Cardiomyocyte and stromal cell cross-talk influences the pathogenesis of arrhythmogenic cardiomyopathy: a multi-level analysis uncovers dlk1-notch pathway role in fibro-adipose remodelling
Cardiomyocyte and stromal cell cross-talk influences the pathogenesis of arrhythmogenic cardiomyopathy: a multi-level analysis uncovers dlk1-notch pathway role in fibro-adipose remodelling"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Arrhythmogenic Cardiomyopathy (ACM) is a life-threatening, genetically determined disease primarily caused by mutations in desmosomal genes, such as _PKP2_. Currently, there is no
etiological therapy for ACM due to its complex and not fully elucidated pathogenesis. Various cardiac cell types affected by the genetic mutation, such as cardiomyocytes (CM) and cardiac
mesenchymal stromal cells (cMSC), individually contribute to the ACM phenotype, driving functional abnormalities and fibro-fatty substitution, respectively. However, the relative importance
of the CM and cMSC alterations, as well as their reciprocal influence in disease progression remain poorly understood. We hypothesised that ACM-dependent phenotypes are driven not only by
alterations in individual cell types but also by the reciprocal interactions between CM and cMSC, which may further impact disease pathogenesis. We utilized a patient-specific, multicellular
cardiac system composed of either control or _PKP2_-mutated CM and cMSC to assess the mutation’s role in fibro-fatty phenotype by immunofluorescence, and contractile behaviour of
co-cultures using cell motion detection software. Additionally, we investigated reciprocal interactions both in silico and via multi-targeted proteomics. We demonstrated that ACM CM can
promote fibro-adipose differentiation of cMSC. Conversely, ACM cMSC contribute to increasing the rate of abnormal contractile events with likely arrhythmic significance. Furthermore, we
showed that an ACM-causative mutation alters the CM-cMSC interaction pattern. We identified the CM-sourced DLK1 as a novel regulator of fibro-adipose remodelling in ACM. Our study challenges
the paradigm of exclusive cell-specific mechanisms in ACM. A deeper understanding of the cell-cell influence is crucial for identifying novel therapeutic targets for ACM, and this concept
is exploitable for other cardiomyopathies. SIMILAR CONTENT BEING VIEWED BY OTHERS PATIENT-SPECIFIC PRIMARY AND PLURIPOTENT STEM CELL-DERIVED STROMAL CELLS RECAPITULATE KEY ASPECTS OF
ARRHYTHMOGENIC CARDIOMYOPATHY Article Open access 27 September 2023 THE EXTRACELLULAR MATRIX GLYCOPROTEIN ADAMTSL2 IS INCREASED IN HEART FAILURE AND INHIBITS TGFΒ SIGNALLING IN CARDIAC
FIBROBLASTS Article Open access 05 October 2021 SINGLE-CELL TRANSCRIPTOMICS REVEAL EXTRACELLULAR VESICLES SECRETION WITH A CARDIOMYOCYTE PROTEOSTASIS SIGNATURE DURING PATHOLOGICAL REMODELING
Article Open access 21 January 2023 INTRODUCTION The heart is composed of different cell types that interact closely to maintain cardiac homeostasis. Although cardiomyocytes (CM) occupy the
largest volume, other cell types are numerically predominant [1,2,3]. This suggests the presence of cell-specific functions and numerous interactions that coordinate cellular activity and
support their functional cooperation, thereby regulating overall heart function. These complex interactions occur both under physiological conditions and during disease development [4].
Arrhythmogenic Cardiomyopathy (ACM) is a rare genetic heart condition that affects predominantly young adults. In ACM, the cardiac tissue is pathologically remodelled, and ventricular
arrhythmias are the major causes of sudden death [5]. Historically, CM have been the primary focus of ACM pathogenesis studies as they are the main determinants of electrical and mechanical
cardiac activities, which are impaired in ACM [6]. ACM is primarily associated with mutations in desmosomal genes such as _PKP2_, which encodes for Plakophilin-2 [7]. Mechanical continuity,
provided by desmosomes, is preferentially located at CM intercalated discs. In addition to impaired ventricular systolic function, ACM is characterized by fibro-adipose replacement of the
myocardium. Among the different hypotheses regarding its origin, most agree that this replacement is sustained by enhanced differentiation of cardiac Mesenchymal Stromal Cells (cMSC)
[8,9,10,11,12,13]. Under normal physiological conditions, cMSC support the structural and functional integrity of the myocardium [14]. It has been shown that these cells express desmosomal
genes [8], and therefore they are subject to the desmosomal mutation effects, being key determinants of ACM pathogenesis [8]. Previous data from our group have highlighted that ACM cMSC
mediate the fibro-adipose accumulation process based on their commitment to differentiate into myofibroblasts and adipocytes [8, 9]. Despite the presence of dead or impaired CM and the
abundance of neighbouring cMSC genetically predisposed to aberrant differentiation in the ACM heart, an in-depth study clarifying how these cells reciprocally affect each other during ACM
progression is lacking. Here, we hypothesise that the reciprocal interaction between CM and cMSC may have an additional impact on disease pathogenesis, beyond the individual cell type
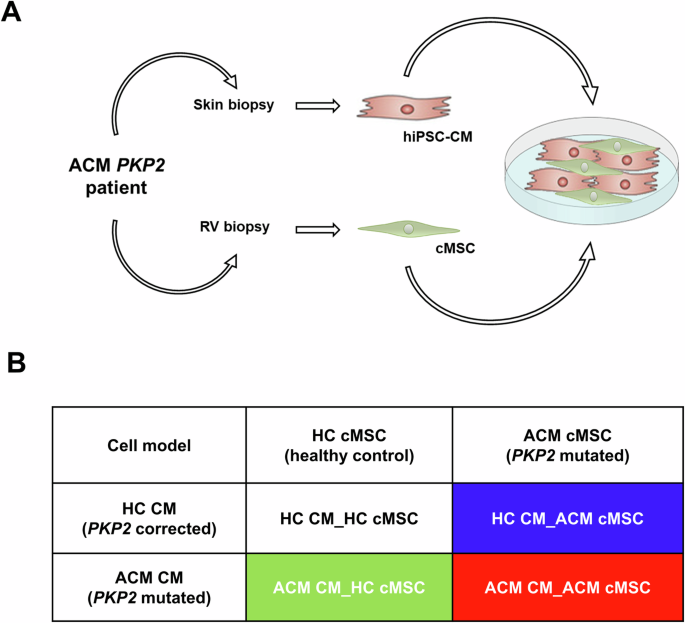
contribution. To address this, we generated a patient-specific co-culture model combining induced pluripotent stem cells derived CM (hiPSC-CM) and primary cMSC. The ability to replace each
healthy cell type (CM or cMSC) with a counterpart carrying an ACM genotype allowed us to assess the specific contribution of each cell population to the alterations typical of the disease.
We chose to use, as representative of the ACM genotype, primary cells and hiPSC from a carrier of a _PKP2_ mutation, the most frequently mutated gene in ACM patients, with a prevalence
ranging between 10 and 45% [15, 16]. Specifically, we: (i) provided evidence that ACM CM can influence the fibro-adipose commitment of cMSC; (ii) validated that ACM cMSC can affect CM
contraction by increasing the incidence of events of arrhythmic significance; (iii) demonstrated that the ACM-causative mutation results in variations in the interaction pattern between CM
and cMSC; (iv) confirmed, through a secretome profiling, that CM-cMSC interplay and regulation in ACM also occur by paracrine signalling; and (v) identified a novel CM-derived factor that
regulates cMSC fibro-adipose differentiation. Given the crucial role of interactions between cMSC and CM in maintaining cardiac tissue integrity, defining the heterotypic cellular
interaction network in ACM is of critical translational importance. Indeed, elucidating the cellular pathways triggered by direct and paracrine interactions could represent a valuable tool
for identifying potential pharmacological targets. RESULTS ACM CARDIOMYOCYTES PROMOTE FIBRO-ADIPOSE DIFFERENTIATION ABILITY OF MESENCHYMAL STROMAL CELLS ACM hearts undergo fibro-adipose
remodelling, primarily due to the alteration of cMSC and their increased propensity to differentiate into adipocytes and myofibroblasts [8, 9, 17]. To verify whether CM contribute to cMSC
fibro-adipogenic differentiation, four different co-cultures were assembled by combining control (HC) or mutated (ACM) hiPSC-CM with primary cMSC, as described in Fig. 1 and detailed in the
methods section. The co-cultures were maintained in BPEL for ten days, after which immunofluorescence and fluorescent staining analyses of Collagen I and Nile Red were performed to evaluate
the fibrotic and adipose accumulation, respectively. The analysis revealed that the production of Collagen I was higher in ACM co-culture (ACM CM_ACM cMSC) compared to the HC (HC CM_HC cMSC)
co-culture (Fig. 2A, B). Intermediate levels of Collagen I were observed in the mixed co-cultures (ACM CM_HC cMSC and HC CM_ACM cMSC) (Fig. 2A, B). Similarly, Nile Red staining revealed
that the ACM co-culture (ACM CM_ACM cMSC) accumulated lipid droplets to a greater extent than the HC (HC CM_HC cMSC) co-culture (Fig. 2C, D). Intermediate and comparable levels of lipid
accumulation were observed in the mixed co-cultures (ACM CM_HC cMSC and HC CM_ACM cMSC) (Fig. 2C, D). These data collectively demonstrate that the presence of ACM CM in co-culture promotes
the accumulation of lipids and collagen by HC cMSC, indicating that CM can influence stromal-dependent fibro-adipose remodelling. ACM STROMAL CELLS CONTRIBUTE TO CARDIOMYOCYTE CONTRACTILE
DYSFUNCTION AND ARRHYTHMIC SUSCEPTIBILITY To assess the influence of each cell type on contractile function, high-speed movies of the four different co-cultures were analysed using
MUSCLEMOTION [18] to extract the contractile patterns at various pacing rates. An increase in the occurrence of contractile anomalies, likely of arrhythmic significance, was observed when
transitioning from purely HC genotypes to purely ACM genotypes. The inclusion of ACM CM in co-cultured monolayers significantly increased the percentage of these events compared to
co-cultures containing HC CM; particularly at pacing rates of 0.5 Hz (Fisher’s exact test, _p_ = 0.001095) and 1 Hz (Fisher’s exact test, _p_ = 0.03211), while no significant changes
observed at 2 Hz (Fig. 3A, B; Supplementary Fig. S2; Supplementary File Video S1–4, Fisher’s exact test, _p_ = 0.2039). Overall, the inclusion of ACM cMSC in co-cultured monolayers did not
increase the percentage of contractile anomalies of likely arrhythmic significance compared to co-cultures with HC cMSC at any frequency (Fisher’s exact test, _p_ = 0.8051 at 0.5 Hz, _p_ =
0.2887 at 1 Hz, _p_ = 1 at 2 Hz). However, the presence of ACM cMSC alone in the co-culture (HC CM_ACM cMSC) increased the percentage of contractile dysfunctions of likely arrhythmic
significance at 1 Hz (Fig. 3A, B; Supplementary Fig. S2; Supplementary File Video S1–4, Fisher’s exact test, _p_ = 0.0482) but not at 0.5 Hz or 2 Hz (Fisher’s exact test, _p_ = 1) when
compared to the HC CM_HC cMSC model. Qualitative stratification of proarrhythmic events by type (Fig. 3C; Supplementary File Video S1–4) revealed a progressive decrease in the fraction of
monolayers exhibiting a normal contractile phenotype, with a higher incidence of monolayers displaying an upward drift at baseline (i.e., incomplete and defective relaxation),
aftercontractions, or monolayers that escaped the imposed pacing frequency (a representation of the observed abnormal contractile phenotype is shown in Supplementary Fig. S1A–F).
Quantitative analyses of temporal contractile parameters were performed only on co-cultures exhibiting normal contractile patterns (Supplementary Fig. S3C, D). Overall, these results suggest
that both cell types contribute to the pro-arrhythmic phenotype observed in ACM. CARDIOMYOCYTE AND STROMAL CELL COMMUNICATION DEPENDS ON THEIR ACM / HC GENOTYPE Our study aimed to highlight
cell-cell communication dynamics by uncovering the reciprocal influence between CM and cMSC. Specifically, we sought to identify how ACM CM could potentially influence healthy stromal cells
and how the ACM stromal cells might pathogenetically impact healthy cardiomyocytes. To achieve this, we performed an in-silico cell-cell communication analysis, leveraging the RNA-Seq data
to detect relevant ligand-receptor pairs. We performed RNA-seq on 12 samples (3 ACM CM, 3 ACM cMSC, 3 HC CM, and 3 HC cMSC). Out of 21,071 expressed genes, 7,864 were found to be highly
differentially expressed (ANOVA adj.p-value < 0.01) in at least one of the following comparisons: 1) ACM CM vs. HC CM (2,611 genes); 2) ACM cMSC vs. HC cMSC (5,253 genes) (Supplementary
Fig. S4A). The entire transcriptomes were then used to identify potential crosstalk between CM and cMSC with CellPhoneDB. We predicted interactions between ligands and receptors
(ligand_receptor) for each pair of cell types based on the expression of a receptor by one cell type and a ligand by the other. Each ligand_receptor pairs received an interaction score based
on the total number of predicted interactions. The enrichment of these interactions for specific comparisons, depending on the genotype, is depicted in Fig. 4. Among the 1,630
ligand-receptor pairs annotated in the CellPhoneDB database, 142, 132, 90 and 81 were found to be significantly interacting (|log2(FC)| > 0.5 and _p_ < 0.01) when considering the
interactions between HC cMSC vs HC CM (Fig. 4A, orange and green dots; Supplementary File Data S1), ACM cMSC vs. HC CM (Fig. 4A, red and green dots; File Data S1), HC CM vs. HC cMSC (Fig.
4B, magenta and green dots; Supplementary File Data S2), ACM CM vs. HC cMSC (Fig. 4B, purple and green dots; Supplementary File Data S2), respectively. In evaluating the interaction of HC or
ACM cMSC ligands with healthy CM receptors, we identified 108 common interactions, of which 72 exhibited differential score patterns. Additionally, HC cMSC had 34 distinct interactions that
were absent in ACM cMSC (indicated by orange dots in Fig. 4A and Supplementary Fig. S5), whereas ACM cMSC showed 24 unique interactions with healthy CM (indicated by red dots in Fig. 4A;
Supplementary Fig. S5). For example, high score interactions specific to healthy cMSC included PTN_PLXNB2 or other receptors and WNT1-4_FZD8. In contrast, IL6_IL6R, TGFB3_TGFBR3, and
WNT5A_ROR2 interactions were specifically present when the cMSC were ACM (Supplementary File Data S1; Supplementary Fig. S5). Our analysis revealed 56 interactions common to both HC and ACM
CM ligands with healthy cMSC receptors. However, 20 of these interactions exhibited differential score patterns. For example, the interaction between DLK1 CM ligand and the NOTCH1-2-3-4
(DLK1_NOTCH1-2-3-4) receptors displayed lower scores when ACM CM ligands were present compared to HC CM ligands. Furthermore, HC CM ligands demonstrated 34 distinct interactions (indicated
by magenta dots in Fig. 4B; Supplementary Fig. S6) that were absent when ACM CM ligands were involved, while ACM CM ligands exhibited 25 unique interactions directed towards healthy cMSC
receptors (indicated by purple dots in Fig. 4B; Supplementary Fig. S6). Notably, the communication between ACM CM ligands and healthy cMSC receptors implied a loss of interactions involving
the COL3A1_a2b1 and COL5A1_a11b1 complexes, and the EFNA4-5_EPHA2-3-4, compared to HC CM ligands and cMSC receptors. Conversely, ACM CM developed new interactions with healthy cMSC, such as
TGFB2_TGFBR1, FGF1-18_FGFR2 and IL6_IL6R (Supplementary File Data S2; Supplementary Fig. S6). CARDIOMYOCYTES AND STROMAL CELLS ACM-SPECIFIC REGULATION ALSO OCCURS THROUGH PARACRINE SIGNALS
The in silico study, which simulated CM-cMSC cell interactions, revealed potential cell-cell communication pathways and unique interactions (Fig. 4; Supplementary File Data S1-2). Cell-cell
communication can occur through paracrine signalling, which involves soluble mediators. To address this issue from the perspective of the effective release of these mediators, we performed a
secretome analysis of our co-cultures. Conditioned media were collected and analysed using Olink technology. Ninety-two factors involved in cardiovascular alterations were screened
(Supplementary File Data S3), and 36 of these were found to be differentially secreted among the four co-cultures. The diversity of the secretomes from the four co-cultures is graphically
shown in Fig. 5A, while the secretion levels of all 36 factors are shown in Supplementary Fig. S7. We focused on factors that could be involved in ACM-dependent phenotypic alterations. For
instance, we identified factors related to cardiac remodelling and the balance between matrix synthesis and deposition, such as Collagen 1A1 (Col1a1), Matrix Metalloproteinases 9 (MMP9), and
Metalloproteinase inhibitor 4 (TIMP4). The levels of secreted Col1a1, MMP9, and TIMP4 were increased in co-cultures containing ACM cMSC (Fig. 5B–D; Supplementary Fig. S7, Supplementary File
Data S3). Additional factors differentially secreted and known to be involved in fibrosis and mechanosensory functions were urokinase-type plasminogen activator receptor (uPAR) and platelet
endothelial cell adhesion molecule (PECAM-1) respectively. The levels of uPAR and PECAM-1 were elevated in the presence of ACM cMSC and HC cMSC respectively (Fig. 5E, F; Supplementary Fig.
S7, Supplementary File Data S3). Markers associated with muscle damage or ACM severity, such as myoglobin (MB), Growth Differentiation factor-15 (GDF-15), and factors involved in cardiac
lipid metabolism, such as LDL receptor, were highly expressed in the conditioned media and differentially represented among the four co-cultures. GDF-15 and LDL receptor were increased in
co-cultures containing ACM cMSC, whereas MB was more abundant in co-cultures with ACM CM (Fig. 5G–I; Supplementary Fig. S7, Supplementary File Data S3). Notably, the DLK1 protein, previously
identified in the in silico interactome studies, was found to be heavily regulated in the different conditioned media. Specifically, DLK1 was secreted exclusively by co-cultures containing
HC CM (Fig. 5L; Supplementary Fig. S7, Supplementary File Data S3). DLK1 MODULATES FIBRO-ADIPOSE ACCUMULATION OF ACM STROMAL CELLS In silico interactome studies of CM-cMSC interactions have
highlighted that the DLK1_NOTCH1 interaction was predicted only when the ligand was derived from CM and the interaction was reduced when CM were from ACM (Supplementary File Data S2-S3).
Furthermore, data from secretome analysis indicated that DLK1 protein was present only in the supernatants of co-cultures including HC CM (Fig. 5L). Given the consistency between the two
analyses, we decided to further investigate DLK1_NOTCH1 pathway. Transcriptome data confirmed that DLK1 expression was restricted to CM and was higher in HC CM compared to ACM CM (Fig. 6A;
Supplementary Fig. S4B). In contrast, _DLK1_ expression levels were extremely low in stromal cells (Fig. 6A; Supplementary Fig. S4C). Notably, both CM and cMSC expressed the gene encoding
for NOTCH1, a known receptor of DLK1 (Fig. 6B; Supplementary Fig. S4B, C). DLK1 is recognized as a negative regulator of adipogenesis [19] and has been implicated in the transition from
fibroblasts to myofibroblasts [20]. We therefore hypothesized that the absence of DLK1 may contribute to the enhanced propensity of ACM cMSC to differentiate into adipocytes and
myofibroblasts in vitro. First, ACM cMSC were cultured in adipogenic medium in the presence or absence of recombinant DLK1. Following the treatment, Nile Red staining was performed to mark
neutral lipids (Fig. 6C, D). The presence of DLK1 reduced lipid accumulation in ACM cMSC compared to adipogenic medium only (Fig. 6C, D). Next, we investigated DKL1’s involvement in ACM
cMSC-mediated collagen production by culturing ACM cMSC in a pro-fibrotic medium and treated with DLK1 (Fig. 6E, F). Immunofluorescence analysis demonstrated that DLK1 treatment reduced the
production of Collagen I in ACM cMSC (Fig. 6E, F). These results underscore the crucial inhibitory role of DLK1 typically secreted by HC CM, in regulating the adipose and pro-fibrotic
differentiation of ACM cMSC. To further validate that DLK1 modulates fibro-adipose accumulation through inhibition of the NOTCH pathway, we treated ACM cMSC with the gamma secretase
inhibitor DAPT, known as an inhibitor of the NOTCH signalling pathway [21]. DAPT treatment, similarly to DLK1 treatment, was able to reduce fibro-adipose accumulation in ACM cMSC (Fig.
6G–L). We believe this effect is particularly relevant in ACM cMSC, where there is a genetic predisposition towards fibro-adipose differentiation. These results could explain the greater
differentiation capacity observed in co-cultures containing both ACM CM and ACM cMSC, compared to those where CM were HC. DISCUSSION Recent advances in understanding the mechanisms of ACM
suggest that it is a multifaceted condition involving different cell types. Our study significantly contributes to the knowledge of ACM pathogenesis by demonstrating that cell-specific
phenotypes are not solely attributable to the intrinsic properties of each cell type. Instead, these phenotypes are fundamentally influenced by interactions with surrounding cardiac cells.
To fully understand the crosstalk between CM and cMSC, we used different combinations of mutated or healthy cells (Fig.1). The genetically homogeneous co-cultures replicate naturally
occurring conditions (all HC and ACM), while the other two are artificial combinations that would never occur in a patient but are instrumental in understanding the relative contribution of
mutated cells to overall derangements. Our multicellular model was intentionally kept as simple as possible, facilitating both direct and paracrine interactions, and ensuring easy-to-read
and reproducible 2D readouts. The limitation posed by the immaturity of hiPSC CM in this model is mitigated by the presence of cMSC and longer culturing [22]. Other in vitro ACM models have
been previously published but they only partially recapitulate the disease phenotypes [22, 23]. There are limited insights in the literature on the role of extracellular factors in the
pathogenicity of cell behaviour, particularly in how they facilitate disease progression in cMSC. Various molecules- such as TGFβ1, oxLDL, NPY, inflammatory cytokines, cyclophilin A- have
been shown to exacerbate the cMSC phenotype [9, 24,25,26,27]. We demonstrated that co-culture with ACM CM can trigger fibro-adipose differentiation in stromal cells (Fig. 2). Importantly,
ACM cMSC, which are sometimes overlooked in the context of ACM pathogenesis [28], can contribute to CM dysfunctions (Fig. 3). Therefore, cMSC play both an active direct role on cardiac
substrate remodelling and an active indirect role on CM-dependent functions. Our models were capable of detecting rhythm dysfunctions through video recordings of culture movement, reflecting
both cardiomyocyte-dependent arrhythmias and the influence of surrounding cMSC. However, we acknowledge that we did not use a direct method of arrhythmia analysis (Fig. 3). In ACM, two
primary mechanisms of arrhythmia are described: “focal” (cell-based) and “re-entry” (tissue-based) arrhythmogenesis. The former, prevalent during the early, ‘concealed’ phase of ACM,
primarily involves intrinsic calcium-handling defects in cardiomyocytes, leading to early or delayed after-depolarization (EADs and DADs) [29,30,31,32]. The latter mechanism becomes evident
in advanced stages of ACM, where extensive structural alterations (fibro-fatty accumulation, mainly caused by cMSC) slow down electrical signal conduction, contributing to re-entrant
circuits [33]. Accordingly, our co-cultures exhibited a higher number of aftercontractions (the contractile counterpart of afterpotentials) in the ACM CM_ACM cMSC group compared to the ACM
CM_HC cMSC group (Fig. 3C). Additionally, incomplete relaxation was observed in our monolayers when HC stromal cells were replaced with ACM stromal cells, possibly due to calcium
accumulation (Fig. 3C). This suggests that ACM cMSC dysregulation may play a fundamental role both in focal triggered activity and, over the long term, as a source of tissue discontinuity.
Cell-cell influence occurs either through direct or paracrine interaction between ligands and receptors [34]. In silico interactome theoretically identifies both direct and paracrine
signals. Our results indicated that the most differentially identified communications were paracrine (ligand-receptor) rather than direct (between membrane proteins; Supplemental data file
S1-2). Regarding direct interactions, it has been reported that CX43 expression and localization are altered in ACM [22, 35, 36]. CX43 mediates both electric coupling, potentially leading to
crucial excitation and conduction disturbances of pro-arrhythmic significance [37], and the direct passage of small molecules. However, our analyses did not identify mutation-induced
differential interactions of hemichannels between cells pairs (Supplemental data file S1-2). Other known mechanisms of direct cell-cell interaction in ACM [16] may arise from mechanical
stimuli, resulting in mechanotransduction signals that eventually regulate cell conduct. We cannot exclude the possibility that in our co-cultures, differences in substrate stiffness or
altered contractile patterns caused by the ACM genotype in one cell type do not influence the other cell type. Among the detected paracrine interactions, significant interest was raised by
the DLK1_NOTCH1-2-3-4 pathway, which was more pronounced in conditions where CM were healthy and inhibited fibro-adipose remodelling (Fig. 4, Supplementary Figs. S5, 6, Supplemental data
file S1-2). This protein is known to regulate adipogenesis, specifically the transition of cMSC to adipocytes [19]. DLK1 acts through different pathways, during stromal cell clonal
expansion, depending on whether it is secreted or retained on the cell membrane, leading to a differentiation block of stromal cells into adipocytes [19]. The role of DLK1 in fibrosis is
controversial, but in cardiac cells, it has been reported to inhibit the process [20], which aligns with our data. As shown in Fig. 4B and Supplementary Fig. S6 and evident from Supplemental
data file S2, ACM CM lose communication with HC cMSC mediated by Collagen 3 and 5 and different integrin receptors, which are abundant in HC CM. However, a large set of signals involving
different minor forms of collagens and a1b1 or a11b1 integrin receptors were established. This reframing suggests a different role for these proteins as signalling molecules versus their
function in extracellular matrix reorganization. ACM CM developed new interactions with HC cMSC, such as TGFB2_TGFBR1 and IL6_IL6R. Both the TGFβ pathway [9, 38] and the inflammatory
cytokines response [39, 40] are known mediators of pro-fibrotic messages. Accordingly, ACM CM send novel signals to cMSC, such as FGF1 and FGF18, which typically mediate fibroblast and
pre-adipocyte proliferation [41, 42]. Notably, ephrin-ephrin receptor interactions were lost in ACM CMs. Dysregulation of this signalling pathway has already been described in ACM cells
[43]. Regarding the signals sent from cMSC (Fig. 4A, Supplementary Fig. S5, Supplemental data file S1), ACM-specific communications included IL6 and its receptor, highlighting the importance
of inflammatory cytokines, and TGFB3_TGFBR3, which may promote fibrosis. In addition, WNT5A ligand, interacting with EPHA7 or ROR2, was exclusively present in ACM cMSC, while the
interaction of WNT1 and WNT4 with FZD8, observed in HC cMSC, was abolished. Indeed, both canonical and non-canonical WNT signalling pathways have been extensively characterized as mediators
of ACM [17, 44,45,46]. Furthermore, ACM cMSC exhibited a loss of communication between PTN (Pleiotrophin) and different receptors such as PLXNB2, PTPRS, PTPRZ1 and ALK, which are likely
involved in regulating CM proliferation and survival [47,48,49]. A targeted proteomic approach allowed us to validate some of the predicted communications by identifying factors that were
effectively secreted by the different co-cultures (Fig. 5A and Supplementary Fig. S7). COL1A1, a stiff collagen [50], was mainly secreted by the ACM stromal cells in the co-cultures, with
the highest levels observed in the ACM cMSC combinations (Fig. 5B). This aligns with our immunostaining results shown in Fig. 2A and previously published data [9, 51]. MMP-9, an enzyme
involved in the remodelling of the extracellular matrix through the degradation of collagen, elastin, fibronectin, gelatin, and laminin [52], was abundant in co-cultures where stromal cells
were of the ACM type (Fig. 5C). TIMP4, a metalloproteinase inhibitor that regulates extracellular matrix balance [53], was also abundant in ACM co-cultures, likely secreted primarily by cMSC
(Fig. 5D). TIMP4 was found to be poorly expressed in ACM heart tissue, as well as in other failing hearts, but is abundant in control hearts [54]. Conversely, its circulating expression is
higher than in controls in patients with pro-fibrotic conditions such as idiopathic pulmonary fibrosis [55], though it has never been tested in ACM. uPAR, part of a proteolytic system
relevant for fibrinolysis and extracellular matrix homeostasis, plays a key role in the progression or degradation of fibrosis and was also found to be abundant in ACM co-cultures (Fig. 5E)
[56]. Platelet endothelial cell adhesion molecule (PECAM-1), a regulator of mechanosensory functions, is associated with left ventricular dilation and systolic dysfunction in mice when
absent [57] (Fig. 5F). GDF-15 [58], member of the TGF-β superfamily, is prominently induced by “injury” [59] and has been previously reported to predict biventricular involvement in ACM
[60]. In line with these findings, it was found to be abundant in co-cultures containing ACM cMSC (Fig. 5G). Among the proteins enriched in the co-cultures containing ACM CM, MB is
particularly noteworthy (Fig. 5H). MB is one of the most abundant proteins in muscle cells and is released upon injury, serving as a marker of myocardial damage in cardiomyopathies and
myocarditis [61,62,63]. Its increase suggests a higher rate of cell death in co-cultures including ACM CMs. The receptor for LDL was highly expressed in the conditioned media when ACM cMSC
were present (Fig. 5I). While its role in ACM has never been studied, lipid metabolism derangements in ACM [26] suggests a possible involvement. The identification of factors deemed relevant
in the in silico interactome converged in the secretome analysis, as seen, for instance, with DLK1 (Fig. 5L). The decision to validate this pathway is due to its recognised role in the
regulation of adipogenesis and fibrosis, known hallmarks of ACM, and the novelty of its possible involvement in the disease pathogenesis. Both ectopic DLK1 administration and NOTCH
inhibition by DAPT led to a reduction in fibro-adipose accumulation (Fig. 6), suggesting that the modulation of the newly discovered DLK1-NOTCH pathway may regulate fibro-adipose
differentiation of stromal cells in ACM. Future gene-editing experiments could confirm these results and the action of DLK1 through NOTCH. Given the progressive propensity toward
fibro-adipose differentiation of co-cultures as HC cMSC and/or HC CM are replaced by ACM counterparts (Fig. 2), we speculate that the mechanisms determined by the mutation in _PKP2_ and
those due to the absence of DLK1 are independent and additive phenomena. It is known that inflammatory components contribute to ACM pathogenesis. We acknowledge that our simplified model
cannot account for the contribution of inflammatory cells. However, we were able to observe the effect of pro-inflammatory mediators secreted either by CM or cMSC. In conclusion, we
developed a new, simple, multicellular ACM model to simulate the early/intermediate stages of phenotype development. This model has proven valuable in understanding both cell-autonomous and
crosstalk-mediated cell behaviours, as well as the underlying pathways in ACM. Deciphering the factors contributing to disease progression is crucial for devising effective therapeutic
strategies. Indeed, DLK1 can be considered as a potential therapeutic target to moderate ACM pathogenesis. This hypothesis will require further preclinical studies on complex cultures and
mice. Furthermore, this approach can be extended to investigate other genetic cardiomyopathies or other cell type pairings, aiding in the unravelling of genotype-specific cell
characteristics and cell-cell interactions. MATERIALS AND METHODS ETHICAL STATEMENT This study complies with the declaration of Helsinki and was approved by the Istituto Europeo di Oncologia
and Centro Cardiologico Monzino IRCCSs Ethics Committee (R1020/19-CCM1072; date of approval: 3/7/2019). Written and informed consent was obtained from all participants. Supplementary Tables
S1, S2 summarize the features of cMSC and hiPSC cell lines, respectively. PATIENT CELLS A right ventricle (RV) biopsy was obtained during a diagnostic procedure [64] from a patient affected
by ACM and carrying the heterozygous c.2013delC _PKP2_ mutation as previously described [8]. Fibroblasts were obtained from a skin biopsy of the same ACM patient and reprogrammed into
hiPSC. _The PKP2_ mutation was corrected using CRISPR/Cas9 technology to create an isogenic hiPSC line, as described in [65]. ISOLATION AND CULTURE OF PRIMARY CARDIAC STROMAL CELLS cMSC were
isolated from RV biopsy and cultured as previously described [8, 66]. Briefly, ventricular biopsies were washed with PBS, cut into 2–3 mm pieces, and incubated at 37 °C for 1.5 h under
continuous agitation in Iscove’s Modified Dulbecco’s media (IMDM; Gibco/Thermo-Fisher, Waltham, MA, USA) containing 3 mg/mL collagenase NB4 (Serva, Heidelberg, Germany). The digested
solution was then centrifuged at 400 g for 10 min, washed with PBS, and centrifuged again. The obtained pellet was resuspended in growth medium (GM) consisting of IMDM supplemented with 20%
fetal bovine serum (FBS; Euroclone, Pero (MI), Italy), 10 ng/mL basic fibroblast growth factor (R&D Systems, Minneapolis, MN, USA), 10,000 U/mL penicillin (Invitrogen/Thermo-Fisher,
Waltham, MA, USA), 10,000 µg/mL streptomycin (Invitrogen/Thermo-Fisher, Waltham, MA, USA), and 20 mmol/L L-Glutamine (Sigma-Aldrich, Saint Louis, MO, USA). The cells were seeded onto
uncoated Petri dishes (Corning, New York, NY, USA) and non-adherent cells were removed after 24 h. cMSC were used for the experiments between passage 3 and 8, and mycoplasma contamination
was excluded. DIFFERENTIATION OF HIPSC INTO CARDIOMYOCYTES hiPSC-CM cells were generated from undifferentiated hiPSC through the induction cardiac mesoderm as previously described [67].
Mycoplasma-free hiPSC were used to induce CM differentiation. Briefly, 25,000 cells per cm2 were seeded on Matrigel on day −1. On day 0, cardiac mesoderm was induced by BPEL medium [68]
supplemented with a mixture of cytokines [20 ng/mL BMP4, (R&D Systems, Minneapolis, MN, USA); 20 ng/mL ACTIVIN A, (Miltenyi Biotec, Bergisch Gladbach, Germany); 1.5 μM GSK3 inhibitor
CHIR99021 (Selleckchem, Houston, TX, USA)]. After 3 days, the cytokines were removed and the WNT inhibitor XAV939 (5 μM, Tocris, Bristol, UK) was added for 3 days. On day 6, medium was
replaced with BPEL medium without supplement. The medium was refreshed every 2-3 days until the beating cells appeared. Metabolic selection of hiPSC-CM with 4 mM sodium-L-lactate
(Sigma-Aldrich, Saint Louis, MO, USA) was performed twice, for 2 days each, to maximize CM enrichment [67]. After selection, the lactate medium was replaced with BPEL. CO-CULTURE ASSEMBLY On
the day of co-culture formation (day 0), cMSC were detached using Trypsin 1X for 5 min at 37 °C, 5% CO2, centrifuged for 5 min at 400 × _g_, resuspended in BPEL medium and counted. hiPSC-CM
at day 14–21 (HC CM, ACM CM) were dissociated using the Multi Tissue Dissociation Kit 3 (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer’s instructions,
resuspended in BPEL medium and counted. Cardiac co-cultures (Fig. 1A) were assembled by combining 85% hiPSC-CM and 15% primary cMSC, as previously tested [22] to a total of 150,000 cells per
1 mL BPEL in four different combinations (Fig. 1B). To differentiate the four co-cultures graphically, a colour code was assigned to each: white for the co-culture composed of healthy
control-cardiomyocytes and healthy control-stromal cells (HC CM_HC cMSC); blue for the co-culture composed of healthy control-cardiomyocytes and arrhythmogenic cardiomyopathy-stromal cells
(HC CM_ACM cMSC), green for the co-culture composed by arrhythmogenic cardiomyopathy-cardiomyocytes and healthy control-stromal cells (ACM CM_HC cMSC); and red for the co-culture composed by
arrhythmogenic cardiomyopathy-cardiomyocytes and arrhythmogenic cardiomyopathy-stromal cells (ACM cMSC_ACM CM). For all co-cultures, cell suspensions were seeded on Matrigel-coated
coverslips placed in 24-well microplates and incubated at 37 °C, 5% CO2 for 10 days with half of the media refreshed on day 5. Functional analysis of the co-cultures was performed on day 10.
STROMAL CELL DIFFERENTIATION AND TREATMENT To induce adipogenic differentiation, cells were plated at a density of 50,000 cells/cm2 and maintained in adipogenic medium, consisting of IMDM
supplemented with 10% FBS (Euroclone, Milan, Italy), 0.5 mmol/L of 3-isobutyl-1-methylxanthine (Sigma-Aldrich, Saint Louis, MO, USA), 1 µmol/L of hydrocortisone (Sigma-Aldrich, Saint Louis,
MO, USA), 0.1 mmol/L of indomethacin (Sigma-Aldrich, Saint Louis, MO, USA), 10,000 U/mL of penicillin (Invitrogen/Thermo-Fisher, Waltham, MA, USA), 10,000 µg/mL of streptomycin
(Invitrogen/Thermo-Fisher, Waltham, MA, USA), and 20 mmol/L of L-Glutamine (Sigma-Aldrich, Saint Louis, MO, USA) [51]. To induce the pro-fibrotic differentiation, cells were plated at a
density of 50,000 cells/cm2 and treated with 5 ng/mL of TGF-β1 (PeproTech, Cranbury, NJ, USA) following overnight growth in low-serum growth medium (2% FBS) [51]. Adipogenic and pro-fibrotic
differentiation were assessed after 3 days. For experiments involving DLK1 and DAPT (Abcam, Cambridge, UK) the cells were treated with serial dilutions (0–100 ng/mL range) of recombinant
DLK1 [69] or 10 μmM of DAPT [21]. DLK1 or DAPT was added daily for 3 days. DETECTION OF CELL FIBRO-ADIPOSE ACCUMULATION To detect fibro-adipose accumulation, co-cultured monolayers or single
cell population were fixed using 4% paraformaldehyde (Santa Cruz biotechnology, Dallas, TX, USA) for 10 min. This was followed by incubation with PBS supplemented with 5% BSA and 0.1%
Triton X-100 (PBS-T/BSA) for 60 min to block nonspecific binding sites. All co-culture monolayers were incubated overnight at 4 °C with specific primary antibody against α-actinin to
visualize CM population. To detect fibrotic accumulation, co-culture monolayers were co-stained with a collagen antibody (as reported in Supplementary Table S3). Subsequently, the slides
were incubated for 1 h at room temperature (RT) with specific fluorescence-labelled secondary antibodies (Invitrogen/Thermo-Fisher, Waltham, MA, USA). For the detection of lipid
accumulation, co-cultured monolayers were co-stained with 12.5 ng/mL of Nile Red (Invitrogen/Thermo-Fisher, Waltham, MA, USA) for 1 h at RT during the incubation with secondary antibody.
Nuclei were stained using Hoechst 33342 (Sigma-Aldrich, Saint Louis, MO, USA). To detect fibro-adipose accumulation in cMSC following specific differentiation induction and treatments
(described in the preceding section), slides were incubated with either collagen or Nile Red as described above. Images were acquired using a confocal microscope in Z-stack mode with a 40×
oil immersion objective (LSM710-ConfoCor3 LSM, Zeiss, Oberkochen, Germany) and the Zen 2008 software (Zeiss, Oberkochen, Germany). Optical sections were captured at a resolution of 1,024 ×
1,024 pixels, with the pinhole diameter adjusted to 1 Airy unit for each emission channel. Quantitative image analysis was performed by scanning 5-10 randomly selected fields, each
containing 15-30 cells each, while maintaining consistent acquisition parameters (laser power and detector amplification). These parameters were below the pixel saturation between the
samples of interest and the negative controls. Fluorescence signal quantification was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA) on Z-Stack images.
Each image was separated into individual channels, and converted into 8-bit grayscale images to subtract background noise. The fluorescence intensity (relative to each channel) and the
nuclei count for each field were measured using ImageJ tools. The fluorescence intensity of each channel in each field was normalized to the corresponding number of nuclei in the field.
CONTRACTILITY MEASUREMENTS Movies of beating monolayers of co-cultured hiPSC-CM and cMSC were recorded at 100 frames per second with a Kiralux CS135MUN camera (Thorlabs, Newton, NJ, USA) at
full resolution. The camera was mounted on an ECLIPSE TE-200 microscope (Nikon, Tokyo, Japan) and a 40X air objective was used. The measurements were performed at physiological temperature
(~37 °C), with temperature control provided by an in-line solution heater (SH-27B, Warner Instruments, Holliston, MA, USA) positioned in close proximity to the monolayers. The monolayers
were stimulated through a pair of gold electrodes positioned near -but not touching- the cell monolayer. The pulses were triggered by an external stimulator (3165 Multiplexing Pulse Booster
– Ugo Basile, Gemonio (VA), Italy) connected to a Molecular Devices Digidata 1440A controlled by pClamp 10 (Molecular Devices, San Jose, CA, USA). Different pacing frequencies were used:
Spontaneous, 0.5 Hz, 1 Hz, 2 Hz. The extracellular modified Tyrode’s solution used for contraction analysis contained (in mM): D-(+)-glucose 10, HEPES 5.0, NaCl 140, KCl 5.4, MgCl2 1.2,
CaCl2 1.8, pH adjusted to 7.3 with NaOH (Tyrode’s solution reagents were purchased from Sigma-Aldrich, Saint Louis, MO, USA). CONTRACTILITY ANALYSIS Contractility was analysed from a total
of n = 322 raw AVI movies, distributed over three independent batches of differentiation, using MUSCLEMOTION [18, 70]. The movies were combined through a custom-made R script
(https://github.com/l-sala/Thorlabs_Kiralux_Concatenate_Files, Thorlabs, Newton, NJ, USA). The protocol for acquiring movies for contraction analyses was as follows: 20 s of electrical
stimulation prior to recording to allow the monolayers to reach a steady-stable contractions, followed by 20 s of recording. Initially, we qualitatively evaluated the contraction patterns in
cell monolayers, observing several abnormalities. Although it is impossible to relate these abnormalities directly to arrhythmias emerging in clinical settings, these phenomena are likely
of arrhythmic significance. Such events include the following: * Failure to follow the imposed pacing frequency, due to overriding spontaneous firing. * Incomplete relaxation or increased
diastolic tension, likely indicating intracellular Ca2+ accumulation. * Presence of aftercontractions, which are indicators of spontaneous Ca2+ release events and proxies of arrhythmogenic
afterpotentials. * Presence of alternating contraction amplitude, which is commonly associated with arrhythmogenic electrical alternans or decreasing peak amplitude. * Presence of chaotic
contraction patterns, suggestive of fibrillation. * Different combinations of the above. Representative examples from each subtype are presented in Supplementary Fig. S1. Next, in the
monolayers that displayed a “normal” contractile pattern, we performed a quantitative analysis of their temporal parameters. Specifically, Contraction Duration and Normalized Contraction
Amplitude were evaluated at several pacing rates. RNA-SEQ DATA AND ANALYSIS OF CELL−CELL INTERACTIONS Homogeneous cell cultures (ACM CM, HC CM, ACM cMSC, HC CM, separately, 3 batches of
differentiation for CM and 3 batches of cMSC, each type) were lysed in RL lysis buffer (Norgen Biotek corp., Thorold, Canada). RNA was isolated from cells using a Total RNA Purification kit
(Norgen Biotek corp., Thorold, Canada). The quantification of the isolated RNA was determined by Qubit™ 4 Fluorometer (Invitrogen/Thermo-Fisher, Waltham, MA, USA) and a quality check was
performed using the Bioanalyzer (Agilent, Santa Clara, CA, USA). The RNA samples that passed the quality check were then sent to Eurofins Ltd Genomic Service for sequencing (Eurofins, Milan,
Italy). RNA libraries were prepared for transcriptome sequencing using standard Illumina protocols as part of the INVIEW Transcriptome Discover protocol. For library preparation, mRNA was
fragmented, and random hexamer-primed cDNA synthesis was performed. Paired-end read sequencing (2 ×150 bp) was performed on the Illumina HiSeq 4000. Reads were aligned to the human genome
reference (GRCh38/hg38) using BWA software (v0.7.1), and mapped reads were used to quantify gene expression with FeatureCounts (v2.0.0). The resulting gene expression matrix and the vector
containing sample information were imported into the R environment (v4.1.1). Using the DaMiRseq package [71], genes with read counts lower than 5 for at least 50% of samples were filtered
out, and the data converted to log2 counts per million mapped reads (CPM). Differential analyses were performed using the ‘limma’ R package [72]. Cell-cell communication analysis was
conducted using the CellPhoneDB package (version 3.1.0) [73]. The investigation focused on discerning enriched interactions between two distinct cell types (CM and cMSC) either healthy or
ACM. CellPhoneDB outputs included interaction scores based on the expression levels of ligands and receptors, as well as _p_-values indicating the likelihood of cell-type specificity of a
given receptor-ligand complex. These _p_-values were calculated after random permutation, based on the proportion of means that are greater than or equal to the actual mean. We reported in
Fig. 4, Supplementary Data file S1, S2 and Supplementary Figs. S5, S6 only the statistically significant interactions. The selection of biologically relevant receptor-ligand or
receptor-receptor pairs was carried out through a manual curation process based on the significance of pairs and their possible relevance in ACM pathogenesis. PROTEOMIC PROFILING OF SOLUBLE
FACTORS IN CONDITIONED MEDIA Conditioned media were generated by harvesting medium from co-cultures on day 10 to collect all soluble factors released during hiPSC-CM-cMSC interactions. The
conditioned media were quantified using the Olink multiplex proximity technology Cardiovascular III panel, which includes 92 targets (Bioscience AB, Uppsala Sweden). Briefly, each protein
was detected by oligonucleotide-labelled antibodies binding to their complementary target proteins. When two antibodies are in close proximity, a new protein-specific DNA reporter sequence
is formed through a proximity-dependent DNA polymerization reaction. The resulting sequence is then amplified and quantified by standard real-time PCR. The relative amounts of protein were
quantified as normalized protein expression (NPX), where an increase of 1 NPX represents a doubling of the relative protein concentration. Further information on the Olink assay is available
at http://www.olink.com (Olink Bioscience AB, Uppsala Sweden). Figure 5A show the results of the analysis in terms of the two most informative projections from a multiple-dimensional
scaling analysis of the samples and represents the diversity of the secretomes from the four co-cultures. Specifically, Multi-Dimensional Scaling (MDS) allow to represent the dissimilarities
between the sample proteomes in a two-dimensional space. Therefore, to avoid introducing noise by data imputation, only the samples with complete proteomes were plotted and to exclude the
samples with more than 20% of missing values randomly distributed. Instead, for the statistical analysis on a single protein (Fig. 5B–L), all samples with an expression value were used.
STATISTICAL ANALYSIS Continuous variables are presented as mean ± standard error (SEM), and categorical data as counts and proportions. A normality test was performed for each sample
variable. Normally distributed continuous variables were compared using Student’s _t_ test for independent samples. Comparisons among three or more groups were conducted with one-way ANOVA
or Kruskal-Wallis test, as appropriate, in conjunction with Tukey’s multiple comparison post-hoc tests. The proportions of the frequencies of categorical variables was compared using
Fisher’s exact test, given the sample size, with the ‘fisher.test()‘ function. Pairwise comparisons were made between the HC CM_HC cMSC group and the other co-culture subtypes. _P_-values
were adjusted for multiple comparisons using the ‘p.adjust()‘ function with the Holm method [74]. A _p_-value < 0.05 was considered statistically significant, unless otherwise indicated.
The statistical evaluation of outliers was performed before exclusion. Statistical analyses and graphics were done with either R (version 4.3.2) or GraphPad Prism 9. DATA AVAILABILITY The
data supporting the findings of this study are available within the article and its Supplementary Materials. Additional supporting data are available from the corresponding author upon
reasonable request. The transcriptome data are available in the GEO repository (ID: GSE249301). REFERENCES * Stadiotti I, Piacentini L, Vavassori C, Chiesa M, Scopece A, Guarino A, et al.
Human Cardiac Mesenchymal Stromal Cells From Right and Left Ventricles Display Differences in Number, Function, and Transcriptomic Profile. Front Physiol. 2020;11:604. Article PubMed
PubMed Central Google Scholar * Gray GA, Toor IS, Castellan R, Crisan M, Meloni M. Resident cells of the myocardium: more than spectators in cardiac injury, repair and regeneration. Curr
Opin Physiol. 2018;1:46–51. Article CAS PubMed PubMed Central Google Scholar * Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, et al. Revisiting Cardiac Cellular
Composition. Circ Res. 2016;118:400–9. Article CAS PubMed Google Scholar * Hall C, Gehmlich K, Denning C, Pavlovic D. Complex Relationship Between Cardiac Fibroblasts and Cardiomyocytes
in Health and Disease. J Am Heart Assoc. 2021;10:e019338. Article CAS PubMed PubMed Central Google Scholar * Pilichou K, Thiene G, Bauce B, Rigato I, Lazzarini E, Migliore F, et al.
Arrhythmogenic cardiomyopathy. Orphanet J Rare Dis. 2016;11:33. Article PubMed PubMed Central Google Scholar * El-Battrawy I, Zhao Z, Lan H, Cyganek L, Tombers C, Li X, et al. Electrical
dysfunctions in human-induced pluripotent stem cell-derived cardiomyocytes from a patient with an arrhythmogenic right ventricular cardiomyopathy. Europace. 2018;20:f46–f56. Article PubMed
Google Scholar * Gerull B, Heuser A, Wichter T, Paul M, Basson CT, McDermott DA, et al. Mutations in the desmosomal protein plakophilin-2 are common in arrhythmogenic right ventricular
cardiomyopathy. Nat Genet. 2004;36:1162–4. Article CAS PubMed Google Scholar * Sommariva E, Brambilla S, Carbucicchio C, Gambini E, Meraviglia V, Dello Russo A, et al. Cardiac
mesenchymal stromal cells are a source of adipocytes in arrhythmogenic cardiomyopathy. Eur Heart J. 2016;37:1835–46. Article CAS PubMed Google Scholar * Maione AS, Stadiotti I, Pilato
CA, Perrucci GL, Saverio V, Catto V, et al. Excess TGF-beta1 Drives Cardiac Mesenchymal Stromal Cells to a Pro-Fibrotic Commitment in Arrhythmogenic Cardiomyopathy. Int J Mol Sci.
2021;22:2673. * Lombardi R, da Graca Cabreira-Hansen M, Bell A, Fromm RR, Willerson JT, Marian AJ. Nuclear plakoglobin is essential for differentiation of cardiac progenitor cells to
adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ Res. 2011;109:1342–53. Article CAS PubMed PubMed Central Google Scholar * Soliman H, Paylor B, Scott RW, Lemos DR,
Chang C, Arostegui M, et al. Pathogenic Potential of Hic1-Expressing Cardiac Stromal Progenitors. Cell Stem Cell. 2020;26:205–20 e8. Article CAS PubMed Google Scholar * Lombardi R, Chen
SN, Ruggiero A, Gurha P, Czernuszewicz GZ, Willerson JT, et al. Cardiac Fibro-Adipocyte Progenitors Express Desmosome Proteins and Preferentially Differentiate to Adipocytes Upon Deletion of
the Desmoplakin Gene. Circ Res. 2016;119:41–54. Article CAS PubMed PubMed Central Google Scholar * Kohela A, van Kampen SJ, Moens T, Wehrens M, Molenaar B, Boogerd CJ, et al.
Epicardial differentiation drives fibro-fatty remodeling in arrhythmogenic cardiomyopathy. Sci Transl Med. 2021;13:eabf2750. Article CAS PubMed Google Scholar * Brown RD, Ambler SK,
Mitchell MD, Long CS. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharm Toxicol. 2005;45:657–87. Article CAS Google Scholar * Lippi M, Chiesa
M, Ascione C, Pedrazzini M, Mushtaq S, Rovina D, et al. Spectrum of Rare and Common Genetic Variants in Arrhythmogenic Cardiomyopathy Patients. Biomolecules. 2022;12:1043. * Beffagna G,
Sommariva E, Bellin M. Mechanotransduction and Adrenergic Stimulation in Arrhythmogenic Cardiomyopathy: An Overview of in vitro and in vivo Models. Front Physiol. 2020;11:568535. Article
PubMed PubMed Central Google Scholar * Maione AS, Faris P, Iengo L, Catto V, Bisonni L, Lodola F, et al. Ca(2+) dysregulation in cardiac stromal cells sustains fibro-adipose remodeling in
Arrhythmogenic Cardiomyopathy and can be modulated by flecainide. J Transl Med. 2022;20:522. Article CAS PubMed PubMed Central Google Scholar * Sala L, van Meer BJ, Tertoolen LGJ,
Bakkers J, Bellin M, Davis RP, et al. MUSCLEMOTION: A Versatile Open Software Tool to Quantify Cardiomyocyte and Cardiac Muscle Contraction In Vitro and In Vivo. Circ Res. 2018;122:e5–e16.
Article CAS PubMed PubMed Central Google Scholar * da Silva C, Durandt C, Kallmeyer K, Ambele MA, Pepper MS. The Role of Pref-1 during Adipogenic Differentiation: An Overview of
Suggested Mechanisms. Int J Mol Sci. 2020;21:4041. * Rodriguez P, Sassi Y, Troncone L, Benard L, Ishikawa K, Gordon RE, et al. Deletion of delta-like 1 homologue accelerates
fibroblast-myofibroblast differentiation and induces myocardial fibrosis. Eur Heart J. 2019;40:967–78. Article CAS PubMed Google Scholar * Dees C, Zerr P, Tomcik M, Beyer C, Horn A,
Akhmetshina A, et al. Inhibition of Notch signaling prevents experimental fibrosis and induces regression of established fibrosis. Arthritis Rheum. 2011;63:1396–404. Article CAS PubMed
PubMed Central Google Scholar * Giacomelli E, Meraviglia V, Campostrini G, Cochrane A, Cao X, van Helden RWJ, et al. Human-iPSC-Derived Cardiac Stromal Cells Enhance Maturation in 3D
Cardiac Microtissues and Reveal Non-cardiomyocyte Contributions to Heart Disease. Cell Stem Cell. 2020;26:862–79.e11. Article CAS PubMed PubMed Central Google Scholar * Bliley JM,
Vermeer M, Duffy RM, Batalov I, Kramer D, Tashman JW, et al. Dynamic loading of human engineered heart tissue enhances contractile function and drives a desmosome-linked disease phenotype.
Sci Transl Med. 2021;13:eabd1817. * Van Linthout S, Miteva K, Tschope C. Crosstalk between fibroblasts and inflammatory cells. Cardiovasc Res. 2014;102:258–69. Article PubMed Google
Scholar * Rurali E, Pilato CA, Perrucci GL, Scopece A, Stadiotti I, Moschetta D, et al. Cyclophilin A in Arrhythmogenic Cardiomyopathy Cardiac Remodeling. Int J Mol Sci. 2019;20:2403. *
Sommariva E, Stadiotti I, Casella M, Catto V, Dello Russo A, Carbucicchio C, et al. Oxidized LDL-dependent pathway as new pathogenic trigger in arrhythmogenic cardiomyopathy. EMBO Mol Med.
2021;13:e14365. Article CAS PubMed PubMed Central Google Scholar * Stadiotti I, Di Bona A, Pilato CA, Scalco A, Guarino A, Micheli B, et al. Neuropeptide Y promotes adipogenesis of
human cardiac mesenchymal stromal cells in arrhythmogenic cardiomyopathy. Int J Cardiol. 2021;342:94–102. Article PubMed Google Scholar * Reisqs JB, Moreau A, Sleiman Y, Boutjdir M,
Richard S, Chevalier P. Arrhythmogenic cardiomyopathy as a myogenic disease: highlights from cardiomyocytes derived from human induced pluripotent stem cells. Front Physiol. 2023;14:1191965.
Article CAS PubMed PubMed Central Google Scholar * Gomes J, Finlay M, Ahmed AK, Ciaccio EJ, Asimaki A, Saffitz JE, et al. Electrophysiological abnormalities precede overt structural
changes in arrhythmogenic right ventricular cardiomyopathy due to mutations in desmoplakin-A combined murine and human study. Eur Heart J. 2012;33:1942–53. Article CAS PubMed PubMed
Central Google Scholar * Cerrone M, Montnach J, Lin X, Zhao YT, Zhang M, Agullo-Pascual E, et al. Plakophilin-2 is required for transcription of genes that control calcium cycling and
cardiac rhythm. Nat Commun. 2017;8:106. Article PubMed PubMed Central Google Scholar * Brodehl A, Belke DD, Garnett L, Martens K, Abdelfatah N, Rodriguez M, et al. Transgenic mice
overexpressing desmocollin-2 (DSC2) develop cardiomyopathy associated with myocardial inflammation and fibrotic remodeling. PloS One. 2017;12:e0174019. Article PubMed PubMed Central
Google Scholar * Song Z, Ko CY, Nivala M, Weiss JN, Qu Z. Calcium-voltage coupling in the genesis of early and delayed afterdepolarizations in cardiac myocytes. Biophysical J.
2015;108:1908–21. Article CAS Google Scholar * van der Voorn SM, Te Riele A, Basso C, Calkins H, Remme CA, van Veen TAB. Arrhythmogenic cardiomyopathy: pathogenesis, pro-arrhythmic
remodelling, and novel approaches for risk stratification and therapy. Cardiovasc Res. 2020;116:1571–84. Article PubMed PubMed Central Google Scholar * Segers VFM, De Keulenaer GW.
Autocrine Signaling in Cardiac Remodeling: A Rich Source of Therapeutic Targets. J Am Heart Assoc. 2021;10:e019169. Article CAS PubMed PubMed Central Google Scholar * Fidler LM, Wilson
GJ, Liu F, Cui X, Scherer SW, Taylor GP, et al. Abnormal connexin43 in arrhythmogenic right ventricular cardiomyopathy caused by plakophilin-2 mutations. J Cell Mol Med. 2009;13:4219–28.
Article CAS PubMed Google Scholar * Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M. Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells.
Circulation Res. 2007;101:703–11. Article CAS PubMed Google Scholar * Severs NJ, Dupont E, Coppen SR, Halliday D, Inett E, Baylis D, et al. Remodelling of gap junctions and connexin
expression in heart disease. Biochim Biophys Acta. 2004;1662:138–48. Article CAS PubMed Google Scholar * Lu W, Li Y, Dai Y, Chen K. Dominant Myocardial Fibrosis and Complex Immune
Microenvironment Jointly Shape the Pathogenesis of Arrhythmogenic Right Ventricular Cardiomyopathy. Front Cardiovasc Med. 2022;9:900810. Article CAS PubMed PubMed Central Google Scholar
* Chelko SP, Asimaki A, Lowenthal J, Bueno-Beti C, Bedja D, Scalco A, et al. Therapeutic Modulation of the Immune Response in Arrhythmogenic Cardiomyopathy. Circulation. 2019;140:1491–505.
Article CAS PubMed PubMed Central Google Scholar * Campian ME, Verberne HJ, Hardziyenka M, de Groot EA, van Moerkerken AF, van Eck-Smit BL, et al. Assessment of inflammation in
patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Eur J Nucl Med Mol Imaging. 2010;37:2079–85. Article PubMed PubMed Central Google Scholar * Hu MC, Wang YP, Qiu
WR. Human fibroblast growth factor-18 stimulates fibroblast cell proliferation and is mapped to chromosome 14p11. Oncogene. 1999;18:2635–42. Article CAS PubMed Google Scholar * Hutley L,
Shurety W, Newell F, McGeary R, Pelton N, Grant J, et al. Fibroblast growth factor 1: a key regulator of human adipogenesis. Diabetes. 2004;53:3097–106. Article CAS PubMed Google Scholar
* Rainer J, Meraviglia V, Blankenburg H, Piubelli C, Pramstaller PP, Paolin A, et al. The arrhythmogenic cardiomyopathy-specific coding and non-coding transcriptome in human cardiac
stromal cells. BMC Genomics. 2018;19:491. Article PubMed PubMed Central Google Scholar * Garcia-Gras E, Lombardi R, Giocondo MJ, Willerson JT, Schneider MD, Khoury DS, et al. Suppression
of canonical Wnt/beta-catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. J Clin Invest. 2006;116:2012–21. Article CAS
PubMed PubMed Central Google Scholar * Parrotta EI, Procopio A, Scalise S, Esposito C, Nicoletta G, Santamaria G, et al. Deciphering the Role of Wnt and Rho Signaling Pathway in
iPSC-Derived ARVC Cardiomyocytes by In Silico Mathematical Modeling. Int J Mol Sci. 2021;22:2004. * van der Voorn SM, Bourfiss M, Muller SA, Cimen T, Saguner AM, Duru F, et al. Circulating
Biomarkers of Fibrosis Formation in Patients with Arrhythmogenic Cardiomyopathy. Biomedicines. 2023;11:813. * Chen HW, Yu SL, Chen WJ, Yang PC, Chien CT, Chou HY, et al. Dynamic changes of
gene expression profiles during postnatal development of the heart in mice. Heart. 2004;90:927–34. Article CAS PubMed PubMed Central Google Scholar * Stoica GE, Kuo A, Aigner A, Sunitha
I, Souttou B, Malerczyk C, et al. Identification of anaplastic lymphoma kinase as a receptor for the growth factor pleiotrophin. J Biol Chem. 2001;276:16772–9. Article CAS PubMed Google
Scholar * Wellstein A, Fang WJ, Khatri A, Lu Y, Swain SS, Dickson RB, et al. A heparin-binding growth factor secreted from breast cancer cells homologous to a developmentally regulated
cytokine. J Biol Chem. 1992;267:2582–7. Article CAS PubMed Google Scholar * Frangogiannis NG. The Extracellular Matrix in Ischemic and Nonischemic Heart Failure. Circulation Res.
2019;125:117–46. Article CAS PubMed Google Scholar * Maione AS, Meraviglia V, Iengo L, Rabino M, Chiesa M, Catto V, et al. Patient-specific primary and pluripotent stem cell-derived
stromal cells recapitulate key aspects of arrhythmogenic cardiomyopathy. Sci Rep. 2023;13:16179. Article CAS PubMed PubMed Central Google Scholar * Cancemi P, Aiello A, Accardi G,
Caldarella R, Candore G, Caruso C, et al. The Role of Matrix Metalloproteinases (MMP-2 and MMP-9) in Ageing and Longevity: Focus on Sicilian Long-Living Individuals (LLIs). Mediators
Inflamm. 2020;2020:8635158. Article PubMed PubMed Central Google Scholar * Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, Ramirez-Acuna JM, Perez-Romero BA, Guerrero-Rodriguez
JF, et al. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int J Mol Sci. 2020;21:9739. Article PubMed PubMed Central Google Scholar * Wei Y, Cui C,
Lainscak M, Zhang X, Li J, Huang J, et al. Type-specific dysregulation of matrix metalloproteinases and their tissue inhibitors in end-stage heart failure patients: relationship between
MMP-10 and LV remodelling. J Cell Mol Med. 2011;15:773–82. Article CAS PubMed Google Scholar * Todd JL, Vinisko R, Liu Y, Neely ML, Overton R, Flaherty KR, et al. Circulating matrix
metalloproteinases and tissue metalloproteinase inhibitors in patients with idiopathic pulmonary fibrosis in the multicenter IPF-PRO Registry cohort. BMC Pulm Med. 2020;20:64. Article CAS
PubMed PubMed Central Google Scholar * Kanno Y. The uPA/uPAR System Orchestrates the Inflammatory Response, Vascular Homeostasis, and Immune System in Fibrosis Progression. Int J Mol Sci.
2023;24:1796. * Allbritton-King JD, Garcia-Cardena G. Endothelial cell dysfunction in cardiac disease: driver or consequence? Front Cell Dev Biol 2023;11:1278166. Article PubMed PubMed
Central Google Scholar * Kaya SI, Cetinkaya A, Ozcelikay G, Samanci SN, Ozkan SA. Approaches and Challenges for Biosensors for Acute and Chronic Heart Failure. Biosensors. 2023;13:282. *
Rochette L, Dogon G, Zeller M, Cottin Y, Vergely C. GDF15 and Cardiac Cells: Current Concepts and New Insights. Int J Mol Sci. 2021;22:8889. * Akdis D, Chen L, Saguner AM, Zhang N, Gawinecka
J, Saleh L, et al. Novel plasma biomarkers predicting biventricular involvement in arrhythmogenic right ventricular cardiomyopathy. Am Heart J. 2022;244:66–76. Article CAS PubMed Google
Scholar * Horike K, Fujiwara H, Matsuda M, Kawamura A, Ishida M, Takemura G, et al. Relation between myoglobin and cardiac dysfunction in myocarditis-immunohistochemical study of
endomyocardial biopsy specimens. Jpn Circulation Journay. 1991;55:24–32. Article CAS Google Scholar * Kottwitz J, Bruno KA, Berg J, Salomon GR, Fairweather D, Elhassan M, et al. Myoglobin
for Detection of High-Risk Patients with Acute Myocarditis. J Cardiovascular Transl Res. 2020;13:853–63. Article Google Scholar * Li X, Luo R, Jiang R, Kong H, Tang Y, Shu Y, et al. The
prognostic use of serum concentrations of cardiac troponin-I, CK-MB and myoglobin in patients with idiopathic dilated cardiomyopathy. Heart Lung. 2014;43:219–24. Article PubMed Google
Scholar * Casella M, Dello Russo A, Vettor G, Lumia G, Catto V, Sommariva E, et al. Electroanatomical mapping systems and intracardiac echo integration for guided endomyocardial biopsy.
Expert Rev Med Devices. 2017;14:609–19. Article CAS PubMed Google Scholar * Meraviglia V, Arendzen CH, Tok M, Freund C, Maione AS, Sommariva E, et al. Generation of human induced
pluripotent stem cell line LUMCi027-A and its isogenic gene-corrected line from a patient affected by arrhythmogenic cardiomyopathy and carrying the c.2013delC PKP2 mutation. Stem Cell Res.
2020;46:101835. Article CAS PubMed Google Scholar * Pilato CA, Stadiotti I, Maione AS, Saverio V, Catto V, Tundo F, et al. Isolation and Characterization of Cardiac Mesenchymal Stromal
Cells from Endomyocardial Bioptic Samples of Arrhythmogenic Cardiomyopathy Patients. J Vis Exp. 2018;132:57263. * Campostrini G, Meraviglia V, Giacomelli E, van Helden RWJ, Yiangou L, Davis
RP, et al. Generation, functional analysis and applications of isogenic three-dimensional self-aggregating cardiac microtissues from human pluripotent stem cells. Nat Protoc.
2021;16:2213–56. Article CAS PubMed PubMed Central Google Scholar * Ng ES, Davis R, Stanley EG, Elefanty AG. A protocol describing the use of a recombinant protein-based, animal
product-free medium (APEL) for human embryonic stem cell differentiation as spin embryoid bodies. Nat Protoc. 2008;3:768–76. Article CAS PubMed Google Scholar * Grassi ES, Jeannot P,
Pantazopoulou V, Berg TJ, Pietras A. Niche-derived soluble DLK1 promotes glioma growth. Neoplasia. 2020;22:689–701. Article CAS PubMed PubMed Central Google Scholar * van Meer BJ, Sala
L, Tertoolen LGJ, Smith GL, Burton FL, Mummery CL. Quantification of Muscle Contraction In Vitro and In Vivo Using MUSCLEMOTION Software: From Stem Cell-Derived Cardiomyocytes to Zebrafish
and Human Hearts. Curr Protoc Hum Genet. 2018;99:e67. Article PubMed Google Scholar * Chiesa M, Colombo GI, Piacentini L. DaMiRseq-an R/Bioconductor package for data mining of RNA-Seq
data: normalization, feature selection and classification. Bioinformatics. 2018;34:1416–8. Article CAS PubMed Google Scholar * Smyth GK, Michaud J, Scott HS. Use of within-array
replicate spots for assessing differential expression in microarray experiments. Bioinformatics. 2005;21:2067–75. Article CAS PubMed Google Scholar * Efremova M, Vento-Tormo M, Teichmann
SA, Vento-Tormo R. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat Protoc. 2020;15:1484–506. Article CAS PubMed
Google Scholar * Holm S. A Simple Sequentially Rejective Multiple Test Procedure. Scand J Stat. 1977;6:65–70. Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to the
patients who consented to participate in this study. We extend our special thanks to Niyati Raj for her assistance with English editing. FUNDING The research was funded by the Italian
Ministry of Health, Ricerca Corrente to Centro Cardiologico Monzino IRCCS (project n. 2775033), the Transnational Research Projects on Cardiovascular Diseases (ACM-HF JTC2016_FP-40-021) to
Giulio Pompilio, Fondazione di Comunità Milano from funds of Fondazione Giacomo Ponzone (2023) to Elena Sommariva and Angela Serena Maione. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Unit
of Vascular Biology and Regenerative Medicine, Centro Cardiologico Monzino IRCCS, 20138, Milan, Italy Angela Serena Maione, Lara Iengo, Melania Lippi, Giulio Pompilio & Elena Sommariva
* Center for Cardiac Arrhythmias of Genetic Origin and Laboratory of Cardiovascular Genetics, Istituto Auxologico Italiano IRCCS, 20095, Milan, Italy Luca Sala * Department of Biotechnology
and Biosciences, University of Milano-Bicocca, Milan, 20126, Italy Luca Sala, Chiara Florindi, Francesco Lodola & Antonio Zaza * Unit for the Study of Aortic, Valvular and Coronary
Pathologies, Centro Cardiologico Monzino IRCCS, 20138, Milan, Italy Ilaria Massaiu & Paolo Poggio * Bioinformatics and Artificial Intelligence Facility, Centro Cardiologico Monzino
IRCCS, 20138, Milan, Italy Mattia Chiesa * Department of Electronics, Information and Biomedical Engineering, Politecnico di Milano, 20133, Milan, Italy Mattia Chiesa * Unit of Functional
Proteomics, Metabolomics, and Network Analysis, Centro Cardiologico Monzino IRCCS, 20138, Milan, Italy Stefania Ghilardi & Cristina Banfi * Department of Clinical Electrophysiology and
Cardiac Pacing, Centro Cardiologico Monzino IRCCS, 20138, Milan, Italy Claudio Tondo & Marco Schiavone * Department of Biomedical, Surgical and Dental Sciences, Università degli Studi di
Milano, 20122, Milan, Italy Claudio Tondo, Giulio Pompilio & Paolo Poggio * Department of Systems Medicine, University of Rome Tor Vergata, 00133, Rome, Italy Marco Schiavone Authors *
Angela Serena Maione View author publications You can also search for this author inPubMed Google Scholar * Lara Iengo View author publications You can also search for this author inPubMed
Google Scholar * Luca Sala View author publications You can also search for this author inPubMed Google Scholar * Ilaria Massaiu View author publications You can also search for this author
inPubMed Google Scholar * Mattia Chiesa View author publications You can also search for this author inPubMed Google Scholar * Melania Lippi View author publications You can also search for
this author inPubMed Google Scholar * Stefania Ghilardi View author publications You can also search for this author inPubMed Google Scholar * Chiara Florindi View author publications You
can also search for this author inPubMed Google Scholar * Francesco Lodola View author publications You can also search for this author inPubMed Google Scholar * Antonio Zaza View author
publications You can also search for this author inPubMed Google Scholar * Claudio Tondo View author publications You can also search for this author inPubMed Google Scholar * Marco
Schiavone View author publications You can also search for this author inPubMed Google Scholar * Cristina Banfi View author publications You can also search for this author inPubMed Google
Scholar * Giulio Pompilio View author publications You can also search for this author inPubMed Google Scholar * Paolo Poggio View author publications You can also search for this author
inPubMed Google Scholar * Elena Sommariva View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.S.M. and E.S. conceived the experiments,
A.S.M., L.I., L.S., M.L., S.G., C.F. conducted the experiments, C.T. and M.S. selected patients and took care of the clinical characterization, A.S.M., L.S., I.M., C. B., and M.C. analysed
the results, A.S.M, E.S., P.P., A.Z., F.L., and G.P. helped with the interpretation of the results, C.T., G.P., and E.S managed funds, all authors reviewed the manuscript. CORRESPONDING
AUTHOR Correspondence to Angela Serena Maione. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature
remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION VIDEO S1 VIDEO S2 VIDEO S3 VIDEO S4 SUPPLEMENTARY MATERIALS
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Maione, A.S., Iengo, L., Sala, L. _et al._ Cardiomyocyte and stromal cell cross-talk influences the pathogenesis of arrhythmogenic cardiomyopathy: a multi-level analysis uncovers
DLK1-NOTCH pathway role in fibro-adipose remodelling. _Cell Death Discov._ 10, 484 (2024). https://doi.org/10.1038/s41420-024-02232-8 Download citation * Received: 30 April 2024 * Revised:
21 October 2024 * Accepted: 29 October 2024 * Published: 28 November 2024 * DOI: https://doi.org/10.1038/s41420-024-02232-8 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
Trending News
Construction of axial chirality via palladium/chiral norbornene cooperative catalysisABSTRACT Axially chiral biaryls are common structural motifs in functional materials, bioactive natural products, pharma...
Exploring differentiated instructionCAROLTOMLINSON: Differentiation would suggest that fairness happens not when we treat everyone as though they were the s...
Delhi bomb scare: Around 100 schools get threat mails, cops say nothing foundNewsletters ePaper Sign in HomeIndiaKarnatakaOpinionWorldBusinessSportsVideoEntertainmentDH SpecialsOperation SindoorNew...
What are france services points and how can they help you?HERE IS HOW TO FIND THE SITE NEAREST YOU AS MANY MORE OPEN AROUND THE COUNTRY France Services is a network of informatio...
Medicare needs to cover the full spectrum of care for substance use disordersImagine if Medicare covered treatments for stage 1 or stage 4 cancers, but nothing in between. Absurd, right? Yet that i...
Latests News
Cardiomyocyte and stromal cell cross-talk influences the pathogenesis of arrhythmogenic cardiomyopathy: a multi-level analysis uncovers dlk1-notch patABSTRACT Arrhythmogenic Cardiomyopathy (ACM) is a life-threatening, genetically determined disease primarily caused by m...
Training camp for aspiring youth to join security forces held in handwaraKupwara, Jan 30: A special training camp has been organised with joint efforts of civil administration, education depart...
Novak djokovic 'concern' raised after first-round win at wimbledonNovak Djokovic’s first-round win over Jack Draper at Wimbledon on Monday was fairly straightforward but there was one co...
In pictures: police implement corona curfew in kashmirIn Pictures: Police Implement Corona Curfew in Kashmir GK Photo Desk April 25, 2021 2:52 am No Comments A security pers...
A lazy afternoon in the charming lipp of luxuryThe best time to visit a Paris brasserie might be midafternoon, the magic hours between lunch and dinner, when there is ...