Integrating whole-genome sequencing within the national antimicrobial resistance surveillance program in the philippines
Integrating whole-genome sequencing within the national antimicrobial resistance surveillance program in the philippines"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT National networks of laboratory-based surveillance of antimicrobial resistance (AMR) monitor resistance trends and disseminate these data to AMR stakeholders. Whole-genome
sequencing (WGS) can support surveillance by pinpointing resistance mechanisms and uncovering transmission patterns. However, genomic surveillance is rare in low- and middle-income
countries. Here, we implement WGS within the established Antimicrobial Resistance Surveillance Program of the Philippines via a binational collaboration. In parallel, we characterize
bacterial populations of key bug-drug combinations via a retrospective sequencing survey. By linking the resistance phenotypes to genomic data, we reveal the interplay of genetic lineages
(strains), AMR mechanisms, and AMR vehicles underlying the expansion of specific resistance phenotypes that coincide with the growing carbapenem resistance rates observed since 2010. Our
results enhance our understanding of the drivers of carbapenem resistance in the Philippines, while also serving as the genetic background to contextualize ongoing local prospective
surveillance. SIMILAR CONTENT BEING VIEWED BY OTHERS WHOLE GENOME SEQUENCING ANALYSIS OF_ MYCOBACTERIUM TUBERCULOSIS_ REVEALS CIRCULATING STRAIN TYPES AND DRUG-RESISTANCE MUTATIONS IN THE
PHILIPPINES Article Open access 23 August 2024 HARMONISATION OF IN-SILICO NEXT-GENERATION SEQUENCING BASED METHODS FOR DIAGNOSTICS AND SURVEILLANCE Article Open access 23 August 2022
NATIONAL GENOMIC SURVEILLANCE INTEGRATING STANDARDIZED QUANTITATIVE SUSCEPTIBILITY TESTING CLARIFIES ANTIMICROBIAL RESISTANCE IN ENTEROBACTERALES Article Open access 05 December 2023
INTRODUCTION Antimicrobial resistance (AMR) is an increasingly serious threat to global public health and the economy that requires concerted action across countries, government sectors and
non-government organizations1. Without AMR containment, an adverse impact on medical costs, global gross domestic product, livestock production and international trade is expected by 2050,
and the sustainable development goals for 2030 are less likely to be attained2. The Global Action Plan developed by the World Health Organization (WHO) to tackle AMR highlights the need to
strengthen our understanding of how resistance develops and spreads, and the underlying resistance mechanisms3. One of the pillars of this objective is the national surveillance system for
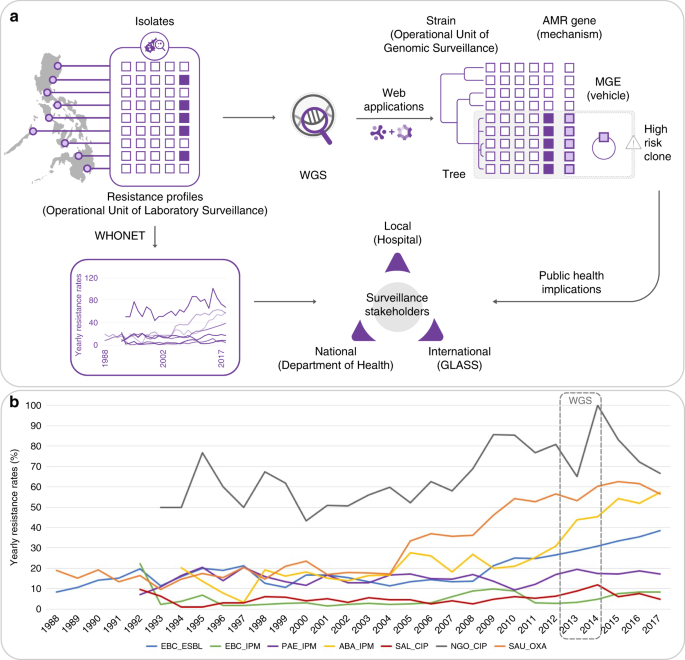
AMR. An advanced example of a national surveillance system is the Antimicrobial Resistance Surveillance Program (ARSP), the national laboratory-based surveillance of the Philippine
Department of Health (DOH). The ARSP began in 1988 with eight hospital-based laboratories (sentinel sites) and the Antimicrobial Resistance Surveillance Reference Laboratory (ARSRL) in Metro
Manila and has since expanded to 24 sentinel sites and two gonorrhoea surveillance sites in 16 regions of the country (Supplementary Fig. 1). Surveillance encompasses common pathogens of
public health importance in the Philippines, where infectious diseases represented nine out of the ten leading causes of morbidity4, and four out of the ten leading causes of infant
mortality5 in 2013–2014. Results of culture-based identification and antimicrobial susceptibility are stored centrally at the ARSRL within the clinical microbiology management software
WHONET (Fig. 1a6). A report summarizing the resistance trends is published annually and disseminated to local, national and international surveillance stakeholders7. For policy change,
surveillance data generated by ARSP are used by the DOH to develop clinical practice guidelines and determine the panel of antibiotics to be included in the national formulary. Furthermore,
the ARSP has been contributing data to international AMR surveillance programmes since the 1980s, including the Global Antimicrobial Surveillance System (GLASS)8 since 2015. Importantly, the
ARSP data has informed the Philippine National Action Plan to combat AMR, one of the key requirements for alignment with the WHO Global Action Plan. The Philippines has seen a steady
increase in resistance rates for several key pathogen–antibiotic combinations in the last 10 years, including carbapenem-resistant organisms (Fig. 1b). The genetic mechanisms underlying
carbapenem resistance, one of the biggest therapeutic challenges in the treatment of antimicrobial-resistant infections, include increased upregulation of efflux pumps, decreased uptake by
altered expression/loss of porin function, and acquisition of hydrolytic enzymes—carbapenemases9. Whole-genome sequencing (WGS) of bacterial pathogens can identify distinct clonal lineages
(strains) on phylogenetic trees, known AMR mechanisms (genes or mutations) and the vehicles for the acquired AMR genes (mobile genetic elements, MGEs) which, in turn, enables enhanced
detection and characterization of high-risk clones (Fig. 1a). WGS is routinely used for infectious disease epidemiology in several high-income countries around the world, where it has
improved outbreak investigations and epidemiological surveillance10,11 and enhanced our knowledge of the spread of antimicrobial-resistant strains and their resistance mechanisms12,13.
Elucidation of resistance mechanisms and their context can be critical for effective infection control. For example, upregulation of efflux pumps and loss of porin function by mutation are
vertically transmitted, while acquired carbapenemases carried in transmissible plasmids or integrative conjugative elements have the potential for horizontal dissemination between strains
and species, thus necessitating enhanced infection control measures14. Moreover, diverse carbapenemase classes, variants, flanking MGEs and associated plasmid incompatibility groups have
been described, requiring multiple biochemical and molecular tests for identification. For example, the class B New Delhi metallo-beta-lactamase (NDM) is found worldwide, represented by over
ten different variants that are usually upstream of intact or truncated IS_Aba125_ elements, within plasmid backbones of different incompatibility groups, across multiple lineages of _E.
coli_—as well as of other carbapenem-resistant organisms15,16. WGS can determine the interplay between these different components, i.e. the gene, the vehicle and the strain, thus maximizing
the epidemiological benefit derived from its cost. Implementation of WGS within existing national surveillance systems in low- and middle-income countries (LMICs) has the potential to
enhance local prevention and control of resistant infections in a sustainable and equitable manner. Integration of WGS into routine surveillance of AMR can be facilitated by international
collaboration focused on transfer of expertize and ownership. Our collaboration (See and Sequence, Fig. 1a) aimed to implement WGS within the ARSP via a multi-faceted approach that included
a large initial retrospective sequencing survey, technology transfer, utilization of user-friendly web applications and capacity building in laboratory and bioinformatic procedures for local
prospective sequencing. We linked what has traditionally been used as the operational unit of laboratory surveillance, the resistance profile (RP), with the operational unit of genomic
epidemiology now provided by WGS, genetic relatedness. Here we provide exemplars from the retrospective sequencing survey that highlight how the granular view of strain-gene-vehicle in
carbapenem-resistant populations at the local, national and global operational scales can be leveraged for surveillance of AMR and public health. RESULTS INTEGRATION OF LABORATORY AND
GENOMIC SURVEILLANCE The Philippine ARSP collects bacterial isolates and stores the associated clinical and epidemiological data in the WHONET software6. Antibiograms are stored as RPs,
which represent the diversity of AMR phenotypes in the country. We implemented WGS within the ARSP via ongoing technology transfer, capacity building in and binational collaboration. In
parallel, we conducted a large retrospective sequencing survey to characterize bacterial populations, dissect resistance phenotypes of key bug–drug combinations and provide genomic context
for local prospective surveillance. Here, we focus on the carbapenem-resistant organisms, which have been classified as of critical priority for the development of new antibiotics by the
WHO. OPERATIONAL UNIT OF LABORATORY SURVEILLANCE—INCREASING THE BURDEN OF SPECIFIC RESISTANCE PHENOTYPES Carbapenem-resistant _P. aeruginosa, A. baumannii, K. pneumoniae_ and _E. coli_ were
all first isolated by the ARSP between 1992 and 1994. Since the year 2000, resistance rates to imipenem and meropenem have remained below 30% for all organisms except for _A. baumannii_,
which has seen a steady rise in resistance rates between 2009 and 2017, reaching 56% (Fig. 2a). This coincides with the expansion of two RPs, first a striking expansion of true extremely
drug-resistant (XDR) and possible pan drug-resistant (PDR) RP-1, and later of true-XDR RP-2 (full profiles can be found in Fig. 2b). Yearly resistance rates for _P. aeruginosa_ have
oscillated between 10 and 25% since the year 2000, and have doubled between 2010 and 2013 (Fig. 2a). Two RPs have seen the largest combined expansion between 2011 and 2013, possible-PDR RP-3
and true-XDR RP-4 (Fig. 2b). The yearly resistance rates to imipenem for Enterobacteriaceae were ~3% in 2011 but have since steadily increased to 5% for _E. coli_ and 11% for _K.
pneumoniae_, respectively (Fig. 2a). This coincides with the expansion of three possible-XDR RPs, RP-5, RP-6 and RP-7 (Fig. 2b). The observed expansion of specific RPs could be driven by the
rapid dissemination of a discrete genetic lineage (strain) carrying AMR determinants or, alternatively, of transmissible MGEs, which shuttle resistance determinants across different genetic
lineages. OPERATIONAL UNIT OF GENOMIC SURVEILLANCE—GENETIC DIVERSITY AND MECHANISMS UNDERPINNING CARBAPENEM RESISTANCE PHENOTYPES The sequences of 805 genomes linked to WHONET data were
obtained from isolates belonging to the four bacterial pathogens in the WHO critical list (Table 1), which were collected in 2013 and 2014 by between 13 and 18 ARSP sites. The isolates
sequenced were non-susceptible to carbapenems and/or to extended-spectrum cephalosporins (Table 1), with the exception of _P. aeruginosa_, for which susceptible isolates were available and
also sequenced. The distribution of the carbapenem RPs highlighted in Fig. 2 was not concordant with the major clades observed on the phylogenetic trees (Fig. 3). Instead, the same RP was
usually observed in multiple genetic lineages across the tree. Yet, the distribution of the multi-locus sequence types (STs), a molecular genotyping method inferred from the sequences of
seven house-keeping genes, was largely concordant with the major clades observed on the trees inferred from whole-genome single-nucleotide polymorphisms (SNPs) (Fig. 3), confirming that the
homoplasic distribution of RPs was not an artefact of the tree topology. The distribution of the same carbapenem RPs across multiple genetic lineages was observed both at the national and
local levels (Supplementary Fig. 2), as the number of ST assignments increased with the number of isolates representing a RP. Thus, the genomic data suggest that the expansion of specific
carbapenem RPs in the Philippines is driven by the horizontal dissemination of resistance determinants across diverse genetic backgrounds, rather than by the sole expansion of single
resistant genetic clones, and that resistance to multiple antibiotics is acquired en bloc via MGEs, as previously observed for carbapenem-resistant organisms16. Carbapenemases are a diverse
collection of hydrolytic enzymes16, and we identified representatives of classes A, B and C carbapenemases in the Philippines (Supplementary Fig. 3). Class B NDM-1 and Verona integron-borne
metallo-beta-lactamase VIM-2 were the most prevalent and geographically disseminated in the Enterobacteriaceae and _P. aeruginosa_, respectively, while the same was true for class D OXA-23
in _A. baumannii_. Importantly, different carbapenemase genes/variants (or combinations thereof) were found underlying the same RP in all four organisms (Fig. 3). LOCAL SCALE OF
SURVEILLANCE—PLASMID-DRIVEN HOSPITAL OUTBREAK OF CARBAPENEM-RESISTANT _K. PNEUMONIAE_ Fifty-seven percent (_N_ = 33) of the _K. pneumoniae_ genomes with the possible-XDR resistance phenotype
AMP FOX CAZ CRO FEP IPM AMC TZP GEN AMK CIP SXT (RP-6) were isolated from a single hospital (MMH) but belonged to 12 different STs, with almost half of them (_N_ = 15) placed within ST340
(Supplementary Fig. 4a). The phylogenetic analysis of these 15 genomes in the wider context of ST340 showed three main lineages, with the 15 possible-XDR isolates from MMH forming a tight
cluster (clade III, Fig. 4a). The shorter branch lengths in the imipenem-resistant clade III (6.5 ± 3.7 pairwise SNP differences) compared with the imipenem-susceptible clade II (26.8 ± 6.9
pairwise SNP differences, Mann–Whitney _U_ test _z_-score −6.265, _p_ value = 3.71 × 10−10, Supplementary Fig. 4b), and the isolation dates spanning 12 months, suggest a rapid expansion of
this clone, which coincides with the acquisition of the _bla_NDM-1 gene (Fig. 4a). Epidemiological data showed that the samples were mostly from hospital-acquired infections (HAI, _N_ = 14)
and from neonates (_N_ = 12) (Fig. 4a). These observations triggered a retrospective epidemiological investigation that revealed that ten of the isolates originated from patients of the
neonatal intensive care unit (NICU), all of which had umbilical catheters and were on mechanical ventilators. The remaining cases were either from paediatric wards (_N_ = 4) or the male ward
(_N_ = 1), suggesting wider dissemination of this high-risk clone within the hospital environment. The genetic diversity underlying the 33 possible-XDR isolates from MMH (12 STs) prompted
us to investigate the hypothesis of dissemination of carbapenem resistance in the hospital by a plasmid carrying _bla_NDM-1. We identified a 101,540 bp IncFII plasmid with _bla_NDM-1, _rmtC_
and _sul1_ (p13ARS_MMH0112-3, Table 2, Supplementary Note 1 and Supplementary Fig. 5a, b) in a representative isolate from ST340 clade III. We found the entire p13ARS_MMH0112-3 plasmid
sequence (i.e., ≥95% of the length) represented in 27 genomes from 9 different STs, including 20 samples from 6 different STs isolated from patients under 1 year old (Fig. 4b). A second
plasmid identified in the representative isolate carried 13 AMR genes, including the extended-spectrum beta-lactamase gene _bla_CTX-M-15 (p13ARS_MMH0112-2, Table 2, Supplementary Note 2 and
Supplementary Fig. 5a, c). Altogether, our findings suggest that the burden of carbapenem-resistant _K. pneumoniae_ infections in hospital MMH was largely linked to plasmid p13ARS_MMH0112-3
with _bla_NDM-1, circulating within diverse genetic lineages, and leading to outbreaks in high-risk patient populations. Hospital authorities were informed of these findings, and measures
for infection control were implemented, including designating a separate multi-drug resistance organism room for cohorting, active surveillance upon identification of any new
carbapenem-resistant _K. pneumoniae_ from the NICU, and referral of any new carbapenem-resistant _K. pneumoniae_ from the NICU to ARSRL for sequencing. NATIONAL SCALE OF
SURVEILLANCE—REGIONAL CIRCULATION OF A SUCCESSFUL _K. PNEUMONIAE_ LINEAGE The possible-XDR resistance phenotype AMP FOX CAZ CRO FEP IPM AMC TZP CIP SXT (RP-5) was represented by 76 _K.
pneumoniae_ isolates from 14 different STs. Seventy-one percent (_N_ = 54) belonged to ST147, an international epidemic clone that was found in 11 sentinel sites in all three island groups
in the Philippines. The phylogeny of the ST147 genomes showed that carbapenem non-susceptible isolates (78.8%, _N_ = 63) were found in clades arising from three out of four deep branches of
the tree (Fig. 5), which represent separate groups of the global population (Supplementary Note 3 and Supplementary Fig. 6). Carbapenem resistance was largely explained by the presence of
_bla_NDM-1 in clade IV genomes, while clade III-B showed geographically distinct clusters with either _bla_NDM-1 or _bla_NDM-7 (Fig. 5, Supplementary Note 4 and Supplementary Fig. 7). Five
plasmid sequences obtained from five different isolates representing carbapenem-resistant clusters in clades III and IV showed that the NDM genes were carried within different variants of
the insertion sequence IS_Aba125_ (Supplementary Fig. 8a) on different plasmid backbones, two of which showed high sequence similarity to international plasmids (Table 3 and Supplementary
Figs. 5b and 8b). The distribution of plasmids harbouring _bla_NDM-1 and _bla_NDM-7 matched the strong phylogeographic signal in the terminal branches of the tree (Fig. 5). Within clade
III-B, a cluster of genomes from sentinel sites MMH and STU was characterized by plasmid p14ARS_MMH0055-5 carrying _bla_NDM-7, while another cluster of genomes from VSM and NMC was
distinguished by plasmid p13ARS_VSM0593-1 with _bla_NDM-1. Plasmids p13ARS_GMH0099 and p14ARS_VSM0843-1, both carrying _bla_NDM-1, were found in different subclades of clade IV, one of local
circulation in hospital VSM, and another one showing regional expansion across five sentinel sites (Fig. 5). Taken together, the phylogeographic signal (strains) in combination with the
distribution of _bla_NDM variants (mechanism) and plasmids (vehicle) revealed local and regional patterns of circulation of carbapenem non-susceptible ST147 within the Philippines, which
could not be identified based solely on the RPs and MLST information. Furthermore, comparison with global ST147 genomes indicated that clade IV may be unique to the Philippines
(Supplementary Fig. 6). INTERNATIONAL SCALE OF SURVEILLANCE—HIGH-RISK CLONE OF _E. COLI_ ST410 WITH NDM-1 AND OXA-181 Recent reports of _E. coli_ ST410 international high-risk clones
carrying class D and class B carbapenemases17,18 are particularly alarming in light of their wide geographic distribution and the broad variety of niches this clone can occupy19,20. ST410
was the second most prevalent ST in the retrospective collection of _E. coli_ (13.2%, _N_ = 24), encompassing a large proportion of imipenem-resistant isolates (_N_ = 15), and the three
expanding possible-XDR RP-5,6,7 (Supplementary Fig. 9). The phylogenetic tree of 24 ST410 genomes showed that isolates with the possible-XDR profiles clustered within one clade (Fig. 6a),
which can be further delineated by the distribution of carbapenemase genes and variants into three different clones carrying _bla_NDM-4 (_N_ = 1), _bla_NDM-1 (_N_ = 8) or both _bla_NDM-1 and
_bla_OXA-181 (_N_ = 5). The phylogenetic analysis with global _E. coli_ ST410 genomes confirmed that these are independent clones, as they were found interspersed with international genomes
within a major high-risk lineage (Fig. 6b and Supplementary Note 5). _K. pneumoniae_ carrying _bla_NDM-1 and _bla_OXA-181 have been previously reported21,22, as well as _E. coli_ ST410
harbouring _bla_NDM-5 and _bla_OXA-181 from Egypt23, Denmark and the UK18, but, to our knowledge, this is the first report of _E. coli_ carrying both _bla_NDM-1 and _bla_OXA-181, which is
likely to have disseminated between two sentinel sites (NMC and VSM). We identified five plasmids in the representative strain 14ARS_NMC0074 (Table 4 and Supplementary Fig. 10). The IncX3
plasmid carrying _bla_OXA-181 (p14ARS_NMC0074-5) was identical to plasmid pAMA1167-OXA-181 isolated previously from an _E. coli_ ST410 strain24 (Supplementary Fig. 10). Mapping short reads
to p14ARS_NMC0074-5 showed that this plasmid is the main vehicle of _bla_OXA-181 in the Philippines, as in the international genomes (Fig. 6b18). However, we did not identify any known
plasmids similar to the IncA/C2 plasmid with _bla_NDM-1 (p14ARS_NMC0074-2), though it shared ~90% of its backbone with the IncA/C2 plasmid described above from _K. pneumoniae_ ST147 strain
13ARS_VSM0593 (Supplementary Fig. 10). This plasmid backbone was found in _E. coli_ ST410 genomes from the Philippines, but not in international genomes (Fig. 6b). Altogether, our results
show that that the Philippine _E. coli_ ST410 genomes represent diverse lineages of the global circulating population, with evidence of frequent international introductions. These lineages
are characterized by a diverse repertoire of carbapenemase genes and variants, amassed via a combination of conserved international plasmids and locally circulating plasmids. DISCUSSION
National networks of laboratory-based surveillance are a key pillar within the Global Action Plan to combat AMR, with the reference laboratory playing a central role in the dissemination of
surveillance data to local, national and international stakeholders. The Philippines ARSP surveillance data have shown increasing resistance trends for key bug–drug combinations (Figs. 1b
and 2a). In this study, the analysis of the dynamics of RPs showed the concomitant expansion of specific resistance phenotypes (Fig. 2b). By complementing laboratory data with WGS and
linking the operational units of laboratory and genomic surveillance, we revealed a diversity of genetic lineages, AMR mechanisms and vehicles underlying the expansion of carbapenem
resistance phenotypes (Figs. 1a and 3). The combination of these three components vastly improved our understanding of the drivers of carbapenem resistance in the Philippines at different
geographical scales, from an plasmid-driven local outbreak of _K. pneumoniae_ ST340 (Fig. 4), to the expansion of a _K. pneumoniae_ ST147 clone carrying NDM-1 in an IncA/C2 plasmid without
any close match in international genomes (Fig. 5), to the independent introductions of international epidemic clone _E. coli_ ST410 that can acquire plasmids of local circulation (Fig. 6). A
crucial aspect of AMR surveillance is the detection of the emergence and spread of high-risk clones. In traditional laboratory surveillance, RPs can be analyzed with spatiotemporal
algorithms to detect hospital outbreaks25 or dissemination of phenotypic subpopulations between hospitals26. Cluster detection based on RPs depends on consistent and complete antibiograms
within and across sentinel sites. During the course of our project it became apparent that there were inconsistencies in the panels of antibiotics tested by the sentinel sites (missing data
or different antibiotics tested), which led to the exclusion of samples from the analysis of the dynamics of RPs (Fig. 2). These inconsistencies would also hamper cluster detection based on
RPs, or the early detection of novel emerging RPs that could act as indicators of novel high-risk clones. The joint discussions of these observations by the two collaborating teams led to an
effort to reinforce the standardization of antibiotic testing across the ARSP sentinel sites, which was coordinated by the ARSRL. Even with complete and comprehensive susceptibility
testing, RPs can only provide a coarse view of the spread of AMR, and sometimes lead to clusters of disparate genetic relatedness (Fig. 3 and Supplementary Fig. 2). Integration of WGS into
laboratory-based AMR surveillance can substantially improve the detection of high-risk clones by providing a high-resolution picture of genetic lineages (strains), AMR mechanisms
(genes/mutations) and vehicles (MGEs, Fig. 1a). We identified high-risk clones within known international epidemic clones at the local, national and international scales by linking clonal
relatedness, geographic clustering, epidemiological data and gene and plasmid content with interactive web tools27. At the local scale, we identified a plasmid-driven NICU outbreak of
carbapenem-resistant _K. pneumoniae_. The epidemiological data captured in WHONET was key to support the interpretation of the genomic findings, triggering a retrospective investigation.
This previously undetected outbreak was traced to an IncFII plasmid carrying _bla_NDM-1 in the genetic context of ST340 (Fig. 4a). Yet, the endemic IncFII plasmid was found across multiple
wards, and genetic backgrounds (STs, Fig. 4b), indicating a major role in the burden of carbapenem resistance _K. pneumoniae_ in this hospital. A second IncFIB-IncFII plasmid found only
within the ST340 lineage might have contributed to the persistence and transmission of this clone in the NICU, by carrying several genomic islands with a role in survival in the host and in
the environment (Supplementary Fig. 5). ST340 is a member of the drug-resistant clonal complex 258 and has been reported to cause outbreaks worldwide28. Our findings were disseminated to the
local stakeholders (i.e., hospital) via a forum with NICU staff, paediatricians and ARSRL representatives. Infection control strategies were implemented, which ultimately bolstered the
infection control team of this hospital. Control of healthcare-associated infections is crucial to containing the spread of AMR29. The roadmap from sequence data to actionable data for
infection control and prevention within a hospital has been mapped by multiple use cases30,31 and supported by recent studies on implementation and cost-effectiveness in high-resource
settings32,33. This study makes a case for extending this roadmap to LMICs that have infection control capacity in place. At the national scale, the combined information on strain,
carbapenemase gene/variant and vehicle shows a granular picture of carbapenem-resistant _K. pneumoniae_ ST147 that uncovers the geographic distribution of high-risk clones. ST147 is an
international epidemic clone that causes carbapenem-resistant infections mediated by NDM, OXA-48-like and VIM carbapenemases28,34. Notably, ST147 was not reported in a recent study of _K.
pneumoniae_ from seven healthcare facilities across South and South East Asia35, which did not include the Philippines. Within the seemingly well-established genetic background of clade IV
(Fig. 5), a high-risk clone characterized by the presence of _bla_NDM-1 within plasmid p14ARS_VSM0843-1 displayed local clonal expansion in one sentinel site, while another high-risk clone
characterized by the presence of _bla_NDM-1 within plasmid p13ARS_GMH0099 had also disseminated geographically across five different sites (Fig. 5). Clonal expansion followed by geographical
dissemination is usually attributed to the acquisition of an AMR determinant36. The extent of the geographical dissemination of the different high-risk clones could be attributed to the
different plasmid backbones (vehicle), but differences in the genetic background (strain) could also be at play. The presence of a robust AMR surveillance network such as the ARSP is key for
the detection of geographical dissemination of high-risk clones at a regional/national level, and for the dissemination of the information to national stakeholders. In this respect, the
ARSRL is currently evaluating formats for the inclusion of genomic data into their annual surveillance reports. The reference laboratory directors can also alert hospitals across the country
to establish concerted infection control measures, as well as relay this information to the national DOH for the formulation of evidence-based guidelines. At the global scale, we identified
several high-risk clones of _E. coli_ ST410 circulating the in the Philippines, with the evidence of frequent international transmission, in agreement with a previous report18. Previous
reports of carbapenem-resistant _E. coli_ ST410 from the Western Pacific and South East Asia regions described the presence of _bla_OXA-181 in China17,37, _bla_NDM-1 in Singapore38,
_bla_NDM-5 in South Korea39, or the combination of _bla_NDM-5 and _bla_OXA-181 in Myanmar40 and South Korea39. The repertoire of carbapenemases in the retrospective collection of ST410 from
the Philippines seemed to be in tune with the genes/variants circulating locally, as most isolates carried the prevalent variant _bla_NDM-1. Of note was the high-risk clone carrying both
_bla_NDM-1 and _bla_OXA-181, a combination hitherto unreported in _E. coli_ ST410, and acquired through the combination of a plasmid of local circulation and a conserved international
plasmid, respectively. This suggests that _E. coli_ ST410, a successful pandemic lineage, can not only easily disseminate, but it can also adjust the complement of carbapenemases by
acquiring endemic plasmids along the way. Global AMR surveillance networks, such as the WHO GLASS8, are paramount to detect the emergence and monitor the spread of resistance at the
international level and inform the implementation of targeted prevention and control programmes. The ARSRL has been supplying laboratory surveillance data to GLASS since 2015, and presented
their experience with WGS at a WHO technical consultation on the application of WGS for national, regional and global surveillance in 2020, thus becoming an international contributor in both
fields. Collecting information on AMR that can be rapidly transformed into action requires harmonized standards, especially at the national and international levels8. For laboratory data,
WHONET serves this purpose in the ARSP and in over 2000 clinical, public health, veterinary and food laboratories in over 120 countries worldwide26, while GLASS serves this purpose at the
global level. Genomic data are amenable to standardization29, and national public health agencies41 and international surveillance networks42 are adopting diverse schema to identify and name
genetic lineages. However, a global standard system for defining a cluster has not been implemented beyond the level of discrimination of MLST. Likewise, different databases of AMR
mechanisms43,44,45 may differ in content and nomenclature. Thus, the standardization of genomic data, as well as the provision of platforms, for the uptake of genomic information is
crucially moving forward, and for ongoing AMR surveillance. Containment of AMR at a global level requires an international concerted effort. High-risk clones have the propensity to
disseminate rapidly, and genomics can improve the detection of their emergence and spread. This highlights the increasing need to build equitable partnerships to facilitate ownership
transfer of genomic epidemiology capacity (operational, analytical and interpretational) to enhance national AMR surveillance programmes within low-resource settings. The binational
partnership of this project led to a large retrospective survey of bacterial pathogens that has provided the first in-depth genomic view of the AMR landscape in the Philippines and
established contextual data for ongoing local prospective sequencing. In parallel, through training and transfer of expertize in laboratory procedures and bioinformatics, the open exchange
of data, and collective interpretation of results, we have expanded the existing capacity of a national reference laboratory with WGS focused on action for public health. WGS commenced at
the ARSRL in 2018 with the Illumina MiSeq equipment available locally. A new dedicated bioinformatics server was installed at ARSRL for sequence data storage and analysis. Collective data
interpretation was aided by data sharing via interactive web tools such as Microreact (www.microreact.org)27 and Pathogenwatch (www.pathogen.watch) to leverage the expertize from both CGPS
and ARSRL. The results of the retrospective survey were presented to the representatives of the sentinel sites during the ARSP annual meetings and at international conferences, at first by
the members of CGPS, followed by presentations by the members of the ARSRL in subsequent years46,47. The ARSRL staff conducted its first outbreak investigation using WGS in July 2019, with
sequencing, bioinformatics, and reporting to the DOH conducted locally, with a turnaround time of 8 days from sample receipt to report preparation. While progress has been made, significant
challenges remain to bring genomics into routine surveillance in low-resource settings. These include, but are not limited to, challenges in the supply chain and procurement of WGS reagents
and equipment, vastly differing costs between high- and low-income settings, shortage of skilled local bioinformaticians due to both limited access to training and difficulties in staff
retention, and the lack of platforms to feedback the actionable genomic data to sentinel sites. In addition, it was expected that the timeline from implementation of a disruptive technology
such as WGS to full adoption by all the relevant stakeholders was to exceed the period of one academically funded project. The continued collaboration with the ARSRL (via an NIHR-funded
project) is building on the foundations laid by this study to tackle some of the challenges mentioned above, and to improve on the translation of genomic data into public-health action and
policy. We laid the foundations for the sustainable implementation of WGS and genomic epidemiology within national surveillance networks in LMICs, which can be extended to other locations to
tackle the global challenge of AMR. METHODS ETHICS STATEMENT Ethical approval is not applicable as per the Research Institute for Tropical Medicine—Institutional Review Board. This study
uses archived bacterial samples processed by ARSP. No identifiable data were used in this study. ARSP WORKFLOW AND DATA The ARSP implements AMR surveillance on aerobic bacteria from clinical
specimens. The programme collects culture and antimicrobial susceptibility data from its 24 sentinel sites and 2 gonorrhoeae surveillance sites. The sentinel sites participate in an
external quality assessment scheme conducted by the reference laboratory to ensure quality of laboratory results. Case finding is based on priority specimens sent routinely to the hospitals’
laboratories for clinical purposes, and is thus based on the diagnostic practices of the clinicians, as well as the resources available to the patient to cover the culture and
susceptibility testing. Sentinel sites submit monthly results of all culture and susceptibility tests to the ARSRL via WHONET, a free Windows-based database software for the management and
analysis of microbiology laboratory data with a special focus on the analysis of antimicrobial susceptibility test results6. Multi-drug-resistant phenotypes are classified according to
recommended standard definitions48. Select isolates are referred to the ARSRL for confirmatory testing if they fall in one of the three following categories: (1) organisms of public health
importance regardless of their resistance phenotype, such as _Salmonella enterica_ ser. Typhi, _Streptococcus pneumoniae_ and _Vibrio cholerae_; (2) organisms with emerging resistance, such
as carbapenem resistance; (3) organisms with rare susceptibility patterns, such as vancomycin-resistant _Staphylococcus aureus_ or ceftriaxone-resistant _Neisseria gonorrhoeae_. At the
ARSRL, all referred isolates are re-identified using standardized culture, and susceptibility tested by disc diffusion or minimum inhibitory concentration (MIC) determined using the most
current guidance for antibiotic test panels and breakpoints recommended by the Clinical and Laboratory Standards Institute. Serotyping for _S. pneumoniae, H. influenzae_, Salmonellae and _V.
cholerae_ is conducted to further characterize the isolates. ARSP DATA AND BACTERIAL ISOLATES INCLUDED IN THIS STUDY Phenotypic data collected since 1988 from antimicrobial susceptibility
tests were summarized with WHONET v5.6 to compute yearly resistance rates for key pathogen–antibiotic combinations. Only the first bacterial isolate per patient per species per year was
included in the calculations. RPs derived from the antibiograms were summarized per organism with WHONET v5.66, and their relative abundance visualized with Tableau Desktop. Data collected
between 2009 and 2017 were included in the analysis as the number of sentinel sites collecting data remained relatively stable during this period (22–24 sites). Isolates with missing data
for any antibiotic on the panels listed on Table 1 were excluded. The bacterial pathogens included in the See and Sequence study were those responsible for the majority of
antimicrobial-resistant infections in the Philippines. These included carbapenemase-producing _Pseudomonas aeruginosa_ and _Acinetobacter baumanii_, carbapenemase-producing and ESBL-suspect
_Escherichia coli_ and _Klebsiella pneumoniae_, methicillin-resistant _Staphylococcus aureus_, _Neisseria gonorrhoeae_, non-typhoidal _Salmonella_, and _Salmonella enterica_ ser. Typhi.
Linked epidemiological data included location and date of specimen collection, type of specimen, type of patient (in or outpatient) and sex and age of the patient. We utilized a proxy
definition for infection origin whereby patient first isolates collected in the community or on either of the first 2 days of hospitalization were categorized as community infection
isolates, while isolates collected on hospital day 3 or later were categorized as HAI isolates8. In this article, we focus on carbapenemase-producing organisms. All carbapenemase-producing
_K. pneumoniae_ and _E. coli_ isolates referred to, confirmed, and banked by the ARSRL in 2013–2014 were selected for the retrospective sequencing survey. Approximately 100 isolates of
carbapenemase-producing _P. aeruginosa_ and _A. baumannii_ each were selected according to the following criteria: (1) referred to ARSRL in 2013–2014; (2) complete RP (i.e., no missing
data); (3) overall prevalence of the RP in the ARSP data (including both referred and non-referred isolates); (4) geographical representation of different sentinel sites. The number of
isolates included from each sentinel site was proportional to their relative abundance and estimated from (_n_/_N_) × 100 (rounded up), where _n_ is the total number of isolates from one
site, and _N_ is grand total of isolates; (5) when both invasive and non-invasive isolates representing a combination of RP, sentinel site and year of collection were available, invasive
isolates (i.e., from blood, or cerebrospinal, joint, pleural and pericardial fluids) were given priority. In addition, ~100 isolates of ESBL-producing _E. coli_ and _K. pneumoniae_ each were
included as per the criteria above. Pan-susceptible isolates were only available for _P. aeruginosa_ isolates and were also included in the retrospective sequencing survey. Lyophilisates of
the selected bacterial isolates were re-suspended in 0.5 mL of tryptic soy broth, plated onto MacConkey agar plates and incubated at 35–37 °C for 18–24 h. The species identity of the
revived bacterial isolates was confirmed by characterization of the colony morphology and conventional biochemical tests (e.g., oxidase, motility and sugar fermentation using triple sugar
iron agar test). Phenotypic re-testing was performed on the resuscitated isolates based on the phenotype stored in WHONET. Carbapenemase production in resuscitated isolates of
Enterobacteriaceae was tested with the modified Hodge test, and confirmed using the imipenem MIC method with imipenem Etest strips (BioMerieux). _P. aeruginosa_ and _A. baumanii_ were
screened for metallo-beta-lactamase production using the EDTA synergy test between imipenem disk (10 µg) and EDTA (750 µg/mL), which was confirmed by imipenem/imipenem inhibitor Etest
strips. _E. coli_ and _K. pneumoniae_ isolates were screened for ESBL with the double-disk synergy test between aztreonam (30 µg) and amoxicillin/clavulanic acid (20/10 µg) and confirmed by
using the gradient diffusion method with cefotaxime/cefotaxime with clavulanic acid or ceftazidime/ceftazidime with clavulanic acid Etest strips. WHOLE-GENOME SEQUENCING, ASSEMBLY AND
ANNOTATION _A. baumanni_, _E. coli_, _K. pneumoniae_ and _P. aeruginosa_ bacterial strains were shipped to the Wellcome Sanger Institute streaked onto Nutrient Agar butts contained in
screw-cap cryovials, where DNA was extracted from a single colony of each isolate with the QIAamp 96 DNA QIAcube HT kit and a QIAcube HT (Qiagen; Hilden, Germany). DNA extracts were
multiplexed and sequenced on the Illumina HiSeq platform (Illumina, CA, USA) with 100-bp paired-end reads. The quality of the sequence data was assessed for median base quality >30,
mapping coverage >80% of the length of a reference genome sequence of the same species (_A. baumannii_ ATCC 17978, _E. coli_ UPEC_ST131, _K. pneumoniae_ subsp. _pneumoniae_ Ecl8 and _P.
aeruginosa_ LESB58), and >80% of the sequence reads assigned to the corresponding species with Kraken v0.10.6-a2d113dc8f49 and a reference a database of all viruses, archaea and bacteria
genomes in RefSeq50 and the mouse and human reference. Annotated assemblies were produced using the pipeline described in51. For each sample, sequence reads were used to create multiple
assemblies using VelvetOptimiser v2.2.552 and Velvet v1.253. An assembly improvement step was applied to the assembly with the best N50 and contigs were scaffolded using SSPACE v2.054 and
sequence gaps were filled using GapFiller v1.1155. Automated annotation was performed using PROKKA v1.556 and a genus-specific database from RefSeq50. The quality of the assemblies was
assessed for total size, number of contigs, N50 and GC content appropriate for each species, with all genomes passing quality control characterized by assemblies of <150 contigs and N50
> 60,000. PHYLOGENETIC ANALYSIS Evolutionary relationships between isolates were inferred from SNPs by mapping the paired-end reads to the reference genomes of _A. baumannii_ A1
(CP010781), _E. coli_ EC958 (HG941718), _K. pneumoniae_ subsp. _pneumoniae_ (AP006725) or _P. aeruginosa_ LESB58 (FM209186), using the Burrows Wheeler aligner v0.7.1257 to produce a BAM
file. PCR duplicate reads were identified using Picard v1.9258 and flagged as duplicates in the BAM file. Variation detection was performed using samtools mpileup v0.1.1959 with parameters
-d 1000 -DSugBf and bcftools v0.1.1960 to produce a BCF file of all variant sites. The option to call genotypes at variant sites was passed to the bcftools call. All bases were filtered to
remove those with uncertainty in the base call. The bcftools variant quality score was required to be >50 (quality < 50) and mapping quality >30 (map_quality < 30). If not all
reads gave the same base call, the allele frequency, as calculated by bcftools, was required to be either 0 for bases called the same as the reference, or 1 for bases called as an SNP (af1
< 0.95). The majority base call was required to be present in at least 75% of reads mapping at the base (ratio < 0.75), and the minimum mapping depth required was 4 reads, at least two
of which had to map to each strand (depth < 4, depth_strand < 2). Finally, strand_bias was required to be <0.001, map_bias < 0.001 and tail_bias < 0.001. If any of these
filters were not met, the base was called as uncertain. A pseudogenome was constructed by substituting the base call at each site (variant and non-variant) in the BCF file into the reference
genome, and any site called as uncertain was substituted with an N. Insertions with respect to the reference genome were ignored and deletions with respect to the reference genome were
filled with Ns in the pseudogenome to keep it aligned and the same length as the reference genome used for read mapping. For sequence-type-specific phylogenies, MGEs and recombination
regions detected with Gubbins v1.4.1061 were masked from the alignment of pseudogenomes produced by mapping against the genomes of _K. pneumoniae_ strain CAV1217 (ST340, CP018676.1), _K.
pneumoniae_ strain MS6671 (ST147, LN824133.1) or E. coli strain AMA1167 (ST410, CP024801.1). Alignments of only variant positions were inferred with snp-sites v2.4.162 and were also used to
compute pairwise SNP differences between isolates (https://github.com/simonrharris/pairwise_difference_count). Maximum-likelihood phylogenetic trees were generated with RAxML v8.2.863 based
on the generalized time reversible model with GAMMA method of correction for among-site rate variation and 100 bootstrap replications. Trees were midpoint rooted. The phylogenetic trees,
genomic predictions of AMR and genotyping results were visualized together with the ARSP epidemiological data using Microreact27. IN SILICO PREDICTIONS OF AMR DETERMINANTS AND PLASMID
REPLICONS Known antibiotic resistance determinants were identified using Antibiotic Resistance Identification By Assembly v.2.6.1 (ARIBA)64. For acquired genes an in-house curated database
comprising 2316 known resistance gene variants was used (https://figshare.com/s/94437a301288969109c2)65. Full-length assembled matches with identity >90% and coverage >5× were
considered as evidence of the presence of the gene in the query genome. Further manual inspection of the results excluded 13 matches that passed the above filters but that were suspected
low-level contamination. Mutations in _ompK35_ and _ompK36_ genes were detected with ARIBA and the Comprehensive Antimicrobial Resistance Database44 and the output was inspected for
truncations, interruptions and frameshifts. Plasmid replicons were identified with ARIBA and the PlasmidFinder database66. Full-length assembled matches with 100% identity were considered as
evidence of the presence of the replicon gene. IN SILICO MULTI-LOCUS SEQUENCE TYPING Multi-locus STs were determined from assemblies with MLSTcheck v1.00700167 or from sequence reads with
ARIBA64, using species-specific databases hosted at PubMLST (https://pubmlst.org)68 for _E. coli_69, _A. baumannii_70, _P. aeruginosa_71 or at BIGSdb
(https://bigsdb.readthedocs.io/en/latest/#)68 for _K. pneumoniae_72. MLST calls from _P. aeruginosa_ genome sequences were manually curated as we noted the presence of two genes exhibiting
100% sequence identity to different _acsA_ alleles in the pubmlst database (accessed April 2019), which confounded the in silico predictions. This was not privy to the genomes is this study,
since the genome sequence of reference strain NCGM2_S1 (accession AP012280), known to belong to ST235, showed two genes with locus tags NCGM2_1665 (nt position 1808730–1810685) and
NCGM2_0806 (nt positions c879796-881733) that are annotated as _acsA_ and _acsB_, respectively, and are 100% identical to alleles acsA_38 and acsA_225, respectively
(https://pubmlst.org/data/alleles/paeruginosa/acsA.tfa, accessed Apr 2019). Furthermore, the same was only observed for the _acsA_ allele both from assemblies (MLST_check) and raw reads
(ARIBA-MLST), and therefore unlikely the result of contamination with other strains. Allele acsA_38 defines ST235, but allele acsA_225 defines a novel ST. Thus, when two different _acsA_
alleles were detected in a _P. aeruginosa_ genome we selected the one associated with a known ST. CHARACTERIZATION OF CARBAPENEMASE PLASMIDS Select _K. pneumoniae_ (14ARS_CVM0040,
14ARS_MMH0055, 13ARS_GMH0099, 14ARS_VSM0843, 13ARS-VSM0593 and 13ARS_MMH0112) and _E. coli_ (14ARS_NMC0074) isolates were also sequenced with PacBio RS II or Sequel (Pacific Biosciences, CA,
USA) or Oxford Nanopore Gridion (Oxford Nanopore, Oxford, UK) platforms. Hybrid assemblies from short and long reads were obtained with Unicycler v0.4.073 with default parameters, except
for 13ARS_GMH0099 for which the bold assembly mode was used. The sequence of the p13ARS_GMH0099 plasmid was manually curated with Bandage v0.8.174 circularized with Circlator v1.5.375, and
further polished with Quiver (Pacific Biosciences, CA, USA). The presence of AMR genes and plasmid replicons in the plasmid sequences was detected with ResFinder45 and PlasmidFinder66,
respectively. Matches with identity larger than 98% were reported. Detailed annotations of plasmids were obtained with MARA76. Visual comparisons between plasmid sequences were created with
BRIG v1.077 with default BLAST v2.7.0 parameters. The distribution of plasmids across draft genomes was inferred by mapping short reads to the plasmid sequences with smalt v0.7.4 with
identity threshold _y_ = 96% and random alignment of reads with multiple mapping positions of equal score (-r 1). The BAM file was sorted and indexed with samtools v0.1.19–44428cd59, which
was also used to and generate a pileup (mpileup -DSugBf) of the alignment records in BCF format. A pseudo-plasmid sequence in fastq format was constructed with the bcftools view and
vcfutils.pl vcf2fq programmes, and the sequence length coverage (%) of the reference plasmid was computed. REPORTING SUMMARY Further information on research design is available in the Nature
Research Reporting Summary linked to this article. DATA AVAILABILITY Raw sequence data generated during this study have been deposited in the European Nucleotide Archive (ENA) under the
study accession PRJEB17615. Run accessions are provided in the Microreact projects linked throughout the paper and below. The plasmid sequence assemblies generated during this study are
available from Tables 2–4. The resistance database used in this study is available at: https://figshare.com/s/94437a301288969109c2. The multi-locus sequence-type databases used in this study
are available at PubMLST (https://pubmlst.org) or at BIGSdb (https://bigsdb.readthedocs.io/en/latest/#). The data presented in this study are available from the following Microreact
projects: https://microreact.org/project/ARSP_ABA_2013-14 https://microreact.org/project/ARSP_PAE_2013-14 https://microreact.org/project/ARSP_KPN_2013-14
https://microreact.org/project/ARSP_ECO_2013-14 https://microreact.org/project/ARSP_KPN_ST340_2013-14 https://microreact.org/project/ARSP_KPN_ST147_2013-14
https://microreact.org/project/ARSP_KPN_ST147_GLOBAL https://microreact.org/project/ARSP_ECO_ST410 https://microreact.org/project/ARSP_ECO_ST410_GLOBAL REFERENCES * World Health
Organization. Antimicrobial resistance: global report on surveillance http://www.who.int/iris/handle/10665/112642 (2014). * World Bank. Drug-resistant infections: a threat to our economic
future http://documents.worldbank.org/curated/en/323311493396993758/pdf/114679-REVISED-v2-Drug-Resistant-Infections-Final-Report.pdf (2017). * World Health Organization. Global Action Plan
on antimicrobial resistance http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf (2015). * Department of Health, Republic of the Philippines. Morbidity
https://www.doh.gov.ph/morbidity (2014). * Department of Health, Republic of the Philippines. Mortality https://www.doh.gov.ph/mortality (2013). * O’Brien, T. F. & Stelling, J. M.
WHONET: an information system for monitoring antimicrobial resistance. _Emerg. Infect. Dis._ 1, 66 (1995). Article PubMed PubMed Central Google Scholar * Antimicrobial Resistance
Surveillance Reference Laboratory, Research Institute for Tropical Medicine. Annual reports https://arsp.com.ph/publications/ (2019). * World Health Organization. Global antimicrobial
resistance surveillance system (GLASS) report: early implementation 2016–2017 https://www.who.int/glass/resources/publications/early-implementation-report/en/ (2017). * Papp-Wallace, K. M.,
Endimiani, A., Taracila, M. A. & Bonomo, R. A. Carbapenems: past, present, and future. _Antimicrob. Agents Chemother._ 55, 4943–4960 (2011). Article CAS PubMed PubMed Central Google
Scholar * Ashton, P. M. et al. Identification of _Salmonella_ for public health surveillance using whole genome sequencing. _PeerJ_ 4, e1752 (2016). Article PubMed PubMed Central CAS
Google Scholar * Deng, X., den Bakker, H. C. & Hendriksen, R. S. Genomic epidemiology: whole-genome-sequencing-powered surveillance and outbreak investigation of foodborne bacterial
pathogens. _Annu. Rev. Food Sci. Technol._ 7, 353–374 (2016). Article PubMed Google Scholar * Doumith, M. et al. Detection of the plasmid-mediated mcr-1 gene conferring colistin
resistance in human and food isolates of _Salmonella enterica_ and _Escherichia coli_ in England and Wales. _J. Antimicrob. Chemother._ 71, 2300–2305 (2016). Article CAS PubMed Google
Scholar * Wong, V. K. et al. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of _Salmonella_ Typhi identifies inter- and intracontinental transmission events. _Nat.
Genet._ 47, 632–639 (2015). Article CAS PubMed PubMed Central Google Scholar * Koser, C. U., Ellington, M. J. & Peacock, S. J. Whole-genome sequencing to control antimicrobial
resistance. _Trends Genet._ 30, 401–407 (2014). Article CAS PubMed PubMed Central Google Scholar * Dadashi, M. et al. Frequency distribution, genotypes and the most prevalent sequence
types of New Delhi metallo-beta-lactamase-producing _Escherichia coli_ among clinical isolates around the world: a review. _J. Glob. Antimicrob. Resist._ 19, 284–293 (2019). Article PubMed
Google Scholar * Diene, S. M. & Rolain, J. M. Carbapenemase genes and genetic platforms in gram-negative bacilli: Enterobacteriaceae, _Pseudomonas_ and _Acinetobacter_ species. _Clin.
Microbiol. Infect._ 20, 831–838 (2014). Article CAS PubMed Google Scholar * Liu, Y. et al. First report of OXA-181-producing _Escherichia coli_ in China and characterization of the
isolate using whole-genome sequencing. _Antimicrob. Agents Chemother._ 59, 5022–5025 (2015). Article CAS PubMed PubMed Central Google Scholar * Roer, L. et al. _Escherichia coli_
sequence type 410 is causing new international high-risk clones. _mSphere_ 3, e00337–e00318 (2018). Article CAS PubMed PubMed Central Google Scholar * Falgenhauer, L. et al. Circulation
of clonal populations of fluoroquinolone-resistant CTX-M-15-producing _Escherichia coli_ ST410 in humans and animals in Germany. _Int. J. Antimicrob. Agents_ 47, 457–465 (2016). Article
CAS PubMed Google Scholar * Schaufler, K. et al. Clonal spread and interspecies transmission of clinically relevant ESBL-producing _Escherichia coli_ of ST410–another successful pandemic
clone? _FEMS Microbiol. Ecol._ 92, fiv155 (2016). Article PubMed CAS Google Scholar * Dortet, L., Poirel, L., Al Yaqoubi, F. & Nordmann, P. NDM-1, OXA-48 and OXA-181
carbapenemase-producing Enterobacteriaceae in Sultanate of Oman. _Clin. Microbiol. Infect._ 18, E144–E148 (2012). Article CAS PubMed Google Scholar * Szekely, E. et al. First description
of _bla_(NDM-1), _bla_(OXA-48), _bla_(OXA-181) producing Enterobacteriaceae strains in Romania. _Int. J. Med. Microbiol._ 303, 697–700 (2013). Article CAS PubMed Google Scholar * Gamal,
D., Fernandez-Martinez, M., El-Defrawy, I., Ocampo-Sosa, A. A. & Martinez-Martinez, L. First identification of NDM-5 associated with OXA-181 in _Escherichia coli_ from Egypt. _Emerg.
Microbes. Infect._ 5, e30 (2016). Article CAS PubMed PubMed Central Google Scholar * Overballe-Petersen, S. et al. Complete nucleotide sequence of an _Escherichia coli_ sequence type
410 strain carrying _bla_ NDM-5 on an IncF multidrug resistance plasmid and _bla_ OXA-181 on an IncX3 plasmid. _Genome Announc_. 6, e01542–e01517 (2018). Article PubMed PubMed Central
Google Scholar * Abboud, C. S. et al. A space-time model for carbapenemase-producing _Klebsiella pneumoniae_ (KPC) cluster quantification in a high-complexity hospital. _Epidemiol. Infect._
143, 2648–2652 (2015). Article CAS PubMed Google Scholar * Park, R. et al. Statistical detection of geographic clusters of resistant Escherichia coli in a regional network with WHONET
and SaTScan. _Expert Rev. Anti Infect. Ther._ 14, 1097–1107 (2016). Article CAS PubMed PubMed Central Google Scholar * Argimon, S. et al. Microreact: visualizing and sharing data for
genomic epidemiology and phylogeography. _Micro. Genom._ 2, e000093 (2016). Google Scholar * Wyres, K. L. et al. Identification of _Klebsiella_ capsule synthesis loci from whole genome
data. _Micro. Genom._ 2, e000102 (2016). Google Scholar * Gwinn, M., MacCannell, D. R. & Khabbaz, R. F. Integrating advanced molecular technologies into public health. _J. Clin.
Microbiol._ 55, 703–714 (2017). Article CAS PubMed PubMed Central Google Scholar * Epson, E. E. et al. Carbapenem-resistant _Klebsiella pneumoniae_ producing New Delhi
metallo-beta-lactamase at an acute care hospital, Colorado, 2012. _Infect. Control Hosp. Epidemiol._ 35, 390–397 (2014). Article PubMed Google Scholar * Harris, S. R. et al. Whole-genome
sequencing for analysis of an outbreak of meticillin-resistant _Staphylococcus aureus_: a descriptive study. _Lancet Infect. Dis._ 13, 130–136 (2013). Article CAS PubMed PubMed Central
Google Scholar * Peacock, S. J., Parkhill, J. & Brown, N. M. Changing the paradigm for hospital outbreak detection by leading with genomic surveillance of nosocomial pathogens.
_Microbiology_ 164, 1213–1219 (2018). Article CAS PubMed Google Scholar * Dymond, A. et al. Genomic surveillance of methicillin-resistant _Staphylococcus aureus_: a mathematical early
modelling study of cost effectiveness. _Clin. Infect. Dis._ ciz480 https://doi.org/10.1093/cid/ciz480 (2019). Article PubMed Central CAS Google Scholar * Lascols, C., Peirano, G.,
Hackel, M., Laupland, K. B. & Pitout, J. D. Surveillance and molecular epidemiology of _Klebsiella pneumoniae_ isolates that produce carbapenemases: first report of OXA-48-like enzymes
in North America. _Antimicrob. Agents Chemother._ 57, 130–136 (2013). Article CAS PubMed PubMed Central Google Scholar * Wyres, K. L. et al. Genomic surveillance for hypervirulence and
multi-drug resistance in invasive _Klebsiella pneumoniae_ from South and Southeast Asia. _Genome Med_ 12, 11 (2020). Article CAS PubMed PubMed Central Google Scholar * Baker, S.,
Thomson, N., Weill, F. X. & Holt, K. E. Genomic insights into the emergence and spread of antimicrobial-resistant bacterial pathogens. _Science_ 360, 733–738 (2018). Article CAS PubMed
PubMed Central Google Scholar * Qin, S., Cheng, J., Wang, P., Feng, X. & Liu, H. M. Early emergence of OXA-181-producing _Escherichia coli_ ST410 in China. _J. Glob. Antimicrob.
Resist._ 15, 215–218 (2018). Article PubMed Google Scholar * Khong, W. X. et al. Tracking inter-institutional spread of NDM and identification of a novel NDM-positive plasmid, pSg1-NDM,
using next-generation sequencing approaches. _J. Antimicrob. Chemother._ 71, 3081–3089 (2016). Article CAS PubMed Google Scholar * Baek, J. Y. et al. Plasmid analysis of _Escherichia
coli_ isolates from South Korea co-producing NDM-5 and OXA-181 carbapenemases. _Plasmid_ 104, 102417 (2019). Article CAS PubMed Google Scholar * Aung, M. S. et al. Prevalence of
extended-spectrum beta-lactamase and carbapenemase genes in clinical isolates of _Escherichia coli_ in Myanmar: dominance of _bla_ NDM-5 and Emergence of _bla_ OXA-181. _Micro. Drug Resist._
24, 1333–1344 (2018). Article CAS Google Scholar * Dallman, T. J. et al. Use of whole-genome sequencing for the public health surveillance of _Shigella sonnei_ in England and Wales,
2015. _J. Med. Microbiol._ 65, 882–884 (2016). Article PubMed Google Scholar * Nadon, C. et al. PulseNet International: vision for the implementation of whole genome sequencing (WGS) for
global food-borne disease surveillance. _Eur. Surveill._ 22, 23.30544 (2017). Article Google Scholar * Gupta, S. K. et al. ARG-ANNOT, a new bioinformatic tool to discover antibiotic
resistance genes in bacterial genomes. _Antimicrob. Agents Chemother._ 58, 212–220 (2014). Article PubMed PubMed Central CAS Google Scholar * Jia, B. et al. CARD 2017: expansion and
model-centric curation of the comprehensive antibiotic resistance database. _Nucleic Acids Res_ 45, D566–D573 (2017). Article CAS PubMed Google Scholar * Zankari, E. et al.
Identification of acquired antimicrobial resistance genes. _J. Antimicrob. Chemother._ 67, 2640–2644 (2012). Article CAS PubMed PubMed Central Google Scholar * Gayeta, J. et al.
Establishment of whole genome sequencing in the antimicrobial resistance surveillance program in the Philippines: assessment of whole genome sequence quality. In _Applied Bioinformatics and
Public Health Microbiology. _(Advanced Courses and Scientific Conferences, Hinxton, Cambridge, UK, 2019). * Masim, M. A. et al. Genomic surveillance report for methicillin-resistant
_Staphylococcus aureus_ in the Philippines. In _Applied Bioinformatics and Public Health Microbiology._ (Advanced Courses and Scientific Conferences, Hinxton, Cambridge, UK, 2019). *
Magiorakos, A. P. et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired
resistance. _Clin. Microbiol. Infect._ 18, 268–281 (2012). Article CAS PubMed Google Scholar * Wood, D. E. & Salzberg, S. L. Kraken: ultrafast metagenomic sequence classification
using exact alignments. _Genome Biol._ 15, R46 (2014). Article PubMed PubMed Central Google Scholar * Pruitt, K. D., Tatusova, T., Brown, G. R. & Maglott, D. R. NCBI reference
sequences (RefSeq): current status, new features and genome annotation policy. _Nucleic Acids Res._ 40, D130–D135 (2012). Article CAS PubMed Google Scholar * Page, A. J. et al. Robust
high-throughput prokaryote de novo assembly and improvement pipeline for Illumina data. _Micro. Genom._ 2, e000083 (2016). Google Scholar * Gladman, S. & Seemann, T. Velvet optimiser:
for automatically optimising the primary parameter options for the Velvet de novo sequence assembler. Victorian Bioinformatics Consortium
https://github.com/Victorian-Bioinformatics-Consortium/VelvetOptimiser (2008). * Zerbino, D. R. & Birney, E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs.
_Genome Res._ 18, 821–829 (2008). Article CAS PubMed PubMed Central Google Scholar * Boetzer, M., Henkel, C. V., Jansen, H. J., Butler, D. & Pirovano, W. Scaffolding pre-assembled
contigs using SSPACE. _Bioinformatics_ 27, 578–579 (2011). Article CAS PubMed Google Scholar * Boetzer, M. & Pirovano, W. Toward almost closed genomes with GapFiller. _Genome Biol._
13, R56 (2012). Article PubMed PubMed Central Google Scholar * Seemann, T. Prokka: rapid prokaryotic genome annotation. _Bioinformatics_ 30, 2068–2069 (2014). Article CAS PubMed
Google Scholar * Li, H. & Durbin, R. Fast and accurate long-read alignment with Burrows-Wheeler transform. _Bioinformatics_ 26, 589–595 (2010). Article PubMed PubMed Central CAS
Google Scholar * Broad Institute. Picard: a set of command line tools (in Java) for manipulating high-throughput sequencing (HTS) data https://broadinstitute.github.io/picard/ (2020). * Li,
H. et al. The sequence alignment/map format and SAMtools. _Bioinformatics_ 25, 2078–2079 (2009). Article PubMed PubMed Central CAS Google Scholar * Samtools. Bcftools: utilities for
variant calling and manipulating VCFs and BCFs http://samtools.github.io/bcftools/ (2020). * Croucher, N. J. et al. Rapid phylogenetic analysis of large samples of recombinant bacterial
whole genome sequences using Gubbins. _Nucleic Acids Res._ 43, e15 (2015). Article PubMed CAS Google Scholar * Page, A. J. et al. SNP-sites: rapid efficient extraction of SNPs from
multi-FASTA alignments. _Micro. Genom._ 2, e000056 (2016). Google Scholar * Stamatakis, A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies.
_Bioinformatics_ 30, 1312–1313 (2014). Article CAS PubMed PubMed Central Google Scholar * Hunt, M. et al. ARIBA: rapid antimicrobial resistance genotyping directly from sequencing
reads. _Micro. Genom._ 3, e000131 (2017). Google Scholar * David, S. et al. Epidemic of carbapenem-resistant _Klebsiella pneumoniae_ in Europe is driven by nosocomial spread. _Nat.
Microbiol._ 4, 1919–1929 (2019). Article CAS PubMed PubMed Central Google Scholar * Carattoli, A. et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid
multilocus sequence typing. _Antimicrob. Agents Chemother._ 58, 3895–3903 (2014). Article PubMed PubMed Central CAS Google Scholar * Page, A. J., Taylor, B. & Keane, J. A.
Multilocus sequence typing by blast from de novo assemblies against PubMLST. _J. Open Source Softw._ 8, 2 (2016). Google Scholar * Jolley, K. A., Bray, J. E. & Maiden, M. C. J.
Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. _Wellcome Open Res._ 3, 124 (2018). Article PubMed PubMed Central CAS Google
Scholar * Wirth, T. et al. Sex and virulence in _Escherichia coli_: an evolutionary perspective. _Mol. Microbiol._ 60, 1136–1151 (2006). Article CAS PubMed PubMed Central Google Scholar
* Bartual, S. G. et al. Development of a multilocus sequence typing scheme for characterization of clinical isolates of _Acinetobacter baumannii_. _J. Clin. Microbiol._ 43, 4382–4390
(2005). Article CAS PubMed PubMed Central Google Scholar * Curran, B., Jonas, D., Grundmann, H., Pitt, T. & Dowson, C. G. Development of a multilocus sequence typing scheme for the
opportunistic pathogen _Pseudomonas aeruginosa_. _J. Clin. Microbiol._ 42, 5644–5649 (2004). Article CAS PubMed PubMed Central Google Scholar * Diancourt, L., Passet, V., Verhoef, J.,
Grimont, P. A. & Brisse, S. Multilocus sequence typing of _Klebsiella pneumoniae_ nosocomial isolates. _J. Clin. Microbiol._ 43, 4178–4182 (2005). Article CAS PubMed PubMed Central
Google Scholar * Wick, R. R., Judd, L. M., Gorrie, C. L. & Holt, K. E. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. _PLoS Comput. Biol._ 13,
e1005595 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Wick, R. R., Schultz, M. B., Zobel, J. & Holt, K. E. Bandage: interactive visualization of de novo genome
assemblies. _Bioinformatics_ 31, 3350–3352 (2015). Article CAS PubMed PubMed Central Google Scholar * Hunt, M. et al. Circlator: automated circularization of genome assemblies using
long sequencing reads. _Genome Biol._ 16, 294 (2015). Article PubMed PubMed Central CAS Google Scholar * Partridge, S. R. & Tsafnat, G. Automated annotation of mobile antibiotic
resistance in gram-negative bacteria: the multiple antibiotic resistance annotator (MARA) and database. _J. Antimicrob. Chemother._ 73, 883–890 (2018). Article CAS PubMed Google Scholar
* Alikhan, N. F., Petty, N. K., Ben Zakour, N. L. & Beatson, S. A. BLAST ring image generator (BRIG): simple prokaryote genome comparisons. _BMC Genomics_ 12, 402 (2011). Article CAS
PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to the members of the Antimicrobial Resistance Surveillance Program that collected bacterial
isolates and linked epidemiological data. The full list can be found in the Supplementary Information (Supplementary Note 6). This work was funded by the Newton Fund, Medical Research
Council (UK) grant MR/N019296/1, Philippine Council for Health Research and Development project number FP160007. J.S. was partially supported by research grants RR025040 and U01CA207167 from
the National Institutes of Health (NIH). S.A. and D.M.A. were additionally supported by the National Institute for Health Research (UK) Global Health Research Unit on genomic Surveillance
of AMR (16_136_111) and by the Centre for Genomic Pathogen Surveillance (http://pathogensurveillance.net). AUTHOR INFORMATION Author notes * Charmian M. Hufano Present address: St. Luke’s
Medical Center—Global City, Taguig, Metro Manila, The Philippines * These authors contributed equally: David M. Aanensen, Celia C. Carlos. AUTHORS AND AFFILIATIONS * Centre for Genomic
Pathogen Surveillance, Wellcome Genome Campus, Hinxton, UK Silvia Argimón, Victoria Cohen, Benjamin Jeffrey, Khalil Abudahab & David M. Aanensen * Antimicrobial Resistance Surveillance
Reference Laboratory, Research Institute for Tropical Medicine, Muntinlupa, The Philippines Melissa A. L. Masim, June M. Gayeta, Marietta L. Lagrada, Polle K. V. Macaranas, Marilyn T. Limas,
Holly O. Espiritu, Janziel C. Palarca, Jeremiah Chilam, Manuel C. Jamoralin Jr., Alfred S. Villamin, Janice B. Borlasa, Agnettah M. Olorosa, Lara F. T. Hernandez, Karis D. Boehme, Charmian
M. Hufano, Sonia B. Sia & Celia C. Carlos * Brigham and Women’s Hospital, Boston, MA, USA John Stelling * University of St Andrews School of Medicine, St Andrews, Scotland, UK Matthew T.
G. Holden * Big Data Institute, Li Ka Shing Centre for Health Information and Discovery, University of Oxford, Oxford, UK David M. Aanensen Authors * Silvia Argimón View author publications
You can also search for this author inPubMed Google Scholar * Melissa A. L. Masim View author publications You can also search for this author inPubMed Google Scholar * June M. Gayeta View
author publications You can also search for this author inPubMed Google Scholar * Marietta L. Lagrada View author publications You can also search for this author inPubMed Google Scholar *
Polle K. V. Macaranas View author publications You can also search for this author inPubMed Google Scholar * Victoria Cohen View author publications You can also search for this author
inPubMed Google Scholar * Marilyn T. Limas View author publications You can also search for this author inPubMed Google Scholar * Holly O. Espiritu View author publications You can also
search for this author inPubMed Google Scholar * Janziel C. Palarca View author publications You can also search for this author inPubMed Google Scholar * Jeremiah Chilam View author
publications You can also search for this author inPubMed Google Scholar * Manuel C. Jamoralin Jr. View author publications You can also search for this author inPubMed Google Scholar *
Alfred S. Villamin View author publications You can also search for this author inPubMed Google Scholar * Janice B. Borlasa View author publications You can also search for this author
inPubMed Google Scholar * Agnettah M. Olorosa View author publications You can also search for this author inPubMed Google Scholar * Lara F. T. Hernandez View author publications You can
also search for this author inPubMed Google Scholar * Karis D. Boehme View author publications You can also search for this author inPubMed Google Scholar * Benjamin Jeffrey View author
publications You can also search for this author inPubMed Google Scholar * Khalil Abudahab View author publications You can also search for this author inPubMed Google Scholar * Charmian M.
Hufano View author publications You can also search for this author inPubMed Google Scholar * Sonia B. Sia View author publications You can also search for this author inPubMed Google
Scholar * John Stelling View author publications You can also search for this author inPubMed Google Scholar * Matthew T. G. Holden View author publications You can also search for this
author inPubMed Google Scholar * David M. Aanensen View author publications You can also search for this author inPubMed Google Scholar * Celia C. Carlos View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS C.C.C. and D.M.A. conceived the study. S.A., M.A.L.M., J.M.G., M.L.L., P.K.V.M., V.C., M.T.L., H.O.E., J.C.P., J.C., M.C.J.,
A.S.V., J.B.B., A.M.O., L.F.T.H., K.D.B., B.J., K.A., C.M.H., S.B.S., J.S., M.T.G.H, C.C.C. and D.M.A. performed the data analysis. S.A., M.A.L.M., M.T.G.H., C.C.C. and D.M.A. wrote the
paper. All authors read and approved the paper. CORRESPONDING AUTHORS Correspondence to David M. Aanensen or Celia C. Carlos. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution to the peer review of this work. PUBLISHER’S
NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION REPORTING SUMMARY SUPPLEMENTARY
INFORMATION PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Argimón, S., Masim, M.A.L., Gayeta, J.M. _et al._ Integrating whole-genome sequencing within the National Antimicrobial Resistance Surveillance Program in the
Philippines. _Nat Commun_ 11, 2719 (2020). https://doi.org/10.1038/s41467-020-16322-5 Download citation * Received: 06 March 2020 * Accepted: 25 April 2020 * Published: 01 June 2020 * DOI:
https://doi.org/10.1038/s41467-020-16322-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Access to this page has been deniedYour browser appears to have Javascript disabled.For instructions on how to enable Javascript please click here.If you h...
Author correction: ketamine can reduce harmful drinking by pharmacologically rewriting drinking memoriesCorrection to: _Nature Communications_ https://doi.org/10.1038/s41467-019-13162-w, published online 26 November 2019. Th...
Police 'concerned for the welfare' of missing nuneaton manPolice say they are concerned for the welfare of a Nuneaton man who has gone missing. They have released an image of Nei...
Ocado reinforces its robot army with american technology dealsThe pacesetter in British online grocery sales is set to expand into fashion retail after striking deals to buy two Amer...
Emma Frances Bloomfield – The ConversationProfile Articles Activity Dr. Emma Frances Bloomfield (Ph.D., University of Southern California) researches the rhetoric...
Latests News
Integrating whole-genome sequencing within the national antimicrobial resistance surveillance program in the philippinesABSTRACT National networks of laboratory-based surveillance of antimicrobial resistance (AMR) monitor resistance trends ...
Camille thurman is a rare jazz double threatIn the world of jazz, most musicians choose one single thing and get as good as humanly possible at it, but not Camille ...
Aarp livability fact sheet - parkingThe only time many of us think about the cost of parking a car is when we actually have to open our wallets and pay a fe...
Why mouth cancer awareness must be a year-round commitmentAccess through your institution Buy or subscribe Mouth cancer is an escalating concern in the UK, with cases surpassing ...
Notes | NatureABSTRACT WE learn with much satisfaction that the announcement of the death of Prof. I. P. Pavlov is incorrect; and we m...