Acute levodopa dosing around-the-clock ameliorates rem sleep without atonia in hemiparkinsonian rats
Acute levodopa dosing around-the-clock ameliorates rem sleep without atonia in hemiparkinsonian rats"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Rapid-eye-movement (REM) sleep without atonia (RSWA), a marker of REM sleep behavior disorder (RBD), is frequently comorbid with Parkinson’s disease (PD). Although rodent models are
commonly used for studying PD, the neurobiological and behavioral correlates of RBD remain poorly understood. Therefore, we developed a behavior-based criteria to identify RSWA in the
hemiparkinsonian rat model of PD. Video recordings of rats were analyzed, to develop a criteria consisting of behavioral signs that occurred during polysomnographically confirmed epochs of
sleep-wake stages. The _sleep-slouch_, a postural shift of the body or head caused only by gravity, was identified as a unique behavioral sign of REM sleep onset and was altered in
hemiparkinsonian rats during RSWA. There was a significant correlation between the behavior-based criteria and polysomnograms for all sleep-wake stages in control but not hemiparkinsonian
rats indicating a deterioration of sleep-wake architecture in parkinsonism. We then tested the efficacy of levodopa in ameliorating RSWA using intermittent and around-the-clock (ATC) dosing
regimens. ATC levodopa dosing at 4 mg/kg for 48 h caused a significant reduction of RSWA as measured by polysomnography and the behavioral-based criteria along with an amelioration of
forelimb motor deficits. Our findings show that the phenomenological correlates of RSWA can be reliably characterized in the hemiparkinsonian rat model. ATC levodopa administration
ameliorates RSWA in this model without deleterious consequences to the overall sleep-wake architecture and therapeutic benefits for parkinsonian motor deficits. These findings suggest that
further study may allow for the application of a similar approach to treat RBD in PD patients. SIMILAR CONTENT BEING VIEWED BY OTHERS DISRUPTED SLEEP-WAKE REGULATION IN THE MCI-PARK MOUSE
MODEL OF PARKINSON’S DISEASE Article Open access 11 March 2024 _GBA_-AAV MITIGATES SLEEP DISRUPTIONS AND MOTOR DEFICITS IN MICE WITH REM SLEEP BEHAVIOR DISORDER Article Open access 02 August
2024 EARLY ONSET OF SLEEP/WAKE DISTURBANCES IN A PROGRESSIVE MACAQUE MODEL OF PARKINSON’S DISEASE Article Open access 19 October 2022 INTRODUCTION Disturbances of sleep are well documented
in Parkinson’s disease (PD) and are estimated to occur in 60–90% of all PD patients.1,2,3,4 Rapid-eye-movement sleep behavior disorder (RBD) is one of the most common and disabling sleep
disturbances associated with PD and is characterized by a loss of muscle atonia during rapid-eye-movement (REM) sleep and dream enactments.5,6,7,8 Therefore, the occurrence of REM sleep
without atonia (RSWA) is considered the prototypical marker of RBD.9 PD patients with RBD report significant morbidity due to fatigue, excessive daytime sleepiness, injuries related to dream
enactments, and fluctuating efficacy of PD therapeutics.10,11,12,13 Studies have also shown that the morbidity of sleep dysfunction significantly affects the quality of life of PD patients
and may exceed the impact of the motor impairment and other complications.10,14 Finally, RBD symptoms frequently predate the diagnosis of PD motor symptoms and has been investigated as a
putative biomarker for pre-clinical PD.4,5,15,16,17,18 Taken together, these factors underscore the need for a pre-clinical model that recapitulates the phenomenology of RSWA in PD and help
close the major gap in this area of unmet therapeutic need.14,19 Although rodents are commonly used to model PD, few studies have investigated sleep dysfunctions in these pre-clinical
models. Current pre-clinical models of sleep disorders in rodents utilize a combination of electroencephalography (EEG) and electromyography (EMG) to classify sleep-wake stages. Rodent sleep
is typically divided into three principal categories: awake, REM sleep, and non-REM sleep (NREM).20,21 Unlike human polysomnography, most rodent sleep studies do not make use of time-locked
video recordings or well-delineated behavior-based criteria for identifying sleep stages or its dysfunctions. Rodent sleep architecture also lacks many of the characteristic hallmarks
commonly found in human sleep recordings. Therefore, rodent sleep recordings present a unique challenge in identifying sleep disorders that are associated with movement such as RBD, as EMG
becomes unreliable as a marker for RBD-associated movement. Previously, we have shown that the sleep architecture in the unilateral 6-hydroxydopamine (6-OHDA) lesioned hemiparkinsonian rat
model of PD, an animal model that has contributed to the development of most of the medications in current use for PD, resembles that of human PD patients with RBD.22 Here we demonstrate
that this animal model exhibits RBD like behavioral features and validated a set of behavior-based criteria for sleep-wake stages that is correlated with rat sleep architecture to reliably
assess RSWA. Most treatments currently available for RBD in PD patients like clonazepam and melatonin are considered suboptimal due to their variable efficacy and side effects such as
sedation, motor incoordination, confusion, memory dysfunction etc.23 In contrast, levodopa one of the most commonly prescribed anti-PD medication has been shown to be effective in
ameliorating RBD in several studies especially when RBD precedes the onset of the motor symptoms of PD.14,24,25 The effects of levodopa in patients who only present with RBD and do not
develop PD in their lifetime is not discussed here. In such patients, levodopa did not meet evidence-based data to be recommended as a treatment.23 All current PD patients, however, will
receive levodopa in their lifetime and exposure to this medication is unavoidable in contemporary medicine as it is by far the most effective pharmacotherapy for PD. Unfortunately, long-term
use of intermittent oral levodopa causes disabling motor complications in most PD patients. A recent review of clinical trials, however, has shown that levodopa dosing, administration
route, and regimen may be critical in affecting the onset of these medication-induced complications and novel dosing regimens with strict compliance may be able to overcome their side
effects.26 The advent of extended-release oral levodopa formulations and intrajejunal pump-based levodopa administration methods have permitted longer and more continuous dosing regimens.
Such treatments with continuous or around-the-clock (ATC) dosing may help avoid the medication-induced complications of levodopa. However, the efficacy of such dosing regimens remains
largely unexplored in pre-clinical models of PD. Therefore, we investigated whether RSWA in the 6-OHDA hemiparkinsonian rodent model of PD is responsive to levodopa therapy when administered
ATC. Our results indicate that ATC dosing of levodopa mitigates both motor symptoms like forelimb bradykinesia and non-motor symptoms such as RSWA without affecting the overall architecture
of sleep-wake stages in this rat model. Our findings suggest that such an ATC dosing regimen with continuous delivery of levodopa could be a potential experimental therapeutic strategy for
RBD in PD patients. RESULTS ATC LEVODOPA DOSING LEADS TO ACCURATE CLASSIFICATION OF HEMIPARKINSONIAN RATS COMPARABLE TO UNLESIONED CONTROLS The accuracy of the behavior-based criteria
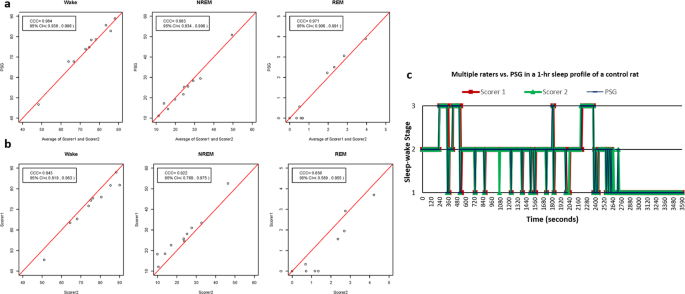
proposed here was tested by comparing the average scores of the two independent blinded raters with polysomnography (PSG) recordings for all sleep-wake stage (Wake, NREM, and REM)
classification. To compute the inter-rater reliability of the criteria, classification was also compared between the two raters for all sleep-wake stages. In unlesioned control rats, the
sleep-wake stage classification using the behavior-based criteria (Average of Scorer 1 and Scorer 2) showed a good agreement (concordance correlation coefficient (CCC) > 0.8) compared
with PSG for all sleep-wake stages (Fig. 1a, Table 1). The inter-rater reliability of the criteria also showed good agreement between the two raters (CCC >0.8) for all sleep-wake stages
(Fig. 1b, Table 1). Graphed sleep profiles between the raters and PSG also showed close matches in the sleep-wake stage classification (Fig. 1c). In untreated hemiparkinsonian rats, there
was poor agreement (CCC <0.8) between the behavior-based criteria compared with the PSG for all sleep-wake stages (Fig. 2a, Table 2). Graphed sleep profiles showed several false-positive
and false-negative identifications of REM periods when comparing the behavior-based criteria to PSG (Fig. 2b). However, following ATC treatment of levodopa at 4 mg/kg, hemiparkinsonian rats
showed good levels of agreement (CCC <0.8) between the behavior-based criteria compared with PSG for all sleep-wake stages (Fig. 2c, Table 2). Graphed sleep profiles also showed close
matches in the sleep-wake stage classification (Fig. 2d). VIDEO RECORDINGS SHOW QUALITATIVE IMPROVEMENTS IN HEMIPARKINSONIAN RAT SLEEP-SLOUCH Evaluation of video recordings of control and
hemiparkinsonian rats showed qualitative differences in the _sleep-slouch_, a unique and characteristic behavior that occurs during the onset of REM sleep. In control rats, the sleep-slouch
was seen in real time (at normal playback speed) as it occurred quickly and the drop of the animal’s body due to gravity was observed. In contrast, in hemiparkinsonian rats, the
sleep-slouches were subtle and cannot be seen in real time as they occur slowly and are therefore less discernible. A closer examination of the video recordings showed that while behaviors
characteristic of REM sleep are preserved in hemiparkinsonian rats, the quality of these behaviors became more subtle as reflected in a decreased accuracy in sleep-classification using the
behavior-based criteria. However, following levodopa treatment, the sleep-slouch qualitatively improved in hemiparkinsonian rats, occurred over faster timescales, was clear and was
comparable to those seen in the control rats (Supplementary Video 1). ACUTE ATC DOSING OF LEVODOPA IMPROVES RSWA IN HEMIPARKINSONIAN RATS ATC dosing of levodopa at 4 mg/kg in
hemiparkinsonian rats (17.3 ± 2.2) compared to baseline (30.2 ± 0.9) resulted in a significant decrease in RSWA (_F_ (3, 18) = 4.543, _p_ = 0.0398) (Fig. 3). In contrast, no statistically
significant differences were observed with 2 mg/kg two times per day (BID) (29.6 ± 6.3), 2 mg/kg three times per day (TID) (31.5 ± 9.5), 2 mg/kg ATC (26.3 ± 3.4), 4 mg/kg BID (22.8 ± 5.3),
and 4 mg/kg TID (25.5 ± 5.0) dosing regimens compared to baseline. Electrooculograph (EOG) was used to confirm the RSWA observed and used to differentiate the tonic and phasic REM epochs.
LEVODOPA DOSING REGIMENS DID NOT CAUSE CHANGES IN OVERALL SLEEP-WAKE STATES Comparison of the mean percentages of all sleep-wake states (Wake, NREM, phasic REM, and tonic REM) between
baseline and the six different levodopa dosing regimens showed no statistically significant differences (_F_ (6, 18) = 0.6305, _p_ = 0.7042). This suggests that the arousals caused by
variability in number of injections used to administer levodopa did not cause any significant alterations to the overall sleep architecture in hemiparkinsonian rats (Fig. 4). ATC DOSING OF
LEVODOPA IMPROVES IMPAIRED LIMB MOTOR FUNCTIONS IN HEMIPARKINSONIAN RATS The vibrissae-evoked forelimb placement following 4 mg/kg ATC dosing (86.4 ± 7.5) compared to the post-lesion
baseline (14.4 ± 8.3) showed a significant improvement in affected limb usage (_F_ (3, 6) = 2.468, _p_ = 0.0075). The 2 mg/kg ATC (52.4 ± 19.5) dosing showed an upward trend but did not
reach levels of statistical significance (Fig. 5). The unaffected forelimb usage showed no changes in usage in any rats. The development of drug-induced dyskinesias interfered with the
ability to conduct the vibrissae-evoked forelimb placement test in hemiparkinsonian rats during the BID and TID testing drug regimens and were therefore excluded from analysis. Control rats
did not show any deficits on the test (data not shown). HEMIPARKINSONIAN RATS SHOW A PROFOUND UNILATERAL LOSS OF NIGROSTRIATAL NEURONS Histological examination of hemiparkinsonian rats
showed greater than 95% depletion of dopaminergic neurons in the substantia nigra on the lesioned side (Fig. 6b) and a dramatic loss of tyrosine hydroxylase positive fibers in the
corresponding striatum (Fig. 6a) as expected from this PD model. Unbiased stereological estimations on the right substantia nigra and Abercombie corrected manual counts on the left
substantia nigra confirmed these histological observations. All rats used in this experiment met the criterion of 95% depletion of nigral neurons. No evidence of histological lesions in the
locus coeruleus, raphe nuclei, sublaterodorsal nucleus, or any areas that have been described previously in models of RBD was found.27 DISCUSSION To our knowledge, this is the first study of
the effect of ATC administration of levodopa on RSWA in the well-studied hemiparkinsonian rat model. Our data provides evidence that RSWA in the hemiparkinsonian rat model of PD is levodopa
responsive if dosed ATC and that such dosing does not result in overall sleep-wake architecture changes. Additionally, we describe here a well-characterized behavior-based criteria that
helps classify sleep-wake stages in both control and treated hemiparkinsonian rats that exhibit RSWA with accuracy comparable to that of the gold standard of PSG. Further, we observe
behavioral changes in hemiparkinsonian rats, during REM sleep which could be potentially combined with PSG to provide a better understanding of RBD-like behavior in PD rodent models. The
sleep-slouch posture described here is a significant marker of the onset of REM sleep and could potentially parallel akinesia observed during REM sleep. This posture was less robust and
slower in lesioned rats compared to unlesioned control rats reflecting a lack of atonia as a consequence of parkinsonism. ATC levodopa treatment of hemiparkinsonian rats led to the
restoration of the sleep-slouch to normal levels suggesting that RWSA is responsive to dopamine replacement therapy in this model of PD. RBD is one of the most understudied aspects of the
sleep-wake alterations in animal models of PD (see review in ref. 28). The unilateral 6-OHDA lesioned hemiparkinsonian rat is a well-described preclinical model of PD that has been
extensively used for obtaining critical preclinical safety and efficacy data for all PD medications in contemporary use.29 This animal model has also led to a better understanding of the
pathophysiology of basal ganglia dysfunction in PD and is increasingly recognized as a useful model to study many of the non-motor features of PD including sleep dysfunctions in PD. Other
rat models using intracerebroventricular or bilateral striatal injections of 6-OHDA have also shown a dysregulation of circadian mechanisms caused by a disruption of dopamine signaling.30,31
However, such models also cause debilitating disabilities, significant morbidity, and variable mortality, which may contribute to the sleep deficits seen.31,32 The rotenone and
cycad-induced PD rat models mimic sleep disturbances seen in humans prior to the onset of clinical symptoms of PD, but produce exceedingly mild locomotor dysfunction that are both bilateral
and symmetric, unlike what is observed in human PD.33,34 Similarly, intranasal or direct intranigral administration of lactacystin, a natural proteasome inhibitor fails to produce stable
parkinsonism while causing RBD in the rat.35 While several studies have shown that inactivation of the glutamatergic neurons of the sublaterodorsal nucleus in rat models results in
RSWA,36,37,38,39 the occurrence of RBD is most often seen in the early stages of PD when there is very little evidence of damage to this region. Moreover, the role of nigrostriatal
degeneration, the dominant pathophysiology seen in PD, on RBD associated with PD is not explained by these models. In contrast, the hemiparkinsonian rat model displays this hallmark
degeneration of the nigrostriatal dopaminergic pathway and the unilaterality of motor deficits, which can be characterized using well-established tests that have been extensively validated.
Since PD always begins with unilateral symptoms (stage I disease), the 6-OHDA lesioned hemiparkinsonian rat simulates this natural disease progression as well. Crucially, we have previously
shown that the sleep architecture in this rat model resembles that of PD patients and may thus be useful to elucidate the pathophysiological basis of PD sleep disturbances and be useful to
test experimental therapies for RBD in early PD.22 While some rudimentary species-specific sleep postures and behaviors have been reported previously, most pre-clinical models of PD lack a
codified rodent equivalent of human behaviors that are commonly associated with RBD.40,41,42,43,44 This issue is exacerbated by the lack of standard PSG recording procedures and sleep stage
classification in stark contrast to the well-accepted methods commonly applied to human sleep studies.45 Our paper provides such a well-defined behavior-based criterion for sleep-stage
classification in rodents with time-locked PSG recordings that has been validated and tested with experimental therapeutics. A review of sleep staging systems in rats21 indicate that with
few exceptions46,47 most sleep studies classify REM sleep as a single stage which could lead to an inaccurate classification of RSWA. In contrast, the use of EOG recordings and a stringent
two standard deviation definition for positive muscle activity in the present study allows for the bifurcation of REM sleep into its tonic and phasic components and consequently accurately
identify RSWA. Further, the use of a behavior-based criteria in conjunction with polysomnography recordings is also in line with newer scales used to detect and quantify RBD in humans such
as the REM Sleep Behavior Disorder Severity Scale.48 There is a plethora of evidence that shown that the dopaminergic neurons present in the substantia nigra pars compacta (SNpc) play an
important role in the regulation of REM sleep in rats.49,50,51 These neurons receive inputs from the locus coeruleus (REM-OFF) and laterodorsal/pedunculopontinetegmentum (REM-ON), neurons
which could modulate the presence of RBD.52 Hemiparkinsonian rats also show reduced ratios of distance and transitions between the resting vs. the activity phase, which could potentially
parallel similar abnormalities seen in RBD associated with PD.36 A variety of underlying mechanisms including dopamine release from the unlesioned hemisphere, disruptions in the mesopontine
dopaminergic neurons, and excessive nigral GABAergic inhibition via the pedunculopontine nucleus (PPN) have been proposed for these REM sleep disturbances seen in hemiparkinsonian
rats.49,53,54,55,56 Number of studies also suggest that divergent midbrain dopaminergic populations have different effects on sleep-wake regulation and RSWA. Dopaminergic cells that
originate in the ventral tegmental area and the dorsal raphe have been implicated as key arousal promoting systems, which are counterbalanced in health by SNpc dopaminergic neurons that
promote sleep.57 The lesioning of SNpc dopaminergic neurons may therefore create an imbalance that contributes to RSWA and the RBD-like features in the hemiparkinsonian rat model. In spite
of several lines of evidence indicating a dopaminergic basis for the development of RBD in PD, few studies have analyzed the effects of dopamine replacement therapy on this sleep dysfunction
in the context of a preclinical model of PD. Studies have suggested that the presence of RBD in PD may put patients at higher risk for levodopa-induced motor fluctuations in
wakefulness.58,59,60,61 This finding may indicate that PD patients with concomitant RBD have a more severe form of neurodegeneration or that RBD is an at-risk biomarker for motor
fluctuations in PD. Recent studies have proposed that many of the deleterious effects of levodopa treatment can be minimized by modifying dosing regimens to provide continuous dopaminergic
stimulation.62,63,64 The varying regimens in the current study were formulated based on pharmacodynamics of levodopa to test the effects of intermittent oscillating versus continuous
non-oscillating dosing schedules. Fluctuations in levodopa concentrations caused by conventional BID and TID dosing regimens have been shown to result in wider oscillations in striatal
dopamine availability causing profound pathophysiological alterations in the basal ganglia which in turn cause motor complications and RBD.65,66,67 We show here that ATC dosing regimen at
4-h intervals potentially overcomes the issue of oscillating levels of striatal dopamine and thereby mitigates daytime motor deficits and night time RSWA. The relationship between these two
beneficial effects of ATC levodopa dosing to mitigate PD-related phenomenology requires further study. Our observations are further strengthened by recent clinical studies where
levodopa/carbidopa intestinal gel was administered via intrajejunal pump continually for 24 h.25,68,69 In these patients, there was a significant overall improvement in their sleep quality
as measured by the Parkinson’s Disease Sleep Scale and Non-Motor Symptoms Scale consistent with improvement of nocturnal akinesia.69 Studies also show that sleep abnormalities are not
adequately addressed with optimized daytime dosing of medication and that untreated sleep disturbances have alarming consequences for daytime function in PD patients. New treatment
approaches in treating PD patients therefore, recommend the utilization of 24-h continuous treatment strategies used here to sufficiently manage and control both daytime and nighttime
symptoms.70,71,72 Advances in formulations and routes of administration have allowed for such treatments in PD patients and suggest that ATC dopamine agonist therapies may provide benefits
to subjective measures of sleep.73 Recent studies of rotigotine, a highly lipophilic dopamine-receptor agonist delivered via transdermal patches has been shown to improve subjective sleep
quality in PD patients with RBD74 and was found to be effective in both early75 and advanced stage PD patients.76 The dopamine agonist, ropinirole in its 24-h prolonged release formulation
has been found to efficacious in improving nocturnal symptoms of PD in double-blind, placebo-controlled trials.77,78 Similarly, intrajejunal infusions of levodopa–carbidopa intestinal gel
(Duodopa) has also been shown to improve sleep quality in PD patients.69,79 These new advances in routes of administration lead to 24-h, continuous, nonfluctuating drug levels and may
therefore help ameliorate RBD in PD. There are some drawbacks to our study that need to be addressed. Only a low-to-medium range of levodopa doses were tested in the current study. This is
in keeping with the dose of levodopa that is typically employed in early PD patients. Testing higher dosages of levodopa may be useful to further clarify its effects on RBD in future
studies. Though animals had well-established baseline recordings and a washout period between dosages of LD our study lacks a cohort of vehicle injected animals. However, our before and
after study design does provide the proof of principle to undertake additional longer studies that include separate control groups. Studies that include longer segments of PSG and video
recordings with multiple camera angles may have additional benefits and help further elucidate the behavior-based criteria described here. Future studies that utilize osmotic minipumps or
other methods for continuous levodopa delivery as shown in a recent study80 may be utilized to further test the feasibility of our ATC levodopa therapeutic approach. The behavior-based
criteria reported here requires well-trained and experienced raters and is labor intensive. Many hours of training were needed for the raters to learn the behavior-based criteria. Future
studies could also try to implement the use of computational software to allow for automatic classification that will permit ease of use. Finally, the use of telemetric implantable devices
that permit the use of cardiac, respiratory, and jaw muscle group monitoring were not available for polysomnographic classification in the current study and may have added advantages in
future studies. In conclusion, our findings support the dopaminergic mechanisms for RBD, an important non-motor aspect of PD. The characterization of RBD as a non-motor aspect of PD does
represent a paradox, despite its continued characterization as such in most PD literature. This is because, movement and tone abnormalities are the key aspects of RBD, and they are indeed
motor phenomena albeit occurring during sleep. Our study in the hemiparkinsonian rat (which has exclusive lesioning of the nigrostriatal pathway with preservation of other relevant monoamine
systems due to the use of desipramine during lesioning) suggests that the nigrostriatal dopaminergic pathway may play a seminal role in RBD and sleep disturbances seen in this PD model as
reported elsewhere.51,81,82 Our findings suggest that dopamine dysregulation contributes to RSWA and that normalizing this with ATC dopaminergic replenishment mitigates this sleep
abnormality. Our results show that ATC levodopa dosing modulates REM sleep disturbances much better than intermittent BID or TID dosing. The use of ATC dopamine administration and its
ability to provide non-fluctuating levels of dopamine replenishment could help cover both daytime and nighttime symptoms of PD. The advent of new drug-delivery systems such as intra-jejunal
levodopa gel infusion therapy and transdermal dopaminergic patches could translate these findings to benefit PD patients. We also propose behavior-based criteria that resembles behavior
rating scales for RBD in humans and could help facilitate studies into the mechanisms underlying RBD in PD using this animal model. Since RBD could potentially be a harbinger of PD, this
animal model-based criteria could have broad applicability for the development of effective neuroprotective therapies. METHODS Twenty-two female Sprague–Dawley rats weighing 220–250 g at the
time of acquisition and housed on a 12-h light:dark cycle (8 am–8 pm) with ad libitum access to food and water were used in the experiment. All procedures were carried out in accordance
with the guidelines in the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Pennsylvania State University Institutional Animal Care and Use Committee. A set of
normal rats were used to create the behavior-based criteria (_n_ = 3) and the remaining rats were randomly assigned to either the hemiparkinsonian (_n_ = 9) or unlesioned control (_n_ = 10)
groups. Rats in the hemiparkinsonian group were further randomly subdivided into an untreated (_n_ = 5) and levodopa-treated (_n_ = 4) groups. LESIONING SURGERY Hemiparkinsonism was induced
in nine rats by lesioning the left nigrostriatal pathway using a modified version of the Ungerstedt model.83 Briefly, 6-hydroxydopamine-hydrogen bromide (12 μg in 4 μl) was stereotactically
injected into the medial forebrain bundle (antero-posterior (AP): −1.5 mm, medio-lateral (ML): +1.8 mm, dorso-ventral (DV): −7.5 mm from bregma, the midline suture and the skull surface,
respectively) at a rate of 0.67 μl/min using a gastight Hamilton syringe, following which the needle was left in place for 5 min. Prior to the surgery, rats received an injection of the
noradrenergic uptake blocker desipramine (15 mg/kg i.p.) to avoid damage to norepinephrine (NE) pathways and to limit the lesion effect to the SNpc in one hemisphere. After 3 weeks of
recovery, the lesioned rats were challenged twice, 2 weeks apart with apomorphine hydrochloride (0.2 mg/kg, s.c.). The apomorphine-induced rotations were counted in an automated ‘rotometer’
(San Diego Instruments). Rats with more than 245 rotations over 35 min on two separate sessions were classified as hemiparkinsonian rats and used in the study.84 Ten rats did not receive the
lesioning surgery and were used as controls. ELECTRODE IMPLANTATION SURGERY Subsequently, all rats were implanted with stainless-steel skull screws over the frontal and parietal cortices
for electroencephalograph (EEG) recordings, a pair of wire electrodes in the nuchal muscles for electromyograph (EMG) recordings and two screws in the supraorbital ridge for
electrooculograph (EOG) recordings under general anesthesia as described previously.85,86 POLYSOMNOGRAPHY RECORDINGS After recovery from surgeries, polysomnography (PSG) comprising of EEG,
EMG, and EOG recordings was obtained, analyzed, and averaged to obtain equal “lights-on” and “lights-off” recording times for all rats. Signals were amplified and filtered with a Grass Model
12 Neurodata amplifier system (Grass Instrument Division of Astro-Med, Inc., West Warwick, RI), and digitized at 128 Hz with a USB-2533 16-bit analog to digital converter (Measurement
Computing Corporation, Norton, MA). The EEG, EMG, and EOG signals were filtered offline in Matlab (Mathworks), segmented into 10-s epochs, and manually rated using the commercial SleepWave
program (Biosoft Studio, Hershey, PA). The behavior criteria development relied on EEG and EMG recordings alone to classify each 10-s epoch as demonstrative of wakefulness, NREM sleep, and
REM sleep.22 Subsequently, to study RSWA, the REM sleep component was further divided into tonic REM sleep and phasic REM sleep using EOG recordings (described below). REM SLEEP
CLASSIFICATION Baseline PSG recordings (24 h each) were obtained from all rats and were aligned to the 12-h light/dark cycles after which the rats received levodopa (described below). The
rats were then recorded to obtain post levodopa PSG. Sleep-wake classification was carried out using only EEG and EOG recordings. EOG recordings were further used to differentiate REM sleep
into its phasic (characterized by bursts of EOG spikes) and tonic components (characterized by periods of little or no EOG activity components) (Fig. 7). EMG was used to evaluate for
positive muscle activity, defined as muscle activity during tonic REM sleep that was two standard deviations above the baseline atonia. The percentage of tonic REM with positive muscle
activity was determined and compared between baseline and each levodopa dosing regimen. LEVODOPA DOSING REGIMENS All levodopa-treated hemiparkinsonian rats (_n_ = 4) received injections of
levodopa and benserazide, on separate occasions at two different doses, 2 mg/kg or 4 mg/kg, of levodopa and 15 mg/kg benserazide (i.p.). Benserazide has been shown to inhibit peripheral
aromatic l-amino acid decarboxylase (AADC) activity, enhance brain bioavailability of levodopa, and increase extracellular striatal dopamine levels in hemiparkinsonian rats.87 Microdialysis
studies in unlesioned rats88,89,90,91 and hemiparkinsonian rats87,92 show that levodopa plus benserazide significantly increases levels of extracellular dopamine in the striatum for up to 4
h post injection compared to baseline levels. Three different dosing regimens were utilized: BID, TID, and ATC every 4 h for 24 h (Supplementary Fig. 1). The first injection of each dosing
regimen was aligned with the lights-on time of 8 am. The BID levodopa injections were administered at 8 am and 4 pm. The TID levodopa injections were administered at 8 am, 12 pm, and 4 pm.
Finally, the ATC levodopa injections were administered at 8 am, 12 pm, 4 pm, 8 pm, 12 am, and 4 am. The overall daily dosage of levodopa that each animal received was constant and was
sub-divided into parts depending on the dosing regimen being used. The BID and TID doses were given only during the 2-h lights-on phases as rats were more likely to be in REM sleep. All rats
in the levodopa treatment group received 2 days of injections of all dosing regimens, which were separated by washout periods of 24 h to analyze the effects of each one independently. PSG
recordings were obtained for all sessions spanning about 48 h of recordings from each rat for each dosing regimen. All rats were acclimatized to injections prior to the start of any
experimentation and after were continually observed after each injection until they resumed their previous behavioral state. BEHAVIOR-BASED CRITERIA DEVELOPMENT To create a set of
behavior-based criteria, video recordings were time-locked with PSG recordings of a cohort of normal rats (_n_ = 3). Video recordings were then reviewed and the behaviors of the animals in
each stage were recharacterized by a blinded rater. Video observations and complementary PSG recordings of rats showed six distinct behaviors characterizing the three sleep-wake stages
(Table 3). _Wakefulness_ was characterized by behaviors including arousal posture (bipedal activity, eating, posture of the extended tail, whisking, and grooming); _NREM_ was characterized
by closed eyes, stationary position, tail not extended, and chin resting on tail or ground; and _REM_ was characterized by the _sleep-slouch_. The _sleep-slouch_ was a movement of the rat’s
body, head, or both to one side of the body caused by gravity rather than active muscle contraction (Supplementary Video 1). This phenomenon served as a behavioral correlate of REM sleep and
was followed by RSWA in hemiparkinsonian rats. A score (Table 3) was assigned to each behavior in correlation with PSG that resulted in a total score at any time point denoting the current
sleep-wake stage of the rats. BEHAVIOR-BASED CRITERIA VS. PSG RECORDINGS AND INTER-RATER AGREEMENT To test the behavior-based criteria against the gold standard of PSG, hour-long video
recordings from the lights-on periods of control rats were scored by a blinded rater (Scorer 1) using the behavior-based criteria. A second rater (Scorer 2) was then used to test the
inter-rater reliability of the behavior-based criteria. The percentage of time spent in each sleep-wake stage was calculated and averaged between the Scorer 1 and Scorer 2, then compared to
that obtained from EEG/EMG alone without the use of EOG recordings. To determine whether any agreement is reflective of the actual sleep-wake patterns, the sleep profiles were graphed and
compared to those obtained from PSG. In all graphing of the stages of wakefulness, the stages were split into 10-s epochs with REM receiving the highest priority in the instance of an epoch
containing more than one sleep-wake stage. This comparison with PSG was again performed on the hemiparkinsonian and the levodopa-treated hemiparkinsonian rats using a blinded rater. Given
the unreliability of EMG in the presence of RSWA-like behaviors in hemiparkinsonian rats (movement artifacts), this comparison was performed using the EEG and EOG recordings against the
rater for the untreated hemiparkinsonian group and the levodopa treated hemiparkinsonian group. CCC with 95% CI was calculated to assess agreement i.e.reliability for all comparisons. The
level of probability for good agreement was set at a CCC >0.8. VIBRISSAE-EVOKED FORELIMB PLACEMENT TEST To evaluate the efficacy of levodopa treatment on forelimb motor deficits, rats
underwent the vibrissae-evoked forelimb placement test93 at the end of each dosing regimen. Previously, we have shown that hemiparkinsonian rats show profound deficits on this test after
lesioning and that these deficits were ameliorated by levodopa/benserazide treatment.94 Since testing in the current study was performed during the lights-on period when the animals are less
active, we chose this evoked test as a measure of forelimb function. The rats were tested immediately following one of the last injections to test the drug at maximum efficacy and separated
from any polysomnographic recordings that were utilized for analysis from these animals. Briefly, animals were held at the torso so that the hind limbs and the forelimb being tested could
hang freely while the forelimb not being tested was carefully restrained. The unrestrained forelimb was evaluated by bringing the rat toward the edge of the tabletop to elicit an evoked
reaching behavior toward the surface due to stimulation of the ipsilateral vibrissae contact with the table surface. This response was tested for each forelimb independently for ten trials
and repeated three times. The number of successful placements for each forelimb onto the tabletop was scored to determine the percentage of affected forelimb usage. HISTOLOGY At the end of
the study, all rats were euthanized via transcardiac perfusion with cold heparinized saline and 4% paraformaldehyde fixative. Brains were removed, cryoprotected, and sectioned coronally at
60 µm. Sections were processed for cresyl violet (Nissl stain) and tyrosine hydroxylase immunohistochemistry to verify unilateral depletion of the nigrostriatal pathway as described
previously.94 STEREOLOGY Estimates of tyrosine hydroxylase positive neurons in the substantia nigra was performed using a design-based optical fractionator method (Stereo Investigator, MBF
Bioscience) on the unlesioned right substantia nigra, using systematic random sampling and unbiased stereological estimation. The Abercrombie correction to manual physical counts was applied
when counting procedures did not meet the criteria for the optical fractionator as was the case with the lesioned left substantia nigra.95 STATISTICAL ANALYSIS All statistical analysis was
performed by an investigator who was blinded to all experiments on animals, polysomnographic and behavioral raters to avoid any bias (A.K.). For additional rigor, histological analysis and
stereology was performed by investigator blinded (K.V.) to all other experimental assessments. CCC with 95% CI was calculated using R software, version 3.5.1 (The R Foundation for
Statistical Computing, Vienna, Austria) to assess inter-rater reliability between raters and to assess face validity between polysomnography and the criteria. The level of good agreement was
set at a CCC >0.8. One-way ANOVA followed by Dunnett’s multiple comparisons test was calculated using GraphPad Prism version 7.05 for Windows, GraphPad Software, La Jolla, California,
USA, www.graphpad.com, that accounts for the within-animal correlation of multiple levodopa doses per rat, was used for analysis of the behavioral assessment, the percentage of tonic REM
epoch with muscle activity between baseline and each levodopa dose and the total REM epoch. Data are expressed as mean ± SEM and the level of probability for statistical significance was set
at 0.05. REPORTING SUMMARY Further information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The data used to support
the findings of this study are available from the corresponding author upon request. REFERENCES * Abbott, R. D. et al. Excessive daytime sleepiness and subsequent development of Parkinson
disease. _Neurology_ 65, 1442–1446 (2005). Article CAS PubMed Google Scholar * Tandberg, E., Larsen, J. P. & Karlsen, K. A community-based study of sleep disorders in patients with
Parkinson’s disease. _Mov. Disord._ 13, 895–899 (1998). Article CAS PubMed Google Scholar * Garcia-Borreguero, D., Larrosa, O. & Bravo, M. Parkinson’s disease and sleep. _Sleep Med.
Rev._ 7, 115–129 (2003). Article PubMed Google Scholar * De Cock, V. C., Vidailhet, M. & Arnulf, I. Sleep disturbances in patients with parkinsonism. _Nat. Clin. Pract. Neurol._ 4,
254–266 (2008). Article PubMed Google Scholar * Iranzo, A. et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study.
_Lancet Neurol._ 5, 572–577 (2006). Article PubMed Google Scholar * Olson, E. J., Boeve, B. F. & Silber, M. H. Rapid eye movement sleep behaviour disorder: demographic, clinical and
laboratory findings in 93 cases. _Brain_ 123, 331–339 (2000). Article PubMed Google Scholar * Larsen, J. P. & Tandberg, E. Sleep disorders in patients with Parkinson’s disease:
epidemiology and management. _CNS Drugs_ 15, 267–275 (2001). Article CAS PubMed Google Scholar * Ferini-Strambi, L. & Zucconi, M. REM sleep behavior disorder. _Clin. Neurophysiol._
111, S136–S140 (2000). Article PubMed Google Scholar * Silvani, A. et al. Muscle activity during sleep in human subjects, rats, and mice: towards translational models of REM sleep without
atonia. _Sleep_ 40, https://doi.org/10.1093/sleep/zsx029 (2017). * Martinez-Martin, P. The importance of non-motor disturbances to quality of life in Parkinson’s disease. _J. Neurol. Sci._
310, 12–16 (2011). Article PubMed Google Scholar * Rolinski, M. et al. REM sleep behaviour disorder is associated with worse quality of life and other non-motor features in early
Parkinson’s disease. _J. Neurol. Neurosurg. Psychiatry_ 85, 560–566 (2014). Article PubMed Google Scholar * Laihinen, A., Alihanka, J., Raitasuo, S. & Rinne, U. K. Sleep movements and
associated autonomic nervous activities in patients with Parkinson’s disease. _Acta Neurol. Scand._ 76, 64–68 (1987). Article CAS PubMed Google Scholar * Lees, A. J., Blackburn, N. A.
& Campbell, V. L. The nighttime problems of Parkinson’s disease. _Clin. Neuropharmacol._ 11, 512–519 (1988). Article CAS PubMed Google Scholar * Chaudhuri, K. R. & Schapira, A.
H. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. _Lancet Neurol._ 8, 464–474 (2009). Article CAS PubMed Google Scholar * Postuma, R. B., Gagnon,
J. F. & Montplaisir, J. Rapid eye movement sleep behavior disorder as a biomarker for neurodegeneration: the past 10 years. _Sleep Med._ 14, 763–767 (2013). Article PubMed Google
Scholar * Maetzler, W., Liepelt, I. & Berg, D. Progression of Parkinson’s disease in the clinical phase: potential markers. _Lancet Neurol._ 8, 1158–1171 (2009). Article CAS PubMed
Google Scholar * Claassen, D. O. et al. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. _Neurology_ 75, 494–499 (2010). Article CAS
PubMed PubMed Central Google Scholar * Boeve, B. F. et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. _Brain_ 130, 2770–2788 (2007).
Article CAS PubMed Google Scholar * Krenzer, M., Lu, J., Mayer, G. & Oertel, W. From bench to bed: putative animal models of REM sleep behavior disorder (RBD). _J. Neural Transm.
(Vienna)_ 120, 683–688 (2013). Article Google Scholar * Low, P. S., Shank, S. S., Sejnowski, T. J. & Margoliash, D. Mammalian-like features of sleep structure in zebra finches. _Proc.
Natl Acad. Sci. USA_ 105, 9081–9086 (2008). Article CAS PubMed PubMed Central Google Scholar * Robert, C., Guilpin, C. & Limoge, A. Automated sleep staging systems in rats. _J.
Neurosci. Methods_ 88, 111–122 (1999). Article CAS PubMed Google Scholar * Vo, Q., Gilmour, T. P., Venkiteswaran, K., Fang, J. & Subramanian, T. Polysomnographic features of sleep
disturbances and REM sleep behavior disorder in the unilateral 6-OHDA lesioned hemiparkinsonian rat. _Parkinsons Dis._ 2014, 852965 (2014). PubMed PubMed Central Google Scholar * Aurora,
R. N. et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD). _J. Clin. Sleep Med._ 6, 85–95 (2010). PubMed PubMed Central Google Scholar * Tan, A., Salgado, M.
& Fahn, S. Rapid eye movement sleep behavior disorder preceding Parkinson’s disease with therapeutic response to levodopa. _Mov. Disord._ 11, 214–216 (1996). Article CAS PubMed
Google Scholar * Olanow, C. W. et al. Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled,
double-blind, double-dummy study. _Lancet Neurol._ 13, 141–149 (2014). Article CAS PubMed Google Scholar * Rascol, O., Perez-Lloret, S. & Ferreira, J. J. New treatments for
levodopa-induced motor complications. _Mov. Disord._ 30, 1451–1460 (2015). Article CAS PubMed Google Scholar * Saponjic, J. [Selective stimulations and lesions of the rat brain nuclei as
the models for research of the human sleep pathology mechanisms]. _Glas. Srp. Akad. Nauka Med_. 51, 85–97 (2011). * Fifel, K., Piggins, H. & Deboer, T. Modeling sleep alterations in
Parkinson’s disease: How close are we to valid translational animal models? _Sleep Med. Rev._ 25, 95–111 (2016). Article PubMed Google Scholar * McDowell, K. & Chesselet, M. F. Animal
models of the non-motor features of Parkinson’s disease. _Neurobiol. Dis._ 46, 597–606 (2012). Article PubMed PubMed Central Google Scholar * Gravotta, L., Gavrila, A. M., Hood, S.
& Amir, S. Global depletion of dopamine using intracerebroventricular 6-hydroxydopamine injection disrupts normal circadian wheel-running patterns and PERIOD2 expression in the rat
forebrain. _J. Mol. Neurosci._ 45, 162–171 (2011). Article CAS PubMed Google Scholar * Ben, V. & Bruguerolle, B. Effects of bilateral striatal 6-OHDA lesions on circadian rhythms in
the rat: a radiotelemetric study. _Life Sci._ 67, 1549–1558 (2000). Article CAS PubMed Google Scholar * Barraud, Q. et al. Sleep disorders in Parkinson’s disease: the contribution of the
MPTP non-human primate model. _Exp. Neurol._ 219, 574–582 (2009). Article CAS PubMed Google Scholar * Yi, P. L. et al. Interleukin-1beta mediates sleep alteration in rats with
rotenone-induced parkinsonism. _Sleep_ 30, 413–425 (2007). Article PubMed Google Scholar * McDowell, K. A. et al. Sleep alterations in an environmental neurotoxin-induced model of
parkinsonism. _Exp. Neurol._ 226, 84–89 (2010). Article CAS PubMed PubMed Central Google Scholar * Ekimova, I. et al. Changes in sleep characteristics of rat preclinical model of
Parkinson’s disease based on attenuation of the ubiquitin—proteasome system activity in the brain. _J. Evol. Biochem. Physiol._ 52, 463–474 (2016). Article CAS Google Scholar * Baier, P.
C. et al. Circadian distribution of motor-activity in unilaterally 6-hydroxy-dopamine lesioned rats. _Exp. Brain Res._ 169, 283–288 (2006). Article CAS PubMed Google Scholar * Valencia
Garcia, S. et al. Genetic inactivation of glutamate neurons in the rat sublaterodorsal tegmental nucleus recapitulates REM sleep behaviour disorder. _Brain_ 140, 414–428 (2017). Article
PubMed Google Scholar * Clement, O., Sapin, E., Berod, A., Fort, P. & Luppi, P. H. Evidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are
glutamatergic. _Sleep_ 34, 419–423 (2011). Article PubMed PubMed Central Google Scholar * Boissard, R. et al. The rat ponto-medullary network responsible for paradoxical sleep onset and
maintenance: a combined microinjection and functional neuroanatomical study. _Eur. J. Neurosci._ 16, 1959–1973 (2002). Article PubMed Google Scholar * Campbell, S. S. & Tobler, I.
Animal sleep: a review of sleep duration across phylogeny. _Neurosci. Biobehav. Rev._ 8, 269–300 (1984). Article CAS PubMed Google Scholar * Kudo, T., Loh, D. H., Truong, D., Wu, Y.
& Colwell, C. S. Circadian dysfunction in a mouse model of Parkinson’s disease. _Exp. Neurol._ 232, 66–75 (2011). Article PubMed Google Scholar * Winson, J. A simple sleep stage
detector for the rat. _Electroencephalogr. Clin. Neurophysiol._ 41, 179–182 (1976). Article CAS PubMed Google Scholar * Neuhaus, H. U. & Borbely, A. A. Sleep telemetry in the rat.
II. Automatic identification and recording of vigilance states. _Electroencephalogr. Clin. Neurophysiol._ 44, 115–119 (1978). Article CAS PubMed Google Scholar * Ruigt, G. S., Van
Proosdij, J. N. & Van Delft, A. M. A large scale, high resolution, automated system for rat sleep staging. I. Methodology and technical aspects. _Electroencephalogr. Clin. Neurophysiol._
73, 52–63 (1989). Article CAS PubMed Google Scholar * Bergmann, B. M., Winter, J. B., Rosenberg, R. S. & Rechtschaffen, A. NREM sleep with low-voltage EEG in the rat. _Sleep_ 10,
1–11 (1987). Article CAS PubMed Google Scholar * Gandolfo, G., Glin, L., Lacoste, G., Rodi, M. & Gottesmann, G. Automatic sleep-wake scoring in the rat on microcomputer APPLE II.
_Int. J. Biomed. Comput._ 23, 83–95 (1988). Article CAS PubMed Google Scholar * Gottesmann, C., Kirkham, P. A., LaCoste, G., Rodrigues, L. & Arnaud, C. Automatic analysis of the
sleep-waking cycle in the rat recorded by miniature telemetry. _Brain Res._ 132, 562–568 (1977). Article CAS PubMed Google Scholar * Sixel-Doring, F., Schweitzer, M., Mollenhauer, B.
& Trenkwalder, C. Intraindividual variability of REM sleep behavior disorder in Parkinson’s disease: a comparative assessment using a new REM sleep behavior disorder severity scale
(RBDSS) for clinical routine. _J. Clin. Sleep Med._ 7, 75–80 (2011). PubMed PubMed Central Google Scholar * Albin, R. L. et al. Decreased striatal dopaminergic innervation in REM sleep
behavior disorder. _Neurology_ 55, 1410–1412 (2000). Article CAS PubMed Google Scholar * Inglis, W. L. & Winn, P. The pedunculopontine tegmental nucleus: where the striatum meets the
reticular formation. _Prog. Neurobiol._ 47, 1–29 (1995). Article CAS PubMed Google Scholar * Lima, M. M., Andersen, M. L., Reksidler, A. B., Vital, M. A. & Tufik, S. The role of the
substantia nigra pars compacta in regulating sleep patterns in rats. _PLoS ONE_ 2, e513 (2007). Article PubMed PubMed Central CAS Google Scholar * Khanday, M. A., Yadav, R. K. &
Mallick, B. N. in _Dopamine and Sleep_ 1–17 (Springer, 2016). * Hutson, P. H., Sarna, G. S. & Curzon, G. Determination of daily variations of brain 5-hydroxytryptamine and dopamine
turnovers and of the clearance of their acidic metabolites in conscious rats by repeated sampling of cerebrospinal fluid. _J. Neurochem._ 43, 291–293 (1984). Article CAS PubMed Google
Scholar * DeLong, M. R. Primate models of movement disorders of basal ganglia origin. _Trends Neurosci._ 13, 281–285 (1990). Article CAS PubMed Google Scholar * Wichmann, T. &
DeLong, M. R. Functional and pathophysiological models of the basal ganglia. _Curr. Opin. Neurobiol._ 6, 751–758 (1996). Article CAS PubMed Google Scholar * Takakusaki, K., Saitoh, K.,
Harada, H., Okumura, T. & Sakamoto, T. Evidence for a role of basal ganglia in the regulation of rapid eye movement sleep by electrical and chemical stimulation for the pedunculopontine
tegmental nucleus and the substantia nigra pars reticulata in decerebrate cats. _Neuroscience_ 124, 207–220 (2004). Article CAS PubMed Google Scholar * Cho, J. R. et al. Dorsal raphe
dopamine neurons modulate arousal and promote wakefulness by salient stimuli. _Neuron_ 94, 1205–1219 e1208 (2017). Article CAS PubMed Google Scholar * Kim, Y. E. et al. REM sleep
behavior disorder: association with motor complications and impulse control disorders in Parkinson’s disease. _Parkinsonism Relat. Disord._ 20, 1081–1084 (2014). Article PubMed Google
Scholar * Arnaldi, D. et al. Nigro-caudate dopaminergic deafferentation: a marker of REM sleep behavior disorder? _Neurobiol. Aging_ 36, 3300–3305 (2015). Article PubMed Google Scholar *
Lee, J. E., Kim, K. S., Shin, H. W. & Sohn, Y. H. Factors related to clinically probable REM sleep behavior disorder in Parkinson disease. _Parkinsonism Relat. Disord._ 16, 105–108
(2010). Article PubMed Google Scholar * Sommerauer, M. et al. Revisiting the impact of REM sleep behavior disorder on motor progression in Parkinson’s disease. _Parkinsonism Relat.
Disord._ 20, 460–462 (2014). Article PubMed Google Scholar * Olanow, C. W. & Obeso, J. A. Pulsatile stimulation of dopamine receptors and levodopa-induced motor complications in
Parkinson’s disease: implications for the early use of COMT inhibitors. _Neurology_ 55, S72–S77 (2000). CAS PubMed Google Scholar * Obeso, J. A., Rodriguez-Oroz, M. C., Rodriguez, M.,
DeLong, M. R. & Olanow, C. W. Pathophysiology of levodopa-induced dyskinesias in Parkinson’s disease: problems with the current model. _Ann. Neurol._ 47, S22–S32 (2000). CAS PubMed
Google Scholar * Nutt, J. G., Obeso, J. A. & Stocchi, F. Continuous dopamine-receptor stimulation in advanced Parkinson’s disease. _Trends Neurosci._ 23, S109–S115 (2000). Article CAS
PubMed Google Scholar * Nunes Junior, G. P., Tufik, S. & Nobrega, J. N. Autoradiographic analysis of D1 and D2 dopaminergic receptors in rat brain after paradoxical sleep
deprivation. _Brain Res. Bull._ 34, 453–456 (1994). Article CAS PubMed Google Scholar * Tufik, S., Lindsey, C. J. & Carlini, E. A. Does REM sleep deprivation induce a
supersensitivity of dopaminergic receptors in the rat brain? _Pharmacology_ 16, 98–105 (1978). Article CAS PubMed Google Scholar * Tufik, S. Changes of response to dopaminergic drugs in
rats submitted to REM-sleep deprivation. _Psychopharmacology (Berl.)_ 72, 257–260 (1981). Article CAS Google Scholar * Cruse, B. et al. 24-hour levodopa-carbidopa intestinal gel may
reduce troublesome dyskinesia in advanced Parkinson’s disease. _NPJ Parkinsons Dis._ 4, 34 (2018). Article PubMed PubMed Central CAS Google Scholar * Ricciardi, L. et al. 24-Hour
infusion of levodopa/carbidopa intestinal gel for nocturnal akinesia in advanced Parkinson’s disease. _Mov. Disord._ 31, 597–598 (2016). Article PubMed Google Scholar * Chaudhuri, K. R.
The basis for day and night-time control of symptoms of Parkinson’s disease. _Eur. J. Neurol._ 9, 40–43 (2002). Article PubMed Google Scholar * Medcalf, P. Good practice in the assessment
and management of nocturnal Parkinson’s disease symptoms. _Age Ageing_ 34, 435–438 (2005). Article PubMed Google Scholar * Leeman, A. L. et al. Parkinson’s disease in the elderly:
response to and optimal spacing of night time dosing with levodopa. _Br. J. Clin. Pharm._ 24, 637–643 (1987). Article CAS Google Scholar * Trotti, L. M. & Bliwise, D. L. Treatment of
the sleep disorders associated with Parkinson’s disease. _Neurotherapeutics_ 11, 68–77 (2014). Article CAS PubMed Google Scholar * Wang, Y. et al. Effects of rotigotine on REM sleep
behavior disorder in parkinson disease. _J. Clin. Sleep Med._ 12, 1403–1409 (2016). Article PubMed PubMed Central Google Scholar * Chen, J. J., Swope, D. M., Dashtipour, K. & Lyons,
K. E. Transdermal rotigotine: a clinically innovative dopamine-receptor agonist for the management of Parkinson’s disease. _Pharmacotherapy_ 29, 1452–1467 (2009). Article CAS PubMed
Google Scholar * Metman, L. V. et al. Continuous transdermal dopaminergic stimulation in advanced Parkinson’s disease. _Clin. Neuropharmacol._ 24, 163–169 (2001). Article CAS PubMed
Google Scholar * Pahwa, R. et al. Ropinirole 24-hour prolonged release: randomized, controlled study in advanced Parkinson disease. _Neurology_ 68, 1108–1115 (2007). Article CAS PubMed
Google Scholar * Ray Chaudhuri, K. et al. Improvements in nocturnal symptoms with ropinirole prolonged release in patients with advanced Parkinson’s disease. _Eur. J. Neurol._ 19, 105–113
(2012). Article CAS PubMed Google Scholar * Zibetti, M. et al. Sleep improvement with levodopa/carbidopa intestinal gel infusion in Parkinson disease. _Acta Neurol. Scand._ 127, e28–e32
(2013). Article CAS PubMed Google Scholar * Mulas, G. et al. Differential induction of dyskinesia and neuroinflammation by pulsatile versus continuous l-DOPA delivery in the 6-OHDA model
of Parkinson’s disease. _Exp. Neurol._ 286, 83–92 (2016). Article CAS PubMed Google Scholar * De Cock, V. C. et al. Restoration of normal motor control in Parkinson’s disease during REM
sleep. _Brain_ 130, 450–456 (2007). Article PubMed Google Scholar * Luppi, P. H. et al. in _Disorders of Sleep and Circadian Rhythms in Parkinson’s Disease_ (eds Birgit, H. &
Videnovic, A.) 35–49 (Springer, Vienna), https://doi.org/10.1007/978-3-7091-1631-9. Google Scholar * Ungerstedt, U. & Arbuthnott, G. W. Quantitative recording of rotational behavior in
rats after 6-hydroxy-dopamine lesions of the nigrostriatal dopamine system. _Brain Res._ 24, 485–493 (1970). Article CAS PubMed Google Scholar * Ungerstedt, U. & Herrera-Marschitz,
M. in _Chemical Neurotransmission: 75 years_ (eds Stjärne, L. et al.) 481–494 (Academic Press, 1981). * Gilmour, T. P., Fang, J., Guan, Z. & Subramanian, T. Manual rat sleep
classification in principal component space. _Neurosci. Lett._ 469, 97–101 (2010). Article CAS PubMed Google Scholar * Gilmour, T. P. et al. The effect of striatal dopaminergic grafts on
the neuronal activity in the substantia nigra pars reticulata and subthalamic nucleus in hemiparkinsonian rats. _Brain_ 134, 3276–3289 (2011). Article PubMed PubMed Central Google
Scholar * Shen, H., Kannari, K., Yamato, H., Arai, A. & Matsunaga, M. Effects of benserazide on L-DOPA-derived extracellular dopamine levels and aromatic L-amino acid decarboxylase
activity in the striatum of 6-hydroxydopamine-lesioned rats. _Tohoku J. Exp. Med._ 199, 149–159 (2003). Article CAS PubMed Google Scholar * Jonkers, N., Sarre, S., Ebinger, G. &
Michotte, Y. Benserazide decreases central AADC activity, extracellular dopamine levels and levodopa decarboxylation in striatum of the rat. _J. Neural Transm. (Vienna)_ 108, 559–570 (2001).
Article CAS Google Scholar * Nakashima, M. et al. In vivo microdialysis to determine the relative pharmacokinetics of drugs. _Biol. Pharm. Bull._ 19, 988–994 (1996). Article CAS PubMed
Google Scholar * Di Stefano, A. et al. Evaluation of rat striatal L-dopa and DA concentration after intraperitoneal administration of L-dopa prodrugs in liposomal formulations. _J.
Control. Release_ 99, 293–300 (2004). Article PubMed CAS Google Scholar * Lindgren, H. S., Andersson, D. R., Lagerkvist, S., Nissbrandt, H. & Cenci, M. A. L-DOPA-induced dopamine
efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: temporal and quantitative relationship to the expression of dyskinesia. _J. Neurochem._ 112, 1465–1476
(2010). Article CAS PubMed Google Scholar * Sarre, S. et al. High-performance liquid chromatography with electrochemical detection for the determination of levodopa, catecholamines and
their metabolites in rat brain dialysates. _J. Chromatogr._ 575, 207–212 (1992). Article CAS PubMed Google Scholar * Woodlee, M. T. et al. Testing forelimb placing “across the midline”
reveals distinct, lesion-dependent patterns of recovery in rats. _Exp. Neurol._ 191, 310–317 (2005). Article PubMed Google Scholar * Lieu, C. A., Kunselman, A. R., Manyam, B. V.,
Venkiteswaran, K. & Subramanian, T. A water extract of Mucuna pruriens provides long-term amelioration of parkinsonism with reduced risk for dyskinesias. _Parkinsonism Relat. Disord._
16, 458–465 (2010). Article PubMed PubMed Central Google Scholar * Abercrombie, M. Estimation of nuclear population from microtome sections. _Anat. Rec._ 94, 239–247 (1946). Article CAS
PubMed Google Scholar Download references ACKNOWLEDGEMENTS The authors thank Erin Handly and Timothy Gilmour for their technical assistance and Danielle Panoz-Brown for useful comments
during the manuscript preparation. This work was supported in part by research grants from the National Institutes of Health National Institute of Neurological Disorders and Stroke (NINDS)
R01NS42402, National Center for Complementary and Alternative Medicine (NCCAM) R21AT001607, Health Resources and Services Administration DIBTH0632, Grace Woodward Fund, Anne M and Phillip
Gladfelter III Foundation and the Pennsylvania Tobacco Settlement Funds Biomedical Research Grant to T.S., Barsumian Trust Grant to K.V. and the American Parkinson’s Disease Association
Grant to Q.V. Additional funding was provided by Penn State University Brain Repair Research Fund. AUTHOR INFORMATION Author notes * These authors contributed equally: Vishakh Iyer, Quynh
Vo, Anthony Mell AUTHORS AND AFFILIATIONS * Program in Neuroscience, Department of Psychological and Brain Sciences, Indiana University, Bloomington, IN, USA Vishakh Iyer * Department of
Neurology, West Virginia University School of Medicine, Morgantown, WV, USA Quynh Vo * Department of Neurology and Neural and Behavioral Sciences, The Pennsylvania State University College
of Medicine, Hershey, PA, USA Anthony Mell, Siven Chinniah, Ashley Zenerovitz, Kala Venkiteswaran & Thyagarajan Subramanian * Department of Public Health Sciences, The Pennsylvania State
University College of Medicine, Hershey, PA, USA Allen R. Kunselman * Department of Psychiatry, The Pennsylvania State University College of Medicine, Hershey, PA, USA Jidong Fang Authors *
Vishakh Iyer View author publications You can also search for this author inPubMed Google Scholar * Quynh Vo View author publications You can also search for this author inPubMed Google
Scholar * Anthony Mell View author publications You can also search for this author inPubMed Google Scholar * Siven Chinniah View author publications You can also search for this author
inPubMed Google Scholar * Ashley Zenerovitz View author publications You can also search for this author inPubMed Google Scholar * Kala Venkiteswaran View author publications You can also
search for this author inPubMed Google Scholar * Allen R. Kunselman View author publications You can also search for this author inPubMed Google Scholar * Jidong Fang View author
publications You can also search for this author inPubMed Google Scholar * Thyagarajan Subramanian View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS V.I., Q.V., A.M., J.F. and T.S. conceptualized and designed the experiments; V.I., Q.V., A.M., K.V. and J.F. performed the experiments; V.I., Q.V., A.M., S.C., A.Z., K.V. and
A.R.K. analyzed the data; V.I., Q.V., A.M., A.R.K., J.F. and T.S. interpreted the results of the experiments; V.I. and Q.V. prepared the figures; V.I., Q.V. and T.S. drafted and edited the
manuscript; all authors approved the final version of manuscript. CORRESPONDING AUTHOR Correspondence to Thyagarajan Subramanian. ETHICS DECLARATIONS COMPETING INTERESTS T.S. is a speaker
board member and scientific advisory board member at Teva, Acadia, Acorda, Adamas, USMedWorlds, and Neurocrine. He has received research funding for clinical trials in which he served as
site PI from Intec, Adamas, Allergan, Pharma 2B, and Eli Lily. These activities do not represent any competing interests regarding the publication of this paper. The remaining authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION FILE SUPPLEMENTARY VIDEO 1 REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Iyer, V., Vo, Q., Mell, A. _et al._ Acute levodopa dosing around-the-clock
ameliorates REM sleep without atonia in hemiparkinsonian rats. _npj Parkinsons Dis._ 5, 27 (2019). https://doi.org/10.1038/s41531-019-0096-2 Download citation * Received: 15 April 2019 *
Accepted: 21 October 2019 * Published: 29 November 2019 * DOI: https://doi.org/10.1038/s41531-019-0096-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Bbc axes 'most perfect film ever' and fans have just days to watchDevotees of the film will be disheartened to discover that it's bidding farewell to the BBC, with only a few days r...
Surrey town named 'best place in england' for new businessesGuildford has been named England's best place for new businesses according to new data, and has managed to beat out...
The better sister finale unveils who killed adam with twist in last scenes_WARNING: THIS ARTICLE CONTAINS MAJOR SPOILERS FROM THE BETTER SISTER. _ The gripping series The Better Sister has been ...
'i'm a gastroenterologist - this is the best time for your first bowel movement'A gastroenterologist has suggested the best time for someone to have their first bowel movement of the day as awareness ...
Fans just discovered how old iconic film that 'defined childhoods' isFans of the beloved Pixar classic Finding Nemo may be surprised to learn that the Oscar-winning movie has reached a mile...
Latests News
Acute levodopa dosing around-the-clock ameliorates rem sleep without atonia in hemiparkinsonian ratsABSTRACT Rapid-eye-movement (REM) sleep without atonia (RSWA), a marker of REM sleep behavior disorder (RBD), is frequen...
How should science funders deal with sexual harassers?US science agencies threaten harsh penalties, but many have yet to take action. Access through your institution Buy or s...
Biospheric works | NatureARTICLE PDF AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Unesco, Paris, France M. BATISSE Authors * M. BATISSE View aut...
Check Please! South Florida | Spaghetto Factory | Season 15 | Episode 10 | PBSCheck Please! South FloridaSpaghetto FactoryClip: Season 15 Episode 10 | 8m 31sVideo has Closed Captions | CCMichelle Be...
Editorial: why is kevin de león trying to stall a transit- and climate-friendly project in eagle rock?In a city trying to reinvent its streets to be more walkable and bike- and transit-friendly and environmentally sustaina...