The light intermediate chain 2 subpopulation of dynein regulates mitotic spindle orientation
The light intermediate chain 2 subpopulation of dynein regulates mitotic spindle orientation"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Cytoplasmic dynein 1 is a multi-protein intracellular motor essential for mediating several mitotic functions, including the establishment of proper spindle orientation. The
functional relevance and mechanistic distinctions between two discrete dynein subpopulations distinguished only by Light Intermediate Chain (LIC) homologues, LIC1 and LIC2 is unknown during
mitosis. Here, we identify LIC2-dynein as the major mediator of proper spindle orientation and uncover its underlying molecular mechanism. Cortically localized dynein, essential for
maintaining correct spindle orientation, consists majorly of LIC2-dynein, which interacts with cortical 14-3-3 ε- ζ and Par3, conserved proteins required for orienting the spindle.
LIC2-dynein is also responsible for the majority of dynein-mediated asymmetric poleward transport of NuMA, helping focus microtubule minus ends. In addition, LIC2-dynein dominates in
equatorially aligning chromosomes at metaphase and in regulating mitotic spindle length. Key mitotic functions of LIC2 were remarkably conserved in and essential for early embryonic
divisions and development in zebrafish. Thus LIC2-dynein exclusively engages with two major cortical pathways to govern spindle orientation. Overall, we identify a novel selectivity of
molecular interactions between the two LICs in mitosis as the underlying basis for their uneven distribution of labour in ensuring proper spindle orientation. SIMILAR CONTENT BEING VIEWED BY
OTHERS DISTINCT DYNEIN COMPLEXES DEFINED BY DYNLRB1 AND DYNLRB2 REGULATE MITOTIC AND MALE MEIOTIC SPINDLE BIPOLARITY Article Open access 27 March 2023 SEQUENTIAL ACCUMULATION OF DYNEIN AND
ITS REGULATORY PROTEINS AT THE SPINDLE REGION IN THE _CAENORHABDITIS ELEGANS_ EMBRYO Article Open access 11 July 2022 KINETOCHORE DYNEIN IS SUFFICIENT TO BIORIENT CHROMOSOMES AND REMODEL THE
OUTER KINETOCHORE Article Open access 21 October 2024 INTRODUCTION Somatic metazoan cells divide via the process of mitosis with high fidelity to generate two daughter cells that contain
the correct complement of chromosomes. The ubiquitous molecular motor cytoplasmic dynein 1 (referred to henceforth as “dynein”) mediates several important mitotic processes1,2,3,4,5. These
include chromosome congression to the cell equator6,7,8, regulation of mitotic spindle assembly, length and spindle pole focusing3, 9,10,11,12, astral microtubule nucleation from spindle
poles13, proper spindle positioning14, 15 and establishment of correct spindle orientation16, 17. Dynein is a large, non-covalent cytoplasmic complex of protein subunits including heavy
chains, intermediate chains, light intermediate chains and light chains1, 2, 18,19,20. Of these, the Light Intermediate Chains (LICs) have been poorly studied in the context of mitosis.
Invertebrates express only a single LIC21,22,23,24, while vertebrates have evolved to express two distinct LIC homologues, LIC1 and LIC2, which occupy cytoplasmic dynein 1 in mutually
exclusive dynein complexes25. Recent studies have illuminated the biochemical basis for assembly of LICs into the dynein complex and suggested cargo-binding functions for the LICs, and also
illuminated their role in spindle pole focusing12, 26, 27. A thorough functional and mechanistic dissection of the two LICs during mitosis is however missing. This knowledge is required to
understand their relative contributions in mitosis as well as to discern the evolutionary significance of the emergence of two LICs in vertebrates. Here, we show that the LIC2 fraction of
cytoplasmic dynein plays a dominant role in orienting the mitotic spindle. LIC2 depletion in cells resulted in prolonged arrest in mitosis and was characterized by severe spindle
mis-orientation as compared to LIC1 depletion. LIC2-dynein, but not LIC1-dynein interacts with Par3 and the 14-3-3 ε and ζ proteins, which constitute key components of the spindle
orientation apparatus in mammalian cells14, 28, 29. Mis-oriented spindles in LIC2-depleted cells showed reduced amounts of NuMA at the defectively anchored spindle pole and a concomitant
accumulation at the corresponding cortex. In addition, LIC2 also plays a dominant role in ensuring chromosome congression at the cell equator during metaphase, while LIC1 plays a larger role
in maintaining spindle pole integrity. The LICs showed overlapping roles in cultured cells and zebrafish embryonic divisions, suggesting that the role of LICs in vertebrate development is
evolutionarily conserved. Overall, our study uncovers an unequal distribution of mitotic functions between the two LIC-containing dynein complexes in vertebrates and reveals the molecular
mechanisms by which LIC2-dynein dominates to ensure proper spindle orientation. RESULTS LIC2 IS REQUIRED FOR MITOTIC PROGRESSION Between the two vertebrate LIC homologues, the mitotic
functions of LIC2 remain less understood. A role for LIC2 in mediating completion of cytokinesis had been reported30. Recently, dynein LICs were reported to have roles in mitosis4 and in
maintaining spindle bipolarity12. LIC2 and LIC1 both prominently decorated mitotic centrosomes, spindle microtubules and the spindle midzone in anaphase31, 32. We confirmed the specificity
of the antibodies used for immunofluorescence by depleting either LIC1 or LIC2 alone, using sequence specific siRNAs (Supplementary Fig. S1A–C 30, 32). The clear and reproducible arrest in
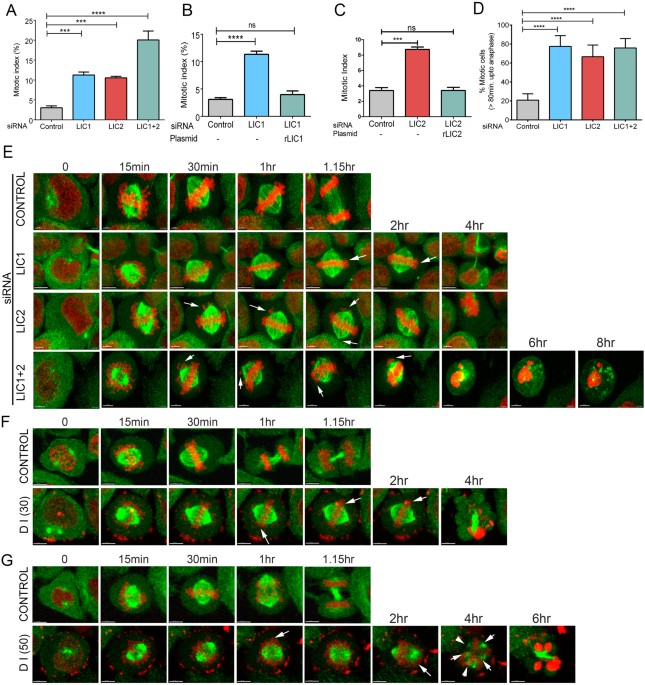
mitosis upon LIC2 depletion (Fig. 1A 4) was independent of LIC1 depletion (Fig. 1A, Supplementary Figs S1A–C and S2A,B), despite using siRNAs of similar potency (Supplementary Fig. S1D).
Only co-depletion of both LICs by treatment with a mixture of both siRNAs led to an additive mitotic arrest (Fig. 1A), suggesting that LIC1 and LIC2 facilitate mitotic progression by largely
independent mechanisms. The mitotic arrest could be rescued by exogenous expression of the corresponding rat LIC orthologs that were not targeted by the respective anti-human LIC siRNAs
(Fig. 1B,C), further verifying the specificity of the depletion phenotype. The mitotic arrest upon LIC2 depletion was also observed in retinal pigment epithelial (hTERT-RPE1) cells and could
be specifically rescued by transgenic expression of rat LIC2 (Supplementary Fig. S1E–G). Time-lapse video imaging (Fig. 1E) showed that LIC1, LIC2 and LIC1-LIC2 co-depleted cells took
significantly longer to proceed from nuclear envelope breakdown (NEB) to anaphase (Supplementary movies 2, 3 and 4 respectively) as compared to control cells (Supplementary movie 1),
confirming the mitotic arrest (Fig. 1D). Treatment with the dynein inhibitor ciliobrevin D as a positive control led to prolonged mitotic arrest (Fig. 1F,G, Supplementary movies 6 and 8)
followed by cell death, as compared to normal mitotic progression in control DMSO (solvent) treated cells (Fig. 1F,G, Supplementary Fig. S2C and movies 5 and 7). We sought to ascertain the
integrity of the dynein complex upon depletion of LIC218, 19. We performed immunoprecipitation assays with the intermediate chain (IC74), a core dynein subunit2, 18, 19, 33 in control siRNA
treated cells, LIC2-depleted cells and cells co-depleted of LIC1 and LIC2. We immunoblotted the precipitates for dynein heavy chain (HC), the scaffold of the dynein complex, to probe for
interaction of HC and IC74 in the same complex. The HC was pulled down with IC74 irrespective of LIC depletion (Fig. 2A), however we see the remnant LICs preferentially pulled down in the
IC74 IPs upon LIC depletion as well, albeit with markedly reduced levels of LICs as compared to control (Supplementary Fig. S10). This suggested that the majority of HC and IC formed
reasonably stable complexes in cells despite the absence of the LICs, consistent with multiple previous studies that show co-sedimentation of the HC (~500 kD) and IC (~74 kD) in the same
density gradient fractions irrespective of LIC knockdown12, 30, 33. The LICs seem to be important for HC dimerization and stability _in vitro_ as shown by reconstitution studies34, 35. It is
possible that the presence of additional binding partners in the cell lysate could serve to partially stabilize the HC-IC interaction even in the absence of the LICs, while complete
stability is achieved in the presence of all subunits in the correct stoichiometric ratios. IC74 also retained its normal mitotic localization at prometaphase kinetochores in a manner
indistinguishable from control cells (Fig. 2B,C). Fluorescence quantification showed that there was no loss of kinetochore IC74 levels upon LIC2 depletion (Fig. 2D). These results show that
the biochemical integrity and localization of cytoplasmic dynein were normal upon LIC2 depletion, consistent with existing literature4, 12, 30, 33. LIC2 GOVERNS MITOTIC SPINDLE ORIENTATION
The predominant mitotic localization of the LICs at spindle poles suggested that they may contribute to spindle orientation, an important spindle pole-related function of cytoplasmic
dynein13, 14, 36,37,38. HeLa cells treated with LIC-specific siRNAs were visualized by time-lapse imaging on gridded cover slips to identify cells that arrested for prolonged periods
(between 80 minutes to 4 hours from NEB) in mitosis to identify LIC-depleted cells. Cells arrested for longer than 4 hours in mitosis often died32. We observed that LIC2 depletion led to
drastic spindle mis-orientation (Fig. 3A) as compared to control cells, with over 40% metaphase cells showing a spindle tilt of greater than 20 degrees with respect to the substratum (Fig.
3B and Supplementary movies 9–control and 10–LIC2 depletion). In contrast, LIC1 depletion showed minimal spindle mis-orientation (Fig. 3A,B), suggesting a stronger role for LIC2-dynein. In
order to ascertain the contribution of adhesion to the substratum, if any, on this phenotype, we performed a similar experiment on collagen-coated cover slips to engage the integrins and
ensure proper adhesion39. We observed a similar trend, with pronounced spindle mis-orientation seen specifically only upon LIC2 depletion, (Supplementary Fig. S3A–D). LIC2-depleted cells
also showed distinctly uneven flattening of daughter cells after anaphase in comparison to control cells that divided parallel to the substratum and thus flattened evenly (Fig. 3C). This
result demonstrated a substantial tilt in the orientation of the division plane (Fig. 3D and Supplementary movies 11–control and 12–LIC2 depletion). The orientation defects were rescued by
exogenous expression of the corresponding rat LIC2 ortholog that was not targeted by the anti-human LIC2 siRNA (Fig. 3E), further verifying the specificity of the LIC2 depletion phenotype.
We also checked whether the prolonged arrest in mitosis upon LIC depletion itself might have caused spindle mis-orientation, by arresting cells in metaphase for prolonged periods upon
treatment with the proteasome inhibitor MG132. We observed that prolonged arrest up to 4 hours by itself did not cause significant spindle mis-orientation in control cells39, 40 and LIC1
depleted cells, but rather only upon LIC2 depletion (Supplementary Fig. S4A,B and Supplementary movies 13, 14 and 15). These results together show a dominant contribution of LIC2-dynein in
maintaining proper mitotic spindle orientation. LIC2-DYNEIN, BUT NOT LIC1-DYNEIN TRANSPORTS NUMA ASYMMETRICALLY TO THE SPINDLE POLES We next aimed to delineate the molecular mechanism(s) by
which LIC2 regulates spindle orientation. NuMA (Nuclear Mitotic Apparatus), a large conserved nuclear protein has been clearly implicated in focusing microtubule minus ends at spindle
poles41, 42. Both NuMA and dynein are also key components of a crucial cortical protein complex responsible for capturing the plus ends of astral microtubules, thus anchoring spindle poles
to the cortex, thereby achieving spindle orientation14,15,16, 36, 43,44,45,46,47. It has also been reported that NuMA is transported by dynein along microtubules to the poles, although this
interaction is weak16, 37, 43, 45, 48. We probed whether LIC2-dynein preferentially influenced intracellular NuMA localization, since the prominent localization of LIC2 to mitotic spindle
poles32 mirrored the polar localization of NuMA. We could distinctly observe the organization of NuMA into well-defined, ring-like structures around mitotic centrosomes in control cells
(Fig. 4A). Upon LIC2 depletion, the “upper” (farther from substratum) poles of mis-oriented cells had less NuMA intensity as compared to the lower poles (Fig. 4A), which we quantified using
3D reconstructed images (Fig. 4B–D). When all centrosomes were analyzed, depletion of LIC2 did not appear to significantly affect the amounts of polar NuMA immunofluorescence with respect to
control cells (Fig. 4B), consistent with a recent report12. However, careful analysis of the 3D quantification of spindle poles revealed that there was a small but significant reduction (by
15–20%) of NuMA intensity preferentially at the “upper” spindle pole upon depleting LIC2 but not LIC1 (Fig. 4C–F). This result indicated that LIC2-dynein is responsible for mitotic
transport of NuMA preferentially to one spindle pole. We observed spindle pole focusing defects upon LIC2 depletion (Supplementary Fig. S8 ), which can partially be attributed to the
defective transport of NuMA to the pole, in addition to the role of LICs in centriole cohesion as recently reported12. The strong spindle orientation defects observed upon LIC2 depletion
prompted us to examine whether there was any change in NuMA localization at the corresponding polar cortex, since cortical NuMA is crucial for ensuring proper spindle orientation. Analysis
of linescan intensities as well as region of interest (ROI) measurements36 revealed a significant accumulation of NuMA at the upper cortex only (corresponding to the upper pole) upon
depletion of LIC2 but not of LIC1 (Supplementary Figs S5A–F and S6). The fold NuMA accumulation at the upper cortex upon LIC2 depletion (Supplementary Fig. S5E) was similar to the fold
reduction of NuMA at the upper pole (Fig. 4D). Thus, we conclude that NuMA is transported preferentially to one spindle pole from the cortex and that this transport is mediated primarily by
LIC2-dynein, but not by LIC1-dynein. LIC2-DYNEIN EXCLUSIVELY INTERACTS WITH KEY PROTEIN COMPLEXES THAT GOVERN SPINDLE ORIENTATION Cortical NuMA serves as a major recruiting factor for
cortical dynein. We next examined whether LIC1- or LIC2-dynein levels were predominant at the cortex. Using a multifunctional GFP-tagged IC74 Hela cell line (mfGFP-IC74 Hela49), we could
visualize the dynamic localization of mitotic dynein on the mitotic spindle, the spindle poles, the kinetochores and at the pole-proximal cortex (Fig. 5A, Supplementary movie 16 and
Supplementary Fig. S7). Quantification of mfGFP-IC74 fluorescence intensity at the cortex from stills of these movies using published methods36 showed that the levels of cortical dynein
dropped significantly upon LIC2 depletion (Supplementary movie 18 and Supplementary Fig. S7). A substantial fraction of LIC1-depleted cells showed normal dynein (mfGFP-IC74) levels at the
cortex (Supplementary movie 17 and Supplementary Fig. S7), while all LIC2 depleted cells analyzed showed reduced cortical IC74 levels (Fig. 5B). Quantitative comparison of fluorescence
intensities revealed significant reduction in mfGFP-IC74 levels at the cortex upon LIC2 depletion, but a minor reduction upon LIC1 depletion (Fig. 5C). The above results together suggest
that LIC2-dynein transports NuMA from the cortex to the spindle pole during mitosis. The other major cortically localized protein complex responsible for maintaining proper spindle
orientation is the Par3-aPKC complex28, 29, 50. Interestingly, Par3 interacts specifically with LIC2 but not with LIC1 in interphase cells, helping to position the centrosome51. Given that
we observed strong spindle orientation defects upon LIC2 depletion (Fig. 3), we probed whether the specific interaction of Par3 with LIC2 is also observed during mitosis. We used mitotically
enriched lysates from cells stably expressing affinity tagged (Streptavidin Binding Protein or SBP-tagged) LIC1 or LIC2 to probe for the interaction of Par3. Affinity purification eluates
from mitotically enriched lysates were analyzed for the presence of Par3 by immunoblotting. Indeed, we observed Par3 (~100 kD isoform) in the affinity pull-downs of LIC2 but not of LIC1 or
control affinity tag alone (Fig. 5D). This result suggested that the exclusive LIC2-Par3 interaction also exists during mitosis and contributes to the spindle orientation functions of
LIC2-dynein. We also probed for the possible interaction of the LICs with the 14-3-3 proteins ε and ζ in the affinity purification eluates, since these proteins are known to interact with
cytoplasmic dynein28, 52 and to biochemically link the Par3 and NuMA spindle orientation pathways to achieve complete spindle orientation28. We observed robust interaction of both 14-3-3 ε
and ζ proteins with LIC2, but no interaction with either LIC1 or with the control empty tag alone (Fig. 5D). Together, the above results revealed exclusive engagement of LIC2-dynein, but not
of LIC1-dynein with both the NuMA and Par3 containing cortical protein complexes that collaborate to achieve proper spindle orientation. LIC2-DYNEIN AND LIC1-DYNEIN DISTRIBUTE OTHER MITOTIC
FUNCTIONS Cytoplasmic dynein facilitates chromosome congression to the metaphase plate53. As described above, we identified cells that had arrested in metaphase for prolonged intervals upon
LIC2 depletion and examined them by immunofluorescence imaging of kinetochores, chromosomes and spindle poles. LIC2-dynein played a major role in ensuring proper chromosome congression at
the cell equator. Over 50% of LIC2 depleted cells that had arrested in metaphase for prolonged periods showed incompletely congressed metaphase plates, an approximately 3-fold defect in
chromosome congression in comparison to control cells. LIC1 played a comparatively minor role in chromosome congression (Supplementary Fig. S9A,B). We also observed that mitotic spindles
were significantly elongated in LIC2 depleted cells, with the spindle poles almost juxtaposed with the cell cortex, an effect much less pronounced for LIC1 depletion (Supplementary Fig.
S9C,D). This observation suggested a dominant role for LIC2-dynein in regulating spindle length. The elongated spindle length is likely due to the loss of dynein function in shortening the
spindle17, 54, 55. As opposed to these phenotypes, LIC1-dynein was the dominant dynein fraction required to prevent major spindle pole fragmentation. LIC1-depleted cells showed a higher
fraction of cells with gamma-tubulin positive foci scattered in the cytoplasm (Supplementary Fig. S8). To test whether pole fragmentation was a consequence of prolonged mitotic arrest
alone40, we arrested cells in metaphase for up to 4 hours using treatment with MG132. The fragmentation phenotype was consistently observed only upon LIC1 depletion (Supplementary Fig.
S4C,D, Supplementary movies 19, 20 and 21). These observations collectively suggested a strong involvement for LIC2 in regulating the length of the mitotic spindle as well as congressing
chromosomes to the equatorial plate. LIC1 makes only minor or negligible contributions in these functions, but plays a larger role in maintaining spindle pole integrity. LIC2 IS REQUIRED FOR
EARLY VERTEBRATE EMBRYONIC DIVISIONS AND DEVELOPMENT The role of dynein during early embryonic development is not well understood. We probed the function of LIC2 in controlling early
divisions in the zebrafish embryo. Immunoblotting and real time PCR analysis revealed that both zebrafish LICs were expressed across early embryonic stages up to the 512-cell stage,
suggesting maternally inherited expression (Fig. 6A,B). We depleted zLICs from zebrafish embryos by injecting sequence-specific morpholinos into one-cell embryos. zLIC2 depleted embryos
(using both translation-blocking and splice-blocking morpholinos specific to zLIC2) exhibited a distinct “furrow” phenotype at the blastula stage (around the 256–512 cell stage, Fig. 6C).
The blastomeres of zLIC2 morphant embryos segregated into two distinct “hemispheres”, with a central furrow separating them. Control morpholino injected embryos (injected with water, p53,
standard control or LIC2 mismatch morpholinos) showed normal embryonic development indistinguishable from uninjected embryos (Fig. 6C). The intensity of the furrow phenotype varied in a
dose-dependent manner with the amount of LIC2 morpholino injected (Fig. 6C,D). We co-injected p53 morpholino to reduce non-specific, off-target effects of the morpholinos56. Immunoblotting
of whole-embryo lysates obtained from furrowed embryos showed a specific decline in the levels of LIC2 protein (Fig. 6E). We also verified specificity of the knockdown by injecting a splice
blocking morpholino against zLIC2, which resulted in alternatively spliced zLIC2 variants (Fig. 6F). We further confirmed the specificity of the zLIC2 depletion phenotypes by performing
functional rescue experiments by introducing an orthologous rat LIC2 mRNA, which ameliorated the gross furrow phenotype in almost all treated embryos (Fig. 6G, top). LIC2 depleted embryos
showed an increased fraction of cells in metaphase by phosphohistone 3 (PH3) staining (6G bottom, 6H) suggesting an arrest in cell division, which was reversed upon rescue with rat LIC2 mRNA
(Fig. 6G,H). Furrowed embryos failed to develop further; however embryos injected with a lower dose of morpholino exhibited slower development around 1 day post fertilization (1 dpf), with
poorly formed head and tailbud and defects in elongation of the body axis (Fig. 6I). The above results demonstrated that LIC2 is required for early development in zebrafish embryos. We
probed the mechanistic basis for the developmental delay upon LIC2 depletion, surmising that like in mammalian cells, LIC2 plays important roles in mitotic progression in zebrafish embryos.
We immunostained embryos for microtubules, spindle poles and chromosomes and performed confocal microscopy followed by 3-dimensional image reconstruction to visualize the surface of whole
embryos. LIC2 depleted embryos were of approximately similar size as control embryos, but were distinctly oblong and deformed (top view), in contrast with control embryos that were spherical
(Fig. 7A). Individual blastomeres of LIC2-depleted embryos were larger and misshapen as compared to control embryos (Fig. 7A). LIC2 depleted furrowed embryos showed a 5–6-fold reduction in
the total number of nuclei compared to control embryos at the same time post injection (Fig. 7B), suggesting a mitotic delay. The mitotic delay was corroborated by a visibly higher fraction
of phosphohistone 3 (PH3) positive surface blastomeres in LIC2 depleted furrowed embryos (Fig. 7C,D). Microscopic analysis revealed that metaphase cells from LIC1 and LIC2 depleted embryos
showed significantly elongated spindles compared to control embryos (Fig. 7E,F), similar to the elongated spindles seen in mammalian cells (Supplementary Fig. S3). LIC2 depletion, but not
LIC1 depletion also led to uncongressed chromosomes in a significant fraction of blastomeres of furrowed embryos (Fig. 7E,G) as seen in mammalian cells (Supplementary Fig. S3). Additionally,
LIC2 depleted embryos showed bipolar spindles with unfocused spindle poles (Fig. 7E,H) similar to the pole dispersion phenotype seen in mammalian cells (Supplementary Fig. S2) while
LIC1-depleted embryos showed fragmented spindle poles (Fig. 7E,I). These results are consistent with the recently demonstrated roles for LICs in maintaining spindle pole integrity12.
Although the gross morphology of the LIC1/2 depleted morphants is similar (Fig. 6C), the underlying mitotic phenotypes appear to be distinct. Together, our results demonstrate that LIC2
plays conserved roles in spindle organization and mitotic fidelity during embryogenesis, and is essential for governing early cell divisions and embryonic development in vertebrate embryos.
DISCUSSION The extensive mitotic localization of LIC2 prompted us to explore its functional repertoire during vertebrate mitosis. Our results reveal pleiotropic functions of LIC2-dynein
during mitosis and uncover protein interaction specificity among the LICs that could explain these functions (Fig. 8). LIC2-dynein is required for spindle orientation via its ability to
anchor astral microtubules at the cortex through its interaction with cortical NuMA, Par3 and 14-3-3 proteins. LIC2 also transports NuMA to spindle poles, which in turn is responsible for
maintaining proper centrosomal focusing. LIC1 plays a stronger, perhaps mechanistically distinct role in this function. LIC2 is responsible for congression of condensed chromosomes to the
equatorial plate and regulates spindle length by shortening it. It is also noteworthy that we quantified the mitotic phenotypes only in cells that had spent abnormally prolonged periods in
mitosis upon LIC1/2 depletion. The mitotic phenotypes of spindle mis-orientation and pole fragmentation were however a direct consequence of LIC2/LIC1 depletion and not of the prolonged
arrest itself (Supplementary Fig. S4). A major advance from this study about our mechanistic understanding of mitotic dynein is the discovery of a dominant role for LIC2-dynein in regulating
spindle orientation (Fig. 8). The orientation of cell division plays key roles in regulating stem cell self-renewal vs. differentiation57,58,59,60, and spindle mis-orientation is suggested
as a cause for disorders like polycystic kidney disease61,62,63,64,65,66. The spindle mis-orientation observed upon LIC2 depletion is stark (Fig. 3), with a 4-fold increase in the fraction
of cells showing appreciable spindle tilt (>20 degrees). In contrast, LIC1 depletion shows a mild defect, highlighting the dominance of LIC2 in influencing spindle orientation. The
orientation defects were not due to any defects in adhesion to the substratum (Supplementary Fig. S3). Both LIC2 and the mitotic regulator NuMA start appearing prominently at spindle poles
simultaneously in prometaphase31, 32, making LIC2 a prime suspect in linking NuMA to cytoplasmic dynein. A major dynein-dependent mechanism regulating spindle orientation is likely to be
through the interaction of LIC2-dynein with NuMA at the pole-proximal cortex (Fig. 8), although a dynein-independent role for NuMA in orienting the spindle has also been recently
demonstrated67. NuMA is a critical component of the cortically localized LGN-Gαi-NuMA complex that attaches to astral microtubules and mechanically anchors the centrosome to the cortex14,
43, thus stabilizing spindle orientation. LIC2 depletion also led to elevated levels of cortical NuMA and a concomitant reduction in polar NuMA levels at the upper cortex and upper pole
respectively of mis-oriented spindles (Fig. 4 and Supplementary Fig. S5). The distribution of cytoplasmic dynein at the cortex in mitotic cells is naturally asymmetric, with more dynein
concentrated at the cortices corresponding to the two poles36. Therefore, dynein-based transport of cortical NuMA towards the pole43 would also be asymmetrically affected upon LIC2
depletion. This could explain the differential NuMA levels upon LIC2 depletion at the upper and lower poles. The altered NuMA levels at these sites upon LIC2 depletion are attributable to
the impaired transport of NuMA from the cortex to the pole due to the inability of dynein to capture cortical NuMA. In support of this hypothesis, the levels of cortical dynein reduced
appreciably upon LIC2 depletion, (Fig. 5), demonstrating that LIC2-dynein is anchored at the cortex at least partially through its interaction with NuMA. This result also indicated that a
larger fraction of cortical dynein consists of the LIC2-dynein subpopulation. Development of more robust methods for quantification of cortical fluorescence would help reveal the levels of
the two dynein subpopulations with greater precision. The majority of pole-localized NuMA is recruited by diffusion from the cytoplasm in a dynein-independent manner, while a smaller but
significant fraction is deposited at poles by dynein mediated transport37, 45, 46, 48. Our results indicate that LIC2-dynein could account for the majority of dynein-mediated poleward
transport of NuMA, which originates primarily from the cortex. The other major cortically localized protein complex that ensures spindle orientation is the Par3-aPKC-LGN complex28, 29. This
pathway has been shown to be partially sufficient for orienting the spindle, by interacting with Dlg and kinesin to capture astral microtubules near the cortex28. Interestingly, Par3 has
been demonstrated to exclusively interact with LIC2 but not with LIC1 in interphase to position the centrosome51. We found that the Par3-LIC2 interaction is also preserved in mitosis (Fig.
5D). The 14-3-3 heterodimer consisting of its ε and ζ proteins is known to interact with dynein and kinesin, thus bridging the NuMa-LGN and Par3-aPKC pathways, two important spindle
orientation pathways that together achieve complete orientation28. Indeed, the 14-3-3 heterodimer interacted very robustly and specifically with LIC2 but not with LIC1 (Fig. 5D),
demonstrating that LIC2-dynein could assist the 14-3-3 proteins in bridging these two pathways. The strong interaction of the 14-3-3 proteins with LIC2 suggests that they may interact with
LIC2-dynein upstream of the other effectors of the two orientation pathways, although this remains to be demonstrated. The specific binding of LIC2-dynein to Par3 and especially to the
14-3-3 heterodimer illuminates the two major molecular networks that LIC2-dynein associates with to mediate spindle orientation. LIC2 depletion also led to elongated spindles, with the poles
juxtaposed with the cortical membrane (Supplementary Fig. S9). In this scenario, the centrosomal NuMA could anchor the poles to the cortex directly through its interaction with membrane
phospholipids68. This attachment may happen at a later stage at random sites on the cortex, leading to mis-oriented spindles. LIC2 depletion also led to chromosome mis-congression in
approximately 50% mitotic cells (Supplementary Fig. S9). The mitotic arrest seen in these cells is likely to be due to an active spindle assembly checkpoint signal, as expected due to their
inability to establish proper inter-kinetochore tension53, 69, 70. LIC2 was crucial for regulating the early divisions in zebrafish embryos. Our zebrafish results mirror the LIC1- and LIC2-
specific phenotypes observed in mammalian cells. We found that both LICs were expressed from the earliest stages of the embryo (Fig. 6) even before zygotic gene expression commences71, 72,
indicating maternal inheritance. The furrow phenotype seen at the mid-blastula transition (MBT) stage (Fig. 6) upon LIC2 depletion could have many implications. The altered cellular and
embryonic shape (Figs 6 and 7) could cause defects in subsequent morphogenetic processes like gastrulation, and alter the cell fate imparted on these blastomeres, possibly resulting in
defects later during development. Severe developmental delays were indeed observed at the blastula stage upon zLIC2 depletion, with stunted head and tail bud regions seen at 1 dpf (Fig. 6).
An important underlying basis for the developmental delay is defective cell proliferation (Fig. 7). Upon LIC2 depletion, we readily observed phenocopy of various mitotic spindle defects seen
in mammalian cells during the blastula stage of control embryos. Mitotic spindles were significantly elongated (Fig. 7), which could result in cell shape changes of individual blastomeres
and by extension, in the oblong morphology of the whole embryo, as opposed to a more spherical shape of control embryos (Fig. 6). The multiple mitotic defects seen in zebrafish embryos are
likely to cumulatively cause the developmental delays observed upon LIC2 depletion at 1 dpf (Fig. 6) and suggested a strong conservation of the mitotic functions of LIC2-dynein across
vertebrates. The long-term developmental consequences of LIC2 depletion and the molecular mechanisms that enable crosstalk with extracellular developmental cues remain to be elucidated. LIC1
and LIC2 are believed to occupy cytoplasmic dynein in mutually exclusive complexes25, conceivably to perform at least a set of distinct functions. In support of this hypothesis, it was
recently reported that LIC1-and LIC2-dynein show differential effects on inactivating the spindle assembly checkpoint32. Here we demonstrate that a prominent share of mitotic dynein
functions is performed by LIC2-dynein, which plays dominant roles in regulating chromosome congression and spindle length and ensures proper spindle orientation. LIC1-dynein plays minor
roles in all of these functions, perhaps through other mechanisms. We show that only LIC2-dynein governs spindle orientation through its exclusive interactions with 14-3-3, Par3 and NuMA.
The binding of mitotic LICs to exclusive cargoes has some precedence; LIC1 exclusively binds to the key mitotic regulator pericentrin25, 73, 74. It is instructive that invertebrates have
only one LIC gene, suggesting that vertebrate LIC1 and LIC2 have evolved in a divergent manner to perform more complex functions. While both LICs have been shown to be important for
maintaining spindle pole integrity in vertebrates12, it is likely that they operate at least partially through distinct biochemical pathways. Given the exclusivity of the LIC1-pericentrin
interaction, it is conceivable that LIC1-dynein contributes to spindle pole integrity by ensuring proper formation of the pericentriolar material, in addition to its role in centriole
cohesion12. LIC1 also appears to be more relevant in some interphase functions, where it localizes to centrosomes and influences Golgi complex organization, a function in which LIC2 plays no
significant role4, 30, 33. It is logical to surmise that the distinct mitotic functions of the two LICs are governed by unique biochemical interactions with other proteins, as shown here
for LIC2-dynein in spindle orientation. Elucidation of these molecular mechanisms would illuminate mechanistic details governing the biology of the two LIC homologues. METHODS CELL CULTURE
AND CELL SYNCHRONIZATION HeLa cells (Sigma Aldrich/ECACC), U2OS cells and hTertRPE1 cells (gifts from Stephen Doxsey) were grown in appropriate culture media. The mfGFP-IC74 Hela stable cell
line (gift from Takashi Murayama) was grown in medium supplemented with hygromycin B. The H2B-mCherry-GFP-α tubulin line (gift from Daniel Gerlich) was grown in medium supplemented with
hygromycin B and puromycin. Nocodazole (Sigma) was used at 150 nM (HeLa) or 400 nM (U2OS) for 12 hours to arrest cells in prometaphase. For obtaining metaphase cells, the prometaphase arrest
was released by washing the cells extensively with PBS (phosphate buffer saline) and harvesting after 1 hour, or by treatment of nocodazole-released cells with MG132 (10 µM). Dynein
inhibitor ciliobrevin D (Millipore) dissolved in DMSO was used as a positive control and added to 60–70% confluent cells at 30 μM and 50 μM concentration in fresh media followed by
time-lapse imaging. PLASMID CONSTRUCTS, SIRNAS AND TRANSFECTION Full length rat LIC2 cDNA was cloned into the pCMV 3Tag 3B vector (Agilent Technologies) using EcoR1 and Xho1 restriction
sites and sequenced. Plasmid transfection was performed using Lipofectamine 2000 (Invitrogen) as described in the manual. SiRNAs against different human genes were synthesized by Dharmacon.
The detailed sequences and working concentrations of individual siRNAs were as follows–LIC1: GAAAGUUUGUACAUGAGAA (100 nM), LIC2a: ACCUCGACUUGUUGUAUAA (100 nM), LIC2b: GCCGGAAGAUGCAUAUGAA
(100 nM), GFP (negative control): CAUGAAGCAGCACGACUUC (100 nM). All the siRNA sequences were previously used and published by different groups30, 33. siRNA transfection was achieved using
Dharmafect 1 (Dharmacon/Thermo Scientific) for 48 hrs. Negative controls for siRNA treatment used either GFP siRNAs or mock transfections. During co-depletion of LIC1 + 2, 100 nM each of the
two siRNAs were used. For rescue experiments in cell lines, plasmids were transfected on day 1 followed by siRNA transfection on day 2 and observation at 48 hrs after siRNA transfection.
Metaphase index was calculated by counting metaphase cells as a fraction of total cells under a fluorescence microscope or confocal microscope to visualize chromosomes, which were stained by
4′, 6-diamidino-2-phenylindole (DAPI) or Syto 13 (Invitrogen) respectively. ANTIBODIES Primary antibodies against the following antigens were used: LIC1 (Thermo Scientific PA5-31644); LIC2
(Abcam ab174895, ab178702), γ-tubulin (A302-631A, Bethyl laboratories); NuMA (Novus Biologicals NB500-174); α-tubulin (DM1α, T9026), β–actin (A3835), Flag M2 (F1804) monoclonal antibodies
from Sigma; IC-74 monoclonal antibody (Abcam ab23905), Par3 (Milllipore, 07-330), 14-3-3 ζ (Abcam, ab124431), 14-3-3 ε (Abcam, ab43057). HRP conjugated anti-mouse (715-035-150) and
anti-rabbit (711-035-152) secondary antibodies for Western blotting and fluorophore attached DyLight 488 (115-485-003), Cy3 (111-165-144), Cy5 (109-175-008) secondary antibodies for
immunofluorescence analyses were purchased from Jackson Immunoresearch. WESTERN BLOTTING The following antibody dilutions were used for Western blotting: LIC1- 1:1000, LIC2–1:1000, β-actin-
1:2000, IC74–1:1000, 14-3-3 ε–1:1000, 14-3-3 ζ–1:200; Par3 1:400, Flag M2 -1:10000, anti-mouse HRP 1:10000, anti-rabbit HRP- 1:10000. Cell lysates were prepared by directly adding Laemmli
buffer into the culture plate and boiling the sample at 95 °C for 5 min. Samples were run on SDS-PAGE, followed by transfer of proteins on to polyvinylidene difluoride (PVDF) membrane
(Millipore). Blots were blocked with 5% skimmed milk followed by incubation in primary antibody for 1 hr at room temperature or at 4 °C overnight, washed and incubated with secondary HRP
conjugated antibodies for 1 hr at room temperature, and washed extensively. The chemiluminescene signal was developed using the Luminata Forte reagent (Millipore) and captured in the Image
Quant 4000 (GE). Densitometric quantification of band intensities was performed using the GE Image Quant 4000 platform. For zebrafish samples, embryos were dechorionated manually and washed
in fresh E3 media. Embryos older than 1dpf were also de-yolked manually and washed in E3 media. The embryos were lysed in Laemmli buffer and boiled at 95 °C for 5 min. Protein estimation was
performed using the CBX protein estimation assay (G-Biosciences). The proteins were resolved by SDS-PAGE, transferred to PVDF membranes and probed with the following antibodies at 4 °C
overnight: LIC2 (1:500, Abcam), β-actin (1:1000, Sigma), anti rabbit (1:10,000, Jackson Laboratories) and anti-mouse (1:10,000, Jackson Laboratories) HRP conjugated secondary antibodies were
used along with Precision Protein strepTactin HRP conjugate (Biorad). The blots were developed and visualized as mentioned above. IMMUNOPRECIPITATION AND AFFINITY PURIFICATION The IC-74
antibody was used for immunoprecipitation experiments. A Streptactin-HP (GE) affinity column was used for purification of the MTAP-mVenus-hLIC1 and –hLIC2 protein from a stably expressing
cell line, using the Streptavidin-binding-protein tag imparted by the MTAP-mVenus vector75. Mitotically enriched cells were suspended in lysis buffer containing 50 mM Tris pH 7.5, 125 mM
sodium chloride, 1mM EGTA, 0.2 NP-40, 5% glycerol, protease inhibitors and phosphatase inhibitors (Roche/Pierce) and incubated for at least 20 minutes on ice. Whole cell lysates were
pre-cleared by incubating with protein G sepharose beads (GE). Protein G sepharose beads (GE) were used for immunoprecipitation. Primary antibodies were crosslinked to protein G sepharose
beads by incubating antibody with beads for 1 hour at 4 °C followed by washing to remove unbound antibody. Pre-cleared cell lysates were subjected to immunoprecipitation by incubating
suitable beads with pre-cleared lysates for 6 to 8 hours at 4 °C with tumbling. Immunoprecipitated samples were resolved by SDS PAGE followed by Western blotting. IMMUNOFLUORESCENCE STAINING
The following antibody dilutions were used: LIC1–1:250, LIC2–1:100, IC74–1:500, NuMA–1:100, α-tubulin–1:1000, γ-tubulin–1:500. HeLa cells were grown on glass coverslips, washed with PBS,
and fixed in 4% formaldehyde or chilled methanol. Fixed coverslips were incubated with blocking buffer (PBS + 1% bovine serum albumin + 0.5% Triton X-100) for 1 hour, followed by incubation
for 1 hour with primary antibody, washed and incubated with secondary antibodies in a humidified chamber. DAPI staining was performed (1:10,000 concentration of a 5 mg/ml stock solution) for
1 minute, cells washed in PBS and water, and mounted on a glass slide using mounting medium (Prolong Gold, Invitrogen). Imaging of fixed coverslips was performed after drying the mounting
medium. For zebrafish embryos: whole embryos were fixed in 4% formaldehyde in PBS at 4 °C overnight followed by post-fixing in 100% methanol until use. Fixed embryos were rehydrated and
washed in PBS, dechorinated and blocked with 1% PBSAT (1% bovine serum albumin in 1X PBS + 0.1% Triton X-100) for 2–4 hours at room temperature. The following primary antibodies were used:
gamma tubulin (1:1000, Bethyl labs), α tubulin (1:250, DSHB) and phosphohistone 3 (1:250, Thermo Scientific). Primary and secondary antibody incubations were carried out in 1% PBSAT at 4 °C
overnight with 1X PBSAT washes between incubations. The embryos were washed in PBS, stained with DAPI, washed again in PBS and mounted for microscopic examination. MICROSCOPY
IMMUNOFLUORESCENCE IMAGING OF CELLS Fixed cell image acquisition was performed on a Leica TCS SP5 II (with Leica DFC 360FX camera, version: FCAM 2 V1.0 in fluorescence mode or using PMTs in
laser illumination mode) or Leica TCS SP8 laser scanning optical confocal microscope using a HCX PL APO CS 63X-1.4 numerical aperture oil immersion objective. All acquisition settings were
identical for control and test samples. Only metaphase cells (judged by DAPI staining) were imaged to analyze spindle orientation, chromosome congression and spindle length. Immunostained
embryos were visualized under a 40X Plan-Apochromat objective of Leica TCS SP5 II confocal microscope. The number of cells and embryos counted per experiment for statistical analysis is
indicated in the figure legends. Error bars depict standard deviation across a minimum of 3 experiments unless otherwise specified. TIME LAPSE IMAGING HeLa cells were grown on coverslips and
suitably treated. The coverslips were placed on custom-designed aluminium slide containing chambers. Time-lapse imaging was performed for 12 hours with a 2.5 or 5-minute time interval
between successive frames at four different positions on each coverslip in bright field or laser fluorescence modes, using a 20X, 40X or 63X Plan-Apochromat objectives. In some experiments,
Labtek chambered cover glass (Nunc) were used. GRIDDED COVERSLIP EXPERIMENTS HeLa cells were grown on gridded coverslips (Electron Microscopy Sciences). After 36 hrs of siRNA transfection
the coverslips were followed by time-lapse imaging as described above. The same gridded coverslips were fixed and used for immunostaining and imaged as described above. The grids on the
coverslips were used to locate the very same cells that got arrested in mitosis for prolonged periods (over 1.5 hours); only these cells were used for further fixed cell imaging following
immunostaining. IMAGE ANALYSIS Fluorescence image analysis was performed on the Imaris software suite (version 5.7, Bitplane), Leica offline image analysis software (LAS) and ImageJ. For
spindle orientation measurements, immunofluorescence images were reconstructed in 3 dimensions using Imaris. The angle made by the spindle axis with the substratum was measured using ImageJ
software by the method described earlier63, 76, 77. Further, to show orientation defects in live cells, we observed the uneven flattening pattern of mitotic cells as they exit mitosis63 by
visual observation of time-lapse movies of dividing cells. Dividing Hela cells round up during mitosis and flatten onto the substratum almost simultaneously as they exit mitosis (movie 11).
The two daughter cells generated from mis-oriented poles (such as upon LIC2 depletion) do not re-flatten at the same time (movie 12); the daughter cell with the pole nearer to the substratum
flattens first followed by the other, which stays rounded for longer and flattens later. NuMA intensity at the cortex was measured by line scans and ROI intensity measurements36 on the LAS
Leica offline analysis software (LAS). For measuring NuMA levels at the poles, images were reconstructed in Imaris, a sphere of diameter 4.5 µm was drawn around the spindle poles and
fluorescence intensity within the sphere measured. Intensities of mf-GFP-IC74 at the cortex were quantified as previously described36 using four rectangular boxes drawn at the cell cortex.
Spindle lengths were measured as the distance between the centers of two spindle poles (visualized using γ-tubulin staining) in either the LAS or Imaris software suites. For zebrafish embryo
imaging, the z-stack images acquired were processed on the LAS Leica offline software. Imaris 3D imaging software suite was used to generate 3D reconstructions of the entire surface of
embryos. The 3D reconstructions were used to count the total number of cells in the embryos, calculate spindle length, chromosome congression and spindle pole-focusing defects. ZEBRAFISH
LINES, MORPHOLINO INJECTION AND CHARACTERIZATION OF PHENOTYPE Tuebingen strain zebrafish were raised according to standard protocols as described earlier78. Embryos were obtained from
natural spawning of adult fish. The embryos were kept at 28.5 °C and staged according to hours post fertilization79. Morpholinos used (all from Gene Tools LLC) were as follows:
TTCTTCTCTAAAACGGGAGCCATCT (zLIC2 translation blocker), GTTGAAGTTTTTGAGTTACCTTTGT (zLIC2 splice blocker), TTgTTgTCTAtAACGGcAcCCATCT (zLIC2 mismatch) and GTGTATTTCTGCCCGTCGTCGCCAT (zLIC1
translation blocker). p53 morpholino and standard control morpholino were used as negative controls. One-cell stage embryos were injected with LIC2 translational blocker (5 ng/15 ng) and 15
ng of other morpholinos. Gross anatomical defects were visualized around 3.3 hpf and at 1 dpf with a Zeiss SMZ Stemi 2000C microscope. Morphant embryos collected around 3.3 hpf
(corresponding to blastula stage of control embryos) were analyzed by high resolution imaging as described above. LIC depletion was assessed by immunoblotting (LIC2 translation blocker) or
reverse transcriptase PCR (LIC2 splice blocker). The following primers used for PCR: CGCTGTAGTGTCTGGATCCTGG (forward primer) and GCCTTAGTGGTGGAGAAGGACG (reverse primer). For rescue
experiments, rLIC2 mRNA was synthesized using the mMESSAGE mMACHINE kit (AM1348, Ambion) and 25 pg of mRNA injected per embryo. For real time PCR, total RNA was isolated from different
developmental stages of wild type Tuebingen zebrafish using a RNA isolation kit (Promega) and cDNA synthesized (using BioRad iScript cDNA synthesis kit). The following primers were used for
the real time PCR for zLIC1 (Fwd GAAGAGTACATGAAGGGACGAG, Rev CACTACAAACAGAATCAGCGAG) and housekeeping gene control ribosomal protein L (zRPL13a): Fwd TCTGGAGGACTGTAAGAGGTATGC and Rev
AGACGCACAATCTTGAGAGCAG. LIC1 real time expression data is normalized to the expression at the 1-cell stage. STATISTICAL ANALYSIS The number of cells counted per experiment for statistical
analysis is mentioned in the respective figure legends. The graphs in all figures are depicted with error bars, mean of at least 3 experiments +/−SD or SEM, unless stated otherwise.
Statistical significance was calculated by Student’s t-test or one-way ANOVA with Tukeys comparison method or Kruskal Wallis test. Scale bars (μm) for images are indicated in the respective
legends. Graphs were created using the GraphPad PRISM software. INSTITUTIONAL APPROVALS All experiments and protocols other than those involving live zebrafish were performed with the
approval of the institutional biosafety committee of the Regional Centre for Biotechnology, India. All live zebrafish experiments were performed according to protocols approved by the
Institutional Animal Ethics Committee (IAEC) of the CSIR-Institute of Genomics and Integrative Biology, India. REFERENCES * King, S. M. Organization and regulation of the dynein microtubule
motor. _Cell Biol Int_ 27, 213–215 (2003). Article CAS PubMed Google Scholar * Vale, R. D. The molecular motor toolbox for intracellular transport. _Cell_ 112, 467–480 (2003). Article
CAS PubMed Google Scholar * Varma, D., Monzo, P., Stehman, S. A. & Vallee, R. B. Direct role of dynein motor in stable kinetochore-microtubule attachment, orientation, and alignment.
_J Cell Biol_ 182, 1045–1054, doi:10.1083/jcb.200710106 (2008). Article CAS PubMed PubMed Central Google Scholar * Raaijmakers, J. A., Tanenbaum, M. E. & Medema, R. H. Systematic
dissection of dynein regulators in mitosis. _J Cell Biol_ 201, 201–215, doi:10.1083/jcb.201208098 (2013). Article CAS PubMed PubMed Central Google Scholar * Tame, M. A. _et al._ Astral
microtubules control redistribution of dynein at the cell cortex to facilitate spindle positioning. _Cell Cycle_ 13, 1162–1170, doi:10.4161/cc.28031 (2014). Article CAS PubMed PubMed
Central Google Scholar * Biggins, S. & Walczak, C. E. Captivating capture: how microtubules attach to kinetochores. _Curr Biol_ 13, R449–460 (2003). Article CAS PubMed Google
Scholar * Bader, J. R. & Vaughan, K. T. Dynein at the kinetochore: Timing, Interactions and Functions. _Semin Cell Dev Biol_ 21, 269–275, doi:10.1016/j.semcdb.2009.12.015 (2010).
Article CAS PubMed PubMed Central Google Scholar * Sharp, D. J., Rogers, G. C. & Scholey, J. M. Cytoplasmic dynein is required for poleward chromosome movement during mitosis in
Drosophila embryos. _Nat Cell Biol_ 2, 922–930, doi:10.1038/35046574 (2000). Article CAS PubMed Google Scholar * Dumont, S. & Mitchison, T. J. Force and length in the mitotic
spindle. _Curr Biol_ 19, R749–761, doi:10.1016/j.cub.2009.07.028 (2009). Article CAS PubMed PubMed Central Google Scholar * Nedelec, F. J., Surrey, T., Maggs, A. C. & Leibler, S.
Self-organization of microtubules and motors. _Nature_ 389, 305–308, doi:10.1038/38532 (1997). Article ADS CAS PubMed Google Scholar * Goshima, G., Nedelec, F. & Vale, R. D.
Mechanisms for focusing mitotic spindle poles by minus end-directed motor proteins. _J Cell Biol_ 171, 229–240, doi:10.1083/jcb.200505107 (2005). Article CAS PubMed PubMed Central Google
Scholar * Jones, L. A. _et al._ Dynein light intermediate chains maintain spindle bipolarity by functioning in centriole cohesion. _J Cell Biol_ 207, 499–516, doi:10.1083/jcb.201408025
(2014). Article CAS PubMed PubMed Central Google Scholar * Gonczy, P., Pichler, S., Kirkham, M. & Hyman, A. A. Cytoplasmic dynein is required for distinct aspects of MTOC
positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo. _J Cell Biol_ 147, 135–150 (1999). Article CAS PubMed PubMed Central Google Scholar *
Kotak, S., Busso, C. & Gonczy, P. Cortical dynein is critical for proper spindle positioning in human cells. _J Cell Biol_ 199, 97–110, doi:10.1083/jcb.201203166 (2012). Article CAS
PubMed PubMed Central Google Scholar * Kotak, S. & Gonczy, P. NuMA phosphorylation dictates dynein-dependent spindle positioning. _Cell Cycle_ 13, 177–178, doi:10.4161/cc.27040
(2014). Article CAS PubMed Google Scholar * Markus, S. M. & Lee, W. L. Microtubule-dependent path to the cell cortex for cytoplasmic dynein in mitotic spindle orientation.
_Bioarchitecture_ 1, 209–215, doi:10.4161/bioa.18103 (2011). Article PubMed PubMed Central Google Scholar * Laan, L. _et al._ Cortical dynein controls microtubule dynamics to generate
pulling forces that position microtubule asters. _Cell_ 148, 502–514, doi:10.1016/j.cell.2012.01.007 (2012). Article CAS PubMed PubMed Central Google Scholar * Pfister, K. K. _et al._
Genetic analysis of the cytoplasmic dynein subunit families. _PLoS Genet_ 2, e1, doi:10.1371/journal.pgen.0020001 (2006). Article PubMed PubMed Central Google Scholar * Pfister, K. K.
_et al._ Cytoplasmic dynein nomenclature. _J Cell Biol_ 171, 411–413, doi:10.1083/jcb.200508078 (2005). Article CAS PubMed PubMed Central Google Scholar * Hook, P. & Vallee, R. B.
The dynein family at a glance. _J Cell Sci_ 119, 4369–4371, doi:10.1242/jcs.03176 (2006). Article CAS PubMed Google Scholar * Mische, S. _et al._ Dynein light intermediate chain: an
essential subunit that contributes to spindle checkpoint inactivation. _Mol Biol Cell_ 19, 4918–4929, doi:10.1091/mbc.E08-05-0483 (2008). Article CAS PubMed PubMed Central Google Scholar
* Yoder, J. H. & Han, M. Cytoplasmic dynein light intermediate chain is required for discrete aspects of mitosis in Caenorhabditis elegans. _Mol Biol Cell_ 12, 2921–2933 (2001).
Article CAS PubMed PubMed Central Google Scholar * Gibbons, I. R. Dynein family of motor proteins: present status and future questions. _Cell Motil Cytoskeleton_ 32, 136–144,
doi:10.1002/cm.970320214 (1995). Article CAS PubMed Google Scholar * Milisav, I. Dynein and dynein-related genes. _Cell Motil Cytoskeleton_ 39, 261–272,
doi:10.1002/(sici)1097-0169(1998)39:4<261::aid-cm2>3.0.co;2-6 (1998). Article CAS PubMed Google Scholar * Tynan, S. H., Purohit, A., Doxsey, S. J. & Vallee, R. B. Light
intermediate chain 1 defines a functional subfraction of cytoplasmic dynein which binds to pericentrin. _J Biol Chem_ 275, 32763–32768, doi:10.1074/jbc.M001536200 (2000). Article CAS
PubMed Google Scholar * Bhabha, G. _et al._ Allosteric communication in the dynein motor domain. _Cell_ 159, 857–868, doi:10.1016/j.cell.2014.10.018 (2014). Article CAS PubMed PubMed
Central Google Scholar * Schroeder, C. M., Ostrem, J. M., Hertz, N. T. & Vale, R. D. A Ras-like domain in the light intermediate chain bridges the dynein motor to a cargo-binding
region. _Elife_ 3, e03351, doi:10.7554/eLife.03351 (2014). Article PubMed PubMed Central Google Scholar * Lu, M. S. & Prehoda, K. E. A NudE/14-3-3 pathway coordinates dynein and the
kinesin Khc73 to position the mitotic spindle. _Dev Cell_ 26, 369–380, doi:10.1016/j.devcel.2013.07.021 (2013). Article CAS PubMed PubMed Central Google Scholar * Hao, Y. _et al._ Par3
controls epithelial spindle orientation by aPKC-mediated phosphorylation of apical Pins. _Curr Biol_ 20, 1809–1818, doi:10.1016/j.cub.2010.09.032 (2010). Article CAS PubMed PubMed Central
Google Scholar * Palmer, K. J., Hughes, H. & Stephens, D. J. Specificity of cytoplasmic dynein subunits in discrete membrane-trafficking steps. _Mol Biol Cell_ 20, 2885–2899,
doi:10.1091/mbc.E08-12-1160 (2009). Article CAS PubMed PubMed Central Google Scholar * Horgan, C. P., Hanscom, S. R. & McCaffrey, M. W. Dynein LIC1 localizes to the mitotic spindle
and midbody and LIC2 localizes to spindle poles during cell division. _Cell Biol Int_ 35, 171–178, doi:10.1042/cbi20100284 (2011). Article CAS PubMed Google Scholar * Mahale, S. P.,
Sharma, A. & Mylavarapu, S. V. Dynein Light Intermediate Chain 2 Facilitates the Metaphase to Anaphase Transition by Inactivating the Spindle Assembly Checkpoint. _PLoS One_ 11,
e0159646, doi:10.1371/journal.pone.0159646 (2016). Article PubMed PubMed Central Google Scholar * Sivaram, M. V., Wadzinski, T. L., Redick, S. D., Manna, T. & Doxsey, S. J. Dynein
light intermediate chain 1 is required for progress through the spindle assembly checkpoint. _EMBO J_ 28, 902–914, doi:10.1038/emboj.2009.38 (2009). Article CAS PubMed PubMed Central
Google Scholar * King, S. J., Bonilla, M., Rodgers, M. E. & Schroer, T. A. Subunit organization in cytoplasmic dynein subcomplexes. _Protein Sci_ 11, 1239–1250, doi:10.1110/ps.2520102
(2002). Article CAS PubMed PubMed Central Google Scholar * Trokter, M., Mucke, N. & Surrey, T. Reconstitution of the human cytoplasmic dynein complex. _Proc Natl Acad Sci USA_ 109,
20895–20900, doi:10.1073/pnas.1210573110 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Kiyomitsu, T. & Cheeseman, I. M. Chromosome- and spindle-pole-derived
signals generate an intrinsic code for spindle position and orientation. _Nat Cell Biol_ 14, 311–317, doi:10.1038/ncb2440 (2012). Article CAS PubMed PubMed Central Google Scholar *
Zimmerman, W. & Doxsey, S. J. Construction of centrosomes and spindle poles by molecular motor-driven assembly of protein particles. _Traffic_ 1, 927–934 (2000). CAS PubMed Google
Scholar * Chen, C. T. _et al._ A unique set of centrosome proteins requires pericentrin for spindle-pole localization and spindle orientation. _Curr Biol_ 24, 2327–2334,
doi:10.1016/j.cub.2014.08.029 (2014). Article CAS PubMed PubMed Central Google Scholar * Toyoshima, F. & Nishida, E. Integrin-mediated adhesion orients the spindle parallel to the
substratum in an EB1- and myosin X-dependent manner. _Embo j _ 26, 1487–1498, doi:10.1038/sj.emboj.7601599 (2007). Article CAS PubMed PubMed Central Google Scholar * Ehrhardt, A. G.
& Sluder, G. Spindle pole fragmentation due to proteasome inhibition. _J Cell Physiol_ 204, 808–818, doi:10.1002/jcp.20335 (2005). Article CAS PubMed Google Scholar * Gaglio, T.,
Saredi, A. & Compton, D. A. NuMA is required for the organization of microtubules into aster-like mitotic arrays. _J Cell Biol_ 131, 693–708 (1995). Article CAS PubMed Google Scholar
* Malikov, V., Kashina, A. & Rodionov, V. Cytoplasmic dynein nucleates microtubules to organize them into radial arrays _in vivo_. _Mol Biol Cell_ 15, 2742–2749,
doi:10.1091/mbc.E03-10-0770 (2004). Article CAS PubMed PubMed Central Google Scholar * Zheng, Z. _et al._ Evidence for dynein and astral microtubule-mediated cortical release and
transport of Galphai/LGN/NuMA complex in mitotic cells. _Mol Biol Cell_ 24, 901–913, doi:10.1091/mbc.E12-06-0458 (2013). Article CAS PubMed PubMed Central Google Scholar * Du, Q.,
Stukenberg, P. T. & Macara, I. G. A mammalian Partner of inscuteable binds NuMA and regulates mitotic spindle organization. _Nat Cell Biol_ 3, 1069–1075, doi:10.1038/ncb1201-1069 (2001).
Article CAS PubMed Google Scholar * Merdes, A., Heald, R., Samejima, K., Earnshaw, W. C. & Cleveland, D. W. Formation of spindle poles by dynein/dynactin-dependent transport of
NuMA. _J Cell Biol_ 149, 851–862 (2000). Article CAS PubMed PubMed Central Google Scholar * Merdes, A., Ramyar, K., Vechio, J. D. & Cleveland, D. W. A complex of NuMA and
cytoplasmic dynein is essential for mitotic spindle assembly. _Cell_ 87, 447–458 (1996). Article CAS PubMed Google Scholar * Sun, Q. Y. & Schatten, H. Role of NuMA in vertebrate
cells: review of an intriguing multifunctional protein. _Front Biosci_ 11, 1137–1146 (2006). Article CAS PubMed Google Scholar * Kisurina-Evgenieva, O. _et al._ Multiple mechanisms
regulate NuMA dynamics at spindle poles. _J Cell Sci_ 117, 6391–6400, doi:10.1242/jcs.01568 (2004). Article CAS PubMed Google Scholar * Kobayashi, T. & Murayama, T. Cell
cycle-dependent microtubule-based dynamic transport of cytoplasmic dynein in mammalian cells. _PLoS One_ 4, e7827, doi:10.1371/journal.pone.0007827 (2009). Article ADS PubMed PubMed
Central Google Scholar * Ananthanarayanan, V. Activation of the motor protein upon attachment: Anchors weigh in on cytoplasmic dynein regulation. _BioEssays: news and reviews in molecular,
cellular and developmental biology_, doi:10.1002/bies.201600002 (2016). * Schmoranzer, J. _et al._ Par3 and dynein associate to regulate local microtubule dynamics and centrosome
orientation during migration. _Curr Biol_ 19, 1065–1074, doi:10.1016/j.cub.2009.05.065 (2009). Article CAS PubMed PubMed Central Google Scholar * Xu, Z. _et al._ 14-3-3 protein targets
misfolded chaperone-associated proteins to aggresomes. _J Cell Sci_ 126, 4173–4186, doi:10.1242/jcs.126102 (2013). Article CAS PubMed PubMed Central Google Scholar * Yang, Z., Tulu, U.
S., Wadsworth, P. & Rieder, C. L. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. _Curr Biol_ 17, 973–980,
doi:10.1016/j.cub.2007.04.056 (2007). Article CAS PubMed PubMed Central Google Scholar * Gaetz, J. & Kapoor, T. M. Dynein/dynactin regulate metaphase spindle length by targeting
depolymerizing activities to spindle poles. _J Cell Biol_ 166, 465–471, doi:10.1083/jcb.200404015 (2004). Article CAS PubMed PubMed Central Google Scholar * Crowder, M. E. _et al._
Dynactin-dependent cortical dynein and spherical spindle shape correlate temporally with meiotic spindle rotation in C. elegans. _Mol Biol Cell_, 10.1091/mbc.E15-05-0290 (2015). * Robu, M.
E. _et al._ p53 Activation by Knockdown Technologies. _PLoS Genet_ 3, e78, doi:10.1371/journal.pgen.0030078 (2007). Article PubMed PubMed Central Google Scholar * Januschke, J. &
Nathke, I. Stem cell decisions: a twist of fate or a niche market? _Semin Cell Dev Biol_ 34, 116–123, doi:10.1016/j.semcdb.2014.02.014 (2014). Article PubMed PubMed Central Google Scholar
* Mora-Bermudez, F., Matsuzaki, F. & Huttner, W. B. Specific polar subpopulations of astral microtubules control spindle orientation and symmetric neural stem cell division. _Elife_ 3,
10.7554/eLife.02875 (2014). * Yamashita, Y. M., Mahowald, A. P., Perlin, J. R. & Fuller, M. T. Asymmetric inheritance of mother versus daughter centrosome in stem cell division.
_Science_ 315, 518–521, doi:10.1126/science.1134910 (2007). Article ADS CAS PubMed PubMed Central Google Scholar * Yamashita, Y. M., Yuan, H., Cheng, J. & Hunt, A. J. Polarity in
stem cell division: asymmetric stem cell division in tissue homeostasis. _Cold Spring Harb Perspect Biol_ 2, a001313, doi:10.1101/cshperspect.a001313 (2010). Article PubMed PubMed Central
Google Scholar * Bacallao, R. L. & McNeill, H. Cystic kidney diseases and planar cell polarity signaling. _Clin Genet_ 75, 107–117, doi:10.1111/j.1399-0004.2008.01148.x (2009).
Article CAS PubMed Google Scholar * Castanon, I. & Gonzalez-Gaitan, M. Oriented cell division in vertebrate embryogenesis. _Curr Opin Cell Biol_ 23, 697–704,
doi:10.1016/j.ceb.2011.09.009 (2011). Article CAS PubMed Google Scholar * Delaval, B., Bright, A., Lawson, N. D. & Doxsey, S. The cilia protein IFT88 is required for spindle
orientation in mitosis. _Nat Cell Biol_ 13, 461–468, doi:10.1038/ncb2202 (2011). Article CAS PubMed PubMed Central Google Scholar * Fischer, E. _et al._ Defective planar cell polarity
in polycystic kidney disease. _Nat Genet_ 38, 21–23, doi:10.1038/ng1701 (2006). Article CAS PubMed Google Scholar * Hildebrandt, F. & Otto, E. Cilia and centrosomes: a unifying
pathogenic concept for cystic kidney disease? _Nat Rev Genet_ 6, 928–940, doi:10.1038/nrg1727 (2005). Article CAS PubMed Google Scholar * Simons, M. & Walz, G. Polycystic kidney
disease: cell division without a c(l)ue? _Kidney Int_ 70, 854–864, doi:10.1038/sj.ki.5001534 (2006). Article CAS PubMed Google Scholar * Seldin, L., Muroyama, A. & Lechler, T.
NuMA-microtubule interactions are critical for spindle orientation and the morphogenesis of diverse epidermal structures 5, doi:10.7554/eLife.12504 (2016). * Kotak, S., Busso, C. &
Gonczy, P. NuMA interacts with phosphoinositides and links the mitotic spindle with the plasma membrane. _EMBO J_ 33, 1815–1830, doi:10.15252/embj.201488147 (2014). Article CAS PubMed
PubMed Central Google Scholar * Murray, A. W. A brief history of error. _Nat Cell Biol_ 13, 1178–1182, doi:10.1038/ncb2348 (2011). Article CAS PubMed Google Scholar * Musacchio, A.
Spindle assembly checkpoint: the third decade. _Philos Trans R Soc Lond B Biol Sci_ 366, 3595–3604, doi:10.1098/rstb.2011.0072 (2011). Article CAS PubMed PubMed Central Google Scholar *
Lee, M. T., Bonneau, A. R. & Giraldez, A. J. Zygotic genome activation during the maternal-to-zygotic transition. _Annu Rev Cell Dev Biol_ 30, 581–613,
doi:10.1146/annurev-cellbio-100913-013027 (2014). Article CAS PubMed PubMed Central Google Scholar * O’Boyle, S., Bree, R. T., McLoughlin, S., Grealy, M. & Byrnes, L. Identification
of zygotic genes expressed at the midblastula transition in zebrafish. _Biochem Biophys Res Commun_ 358, 462–468, doi:10.1016/j.bbrc.2007.04.116 (2007). Article PubMed Google Scholar *
Young, A., Dictenberg, J. B., Purohit, A., Tuft, R. & Doxsey, S. J. Cytoplasmic dynein-mediated assembly of pericentrin and gamma tubulin onto centrosomes. _Mol Biol Cell_ 11, 2047–2056
(2000). Article CAS PubMed PubMed Central Google Scholar * Purohit, A., Tynan, S. H., Vallee, R. & Doxsey, S. J. Direct interaction of pericentrin with cytoplasmic dynein light
intermediate chain contributes to mitotic spindle organization. _J Cell Biol_ 147, 481–492 (1999). Article CAS PubMed PubMed Central Google Scholar * Ma, H. _et al._ A highly efficient
multifunctional tandem affinity purification approach applicable to diverse organisms. _Molecular & cellular proteomics: MCP_ 11, 501–511, doi:10.1074/mcp.O111.016246 (2012). Article
PubMed PubMed Central Google Scholar * Hehnly, H. & Doxsey, S. Rab11 endosomes contribute to mitotic spindle organization and orientation. _Dev Cell_ 28, 497–507,
doi:10.1016/j.devcel.2014.01.014 (2014). Article CAS PubMed PubMed Central Google Scholar * Juschke, C., Xie, Y., Postiglione, M. P. & Knoblich, J. A. Analysis and modeling of
mitotic spindle orientations in three dimensions. _Proc Natl Acad Sci USA_ 111, 1014–1019, doi:10.1073/pnas.1314984111 (2014). Article ADS PubMed Google Scholar * Westerfield, M. The
zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) _4th ed., Univ. of Oregon Press, Eugene_ (2000). * Kimmel, C. B., Ballard, W. W., Kimmel, S. R., Ullmann, B. &
Schilling, T. F. Stages of embryonic development of the zebrafish. _Dev Dyn_ 203, 253–310, doi:10.1002/aja.1002030302 (1995). Article CAS PubMed Google Scholar Download references
ACKNOWLEDGEMENTS The authors thank the following for gifts of reagents: David J Stephens for the human LIC2 construct through Addgene; Daniel Gerlich and Claudia Blaukopf (Institute of
Molecular Biotechnology, Vienna Austria) for the H2B-mCherry and GFP-α tubulin Hela cell line; Stephen Doxsey and Sambra Redick for the RPE1 and U2OS cell lines and the MTAP-mVenus vector,
which was originally obtained from Dannel McCollum (all UMass Medical School, Worcester USA); T. Murayama (Juntendo University School of Medicine, Japan) for the mf-GFP-IC74 Hela cell line
and the human LIC1 construct. We thank members of the Laboratory of Cellular Dynamics, Regional Centre for Biotechnology for critical comments during the work and Deepak T Nair for critical
comments on the manuscript. CS is funded by grant BSC0123 from the Council of Scientific and Industrial Research (CSIR), India; AS by a research fellowship from the Indian Council of Medical
Research (ICMR), India and SVSM by a Rapid Grant for Young Investigators from the Department of Biotechnology, Government of India and by the Regional Centre for Biotechnology. The authors
declare that they have no conflicts of interest. COMPETING INTERESTS The authors declare no competing financial interests. AUTHOR INFORMATION Author notes * Sagar Mahale, Megha Kumar and
Amit Sharma contributed equally to this work. AUTHORS AND AFFILIATIONS * Laboratory of Cellular Dynamics, Regional Centre for Biotechnology, NCR Biotech Science Cluster, 3rd Milestone,
Faridabad-Gurgaon Expressway, Faridabad, Haryana, 121001, India Sagar Mahale, Megha Kumar, Amit Sharma & Sivaram V. S. Mylavarapu * Affiliated to Manipal University, Manipal, Karnataka,
576104, India Sagar Mahale, Amit Sharma & Sivaram V. S. Mylavarapu * CSIR-Institute of Genomics & Integrative Biology, South Campus, New Delhi, 110025, India Aswini Babu, Shashi
Ranjan & Chetana Sachidanandan * Academy of Scientific and Innovative Research (AcSIR), New Delhi, 110025, India Aswini Babu & Chetana Sachidanandan Authors * Sagar Mahale View
author publications You can also search for this author inPubMed Google Scholar * Megha Kumar View author publications You can also search for this author inPubMed Google Scholar * Amit
Sharma View author publications You can also search for this author inPubMed Google Scholar * Aswini Babu View author publications You can also search for this author inPubMed Google Scholar
* Shashi Ranjan View author publications You can also search for this author inPubMed Google Scholar * Chetana Sachidanandan View author publications You can also search for this author
inPubMed Google Scholar * Sivaram V. S. Mylavarapu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS S.P.M. conducted experiments on cell
lines, time-lapse imaging, fluorescence intensity quantification, prepared related figures, partially wrote the manuscript. M.K. conducted all zebrafish experiments, prepared related figures
and the model, partially wrote the manuscript. A.S. conducted experiments on cell lines, prepared related figures, partially wrote the manuscript. A.B. helped set up zebrafish breeding and
embryo collection. S.R. maintained the zebrafish facility. C.S. advised on and provided the facilities for zebrafish experiments. S.V.S.M. conceived, designed and supervised the entire
study, wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Sivaram V. S. Mylavarapu. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY FIGURES AND LEGENDS SUPPLEMENTARY MOVIE 1 SUPPLEMENTARY MOVIE 2
SUPPLEMENTARY MOVIE 3 SUPPLEMENTARY MOVIE 4 SUPPLEMENTARY MOVIE 5 SUPPLEMENTARY MOVIE 6 SUPPLEMENTARY MOVIE 7 SUPPLEMENTARY MOVIE 8 SUPPLEMENTARY MOVIE 9 SUPPLEMENTARY MOVIE 10 SUPPLEMENTARY
MOVIE 11 SUPPLEMENTARY MOVIE 12 SUPPLEMENTARY MOVIE 13 SUPPLEMENTARY MOVIE 14 SUPPLEMENTARY MOVIE 15 SUPPLEMENTARY MOVIE 16 SUPPLEMENTARY MOVIE 17 SUPPLEMENTARY MOVIE 18 SUPPLEMENTARY MOVIE
19 SUPPLEMENTARY MOVIE 20 SUPPLEMENTARY MOVIE 21 RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party
material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons
license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mahale, S., Kumar, M., Sharma, A. _et al._ The Light Intermediate Chain 2 Subpopulation of Dynein Regulates Mitotic Spindle Orientation.
_Sci Rep_ 6, 22 (2016). https://doi.org/10.1038/s41598-016-0030-3 Download citation * Received: 01 August 2016 * Accepted: 03 November 2016 * Published: 23 December 2016 * DOI:
https://doi.org/10.1038/s41598-016-0030-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
1190 effect of human growth hormone (hgh) treatment on fat metabolismABSTRACT Administration of pharmacological quantities of hGH to humans and animals causes a brisk rise in serum free fat...
Reading che guevara in his own wordsChe Guevara was killed on 9 October 1967, but new books by him are still appearing. He was a prolific writer and the Cen...
No fast music or fast running: covid rules in seoul force gym-goers to slow downSome in South Korea may be stuck listening to slow ballads during their next high-energy workout. In an attempt to curb ...
Only 10 minutes of water a day: harsh reality of life in east delhi's dda flatsAnother resident of the housing society, Sameer Sharma, adds, "The water comes at around 6:45 am (every day) and th...
1189 use of biostator glucose controller (closed-loop) to estimate insulin needs on a portable pump (open-loop): effect of previous metabolic controlABSTRACT Fourteen insulinopenic diabetics were treated with a closed-loop Biostator Glucose Controller for 24-48 hours f...
Latests News
The light intermediate chain 2 subpopulation of dynein regulates mitotic spindle orientationABSTRACT Cytoplasmic dynein 1 is a multi-protein intracellular motor essential for mediating several mitotic functions, ...
780 malnutrition in children having solid tumorsABSTRACT Evidence of growth failure at diagnosis and of acute or chronic malnutrition during management was sought in 43...
Confusion as bars that serve 'substantial' meals spared shutdown* ALCOHOL COULD BE SERVED AS PART OF A 'SUBSTANTIAL MEAL' IN A PUB UNDER NEW RULES * IT WILL BE THE POLICE AND...
Doubling down on sirnas in the brainDivalent siRNAs induce widespread gene silencing in the brains of mice and non-human primates. Access through your insti...
Macron announces 11 reforms for french police and gendarmeriePresident Emmanuel Macron announced reforms for the French police and gendarmerie yesterday (September 14), saying he wa...