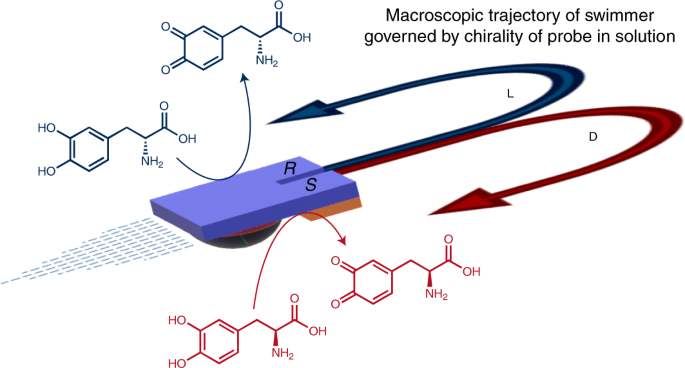
Direct dynamic read-out of molecular chirality with autonomous enzyme-driven swimmers
Direct dynamic read-out of molecular chirality with autonomous enzyme-driven swimmers"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A key approach for designing bioinspired machines is to transfer concepts from nature to man-made structures by integrating biomolecules into artificial mechanical systems. This
strategy allows the conversion of molecular information into macroscopic action. Here, we describe the design and dynamic behaviour of hybrid bioelectrochemical swimmers that move
spontaneously at the air–water interface. Their motion is governed by the diastereomeric interactions between immobilized enantiopure oligomers and the enantiomers of a chiral probe molecule
present in solution. These dynamic bipolar systems are able to convert chiral information present at the molecular level into enantiospecific macroscopic trajectories. Depending on the
enantiomer in solution, the swimmers will move clockwise or anticlockwise; the concept can also be used for the direct visualization of the degree of enantiomeric excess by analysing the
curvature of the trajectories. Deciphering in such a straightforward way the enantiomeric ratio could be useful for biomedical applications, for the read-out of food quality or as a more
general analogue of polarimetric measurements. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access
through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to
this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy
now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer
support SIMILAR CONTENT BEING VIEWED BY OTHERS RECHARGEABLE SELF-ASSEMBLED DROPLET MICROSWIMMERS DRIVEN BY SURFACE PHASE TRANSITIONS Article 15 July 2021 VISCOELASTIC CONTROL OF
SPATIOTEMPORAL ORDER IN BACTERIAL ACTIVE MATTER Article 03 February 2021 BI-ENZYMATIC CHEMO-MECHANICAL FEEDBACK LOOP FOR CONTINUOUS SELF-SUSTAINED ACTUATION OF CONDUCTING POLYMERS Article
Open access 12 October 2023 DATA AVAILABILITY Due to an important number of different source files, the datasets generated and analysed in the frame of the current study are available from
the corresponding author on request. REFERENCES * Paxton, W. F., Sundarajan, S., Mallouk, T. E. & Sen, A. Chemical locomotion. _Angew. Chem. Int. Ed._ 45, 5420–5429 (2006). Article CAS
Google Scholar * Sengupta, S., Ibele, M. E. & Sen, A. Fantastic voyage: designing self-powered nanorobots. _Angew. Chem. Int. Ed._ 51, 8434–8445 (2012). Article CAS Google Scholar
* Pacheco, M., Lopez, M. A., Jurado-Sanchez, B. & Escarpa, A. Self-propelled micromachines for analytical sensing: a critical review. _Anal. Bioanal. Chem._ 411, 6561–6573 (2019).
Article CAS PubMed Google Scholar * Sheng Moo, J. G. et al. Nano/microrobots meet electrochemistry. _Adv. Funct. Mater._ 27, 1604759 (2017). Article Google Scholar * Kong, L., Guan, J.
& Pumera, M. Micro- and nanorobots based sensing and biosensing. _Curr. Opin. Electrochem._ 10, 174–182 (2018). Article CAS Google Scholar * Campuzano, S., Esteban-Fernández De
Ávila, B., Yáñez-Sedeño, P., Pingarrón, J. M. & Wang, J. Nano/microvehicles for efficient delivery and (bio)sensing at the cellular level. _Chem. Sci._ 8, 6750–6763 (2017). Article CAS
PubMed PubMed Central Google Scholar * Duan, W. et al. Synthetic nano- and micromachines in analytical chemistry: sensing, migration, capture, delivery, and separation. _Annu. Rev.
Anal. Chem._ 8, 311–333 (2015). Article Google Scholar * Ismagilov, R. F., Schwartz, A., Bowden, N. & Whitesides, G. M. Autonomous movement and self-assembly. _Angew. Chem. Int. Ed._
41, 652–654 (2002). Article CAS Google Scholar * Ozin, G. A., Manners, I., Fournier-Bidoz, S. & Arsenault, A. Dream nanomachines. _Adv. Mater._ 17, 3011–3018 (2005). Article CAS
Google Scholar * Sanchez, S., Soler, L. & Katuri, J. Chemically powered micro- and nanomotors. _Angew. Chem. Int. Ed._ 54, 1414–1444 (2015). Article CAS Google Scholar * Turner, L.,
Ryu, W. S. & Berg, H. C. Real-time imaging of fluorescent flagellar filaments. _J. Bacteoriol. Res._ 182, 2793–2801 (2000). Article CAS Google Scholar * Darnton, N. C., Turner, L.,
Rojevsky, S. & Berg, H. C. On torque and tumbling in swimming _Escherichia coli_. _J. Bacteriol._ 189, 1756–1764 (2007). Article CAS PubMed Google Scholar * Magdanz, V., Sanchez, S.
& Schmidt, G. Development of a sperm-flagella driven micro-bio-robot. _Adv. Mater._ 25, 6581–6588 (2013). Article CAS PubMed Google Scholar * Hess, H. & Bachand, G. D.
Biomolecular motors. _Mater. Today_ 8, 22–29 (2005). Article Google Scholar * Xu, H., Medina-Sanchez, M. & Schmidt, O. G. Magnetic micromotors for multiple motile sperm cell capture,
transport, and enzymatic release. _Angew. Chem. Int. Ed._ 59, 15029–15037 (2020). Article CAS Google Scholar * Schmidt, C. K., Medina-Sanchez, M., Edmondson, R. J. & Schmidt, O. G.
Engineering microrobots for targeted cancer therapies from a medical perspective. _Nat. Commun._ 11, 5618 (2020). Article CAS PubMed PubMed Central Google Scholar * Wu, J. et al.
Motion-based DNA detection using catalytic nanomotors. _Nat. Commun._ 1, 36 (2010). Article PubMed Google Scholar * Wang, J. _Nanomachines: Fundamentals and Applications_ (Wiley-VCH,
2013). * Sun, J., Mathesh, M., Li, W. & Wilson, D. A. Enzyme-powered nanomotors with controlled size for biomedical applications. _ACS Nano_ 13, 10191–10200 (2019). Article CAS PubMed
PubMed Central Google Scholar * Mathesh, M., Sun, J. & Wilson, D. A. Enzyme catalysis powered micro/nanomotors for biomedical applications. _J. Mater. Chem. B_ 8, 7319–7334 (2020).
Article PubMed Google Scholar * Yuan, H., Liu, X., Wang, L. & Ma, X. Fundamentals and applications of enzyme powered micro/nano-motors. _Bioact. Mater._ 6, 1727–1749 (2021). Article
CAS PubMed Google Scholar * Dey, K. K. et al. Micromotors powered by enzyme catalysis. _Nano Lett._ 15, 8311–8315 (2015). Article CAS PubMed Google Scholar * Pavan Kumar, B. V. V. S.,
Patil, A. J. & Mann, S. Enzyme-powered motility in buoyant organoclay/DNA protocells. _Nat. Chem._ 10, 1154–1163 (2018). Article Google Scholar * Soong, R. K. et al. Powering an
inorganic nanodevice with a biomolecular motor. _Science_ 290, 1555–1558 (2000). Article CAS PubMed Google Scholar * Mano, N. & Heller, A. Bioelectrochemical propulsion. _J. Am.
Chem. Soc._ 127, 11574–11575 (2005). Article CAS PubMed Google Scholar * Pantarotto, D., Browne, W. R. & Feringa, B. L. Autonomous propulsion of carbon nanotubes powered by a
multienzyme ensemble. _Chem. Commun_. 13, 1533–1535 (2008). * Sanchez, S., Solovev, A. A., Mei, Y. F. & Schmidt, O. G. Dynamics of biocatalytic microengines mediated by variable friction
control. _J. Am. Chem. Soc._ 132, 13144–13145 (2010). Article CAS PubMed Google Scholar * Orozco, J. et al. Artificial enzyme-powered microfish for water-quality testing. _ACS Nano_ 7,
818–824 (2013). Article CAS PubMed Google Scholar * Simmchen, J., Baeza, A., Ruiz, D., Esplandiu, M. J. & Vallet-Regí, M. Asymmetric hybrid silica nanomotors for capture and cargo
transport: towards a novel motion-based DNA sensor. _Small_ 8, 2053–2059 (2012). Article CAS PubMed Google Scholar * Zhao, G., Sanchez, S., Schmidt, O. G. & Pumera, M. Poisoning of
bubble propelled catalytic micromotors: the chemical environment matters. _Nanoscale_ 5, 2909–2914 (2013). Article CAS PubMed PubMed Central Google Scholar * Villa, K., Manzanares
Palenzuela, C. L., Sofer, Z., Matějková, S. & Pumera, M. Metal-free visible-light photoactivated C3N4 bubble-propelled tubular micromotors with inherent fluorescence and on/off
capabilities. _ACS Nano_ 12, 12482–12491 (2018). Article CAS PubMed Google Scholar * Wang, K. et al. Fluorescent self-propelled covalent organic framework as a microsensor for nitro
explosive detection. _Appl. Mater. Today_ 19, 100550 (2020). Article Google Scholar * Iamsaard, S. et al. Conversion of light into macroscopic helical motion. _Nat. Chem._ 6, 229–235
(2014). Article CAS PubMed Google Scholar * Lee, K. M. et al. Photodriven, flexural-torsional oscillation of glassy azobenzene liquid crystal polymer networks. _Adv. Funct. Mater._ 21,
2913–2918 (2011). Article CAS Google Scholar * Arnaboldi, S., Grecchi, S., Magni, M. & Mussini, P. Electroactive chiral oligo- and polymer layers for electrochemical
enantiorecognition. _Curr. Opin. Electrochem._ 7, 188–199 (2018). Article CAS Google Scholar * Arnaboldi, S. et al. Absolute chiral recognition with hybrid wireless electrochemical
actuators. _Anal. Chem._ 92, 10042–10047 (2020). Article CAS PubMed Google Scholar * Sengupta, S. et al. Self-powered enzyme micropumps. _Nat. Chem._ 6, 415–422 (2014). Article CAS
PubMed Google Scholar * Gupta, B. et al. Wireless coupling of conducting polymer actuators with light emission. _ChemPhysChem_ 20, 941–945 (2019). Article CAS PubMed Google Scholar *
Gupta, B., Goudeau, B., Garrigue, P. & Kuhn, A. Bipolar conducting polymer crawlers based on triple symmetry breaking. _Adv. Funct. Mater._ 28, 1705825 (2018). Article Google Scholar *
Gupta, B., Goudeau, B. & Kuhn, A. Wireless electrochemical actuation of conducting polymers. _Angew. Chem. Int. Ed._ 56, 14183–14186 (2017). Article CAS Google Scholar * Mano, N.
& de Poulpiquet, A. O2 reduction in enzymatic biofuel cells. _Chem. Rev._ 118, 2392–2468 (2018). Article CAS PubMed Google Scholar * Paxton, W. F. et al. Catalytic nanomotors:
autonomous movement of striped nanorods. _J. Am. Chem. Soc._ 126, 13424–13431 (2004). Article CAS PubMed Google Scholar * Brooks, A. M. et al. Shaped-directed rotation of homogeneous
micromotors via catalytic self-electrophoresis. _Nat. Commun._ 10, 495 (2019). Article CAS PubMed PubMed Central Google Scholar * Zhan, X. et al. Enhanced ion tolerance of
electrokinetic locomotion in polyelectrolyte-coated microswimmer. _Nat. Commun._ 10, 3921 (2019). Article PubMed PubMed Central Google Scholar * Williams, B. J., Anand, S. V.,
Rajagopalan, J. & Saif, M. T. A. A self-propelled biohybrid swimmer at low Reynolds number. _Nat. Commun._ 5, 3081 (2014). Article PubMed Google Scholar * Moran, J. & Posner, J.
Microswimmer with no moving parts. _Phys. Today_ 72, 44–50 (2019). Article CAS Google Scholar * Hamilton, J. J., Bryan, M. T., Gilbert, A. D., Ogrin, F. Y. & Myers, T. O. A new class
of magnetically actuated pumps and valves for microfluidic applications. _Sci. Rep._ 8, 933 (2018). Article PubMed PubMed Central Google Scholar * Mano, N., Kim, H. H., Zhang, Y. &
Heller, A. An oxygen cathode operating in a physiological solution. _J. Am. Chem. Soc._ 124, 6480–6486 (2002). Article CAS PubMed Google Scholar * García-Carmona, L., Moreno-Guzmán, M.,
González, M. C. & Escarpa, A. Class enzyme-based motors for ‘on the fly’ enantiomer analysis of aminoacids. _Biosens. Bioelectron._ 96, 275–280 (2017). Article PubMed Google Scholar *
Rosini, E., D’Antona, P. & Pollegioni, L. Biosensors for D-amino acids: detection methods and applications. _Int. J. Mol. Sci._ 21, 4574 (2020). Article CAS PubMed Central Google
Scholar * Durand, F., Gounel, S., Kjaergaard, C. H., Solomon, E. I. & Mano, N. Bilirubin oxidase from _Magnaporthe oryzae_: an attractive new enzyme for biotechnological applications.
_Appl. Microbiol. Biotechnol._ 96, 1489–1498 (2012). Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The work has been funded by the European
Research Council under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 741251, European Research Council Advanced Grant ELECTRA). S.A. acknowledges
financial support from the Università degli Studi di Milano for a partial postdoc scholarship. We are also very grateful for fruitful discussions with P. Mussini. AUTHOR INFORMATION Author
notes * These authors contributed equally: Serena Arnaboldi, Gerardo Salinas. AUTHORS AND AFFILIATIONS * Institut des Sciences Moléculaires, UMR 5255, University of Bordeaux, CNRS, Bordeaux
INP, Pessac, France Serena Arnaboldi, Gerardo Salinas, Aleksandar Karajić, Patrick Garrigue & Alexander Kuhn * Centre de Recherche Paul Pascal, CNRS UMR 5031, University of Bordeaux,
Pessac, France Aleksandar Karajić, Sabrina Bichon, Sébastien Gounel & Nicolas Mano * Dipartimento di Scienza e Alta Tecnologia, Università degli Studi dell’Insubria, Como, Italy Tiziana
Benincori & Giorgia Bonetti * Centro Nazionale per il Controllo e la Valutazione dei Farmaci, Istituto Superiore di Sanità, Roma, Italy Roberto Cirilli Authors * Serena Arnaboldi View
author publications You can also search for this author inPubMed Google Scholar * Gerardo Salinas View author publications You can also search for this author inPubMed Google Scholar *
Aleksandar Karajić View author publications You can also search for this author inPubMed Google Scholar * Patrick Garrigue View author publications You can also search for this author
inPubMed Google Scholar * Tiziana Benincori View author publications You can also search for this author inPubMed Google Scholar * Giorgia Bonetti View author publications You can also
search for this author inPubMed Google Scholar * Roberto Cirilli View author publications You can also search for this author inPubMed Google Scholar * Sabrina Bichon View author
publications You can also search for this author inPubMed Google Scholar * Sébastien Gounel View author publications You can also search for this author inPubMed Google Scholar * Nicolas
Mano View author publications You can also search for this author inPubMed Google Scholar * Alexander Kuhn View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS S.A. performed the experiments and wrote and edited the manuscript. G.S. performed experiments, wrote and edited the manuscript and treated the data. A. Karajić
performed enzyme characterization and immobilization experiments. P.G. assisted with electron microscopy characterization. T.B. designed the inherently chiral monomers and edited the
manuscript. G.B. synthesized the BT2T4 molecules. R.C. separated the enantiomers by chiral HPLC. S.B. synthesized the redox polymer and tested the BOD under heterogeneous conditions. S.G.
produced, purified and tested the BOD in homogeneous solution. N.M. discussed the results and edited the manuscript. A. Kuhn proposed the research project, provided resources, designed the
experiments and edited the manuscript. CORRESPONDING AUTHOR Correspondence to Alexander Kuhn. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PEER REVIEW INFORMATION _Nature Chemistry_ thanks Alberto Escarpa, Xing Ma and Hong Wang for their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA EXTENDED DATA FIG. 1 ILLUSTRATION OF THE HYDRODYNAMIC FLOW. Illustration
of the hydrodynamic flow around the Ppy swimmer in an experiment where the swimmer is immobilized on a support. The two enantiopure oligomer-modified strips, constituting the anode, are
pointing upwards, and the enzyme covered part is oriented downwards on the image. The swimmer is placed in a 5 mM D-DOPA 0.3 M citrate/phosphate buffer (pH 5) at 22 °C containing only a
small amount of carbon beads, acting as individual tracers of the hydrodynamic flow. EXTENDED DATA FIG. 2 INFLUENCE OF IONIC STRENGTH ON THE MOTION. Z projections of the enantioselective
macroscopic motion obtained by placing swimmers with double arms ((R)-oligomer is deposited on the left arm) in three solutions of 5 mM D-DOPA at pH 5 at room temperature with different
buffer concentrations: 0.15 M (red arrow), 0.3 M (green arrow), 0.5 M (blue arrow). Black arrows indicate the initial direction of motion. The time in seconds refers to the time lapse
between two video frames, which had to be adjusted for the three experiments, as the respective speeds are very different. EXTENDED DATA FIG. 3 CHARACTERIZATION OF THE BIOELECTROCATALYTIC
REDUCTION OF OXYGEN AS A FUNCTION OF IONIC STRENGTH. Cyclic voltammograms recorded at 5 mV/s, using glassy carbon electrodes modified with redox hydrogel and BOD enzyme, in naturally aerated
buffer solutions with different ionic strength (0.15 M, 0.3 M and 0.5 M) at pH 5 and 22 °C. The hydrogel was prepared as described in the experimental procedure. The bioelectrocatalytic
reduction of oxygen is clearly visible for potentials more negative than 0.5 V, but only slightly varies as a function of the ionic strength within standard errors (≈15%). EXTENDED DATA FIG.
4 DPV ENANTIORECOGNITION TESTS FOR L-ASCORBIC ACID. DPV enantiorecognition tests carried out in a 0.3 M citrate/phosphate buffer solution at pH 5 containing 1.25 mM L-ascorbic acid (L-AA).
Measurements were performed on a glassy carbon electrode covered either with (S)-BT2T4 oligomer (red curve) or with (R)-BT2T4 oligomer (green curve). EXTENDED DATA FIG. 5 SEM MICROGRAPHS OF
THE (S)-OLIGO-BT2T4 MODIFIED PPY EXTREMITY. SEM micrographs of the (S)-oligo-BT2T4 modified Ppy extremity: (A) for the pristine swimmer, (B) same swimmer after the swimming experiments
carried out in a 0.3 M citrate/phosphate buffer solution at pH 5 at 22 °C containing 5mM L-DOPA. EXTENDED DATA FIG. 6 SEM MICROGRAPHS OF THE ENZYME HYDROGEL MODIFIED PPY EXTREMITY. SEM
micrographs of the enzyme hydrogel modified PPy extremity: (A) for the pristine swimmer, (B) same swimmer after the swimming experiments carried out in a 0.3 M citrate/phosphate buffer
solution at pH 5 at 22 °C containing 5 mM L-DOPA. EXTENDED DATA FIG. 7 EDS SIGNAL AROUND THE EMISSION PEAK OF SULPHUR. EDS signal around the emission peak of sulphur (S Kα) at the extremity
modified with the enantiopure oligomer. EXTENDED DATA FIG. 8 EDS SIGNAL FOR MEASURING THE EMISSION PEAK OF COPPER. EDS signal for measuring the emission peak of copper (Cu-Kα) of the enzyme
hydrogel modified extremity of the polypyrrole strip. The inset shows the magnification of the EDS signal of copper for the pristine swimmer (black line), and after the swimming experiments
carried out in a 0.3 M citrate/phosphate buffer solution at pH 5 at 22 °C containing 5 mM L-DOPA (red line). The decrease of this signal indicates a loss of enzyme during the swimming
experiment. EXTENDED DATA FIG. 9 EVOLUTION OF THE CURVATURE AND THE STRAIGHTNESS INDEX. Evolution of the curvature (yellow line) and the straightness index (green line) of hybrid swimmers as
a function of the enantiomeric excess. The data analysis is based on the set of experiments reported in Fig. 3, performed in triplicate. SUPPLEMENTARY INFORMATION SUPPLEMENTARY VIDEO 1
Hydrodynamic flow around a Ppy swimmer. The swimmer is placed in a 5 mM d-DOPA 0.3 M citrate/phosphate buffer (pH 5) at 22 °C containing a large concentration of 1 mm carbon beads. Video is
in real time. SUPPLEMENTARY VIDEO 2 Hydrodynamic flow around a Ppy swimmer. The swimmer is placed in a 5 mM d-DOPA 0.3 M citrate/phosphate buffer (pH 5) at 22 °C containing only one carbon
bead. Video is in real time. SUPPLEMENTARY VIDEO 3 Hydrodynamic flow around a Ppy swimmer. The swimmer is placed in a 5 mM d-DOPA 0.3 M citrate/phosphate buffer (pH 5) at 22 °C with three
carbon beads moving along one edge. Video is in real time. SUPPLEMENTARY VIDEO 4 Macroscopic enantiosensitive motion of a swimmer placed at the air–water interface of a 0.15 M
citrate/phosphate buffer (pH 5) at 22 °C containing 5 mM d-DOPA. Video is in real time. SUPPLEMENTARY VIDEO 5 Macroscopic enantiosensitive motion of a swimmer placed at the air–water
interface of a 0.5 M citrate/phosphate buffer (pH 5) at 22 °C containing 5 mM d-DOPA. Video is 16 times accelerated. SUPPLEMENTARY VIDEO 6 Macroscopic enantiosensitive clockwise motion of a
swimmer placed at the air–water interface of a 0.3 M citrate/phosphate buffer (pH 5) at 22 °C containing 5 mM d-DOPA. Video is 2 times decelerated. SUPPLEMENTARY VIDEO 7 Macroscopic
enantiosensitive anticlockwise motion of a swimmer placed at the air–water interface of a 0.3 M citrate/phosphate buffer (pH 5) at 22 °C containing 5 mM l-DOPA. Video is 2 times decelerated.
SUPPLEMENTARY VIDEO 8 Macroscopic enantiosensitive motion of two swimmers with opposite oligomer configurations placed simultaneously at the air–water interface of a 0.3 M citrate/phosphate
buffer (pH 5) at 22 °C containing 5 mM l-DOPA. Video is 2 times decelerated. SUPPLEMENTARY VIDEO 9 Macroscopic enantiosensitive motion of two swimmers with opposite oligomer configurations
placed simultaneously at the air–water interface of a 0.3 M citrate/phosphate buffer (pH 5) at 22 °C containing a racemic mixture of l- and d-DOPA with a total fixed concentration of 10 mM.
Video is 2 times decelerated. SUPPLEMENTARY VIDEO 10 Macroscopic enantiosensitive anticlockwise motion of a swimmer placed at the air–water interface of a 0.3 M citrate/phosphate buffer (pH
5) at 22 °C containing 0.25 mM l-AA. Video is 2 times decelerated. SUPPLEMENTARY VIDEO 11 Macroscopic enantiosensitive clockwise motion of a swimmer placed at the air–water interface of a
0.3 M citrate/phosphate buffer (pH 5) at 22 °C containing 0.25 mM l-AA. Video is 2 times decelerated. SUPPLEMENTARY VIDEO 12 Macroscopic enantiosensitive motion of a swimmer placed at the
surface of a bovine serum solution at 22 °C containing 10 mM l-DOPA. Video is in real time. SUPPLEMENTARY VIDEO 13 Macroscopic enantiosensitive motion of a recycled swimmer placed at the
air–water interface of a 0.3 M citrate/phosphate buffer (pH 5) containing 5 mM d-DOPA at 22 °C after immobilization of a fresh aliquot of BOD redox hydrogel on the swimmer surface. Video is
2 times decelerated. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Arnaboldi, S., Salinas, G., Karajić, A. _et al._ Direct dynamic read-out of
molecular chirality with autonomous enzyme-driven swimmers. _Nat. Chem._ 13, 1241–1247 (2021). https://doi.org/10.1038/s41557-021-00798-9 Download citation * Received: 24 January 2021 *
Accepted: 24 August 2021 * Published: 14 October 2021 * Issue Date: December 2021 * DOI: https://doi.org/10.1038/s41557-021-00798-9 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
Trending News
Genetic analyses of pupation distance in Drosophila melanogasterThe inheritance of Drosophila melanogaster larval pupation behaviour is investigated in sixteen reciprocal crosses betwe...
Something went wrong, sorry. :(Sex differences in biological substrates of drug use and addiction are poorly understood. The present study investigated...
Central nervous system monoamine correlates of social dominance in cynomolgus monkeys (macaca fascicularis)ABSTRACT Social dominance is a fundamental component of both human and nonhuman primate sociality. However, its neurobio...
Chromatin conformation and histone modification profiling across human kidney anatomic regionsABSTRACT The three major anatomic regions of the human kidney include the cortex, medulla and papilla, with different fu...
Selective targeting of irf4 by synthetic microrna-125b-5p mimics induces anti-multiple myeloma activity in vitro and in vivoABSTRACT Interferon regulatory factor 4 (IRF4) is an attractive therapeutic target in multiple myeloma (MM). We here rep...
Latests News
Direct dynamic read-out of molecular chirality with autonomous enzyme-driven swimmersABSTRACT A key approach for designing bioinspired machines is to transfer concepts from nature to man-made structures by...
Simulation program at minneapolis va medical center | veterans affairsMISSION AND GOALS To promote Veteran patient safety and quality of care through simulation-based education with a focus ...
Trump didn’t invent “make america great again”Andrew Kelly/Zuma Get your news from a source that’s not owned and controlled by oligarchs. Sign up for the free _Mother...
Radiation-induced genomic instability and bystander effects: inter-related nontargeted effects of exposure to ionizing radiationABSTRACT The paradigm of genetic alterations being restricted to direct DNA damage after exposure to ionizing radiation ...
… and britain considers impact on life insuranceAccess through your institution Buy or subscribe This is a preview of subscription content, access via your institution ...