NGTDM) features (n = 5)38, and run-length matrix (RLM) features (n
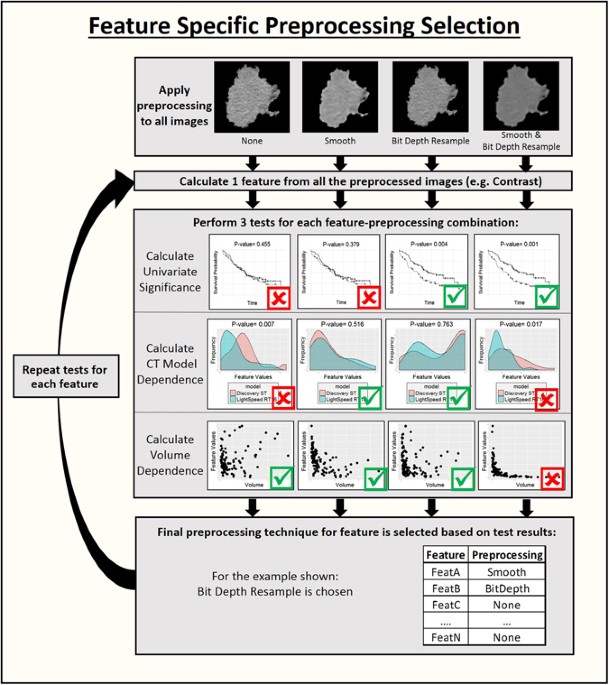
= 11)39. To determine the best parameters for the features, each feature was calculated four times: (1) once with no image preprocessing other than thresholding, (2) once with thresholding
and smoothing using a Butterworth filter with an order of 2 and a cutoff of 125, (3) once with thresholding and an 8-bit depth resample, and (4) once with thresholding, Butterworth
smoothing, and an 8-bit depth resample40. The Butterworth smoothing acts to remove Gaussian noise from the images, which may obscure the lower frequency biological variations that radiomics
features are designed to measure. The 8-bit depth resample is used as an alternative to modifying the binning parameter for the histogram and radiomics matrices. Using 8-bit depth images
results in a bin width of 16 HU and thus is more likely to reflect actual density changes in neighboring pixels than bins with a width of 1 HU, which largely reflect image noise. Optimal
image pre-processing was determined on a feature-specific basis using the following steps which are also illustrated in Fig. 1. First a univariate Cox regression model for overall survival
was fitted for each preprocessed version of each feature using only the pretreatment images for each patient. The significance of the feature in the model was calculated to determine whether
a model built on only this feature was a better fit than the null model. This step identified radiomics features that were predictive and therefore might be useful for calculating
delta-radiomics features. The correlation between each feature and the gross tumor volume was calculated using Spearman’s rank correlation coefficient. The feature values and gross tumor
volumes were calculated from the pretreatment images for this step. Next a Wilcoxon rank sum test was performed for each feature and pre-processing combination to determine if the feature
values were significantly different when images were acquired on the GE Discovery ST versus the GE Lightspeed RT16, as CT scanner model has been demonstrated to be an important factor in
feature reproducibility41. A patient subset that had images available from the first week of treatment was used for this test because at this time point the patients were roughly split
between the two CT scanners used in this study (37 patients imaged with the GE Discovery ST and 44 patients imaged with the GE Lightspeed RT16) and their tumors would not yet have shown any
therapy-induced changes. For each feature, the pre-processed version that was significant in univariate analysis for survival (p-value < 0.10) and did not have a significant value
(p-value > 0.05) for the Wilcoxon rank sum test between CT scanners was included in the final feature set. Features that never met these two criteria regardless of the image
pre-processing used were excluded from the feature set. If a feature met both criteria for more than one image pre-processing type, the version of the feature that had the smallest
correlation with volume was selected. A p-value of 0.10 was used as the threshold for significance in this pre-analysis because the p-values were used only for feature selection, not
hypothesis testing, and thus the filtering need not be overly stringent. This choice was balanced against the need to remain conservative so that the feature dimensionality is decreased
during this step. For the same reason, no multiplicity correction was used at this stage. DELTA-RADIOMICS FEATURES Two tests were conducted to determine which of the optimized features
changed during treatment and thus might be useful indicators of tumor response. First a linear mixed effects model with random intercepts for each patient was built for each feature in the
form equation (1), $${\rm{\Delta }}Feature\sim {\rm{\Delta }}Dose+({1}|{PatID})$$ (1) Here, _ΔFeature_ was the feature value measured from each weekly 4DCT, _ΔDose_ was the total dose
delivered to the tumor at that point in treatment, and _PatID_ was a patient-specific identifier that allows the model to account for the fact that we had multiple, longitudinal measurements
of each feature for each patient by assigning each patient their own intercept. The p-value of the log-likelihood ratio for each model was calculated. P-values were corrected for multiple
comparisons using the Benjamini-Hochberg method42. If the corrected p-value was less than 0.05, the model was considered significant and indicated that the changes in the feature were
significantly associated with the dose delivered to the tumor. For each feature with a significant p-value in this test, simplified measures of the overall change were calculated and defined
as delta-radiomics features. The delta-radiomics features were defined as the relative net change, equation (2), the linear regression slope, and the value of the feature at the last week
of treatment for each patient. $${relativeNetChange}=({Featur}{{e}}_{{WeekFinal}}-{Featur}{{e}}_{{Week}1})/{Featur}{{e}}_{{Week}1}$$ (2) Here, _Feature_ _WeekFinal_ was the value of the
feature at the end of treatment and _Feature_ _Week1_ was the value at the first weekly 4DCT for each patient. A one-sample, two-tailed _t_-test was conducted for each of the delta-radiomics
features to determine whether the overall changes for the group were significantly different from 0, and values were again corrected using the Benjamini-Hochberg method. Features that
passed both the linear mixed effects and _t_-test analyses (corrected p-value < 0.05) were considered to significantly demonstrate radiation therapy-induced changes and were included as
potential covariates in model building. MULTIVARIATE ANALYSIS Multivariate Cox regression models were built for each of the primary endpoints using leave-one-out cross validation (LOOCV) and
Akaike Information Criterion (AIC) with the following procedure, which is also illustrated in Fig. 2. First, one patient was removed from the dataset and a Cox proportional hazards model
was built using all of the clinical factors and the remaining patients. The covariates were reduced using stepwise AIC in both directions. Next, all of the pretreatment radiomics features
were added to this model and stepwise AIC was repeated in both directions with forced nesting of the clinical covariates. Then, all of the delta-radiomics features were added to this model
with forced nesting of the clinical and pretreatment radiomics covariates. The delta-radiomics versions of the features were identified by the suffixes “netPercentChange”, “Slope”, or
“WeekLast”, while the pretreatment radiomics features are indicated by the suffix “Week0”. This process was repeated with each patient left out in turn so that at the end there were three
models for each left-out patient: one with only clinical factors, one with clinical factors and pretreatment radiomics features, and one with clinical factors, pretreatment radiomics
features, and delta-radiomics features. The total number of times each covariate was selected for the three models over all of the LOOCV iterations was calculated. Covariates that were
selected in more than half of the iterations were retained and considered high-performing. Final versions of the three models using only these frequently selected covariates were then
calculated and compared using the log-likelihood ratio to determine whether the radiomics and/or delta-radiomics features significantly (p-value < 0.05) improved the fit of the model to
the data. If no feature was selected in more than half of the iterations for a particular model, then the null model or the nested model from the previous iteration was used. To evaluate the
prognostic potential of these features, a new LOOCV was performed. For this analysis, the three models were built on each iteration using only the high-performing clinical, radiomics, and
delta-radiomics covariates from the original LOOCV. No covariate reduction was performed, but the coefficients were refit on each iteration. On each iteration of the loop, a prediction for
the left-out patient was calculated using each of the three models. Because the patient was left out of the coefficient fitting process, predictions generated for the left out patient were
unbiased. Once the loop was complete, and each patient had a prediction for each model, the Harrel concordance index43 (c-index) was calculated for each model. The c-index is analogous to
the area under the curve but is designed for survival data instead of binary data. Values of the c-index can range from 0 to 1 with a value of 1 indicating perfect prediction and a value ≤
0.5 indicating that a model performs no better or worse than a random guess. Thus the c-indices allowed for the comparison of the predictive accuracy of models that included radiomics and
delta-radiomics features to models incorporating only clinical factors. Finally, patients were stratified as high or low risk based on whether their prediction was above or below the median
prediction for each model. Kaplan-Meier curves were plotted using this patient stratification, and the log-rank test was used to determine whether the stratifications were significant
(p-value < 0.05). All statistical analyses were performed in R language44 using the survival45, lme446, MASS47, and ggplot248 analysis packages. RESULTS FEATURE SELECTION The initial
feature set had 49 texture features measured before treatment, with four different image preprocessing types and 16 shape features, for a total of 212 feature and preprocessing combinations.
Of these, 75 were significant in univariate analysis (p < 0.10), and 123 were not significantly different between different CT scanners (p < 0.05). These results are shown in
Supplementary Figures S1–S3. Using the feature selection process, this feature set was reduced to 31 features. Of these, 9 were calculated with no extra preprocessing, 15 were calculated
with Butterworth smoothing, and 7 were calculated with Butterworth smoothing and 8-bit depth resampling (Supplementary Figure S4). At least one feature from every feature category was
represented in this final feature set. All 31 features had significant p-values for the log-likelihood ratio of their linear mixed effects model, with dose as the covariate and random
intercepts for each patient, even after Benjamini-Hochberg correction for multiplicity. The net changes and slope in each feature were also significant in _t_-tests comparing their means to
0 after multiplicity correction for every feature. Thus a total of 31 features were available for feature selection in the multivariate model building. MULTIVARIATE ANALYSIS For overall
survival, 67 of the 107 patients reached the endpoint of death. The median survival time was 638 days. 50 patients had a distant metastases with a median time until reaching the endpoint or
censoring of 311 days. 23 patients had a local recurrence with a median time until reaching the endpoint or censoring of 420 days. The final results of the multivariate analysis are
summarized in Table 2 for all three outcomes and all three models. The number of times each clinical factor and radiomics feature was selected in the first LOOCV is tabulated in
Supplementary Table S1 for each outcome. Adding the single selected pretreatment feature compactness2 increased the c-index from 0.597 to 0.672 for overall survival. The log-likelihood ratio
between these two models was significant. However, further addition of delta-radiomics features made a negligible difference to the c-index and did not substantially affect the patient
stratification by the Kaplan-Meier curves (Fig. 3). The log-likelihood ratio between model 2 (with clinical factors and pretreatment radiomics features) and model 3 (with clinical factors,
pretreatment radiomics features, and delta-radiomics features) was significant, indicating an improved fit. The clinical factors included in the final model were T stage, patient sex, tumor
histology, and total radiation dose. The pretreatment feature that was included was compactness2 from the shape category. The delta-radiomics features that were included were the slopes in
grey-level non-uniformity from the RLM and texture strength calculated from the NGTDM. For distant metastases, no delta-radiomics features were included in the final model. The final
clinical factors included in the model were tumor T stage, overall disease stage, and patient age, sex, and smoking status. Adding a pretreatment feature, compactness2 from the shape
category, did result in an increase in the c-index from 0.539 to 0.632. The log-likelihood ratio between model 1 (clinical factors only) and model 2 (clinical factors and pretreatment
radiomics features) was highly significant. Furthermore, patient stratification was significant when the pretreatment radiomics feature was added, while it was not significant for the purely
clinical model (Fig. 4). For local-regional recurrence, no clinical factors or pretreatment radiomics features were selected in more than half of the LOOCV iterations. As a result, none
were considered high-performing or were available for use in the final models for local recurrence. However, the delta-radiomics feature texture strength from the NGTDM measured at the end
of treatment was selected in a majority of the LOOCV iterations. As a result, only the model including delta-radiomics features was built. This univariate model resulted in a low value for
the c-index (0.558) but a statistically significant stratification of the patients (p-value = 0.0269; Fig. 5). In lieu of calculating the log-likelihood ratio between this model and model 1
(clinical features only) or model 3 (clinical, pretreatment radiomics, and delta-radiomics factors), the log-likelihood ratio between this model and the null model was calculated (p-value =
0.0725). DISCUSSION While the inclusion of delta-radiomics features had a statistically significant impact on the overall likelihood of a model for overall survival compared to a model with
only clinical and pretreatment radiomics features, the impact on the model’s prognostic abilities was generally negligible. For distant metastases, no delta-radiomics features were selected
in the final round of model building. This suggests that delta-radiomics features do not offer substantially new prognostic information for these outcomes though they were still prognostic
for overall survival. The same pretreatment radiomics feature, compactness2, was selected for both the overall survival and time to distant metastases models and improved their prognostic
potential. For both overall survival and distant metastases, the coefficient for this feature was positive, meaning a patient had a higher predicted risk of experiencing the outcome if the
value for compactness2 from their ROI was relatively large. This feature was related to the volume and shape of the tumor ROI, i.e. how spiculated it may appear. The feature values were also
affected by the tumor location, since a tumor attached to the chest wall was contoured with at least one smooth side compared to a tumor surrounded by lung which ranged anywhere between
fully smooth or fully spiculated. Compactness 2 was also found to be predictive in a radiomics study by Aerts _et al_. where it was included as part of a four feature radiomics signature11.
This study is unique in that it demonstrated that compactness 2 added significant new information to a variety of clinical factors already routinely obtained, as opposed to only TNM staging
and tumor volume. In this study, the clinical model was built first and then radiomics features were added to it rather than building a purely radiomics model and assessing its capabilities.
This is important because the introduction of radiomics features into a routine clinical workflow is unlikely to be accepted unless models built using radiomics features outperform models
built using only routinely acquired clinical factors. Interestingly, in the models for local-regional recurrence, the only covariate that was predictive for outcomes was a radiomics feature,
texture strength from the NGTDM, measured from images acquired during the last week of treatment. This feature was designed to quantify whether an image has clear, perceivable
characteristics that can be considered as texture and the overall strength of that signal38. Further work is needed to identify what this feature may represent in the context of NSCLC tumor
analysis. This result may be evidence that, although it is not possible to predict local-regional recurrence prior to treatment, the state of the tumor at the end of the treatment can be
assessed using radiomics. One possible cause of the poor selection of delta-radiomics features in the models may be due to the initial feature preselection process. The full feature set was
first reduced to features whose pretreatment values were at least prognostic in univariate models for overall survival. It is possible that the results would differ if this requirement was
changed to instead select for delta-radiomics features that are significant in univariate models. The original requirement was chosen for two reasons: first, because several publications
have shown that pretreatment radiomics features have informative value and thus changes in the features that are already prognostic may reflect actual biological changes in the tumor, and
second, if model building was limited to delta-radiomics features that were significant in univariate analyses the results could be biased and overly optimistic. One limitation of this study
was the lack of a dataset for independent model validation due to the fact that patients are not routinely imaged weekly during their treatment. This limitation was mitigated by using cross
validation, which has been shown to be an effective method for creating unbiased patient-specific predictions49, 50. Another limitation of this study was that the median predicted value was
used as the cut-off point for high- and low-risk patients. This is not an optimized approach, and it is very likely that a different model-specific value would yield different results.
However, testing multiple cutoffs to find the best one without an independent validation dataset to test it in has been repeatedly shown to yield overly optimistic results51,52,53. By using
the median, this source of bias is avoided and the conclusions remained conservative. Lastly, because the images used in this analysis were non-contrast CT images, vessels passing through
the lesion could not be segmented from the contour. Thus the contours for the tumor ROIs may contain vasculature along with the solid tumor component we are interested in. The inclusion of
vasculature in the tumor ROIs may affect the radiomics features and the calculated tumor volume. Radiomics is in some ways fundamentally limited because the features are not inherently
descriptive. This is in contrast to clinical covariates which, when selected in prognostic models, lend themselves to hypotheses, e.g., age is likely to affect survival because a younger
person is statistically likelier to live longer than an older person. For radiomics features, this type of reasoning is difficult and instead new studies must be undertaken to correlate
feature values with biological characteristics such as genetic mutations. Radiomics features also suffer from lack of robustness, as they have been demonstrated to vary with imaging
equipment, ROI contouring, and imaging parameters. Thus the implementation of radiomics features in a clinical setting would require substantial effort to standardize both imaging and
measurement parameters. This study identified two features, compactness2 and texture strength, which may be of clinical significance. The first step in determining their robustness will be
to examine the impact of segmentation on both features’ values and prognostic potentials. This is especially critical for compactness2 since it is a shape based feature and thus could be
substantially impacted by segmentation. In conclusion, this study found evidence that radiomics features change during the course of radiation therapy for NSCLC. However, these changes in
features did not significantly outperform features measured before treatment in multivariate models for overall survival and distant metastases. Thus it may be more important to focus
efforts on improving the standardization of features measured before treatment and identifying a biological or molecular explanation for their predictive values. One radiomics feature
measured at the end of treatment did outperform both clinical factors and pretreatment radiomics features for prediction of local-regional recurrence. This feature, texture-strength, could
become an indicator for tumor response since it was only prognostic when measured at the end of treatment. Despite the fact that this study did not find strong evidence supporting the
prognostic potential of delta-radiomics features, the results of this study are important because the potential of tracking radiomics features throughout treatment for NSCLC was
investigated. Furthermore, while other studies have used delta-radiomics features for other treatment sites or for normal tissue toxicity, they have used only the relative net change in
their models21, 22, 54. This study included both the slope of a linear regression for the features of each patient, which may be less susceptible to noise than the relative net change, and
the feature values at the end of treatment, which may reflect tumor response. REFERENCES * Molina, J. R., Yang, P., Cassivi, S. D., Schild, S. E. & Adjei, A. A. Non-small cell lung
cancer: Epidemiology, risk factors, treatment, and survivorship. _Mayo Clin. Proc._ 83, 584–594 (2008). Article PubMed PubMed Central Google Scholar * SEER stat fact sheets: Lung and
bronchus cancer. Available at: http://seer.cancer.gov/statfacts/html/lungb.html (Accessed: 23rd September 2016) (2014). * Kumar, V. _et al_. Radiomics: the process and the challenges. _Magn.
Reson. Imaging_ 30, 1234–1248 (2012). Article PubMed PubMed Central Google Scholar * Lambin, P. _et al_. Radiomics: Extracting more information from medical images using advanced
feature analysis. _Eur. J. Cancer_ 48, 441–446 (2012). Article PubMed PubMed Central Google Scholar * Wang, H. _et al_. Multilevel binomial logistic prediction model for malignant
pulmonary nodules based on texture features of CT image. _Eur. J. Radiol._ 74, 124–129 (2010). Article PubMed Google Scholar * Basu, S. _et al_. Developing a classifier model for lung
tumors in CT-scan images. in _2011 IEEE International Conference on Systems_, _Man_, _and Cybernetics_ 1306–1312, doi:10.1109/ICSMC.2011.6083840 (2011). * Ganeshan, B., Abaleke, S., Young,
R. C. D., Chatwin, C. R. & Miles, K. A. Texture analysis of non-small cell lung cancer on unenhanced computed tomography: Initial evidence for a relationship with tumour glucose
metabolism and stage. _Cancer Imaging_ 10, 137–143 (2010). Article PubMed PubMed Central Google Scholar * Ganeshan, B., Panayiotou, E., Burnand, K., Dizdarevic, S. & Miles, K. Tumour
heterogeneity in non-small cell lung carcinoma assessed by CT texture analysis: A potential marker of survival. _Eur. Radiol._ 22, 796–802 (2012). Article PubMed Google Scholar * Win, T.
_et al_. Tumor heterogeneity and permeability as measured on the CT component of PET/CT predict survival in patients with non-small cell lung cancer. _Clin. Cancer Res._ 19, 3591–3599
(2013). Article CAS PubMed Google Scholar * Balagurunathan, Y. _et al_. Reproducibility and prognosis of quantitative features extracted from CT images. _Transl. Oncol._ 7, 72–87 (2014).
Article PubMed PubMed Central Google Scholar * Aerts, H. J. W. L. _et al_. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. _Nat. Commun._ 5,
1–8 (2014). Google Scholar * Parmar, C. _et al_. Radiomic feature clusters and prognostic signatures specific for Lung and Head & Neck cancer. _Sci. Rep_ 5, 11044 (2015). Article ADS
PubMed PubMed Central Google Scholar * Coroller, T. P. _et al_. Radiomic phenotype features predict pathological response in non-small cell lung cancer. _Radiother. Oncol._ 119, 480–486
(2016). Article PubMed Google Scholar * Coroller, T. P. _et al_. CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. _Radiother. Oncol._ 114, 345–350 (2015).
Article PubMed PubMed Central Google Scholar * Fried, D. V. _et al_. Prognostic value and reproducibility of pretreatment CT texture features in stage III non-small cell lung cancer.
_Int. J. Radiat. Oncol. Biol. Phys._ 90, 834–842 (2014). Article PubMed PubMed Central Google Scholar * Weiss, G. J. _et al_. Noninvasive image texture analysis differentiates K-ras
mutation from pan-wildtype NSCLC and is prognostic. _PLoS One_ 9, e100244 (2014). Article ADS PubMed PubMed Central Google Scholar * Gevaert, O. _et al_. Non–small cell lung cancer:
identifying prognostic imaging biomarkers by leveraging public gene expression microarray data—methods and preliminary results. _Radiology_ 264, 387–396 (2012). Article PubMed PubMed
Central Google Scholar * Miles, K. A. How to use CT texture analysis for prognostication of non-small cell lung cancer. _Cancer Imaging_ 16, 10 (2016). Article PubMed PubMed Central
Google Scholar * Rao, S. X. _et al_. CT texture analysis in colorectal liver metastases: A better way than size and volume measurements to assess response to chemotherapy? _United Eur.
Gastroenterol. J._ 4, 257–263 (2016). Article CAS Google Scholar * Goh, V. _et al_. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a
predictive biomarker. _Radiology_ 261, 165–171 (2011). Article PubMed Google Scholar * Cunliffe, A. _et al_. Lung texture in serial thoracic computed tomography scans: Correlation of
radiomics-based features with radiation therapy dose and radiation pneumonitis development. _Int. J. Radiat. Oncol. Biol. Phys._ 91, 1048–1056 (2015). Article PubMed PubMed Central Google
Scholar * Carvalho, S. _et al_. Early variation of FDG-PET radiomics features in NSCLC is related to overall survival - the ‘delta radiomics’ concept. in. _Radiotherapy and Oncology_ 118,
S20–S21 (2016). Article Google Scholar * Nishino, M. _et al_. New response evaluation criteria in solid tumors (RECIST) guidelines for advanced non–small cell lung cancer: Comparison with
original RECIST and impact on assessment of tumor response to targeted therapy. _Am. J. Roentgenol_ 195, W221–W228 (2010). Article Google Scholar * Jaffe, C. C. Measures of response:
RECIST, WHO, and new alternatives. _J. Clin. Oncol._ 24, 3245–3251 (2006). Article PubMed Google Scholar * Eisenhauer, E. A. _et al_. New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). _Eur. J. Cancer_ 45, 228–247 (2009). Article CAS PubMed Google Scholar * The University of Texas MD Anderson Cancer Center. Image-guided adaptive
conformal photon versus proton therapy. Available at: https://clinicaltrials.gov/ct2/show/record/NCT00915005 (Accessed: 4th September 2015). * Dafni, U. Landmark analysis at the 25-year
landmark point. _Circ. Cardiovasc. Qual. Outcomes_ 4, 363–371 (2011). Article PubMed Google Scholar * Anderson, J., Cain, K. & Gelber, R. Analysis of survival by tumor response. _J.
Clin. Oncol._ 1, 710–719 (1983). Article CAS PubMed Google Scholar * Seppenwoolde, Y. _et al_. Precise and real-time measurement of 3D tumor motion in lung due to breathing and
heartbeat, measured during radiotherapy. _Int. J. Radiat. Oncol_ 53, 822–834 (2002). Article Google Scholar * Fave, X. _et al_. Preliminary investigation into sources of uncertainty in
quantitative imaging features. _Comput. Med. Imaging Graph._ 44, 4–11 (2015). Article Google Scholar * Wang, H. _et al_. Implementation and validation of a three-dimensional deformable
registration algorithm for targeted prostate cancer radiotherapy. _Int. J. Radiat. Oncol. Biol. Phys._ 61, 725–735 (2005). Article PubMed Google Scholar * Chao, K. S. C. _et al_. Reduce
in variation and improve efficiency of target volume delineation by a computer-assisted system using a deformable image registration approach. _Int. J. Radiat. Oncol. Biol. Phys._ 68,
1512–1521 (2007). Article PubMed Google Scholar * Liu, H. H. _et al_. Assessing respiration-induced tumor motion and internal target volume using four-dimensional computed tomography for
radiotherapy of lung cancer. _Int. J. Radiat. Oncol. Biol. Phys._ 68, 531–540 (2007). Article PubMed Google Scholar * Zhang, L. _et al_. IBEX: An open infrastructure software platform to
facilitate collaborative work in radiomics. _Med. Phys._ 42, 1341–1353 (2015). Article PubMed PubMed Central Google Scholar * Zhang, J. & Court, L. IBEX. (2014). * Haralick, R. M.,
Shanmugam, K. & Dinstein, I. Textural features for image classification. _IEEE Trans. Syst. Man. Cybern_ 3, 610–621 (1973). Article Google Scholar * Haralick, R. M. Statistical and
structural approaches to texture. _Proc. IEEE_ 67, 786–804 (1979). Article Google Scholar * Amadasun, M. & King, R. Textural features corresponding to textural properties. _IEEE Trans.
Syst. Man. Cybern_ 19, 1264–1274 (1989). Article Google Scholar * Galloway, M. M. Texture analysis using gray level run lengths. _Comput. Graph. Image Process._ 4, 172–179 (1975). Article
Google Scholar * Fave, X. _et al_. Impact of image preprocessing on the volume dependence and prognostic potential of radiomics features in non-small cell lung cancer. _Transl. Cancer
Res._ 5, 349–363 (2016). Article Google Scholar * Mackin, D. _et al_. Measuring computed tomography scanner variability of radiomics features. _Invest. Radiol._ 50, 757–765 (2015). Article
PubMed PubMed Central Google Scholar * Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate : A practical and powerful approach to multiple testing. _J. R. Stat. Soc._
57, 289–300 (1995). MathSciNet MATH Google Scholar * Harrell, F. E., Califf, R. M., Pryor, D. B., Lee, K. L. & Rosati, R. A. Evaluating the yield of medical tests. _JAMA J. Am. Med.
Assoc._ 247, 2543–2546 (1982). Article Google Scholar * RCoreTeam. R: A language and enviroment for statistical computing. Available at: https://www.r-project.org/ (2015). * Therneau, T. A
package for survival analysis in S. Available at: http://cran.r-project.org/package=survival (2015). * Bates, D., Machler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects
models using lme4. _J. Stat. Softw._ 67, 1–48 (2015). Article Google Scholar * Venables, W. N. & Ripley, B. D. _Modern applied statistics with S_. (Springer, 2002). * Wickham, H.
ggplot2: Elegant graphics for data analysis. Available at: http://ggplot2.org (2009). * Fushiki, T. Estimation of prediction error by using K-fold cross-validation. _Stat. Comput._ 21,
137–146 (2011). Article MathSciNet MATH Google Scholar * Simon, R. M., Subramanian, J., Li, M.-C. & Menezes, S. Using cross-validation to evaluate predictive accuracy of survival
risk classifiers based on high-dimensional data. _Brief. Bioinform._ 12, 203–214 (2011). Article PubMed PubMed Central Google Scholar * Hilsenbeck, S. G., Clark, G. M. & McGuire, W.
L. Why do so many prognostic factors fail to pan out? _Breast Cancer Res. Treat._ 22, 197–206 (1992). Article CAS PubMed Google Scholar * Hilsenbeck, S. G. & Clark, G. M. Practical
p-value adjustment for optimally selected cutpoints. _Stat. Med._ 15, 103–112 (1996). Article CAS PubMed Google Scholar * Chalkidou, A., O’Doherty, M. J. & Marsden, P. K. False
Discovery Rates in PET and CT Studies with Texture Features: A Systematic Review. _PLoS One_ 10, e0124165 (2015). Article PubMed PubMed Central Google Scholar * Tian, F., Hayano, K.,
Kambadakone, A. R. & Sahani, D. V. Response assessment to neoadjuvant therapy in soft tissue sarcomas: Using CT texture analysis in comparison to tumor size, density, and perfusion.
_Abdom. Imaging_ 40, 1705–1712 (2015). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS This project was funded in part by grant 5U19CA021239 from the U.S. National
Institutes of Health and by grant RP110562-P2 from the Cancer Prevention and Research Institute of Texas. The authors would also like to acknowledge Kathryn Hale for help with manuscript
preparation. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Radiation Physics, The University of Texas MD Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX, 77030, USA
Xenia Fave, Lifei Zhang, Jinzhong Yang, Dennis Mackin, Peter Balter, David Followill, Radhe Mohan & Laurence Court * The University of Texas Graduate School of Biomedical Sciences at
Houston, 6767 Bertner Ave, Houston, TX, 77030, USA Xenia Fave & Laurence Court * Department of Radiation Oncology, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe
Blvd, Houston, TX, 77030, USA Daniel Gomez & Zhongxing Liao * Department of Imaging Physics, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX, 77030,
USA Aaron Kyle Jones * Dipartimento Di Statistica, Informatica, Applicazioni, University of Florence, Viale Morgagni, 59, Florence, 50134, Italy Francesco Stingo Authors * Xenia Fave View
author publications You can also search for this author inPubMed Google Scholar * Lifei Zhang View author publications You can also search for this author inPubMed Google Scholar * Jinzhong
Yang View author publications You can also search for this author inPubMed Google Scholar * Dennis Mackin View author publications You can also search for this author inPubMed Google Scholar
* Peter Balter View author publications You can also search for this author inPubMed Google Scholar * Daniel Gomez View author publications You can also search for this author inPubMed
Google Scholar * David Followill View author publications You can also search for this author inPubMed Google Scholar * Aaron Kyle Jones View author publications You can also search for this
author inPubMed Google Scholar * Francesco Stingo View author publications You can also search for this author inPubMed Google Scholar * Zhongxing Liao View author publications You can also
search for this author inPubMed Google Scholar * Radhe Mohan View author publications You can also search for this author inPubMed Google Scholar * Laurence Court View author publications
You can also search for this author inPubMed Google Scholar CONTRIBUTIONS X.F., P.B., D.G., D.F., A.K.J., F.S., and L.C. were responsible for project conception and design. L.Z., J. Y.,
P.B., D.G., D.F., R.M., Z. L., and L.C. provided expertise, guidance, data, and/or analysis tools. X.F. drafted the manuscript and prepared all figures. All authors reviewed the manuscript.
CORRESPONDING AUTHOR Correspondence to Xenia Fave. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION PUBLISHER'S
NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY DATA RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Fave, X., Zhang,
L., Yang, J. _et al._ Delta-radiomics features for the prediction of patient outcomes in non–small cell lung cancer. _Sci Rep_ 7, 588 (2017). https://doi.org/10.1038/s41598-017-00665-z
Download citation * Received: 12 October 2016 * Accepted: 07 March 2017 * Published: 03 April 2017 * DOI: https://doi.org/10.1038/s41598-017-00665-z SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
Trending News
Video Author Angeline Boulley talks about her new book, 'Firekeeper’s Daughter' - ABC NewsABC NewsVideoLiveShowsShopStream onLive UpdatesLive UpdatesTrump 2nd term Ukraine drone attack Trump tariffs Boulder att...
Pardon Our InterruptionPardon Our Interruption As you were browsing something about your browser made us think you were a bot. There are a few ...
Ben shephard’s meltdown after ‘x-rated messages’ slammed by gmb starBen Shephard has been a stalwart of the ITV presenting line-up since his first appearance on the broadcaster two decades...
Olympic exercise: get fit with sam claytonSeven minutes and 21 days: that’s the new goal for fitness according to Sam Clayton who launches a new home fitness prog...
Locations | VA Boise Health Care | Veterans AffairsLocations Main locations 500 West Fort Street Boise, ID 83702-4501 Main phone: VA health connect: Mental health care: He...
Latests News
Delta-radiomics features for the prediction of patient outcomes in non–small cell lung cancerABSTRACT Radiomics is the use of quantitative imaging features extracted from medical images to characterize tumor patho...
Important and useful information | veterans affairsSecretary McDonough often says that VA’s workforce is our number one asset, and he has made clear time and again that in...
Very early to seek status report from cbi on manipur violence: scNEW DELHI: The Supreme Court on Friday said that it will be “very early” to ask former Maharashtra DGP Dattatray Padsalg...
Washington dc va medical center researchers discover new ways to improve kidney health | va washington dc health care | veterans affairsResearchers at the Washington DC VA Medical Center had their experimental findings from a recent study published in the ...
Caregiver support | veterans affairsCARE WE PROVIDE AT VA MANCHESTER HEALTH CARE If you are a caregiver for a Veteran, you can get support by contacting a V...