Biomarker characterization of clinical subtypes of parkinson disease
Biomarker characterization of clinical subtypes of parkinson disease"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The biological underpinnings of the PD clusters remain unknown as the existing PD clusters lacks biomarker characterization. We try to identify clinical subtypes of Parkinson
Disease (PD) in an Asian cohort and characterize them by comparing clinical assessments, genetic status and blood biochemical markers. A total of 206 PD patients were included from a
multi-centre Asian cohort. Hierarchical clustering was performed to generate PD subtypes. Clinical and biological characterization of the subtypes were performed by comparing clinical
assessments, allelic distributions of Asian related PD gene (SNCA, LRRK2, Park16, ITPKB, SV2C) and blood biochemical markers. Hierarchical clustering method identified three clusters:
cluster A (severe subtype in motor, non-motor and cognitive domains), cluster B (intermediate subtype with cognitive impairment and mild non-motor symptoms) and cluster C (mild subtype and
young age of onset). The three clusters had significantly different allele frequencies in two SNPs (Park16 rs6679073 A allele carriers in cluster A B C: 67%, 74%, 89%, _p_ = 0.015; SV2C
rs246814 T allele distribution: 7%, 12%, 25%, _p_ = 0.026). Serum homocysteine (Hcy) and C-reactive protein (CRP) levels were also significantly different among three clusters (Mean levels
of Hcy and CRP among cluster A B C were: 19.4 ± 4.2, 18.4 ± 5.7, 15.6 ± 5.6, adjusted _p_ = 0.005; 2.5 ± 5.0, 1.5 ± 2.4, 0.9 ± 2.1, adjusted _p_ < 0.0001, respectively). Of the 3 subtypes
identified amongst early PD patients, the severe subtype was associated with significantly lower frequency of Park16 and SV2C alleles and higher levels of Hcy and CRP. These biomarkers may
be useful to stratify PD subtypes and identify more severe subtypes. SIMILAR CONTENT BEING VIEWED BY OTHERS A PROTEOGENOMIC VIEW OF PARKINSON’S DISEASE CAUSALITY AND HETEROGENEITY Article
Open access 11 February 2023 MRI SUBTYPES IN PARKINSON’S DISEASE ACROSS DIVERSE POPULATIONS AND CLUSTERING APPROACHES Article Open access 16 August 2024 COMPREHENSIVE SUBTYPING OF
PARKINSON’S DISEASE PATIENTS WITH SIMILARITY FUSION: A CASE STUDY WITH BIOFIND DATA Article Open access 17 September 2021 INTRODUCTION Parkinson’s disease (PD) is the most common hypokinetic
movement disorder with significant heterogeneity in symptoms and outcomes. Non-Motor symptoms (NMS) resulting from various neurotransmitter pathway dysfunctions affects both the central and
peripheral nervous systems, which contribute to PD heterogeneity1,2. Subtype identification has been established as one of the top three clinical research priorities in the field of PD3.
Identification of PD subtype could be valuable in revealing the underlying etiology and understanding the disease course. More importantly, PD subtyping could guide the design of clinical
trial and future personalized PD treatment. Cluster analysis, a data-driven approach could help to define the disease phenotypes. Most studies use cluster analysis to stratify PD subtypes
based on clinical data, such as motor, NMS and demographic features4,5. These studies have however been limited by the inclusion of PD patients from different disease stages; absence of
genetic data that may influence clinical heterogeneity; and limited analysis of Asian cohorts. The biological underpinnings of the PD clusters remain unknown as the existing multidimensional
data-driven derivation of PD clusters lacks biomarker characterisation. PD biomarkers including clinical, blood, cerebrospinal fluid (CSF) and imaging biomarkers have been playing
increasingly important roles in early diagnosis and disease prognostication6. Blood biomarkers may have wider implications than CSF and imaging biomarkers as they are more accessible at
lower cost7. Homocysteine (Hcy) and C-reactive protein (CRP) are two blood biochemical biomarkers that are associated with PD severity. Severe PD subtypes have been found to have
significantly higher levels of CRP8 while elevated plasma Hcy level was found in depressed and cognitively impaired PD patients9. Vitamin D, uric acid(UA) and lipids are thought to play
important neuroprotective roles in PD. Studies have shown that higher serum UA levels were associated with a lower risk of PD development10 and more benign prognosis in PD patients11.
Vitamin D deficiency is common in PD patients12 and lower vitamin D levels have been associated with worse prognosis in PD13. In addition, serum lipid biomarkers were reduced in PD patients
compared to healthy controls14,15,16,17. To understand the clinical heterogeneity of PD, we used cluster analysis to search for subtypes in a multi-centre, Asian early PD cohort. The aims of
our study are: (1) To identify distinct PD clusters from a comprehensive dataset; (2) To provide clinical and biological features of the identified subtypes by comparing the clinical
characteristics, allelic distributions of Asian related PD genes, and blood biochemical markers. RESULTS PATIENT DEMOGRAPHIC AND CLINICAL CHARACTERISTICS A total of 206 PD patients were
enrolled in the study and 122 (59.2%) patients were male. Mean age of diagnosis was 63.5 ± 9.0 years. MCI was presented in 108 (52.4%) patients at baseline in our cohort. A summary of
patient demographics and clinical characteristics was shown in Table 1. The comparison of comorbidities among three clusters can be found in Supplementary Table 1. CLUSTER ANALYSIS RESULTS
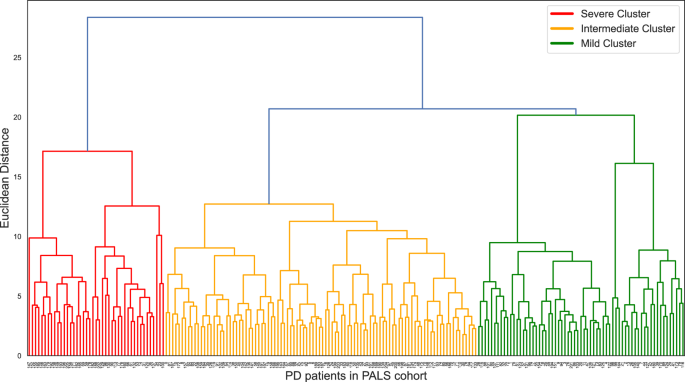
Three independent PD clusters were identified from hierarchical cluster analysis (Fig. 1). The features comparison among three clusters can be seen in Table 2. The three clusters had
significant differences in age of diagnosis (mean age of diagnosis of cluster A, B, C was 69.6 ± 7.9, 63.6 ± 7.4, 59.4 ± 9.7 years, _p_ < 0.001, respectively). The three clusters also
differed significantly in all cognitive domain scores, most of the motor scores (MDS-UPDRS part II, III score, tremor score, PIGD score) and most NMS items (MDS-UPDRS part I score, systolic
BP drop, ESS total score and HADS depression score). There were no significant differences in terms of PRS, HADS anxiety score and RBD1Q among the three clusters. Cluster A was severe
subtype in motor, NMS and cognition, which comprised 43 (20.9%) PD patients. Cluster A was the most severe in terms of motor, NMS and cognition domains as supported by the highest MDS-UPDRS
part I, II, III scores, PIGD score, cognitive domain scores and depression score. Significant BP drop was the most common in cluster A (35% of the patients in cluster A had significant BP
drop vs 21% and 11% in clusters B and C, _p_ = 0.010). The second cluster (cluster B) was the largest cluster with 98 subjects, consisting of 47.6% of PD patients. Cluster B was the
intermediate subtype characterized by cognitive impairment with mild NMS. Cognitive domain scores in this cluster were moderate, ranging between cluster A and C. However, patients in this
cluster had very mild NMS impairment supported by the lowest MDS-UPDRS part I score (2.5 in cluster B vs 6 and 4 in cluster A and C, _p_ < 0.001). There were 65 patients in cluster C,
accounting for 31.6% of the PD patients. Cluster C was a mild subtype with a younger age of onset. The mean age of diagnosis of cluster C was significantly younger than the other two
clusters (59.4 ± 9.7 in cluster C vs 63.6 ± 7.4, 69.6 ± 7.9 in cluster B and A, _p_ < 0.001). Cluster C had good cognitive performance supported by the highest cognitive domain scores.
Significant BP drop was not common in the cluster, with only 7 patients having significant systolic blood pressure drop. The other non-motor and motor profiles in cluster C were relatively
mild. CHARACTERIZATION OF PD SUBTYPES USING CLINICAL BIOMARKERS Clinical variables that were not included in the clustering model were used for post-hoc comparison among the clusters (Table
3). The 3 clusters remained significantly different with regard to MCI rate, MoCA Score, and most of the NMSS domain scores, except domain 4 (perceptual problems) and domain 8 (sexual
function), after correction for multiple comparison. Cluster A consistently had significantly worse performance in all profiles, including highest MCI percentage (81%) and NMSS total score.
Cluster B had obvious cognitive impairment with mild NMS and was characterized by having a moderate percentage of MCI (64%) and the lowest NMSS total score (11 vs 26 and 16 in cluster A and
C, _p_ < 0.001, _q_ < 0.0019). Cluster C was a mild subtype and was characterized by having the lowest MCI percentage (15%, 64%, 81%, for cluster C, B, A respectively, _p_ < 0.001,
_q_ < 0.0019). CHARACTERIZATION OF PD SUBTYPES USING BLOOD BIOMARKERS ALLELIC DISTRIBUTIONS OF ASIAN RELATED PD GENES IN THREE PD CLUSTERS A total of 206 PD patients were genotyped. The
_Park16 rs6679073_ A allele frequency was 76.7% (158 A allele carriers, including 77 patients carried AA and 81 patients harboured AC), the _SV2C rs246814_ T allele frequency was 15.0 % (31
T allele carriers, including 2 patient carried TT and 29 patients carried TC). The three clusters had significantly different effect allele frequency in these two SNPs (distribution of
Park16 rs6679073 A allele carriers in cluster A B C: 67%, 74%, 89%, _p_ = 0.015, _q_ = 0.065; SV2C rs246814 T allele distribution: 7%, 12%, 25%, _p_ = 0.026, _q_ = 0.065; Table 4). Cluster A
(severe subtype in motor, NMS and cognitive domains) had the lowest percentage of both Park16 and SV2C effect allele, while cluster C (mild subtype and young age of onset) contained the
largest number of the carriers of these two SNPs. COMPARISON OF BLOOD BIOCHEMICAL MARKERS AMONG THREE CLUSTERS We found significant differences in Hcy and CRP levels among three clusters in
the generalized linear model after adjustment for age and sex. Highest levels of Hcy and CRP were present in Cluster A (severe subtype in motor, NMS and cognitive domains), while lowest
levels were shown in Cluster C (mild subtype and young age of onset). The differences of Hcy and CRP levels among three cluster were remained significant after adjustment for multiple
comparisons. Mean levels of Hcy among three clusters were: 19.4 ± 4.2, 18.4 ± 5.7, 15.6 ± 5.6, _p_ = 0.001, _q_ = 0.005; while the mean levels of CRP were: 2.5 ± 5.0, 1.5 ± 2.4, 0.9 ± 2.1,
_p_ = 0.000, _q_ < 0.0001 (Table 5). The comparison of Hcy and CRP levels among three clusters remained significant after adjustment for age of diagnosis, sex and significant
comorbidities including hypertension, hyperlipidemia, lipid medication and hypertension medication. Please refer to Supplementary Table 2. DISCUSSION In this study, 206 early PD patients who
were recruited within 1 year from diagnosis were assigned to three clusters by an unbiased data-driven hierarchical cluster analysis: cluster A (severe subtype in motor, NMS and cognitive
domains), cluster B (intermediate subtype with cognitive impairment and mild NMS) and cluster C (mild subtype and young age of onset). Despite similar disease durations, the three clusters
presented with substantially different clinical features and blood biomarker (genetic markers and biochemical markers) profiles. The significantly different allele frequencies in two SNPs
(_Park16 rs6679073_ A allele and _SV2C rs246814_ T allele), suggest that these may be important genetic biomarkers for PD subtypes. We also found Hcy and CRP to be promising biomarkers to
identify the severe PD subtype. These findings contribute to our understanding of PD heterogeneity, especially among Asian PD. Various PD subtypes have been identified through cluster
analysis in previous studies. The diffuse malignant cluster previously reported by Fereshtehnejad in two different studies4,18 is most akin to cluster A in our study. Cluster A was severe in
all disease domains including motor, NMS and cognition. The underlying mechanism of this severe cluster most likely lies in simultaneous involvement of dopaminergic and non-dopaminergic
pathways at an early disease stage18. Previous studies reported that the most critical determinants of PD subtype were UPDRS, cognitive status, RBD, and orthostatic hypotension4,18. In our
study, cluster A was best defined by MDS-UPDRS part I, II, III scores, cognitive impairment and significant BP drop, suggesting that the most critical drivers for PD subtype in our cohort
are consistent with previous reports. However, RBD was not found to be an effective clinical determinant for PD subtyping in our cohort. Our study had a low RBD detection rate, which is
likely attributable to the use of RBD1Q to detect RBD rather than use of gold standard overnight polysomnography assessment. In addition, the MCI percentage in our cohort was higher than
that in PPMI cohort reported by Weintraub19. Older age of diagnosis, lower education year and different ethnic population in our cohort may contribute to the difference. The identification
of the severe cluster and its critical clinical drivers would enable clinicians to identify PD patients with a more severe subtype at an early disease stage. Besides the severe cluster,
there were two comparatively more benign PD clusters in our cohort. Cluster C was characterised by young onset with generally better performance in all disease domains. This finding is
consistent with previous studies5,20 that have identified a mild PD subtype with young onset. Another comparatively benign PD cluster in our cohort was cluster B, comprising 47.6% of the PD
patients, with the key features of cognitive impairment and mild NMS. Cluster B is a unique subtype in our cohort with the cognitive performance and NMS scores found to be in opposing
directions from each other. The mechanism of cognitive impairment in PD is not fully understood. Acetylcholine neurotransmitter dysfunction is one of the possible pathways21. Muller et al
reported that cognitive impairment alone in PD patients was related to isolated cortical cholinergic deficits, while a combination of cognitive decline, falls and RBD correlated with
thalamic and cortical cholinergic deficiency22. The features of cluster B in our cohort suggest that the underlying affected brain areas of cognitive impairment in PD patients might be
heterogenous. When characterizing genetic markers in the three PD subtypes, we found that they had significantly different allele frequencies in two SNPs even though the composite genetic
score was not significantly different among three clusters. The mild cluster had significantly higher frequencies of the Park16 rs6679073 A allele and SV2C rs246814 T allele, indicating that
these two SNPs may have potential neuroprotective effects in our Asian cohort. _Park16_ SNPs has been consistently reported to play a protective role of PD development in different
populations23,24. However, there is little information about the clinical characteristics of _Park16_ carriers. Our study found that the number of _Park16_ carriers was highest in the mild
cluster (_Park16_ rs6679073 A allele frequency in mild cluster was similar with the healthy control group, results not shown), indicating that _Park16_ carriers were likely to have mild
symptoms, which supports the SNP’s neuroprotective effects in PD. The underlying mechanism is not entirely clear. One possible explanation may lie in the interaction between _Park16_ and
_LRRK2_25. MacLeod et al. reported that deficiency of the _Park16_ locus gene _RAB7L1(_RAB29_)_ resulted in neurodegeneration in _LRRK2_ mutant neurons while overexpression of RAB7L1
restored the function of neurons with _LRRK2_ mutation in an animal model25. Future clinical studies are needed to further elucidate the interaction between _Park16 and LRRK2_. Recently, Foo
et al.9 reported that synaptic vesicle glycoprotein 2C (SV2C) was a novel gene having robust association with PD development in various populations. It was also reported that SV2C was a
functional PD candidate gene and an important mediator of dopamine homeostasis. Genetic deletion of SV2C caused a reduction of dopamine release, resulting in a decrease in motor activity26.
Our findings corroborate the possible neuroprotective effects of SV2C, as the severe cluster had the lowest percentage of SV2C, while the mild cluster had the highest number of the SV2C
carriers. Our characterization of blood biochemical markers and PD clusters found Hcy to be a promising biomarker for the severe PD subtype after adjusting for confounders and multiple
comparisons. Previous evidence have found elevated blood Hcy levels to be associated with cognitive impairment in PD patients9,27. However, to our best knowledge, Hcy has not been previously
reported to be associated with PD severity. The robust relationship between elevated Hcy levels and severe PD subtype may open new strategies for PD treatment. Since the accelerated rate of
brain atrophy in the elderly with MCI have been found to be slowed by treatment with homocysteine-lowering B vitamins28, it is worth investigating whether PD severity can be ameliorated by
adding vitamin supplementation to lower the Hcy levels. In addition to Hcy, we found CRP to be another reliable biomarker for the severe PD subtype, a finding that is in agreeable with a
previous report8. These findings lend evidence for the existence of an heightened inflammatory state in severe PD subtypes. Previous studies have reported that lipids have a neuroprotective
effect on PD development. However, it is still controversial if lipid markers are associated with specific PD subtypes. We found that clusters with more than 60% MCI incidence (both clusters
A and B) had significantly higher TG level, consistent with our previous finding that higher TG levels were related to cognitive impairment29. Our results also showed that the severe
cluster (cluster A) had lowest TC levels. However, these associations were not significant after adjusting for multiple comparisons. Lawton et al recently reported that the severe motor
disease phenotype, poor psychological well-being, and poor sleep subtype was associated with reduced Apo A1 levels8. Our study, however, failed to reproduce this association. We were also
unable to find out any significant correlation between our PD subtypes and Vitamin D or UA levels. Our PD clusters were generated from an Asian cohort with all PD patients recruited within 1
year from diagnosis, which ensures that the cluster features were not driven by different disease durations and stages. In addition, we performed cluster analysis by including genetic
status, that enabled us to investigate PD heterogeneity at the genetic level. Our study also tries to assess the association between a broad list of blood biomarkers (genetic markers and
serum biochemical markers) and PD clusters, which provides comprehensive biological characterization for the newly generated clusters. However, some limitations of the study should be noted.
The current study was cross-sectional and blood biomarkers were not monitored overtime. Longitudinal follow-up of these PD subtypes to monitor their biomarkers and disease progression will
be needed. Furthermore, this was a single cohort study with limited sample size that requires further validation in other populations. In summary, we introduced three subtypes of early PD
patients in a multi-centre Asian cohort: ‘severe’, ‘intermediate’ and ‘mild young-onset’ subtypes. The severe subtype was associated with significantly lower frequency of Park16 and SV2C
alleles; and had significantly higher levels of serum Hcy and CRP. Park16, SV2C, Hcy and CRP may be useful biomarkers to stratify PD patients into disease subtypes. Our findings also shed
light on the possible underlying mechanisms that account for PD heterogeneity. This will improve the stratification of PD patients into disease subtypes that will enable more targeted
personalised treatment strategies. Further validation of the genetic and biochemical differences between subtypes in larger cohorts and evaluation of their impact on PD progression is
warranted. METHODS PARTICIPANTS AND ENROLMENT STUDY POPULATION A total of 206 idiopathic early PD patients defined by National Institute of Neurological Disorders and Stroke (NINDS)
diagnostic criteria have been recruited from Early Parkinson’s disease Longitudinal Singapore (PALS) cohort based on the inclusion and exclusion criteria of PALS study protocol30. PALS is an
ongoing prospective cohort study undertaken to investigate the disease course of early PD patients who were recruited within 1 year of diagnosis. ENROLMENT Our study was conducted at two
movement disorder outpatient clinics(Singapore General Hospital and Tan Tock Seng Hospital) in Singapore. Our study has been approved by SingHealth Centralized Institutional Review Board
(CIRB) with Ref 2019/2433 and written informed consent was provided by all participants. DATA COLLECTION Comprehensive clinical features (motor, NMS and cognitive domains) and blood
biomarkers were collected and used in the study. All clinical assessments were performed while patients were on their PD medications. CLINICAL ASSESSMENTS MOTOR MANIFESTATIONS Movement
Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part II score(Motor Aspects of Experiences of Daily Living), part III motor score, tremor score, Postural Instability
and Gait Disorder (PIGD) score31,32 were used to assess motor performance. The calculation of tremor score was based on MDS‐UPDRS items: 2.10, 3.15a, 3.15b, 3.16a, 3.16b, 3.17a, 3.17b,
3.17c, 3.17d, 3.17e, and 3.18, while the mean of MDS‐UPDRS items 2.12, 2.13, 3.10, 3.11, and 3.12 was PIGD score32. NMS MDS-UPDRS Part I score (Non-Motor Aspects of Experiences of Daily
Living) and Non-motor symptom scale (NMSS) total score were used to assess NMS burden; NMSS33 consists of 30 items which are grouped into nine domains (cardiovascular domain, sleep/fatigue,
mood/apathy, perceptual problems/hallucinations, attention/memory, gastrointestinal, urinary, sexual function and miscellaneous). Olfaction impairment and autonomic failures are included in
the miscellaneous domain. Rapid Eye Movement (REM) Sleep Behavior Disorder (RBD), Daytime sleepiness and sleep quality were evaluated by the RBD Single-Question Screen (RBD1Q)34 and Epworth
sleepiness scale (ESS) respectively. Patient depression and anxiety were assessed by Hospital Anxiety Depression Scale (HADS). COGNITIVE IMPAIRMENT Montreal Cognitive Assessment (MoCA) was
performed to monitor the overall cognitive change. Comprehensive neuropsychological tests were performed and 5 cognitive domain scores (executive, visuospatial, memory, attention and working
memory, language) were calculated by using the average of the standardized score of two neuropsychological tests from the same domain. Specifically, the following cognitive tests were
administered to evaluate the cognitive status of the 5 domains: (1) Executive: Frontal Assessment Battery (FAB) total score and Fruit Fluency; (2) Visuospatial: Repeatable Battery for the
Assessment of Neuropsychological Status (RBANS) judgment of Line Orientation and Rey-Osterrieth Complex Figure Test (ROCF) copy total score; (3) Memory: Alzheimer Disease Assessment Scale
(ADAS)-cog delayed recall score and ROCF delayed recall total score; (4) Attention and working memory: Digit Span Backward and Symbol Span total score; (5) Language: Boston Naming Test (BNT)
total score and Wechsler Adult Intelligence Scale | Fourth Edition (WAIS-IV)-Similarities. Mild Cognitive Impairment (MCI) diagnosis was based on International Parkinson and Movement
Disorder Society (MDS) level II criteria35, in which cognitive impairment should be present in at least two neuropsychological tests with 1.5 standard deviations (SDs) worse than norms as
cut offs, either within a single cognitive domain or across different cognitive domains. OTHERS Blood pressure was measured both in the supine position and after 3 min of standing.
Orthostatic drop in Systolic Blood Pressure(SBP) greater than 10 mmHg was considered significant BP drop and viewed as an objective measure of autonomic dysfunction4. We also collected
demographic data including sex, age of diagnosis, ethnicity. BLOOD BIOMARKERS ASSESSMENTS We genotyped variants of SNCA, LRRK2, Park16, ITPKB, SV2C using Illumina Infinium Global Screening
Array − 24 v2.0. The PRS was defined as the sum of the number of risk alleles per individual weighted by their effect size estimate corresponding to the logarithm of the odds ratio36. In the
current study we calculated PRS by comprising 5 Asian GWAS SNPs (_SNCA, LRRK2, Park16, ITPKB, SV2C_) with the highest effect size and p level less than the genome wide significant
association level (5*10−8) from the latest Asian GWAS meta-analysis37 to provide quantitative data of genetic burden individually. The SNPs data being used for PRS calculation can be found
in supplementary data. We tested 10 commercially available blood biomarkers. They are homocysteine (Hcy), C-reactive protein (CRP), vitamin D, uric acid(UA) and lipid markers including
Triglyceride (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) Apolipoprotein A1 (Apo A1) and Apolipoprotein B (Apo B).
Blood biomarkers were measured using overnight fasting venous serum sample and were determined by enzymatic assay in a professional medical laboratory (Quest Laboratories Pte Ltd,
Singapore). STATISTICAL METHODS CLUSTER ANALYSIS Cluster analysis was performed in Python Software version 3 (http://www.python.org). Seventeen variables (Age of diagnosis, PRS, Number of
patients having significant BP drop, MDS-UPDRS Part II score, MDS-UPDRS Part III score, tremor score, PIGD score, MDS-UPDRS Part I score, ESS Total Score, HADS Anxiety Total score, HADS
Depression Total score, RBD1Q, Memory score, Visuospatial score, Attention score, Language score, Executive score) were selected by expert opinion and contemporary evidence18. All variable
measurements were standardized by using the Z-scores for the cluster analysis. Agglomerative hierarchical clustering with Euclidean distance calculation was applied. We selected the
three-cluster solution due to more balanced data distribution and better clinical interpretation. Missing value pattern was identified as missing by random. Hence, single imputation approach
was used to impute 92 (2.6%) missing values in the baseline variables. POST-HOC COMPARISONS OF CLINICAL CHARACTERISTICS AND ALLELIC DISTRIBUTIONS OF RELATED GENES Post-hoc comparisons were
performed in Stata software (Stata/SE 16.1 Stata Corp. 2019. Stata Statistical Software: Release 16. College Station, TX: Stata Corp LLC) and SAS OnDemand for Academics (SAS Institute Inc.
2014. SAS® OnDemand for Academics: User’s Guide. Cary, NC: SAS Institute Inc.). Continuous variables were summarized using mean with standard deviation (SD) or median with first and third
quartile. Categorical variables were summarized by frequencies and percentages. Demographics, clinical characteristics not included in cluster analysis and allelic distributions of related
PD genes were compared among clusters. Fisher’s exact test or Pearson Chi square test (where appropriate) was carried out to compare the categorical variables among different clusters; while
one-way ANOVA or Kruskal-Wallis tests (depends whether normality assumption was tenable) was performed to compare continuous variables among different clusters. BLOOD BIOCHEMICAL MARKERS
COMPARISONS AMONG CLUSTERS Blood biochemical markers comparisons were performed in SAS OnDemand for Academics (SAS Institute Inc. 2014. SAS® OnDemand for Academics: User’s Guide. Cary, NC:
SAS Institute Inc.). All blood biochemical markers except CRP were log-transformed to reduce the right-skewness. Generalized linear model was performed to compare the biomarkers among
different clusters and adjusted for age of diagnosis, sex, using normal distribution assumption for the outcome variable. Gamma distribution was assumed for CRP due to the skewed
distribution even after log-transformation. False discovery rate (FDR) method38 was performed to control for multiple testing comparison and q value was calculated. We set the threshold of
_q_ values as 0.1. DATA AVAILABILITY The data collected during this study are available from the corresponding author upon reasonable request from qualified individuals. CODE AVAILABILITY No
previously unreported custom computer code or algorithm was used to generate results in this study. REFERENCES * Jellinger, K. A. Neuropathology of sporadic Parkinson’s disease: evaluation
and changes of concepts. _Mov. Disord._ 27, 8–30 (2012). Article CAS Google Scholar * Gjerloff, T. et al. Imaging acetylcholinesterase density in peripheral organs in Parkinson’s disease
with 11C-donepezil PET. _Brain_ 138, 653–663 (2015). Article Google Scholar * Sieber, B. A. et al. Prioritized research recommendations from the National Institute of Neurological
Disorders and Stroke Parkinson’s Disease 2014 conference. _Ann. Neurol._ 76, 469–472 (2014). Article Google Scholar * Fereshtehnejad, S. M. et al. New clinical subtypes of Parkinson
disease and their longitudinal progression: a prospective cohort comparison with other phenotypes. _JAMA Neurol._ 72, 863–873 (2015). Article Google Scholar * van Rooden, S. M. et al. The
identification of Parkinson’s disease subtypes using cluster analysis: a systematic review. _Mov. Disord._ 25, 969–978 (2010). Article Google Scholar * Delenclos, M., Jones, D. R., McLean,
P. J. & Uitti, R. J.Biomarkers in Parkinson’s disease: Advances and strategies. _Parkinsonism Relat. Disord._ 22(Suppl.), S106–S110 (2016). Article Google Scholar * Parnetti, L. et
al. CSF and blood biomarkers for Parkinson’s disease. _Lancet Neurol._ 18, 573–586 (2019). Article CAS Google Scholar * Lawton, M. et al. Blood biomarkers with Parkinson’s disease
clusters and prognosis: The oxford discovery cohort. _Mov. Disord._ 35, 279–287 (2020). Article CAS Google Scholar * O’Suilleabhain, P. E. et al. Elevated plasma homocysteine level in
patients with Parkinson disease: motor, affective, and cognitive associations. _Arch. Neurol._ 61, 865–868 (2004). Article Google Scholar * de Lau, L. M., Koudstaal, P. J., Hofman, A.
& Breteler, M. M. Serum uric acid levels and the risk of Parkinson disease. _Ann. Neurol._ 58, 797–800 (2005). Article Google Scholar * Simon, K. C. et al. Mendelian randomization of
serum urate and parkinson disease progression. _Ann. Neurol._ 76, 862–868 (2014). Article CAS Google Scholar * Ding, H. et al. Unrecognized vitamin D3 deficiency is common in Parkinson
disease: Harvard Biomarker Study. _Neurology_ 81, 1531–1537 (2013). Article CAS Google Scholar * Yeshokumar, A. K., Saylor, D., Kornberg, M. D. & Mowry, E. M. Evidence for the
Importance of Vitamin D Status in Neurologic Conditions. _Curr. Treat. Options Neurol._ 17, 51 (2015). Article Google Scholar * Yan, L. Y. et al. Association between carotid plaque and
Parkinson’s disease. _Ann. Transl. Med_ 7, 94 (2019). Article CAS Google Scholar * Swanson, C. R. et al. Lower plasma apolipoprotein A1 levels are found in Parkinson’s disease and
associate with apolipoprotein A1 genotype. _Mov. Disord._ 30, 805–812 (2015). Article CAS Google Scholar * Li, J. et al. Correlations between blood lipid, serum cystatin C, and
homocysteine levels in patients with Parkinson’s disease. _Psychogeriatrics_ 20, 180–188 (2020). Article Google Scholar * Park, J. H. et al. Association of high-density lipoprotein
cholesterol variability and the risk of developing Parkinson disease. _Neurology_ 96, e1391–e1401 (2021). Article CAS Google Scholar * Fereshtehnejad, S. M., Zeighami, Y., Dagher, A.
& Postuma, R. B. Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. _Brain_ 140, 1959–1976 (2017). Article Google Scholar * Weintraub, D. et
al. Cognitive performance and neuropsychiatric symptoms in early, untreated Parkinson’s disease. _Mov. Disord._ 30, 919–927 (2015). Article Google Scholar * Erro, R. et al. The
heterogeneity of early Parkinson’s disease: a cluster analysis on newly diagnosed untreated patients. _PLoS One_ 8, e70244 (2013). Article CAS Google Scholar * Halliday, G. M., Leverenz,
J. B., Schneider, J. S. & Adler, C. H. The neurobiological basis of cognitive impairment in Parkinson’s disease. _Mov. Disord._ 29, 634–650 (2014). Article CAS Google Scholar *
Muller, M. L. et al. Clinical markers for identifying cholinergic deficits in Parkinson’s disease. _Mov. Disord._ 30, 269–273 (2015). Article Google Scholar * Gopalai, A. A. et al. PARK16
is associated with PD in the Malaysian population. _Am. J. Med Genet B Neuropsychiatr. Genet_ 171, 839–847 (2016). Article CAS Google Scholar * He, T. et al. Association between PARK16
and Parkinson’s disease: A meta-analysis. _Neurosci. Lett._ 657, 179–188 (2017). Article CAS Google Scholar * MacLeod, D. A. et al. RAB7L1 interacts with LRRK2 to modify intraneuronal
protein sorting and Parkinson’s disease risk. _Neuron_ 77, 425–439 (2013). Article CAS Google Scholar * Dunn, A. R. et al. Synaptic vesicle glycoprotein 2C (SV2C) modulates dopamine
release and is disrupted in Parkinson disease. _Proc. Natl. Acad. Sci. USA_ 114, E2253–E2262 (2017). Article CAS Google Scholar * de Jager, C. A., Oulhaj, A., Jacoby, R., Refsum, H. &
Smith, A. D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: a randomized controlled trial. _Int. J. Geriatr. Psychiatry_ 27,
592–600 (2012). Article Google Scholar * Smith, A. D. et al. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: a randomized
controlled trial. _PLoS One_ 5, e12244 (2010). Article Google Scholar * Huang, X. et al. Higher serum triglyceride levels are associated with Parkinson’s disease mild cognitive impairment.
_Mov. Disord._ 33, 1970–1971 (2018). Article Google Scholar * Huang, X. et al. Serum uric acid level and its association with motor subtypes and non-motor symptoms in early Parkinson’s
disease: PALS study. _Parkinsonism Relat. Disord._ 55, 50–54 (2018). Article Google Scholar * Jankovic, J. et al. Variable expression of Parkinson’s disease: a base-line analysis of the
DATATOP cohort. The Parkinson Study Group. _Neurology_ 40, 1529–1534 (1990). Article CAS Google Scholar * Stebbins, G. T. et al. How to identify tremor dominant and postural
instability/gait difficulty groups with the movement disorder society unified Parkinson’s disease rating scale: comparison with the unified Parkinson’s disease rating scale. _Mov. Disord._
28, 668–670 (2013). Article Google Scholar * Chaudhuri, K. R. et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot
study. _Mov. Disord._ 22, 1901–1911 (2007). Article Google Scholar * Postuma, R. B. et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation
study. _Mov. Disord._ 27, 913–916 (2012). Article Google Scholar * Litvan, I. et al. Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society
Task Force guidelines. _Mov. Disord._ 27, 349–356 (2012). Article Google Scholar * Nalls, M. A. et al. Large-scale meta-analysis of genome-wide association data identifies six new risk
loci for Parkinson’s disease. _Nat. Genet_ 46, 989–993 (2014). Article CAS Google Scholar * Foo, J. N. et al. Identification of risk loci for Parkinson Disease in Asians and comparison of
risk between Asians and Europeans: A Genome-Wide Association Study. _JAMA Neurol._ 77, 746–754 (2020). Article Google Scholar * Glickman, M. E., Rao, S. R. & Schultz, M. R. False
discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. _J. Clin. Epidemiol._ 67, 850–857 (2014). Article Google Scholar Download references
ACKNOWLEDGEMENTS We would like to thank all participants and their families for their support of the PALS study, and also neurologists from the National Neuroscience Institute, Singapore for
referring their patients for the study. We also thank the National Medical Research Council (LCG Funding: MOH-OFLCG18May-0002 and Translational Clinical Research program in PD). The authors
thank Niu Chenglin for Python coding support. AUTHOR INFORMATION Author notes * These authors jointly supervised this work: Eng-King Tan, Louis C. S Tan. AUTHORS AND AFFILIATIONS *
Department of Neurology, National Neuroscience Institute, Singapore, Singapore Xiao Deng, Bin Xiao, Samuel Yong Ern Ng, Nicole Chia, Yi Jayne Tan, Xinyi Choi, Dede Liana Heng, Yew-long Lo,
Zheyu Xu, Kay-Yaw Tay, Wing-Lok Au, Adeline Ng, Eng-King Tan & Louis C. S. Tan * Duke-NUS Medical School, Singapore, 8 College Rd, Singapore, 169857, Singapore Xiao Deng, Bin Xiao,
Wing-Lok Au, Adeline Ng, Eng-King Tan & Louis C. S. Tan * Centre for Quantitative Medicine, Duke-NUS Medical School, Singapore, Singapore Seyed Ehsan Saffari & John Carson Allen *
Programme in Health Services and Systems Research, Duke-NUS Medical School, Singapore, Singapore Nan Liu Authors * Xiao Deng View author publications You can also search for this author
inPubMed Google Scholar * Seyed Ehsan Saffari View author publications You can also search for this author inPubMed Google Scholar * Nan Liu View author publications You can also search for
this author inPubMed Google Scholar * Bin Xiao View author publications You can also search for this author inPubMed Google Scholar * John Carson Allen View author publications You can also
search for this author inPubMed Google Scholar * Samuel Yong Ern Ng View author publications You can also search for this author inPubMed Google Scholar * Nicole Chia View author
publications You can also search for this author inPubMed Google Scholar * Yi Jayne Tan View author publications You can also search for this author inPubMed Google Scholar * Xinyi Choi View
author publications You can also search for this author inPubMed Google Scholar * Dede Liana Heng View author publications You can also search for this author inPubMed Google Scholar *
Yew-long Lo View author publications You can also search for this author inPubMed Google Scholar * Zheyu Xu View author publications You can also search for this author inPubMed Google
Scholar * Kay-Yaw Tay View author publications You can also search for this author inPubMed Google Scholar * Wing-Lok Au View author publications You can also search for this author inPubMed
Google Scholar * Adeline Ng View author publications You can also search for this author inPubMed Google Scholar * Eng-King Tan View author publications You can also search for this author
inPubMed Google Scholar * Louis C. S. Tan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors have contributed significantly to
this study, have read the manuscript, attest to the validity. E.-K.T. and L.C.S.T.: study concept and design, revising the manuscript, study supervision, obtaining funding. X.D.: statistical
analysis and interpretation of data, drafting/revising the manuscript. S.E.S., N.L., and J.C.A.: methodology support, statistical analysis, revising the manuscript. X.B.: interpretation of
data and revising the manuscript. S.N.Y.E., N.C., J.T., X.Y.C., and D.H.: acquisition of data. Y.-.L.L., Z.X., K.-Y.T., W.-L.A., A.N.: revising the manuscript. CORRESPONDING AUTHOR
Correspondence to Louis C. S. Tan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES SUPPLEMENTARY DATA RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Deng, X., Saffari, S.E., Liu, N. _et
al._ Biomarker characterization of clinical subtypes of Parkinson Disease. _npj Parkinsons Dis._ 8, 109 (2022). https://doi.org/10.1038/s41531-022-00375-y Download citation * Received: 02
March 2022 * Accepted: 05 August 2022 * Published: 29 August 2022 * DOI: https://doi.org/10.1038/s41531-022-00375-y SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
Trending News
ABC.net.au: Page not FoundOpen Sites menu ABC Home News iview TV Radio Kids Shop More...
Access to this page has been deniedYour browser appears to have Javascript disabled.For instructions on how to enable Javascript please click here.If you h...
Page Not Found.Page Not Found. Hopefully you will find what you are looking for here....
Kir genes and kir ligands affect occurrence of acute gvhd after unrelated, 12/12 hla matched, hematopoietic stem cell transplantationABSTRACT Interactions of polymorphic killer Ig-like receptor (KIR) receptors with KIR ligands have been shown to modify ...
Author correction: rpap3 provides a flexible scaffold for coupling hsp90 to the human r2tp co-chaperone complexCorrection to: _Nature Communications_ https://doi.org/10.1038/s41467-018-03942-1; published online 16 April 2018 In the...
Latests News
Biomarker characterization of clinical subtypes of parkinson diseaseABSTRACT The biological underpinnings of the PD clusters remain unknown as the existing PD clusters lacks biomarker char...
Jbl’s party boost turns my bluetooth speakers into surround soundAs a tech writer for the Strategist, Jordan Bowman covers everything from headphones to speakers to computers (and also ...
Hyperornithinemia-hyperammonemia- homocitrullinuria syndrome: low creatine excretion and effect of citrulline, arginine, or ornithine supplementABSTRACT ABSTRACT: Two patients with neonatal onset of hyperornithinemia- hyperammonemia-homocitrullinuria syndrome were...
Priti patel’s cynical immigration statement is an attempt to outflank farage | thearticleIt is instructive that the first major policy announcement of the Conservative election campaign is on immigration. Bori...
Plan to double size of yorkshire glamping park - complete with hot tubs - refusedCouncillors have blocked plans to expand an Yorkshire glamping park including with a manager’s home which nearby locals ...