Construction of axial chirality via palladium/chiral norbornene cooperative catalysis
Construction of axial chirality via palladium/chiral norbornene cooperative catalysis"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
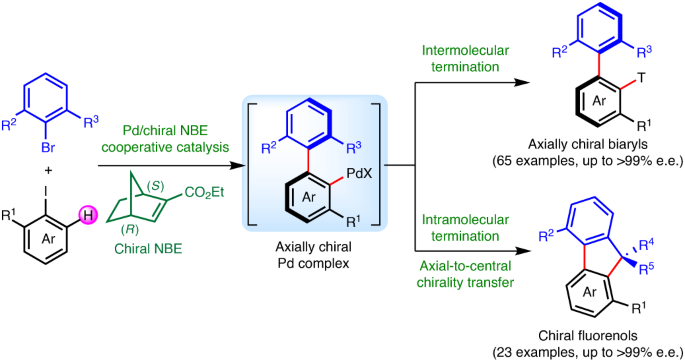
ABSTRACT Axially chiral biaryls are common structural motifs in functional materials, bioactive natural products, pharmaceuticals and chiral catalysts/ligands. As such, efficient preparation
of these privileged scaffolds is an important endeavour in organic chemistry. Herein we report a general and modular platform technology for the construction of axial chirality via
palladium/chiral norbornene cooperative catalysis. It is a three-component cascade process that involves widely available aryl iodides, 2,6-substituted aryl bromides and olefins (or alkynes,
boronic acids and so on) as the reactants. A wide variety of substrates bearing an assortment of functional groups (88 examples) are compatible with this method. Other features include a
distinct stereoinduction model, excellent enantioselectivities, step economy and scalability. This method is also amenable for the synthesis of chiral fluorenols through axial-to-central
chirality transfer in high stereochemical fidelity. We anticipate that this work will have broad synthetic utilities in chiral ligands and catalyst-design for asymmetric catalysis. Access
through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other
Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 digital issues and online
access to articles $119.00 per year only $9.92 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local
taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING
VIEWED BY OTHERS BIS-INDOLE CHIRAL ARCHITECTURES FOR ASYMMETRIC CATALYSIS Article Open access 17 April 2025 CHIRAL DINITROGEN LIGAND ENABLED ASYMMETRIC PD/NORBORNENE COOPERATIVE CATALYSIS
TOWARD THE ASSEMBLY OF C–N AXIALLY CHIRAL SCAFFOLDS Article Open access 08 June 2024 CATALYTIC ATROPOSELECTIVE SYNTHESIS OF AXIALLY CHIRAL BENZONITRILES VIA CHIRALITY CONTROL DURING BOND
DISSOCIATION AND CN GROUP FORMATION Article Open access 10 January 2022 DATA AVAILABILITY Data relating to the materials, optimization studies, experimental procedures and characterization
of the new compounds are available in the Supplementary Information. Crystallographic data for 4g, 4da and 5j are available free of charge from the Cambridge Crystallographic Database Centre
(CCDC) under reference numbers 1946138, 1946094 and 1946139, respectively. All other data are available from the authors on reasonable request. REFERENCES * Kumarasamy, E., Raghunathan, R.,
Sibi, M. P. & Sivaguru, J. Nonbiaryl and heterobiaryl atropisomers: molecular templates with promise for atropselective chemical transformations. _Chem. Rev._ 115, 11239–11300 (2015).
Article CAS Google Scholar * Li, Q. et al. Reversible photoswitchable axially chiral dopants with high helical twisting power. _J. Am. Chem. Soc._ 129, 12908–12909 (2007). Article CAS
Google Scholar * Smyth, J. E., Butler, N. M. & Keller, P. A. A twist of nature–the significance of atropisomers in biological systems. _Nat. Prod. Rep._ 32, 1562–1583 (2015). Article
CAS Google Scholar * Clayden, J., Moran, W. J., Edwards, P. J. & LaPlante, S. R. The challenge of atropisomerism in drug discovery. _Angew. Chem. Int. Ed._ 48, 6398–6401 (2009).
Article CAS Google Scholar * Noyori, R. & Takaya, H. BINAP: an efficient chiral element for asymmetric catalysis. _Acc. Chem. Res._ 23, 345–350 (1990). Article CAS Google Scholar *
Chen, Y., Yekta, S. & Yudin, A. K. Modified BINOL ligands in asymmetric catalysis. _Chem. Rev._ 103, 3155–3211 (2003). Article CAS Google Scholar * Parmar, D., Sugiono, E., Raja, S.
& Rueping, M. Complete field guide to asymmetric BINOL-phosphate derived Brønsted acid and metal catalysis: history and classification by mode of activation; Brønsted acidity, hydrogen
bonding, ion pairing, and metal phosphates. _Chem. Rev._ 114, 9047–9153 (2014). Article CAS Google Scholar * Shirakawa, S., Wu, X. & Maruoka, K. Kinetic resolution of axially chiral
2-amino-1,1′-biaryls by phase-transfer-catalyzed _N_-allylation. _Angew. Chem. Int. Ed._ 52, 4312–4348 (2013). Article CAS Google Scholar * Chen, J. et al. Carbonyl catalysis enables a
biomimetic asymmetric Mannich reaction. _Science_ 360, 1438–1442 (2018). Article CAS Google Scholar * Wencel-Delord, J., Panossian, A., Leroux, F. R. & Colobert, F. Recent advances
and new concepts for the synthesis of axially stereoenriched biaryls. _Chem. Soc. Rev._ 44, 3418–3430 (2015). Article CAS Google Scholar * Loxq, P., Manoury, E., Poli, R., Deydier, E.
& Labande, A. Synthesis of axially chiral biaryl compounds by asymmetric catalytic reactions with transition metals. _Coord. Chem. Rev._ 308, 131–190 (2016). Article CAS Google Scholar
* Yang, H., Yang, X. & Tang, W. Transition-metal catalyzed asymmetric carbon–carbon cross-coupling with chiral ligands. _Tetrahedron_ 72, 6143–6174 (2016). Article CAS Google Scholar
* Hazra, C. K., Dherbassy, Q., Wencel-Delord, J. & Colobert, F. Synthesis of axially chiral biaryls through sulfoxide-directed asymmetric mild C–H activation and dynamic kinetic
resolution. _Angew. Chem. Int. Ed._ 53, 13871–13875 (2014). Article CAS Google Scholar * Gustafson, J. L., Lim, D. & Miller, S. J. Dynamic kinetic resolution of biaryl atropisomers
via peptide-catalyzed asymmetric bromination. _Science_ 328, 1251–1255 (2010). Article CAS Google Scholar * Ma, G. & Sibi, M. P. Catalytic kinetic resolution of biaryl compounds.
_Chem. Eur. J._ 21, 11644–11657 (2015). Article CAS Google Scholar * Liao, G., Zhou, T., Yao, Q.-J. & Shi, B.-F. Recent advances in the synthesis of axially chiral biaryls via
transition metal-catalysed asymmetric C–H functionalization. _Chem. Commun._ 55, 8514–8523 (2019). Article CAS Google Scholar * Giri, R., Shi, B.-F., Engle, K. M., Maugel, N. & Yu,
J.-Q. Transition metal-catalyzed C–H activation reactions: diastereoselectivity and enantioselectivity. _Chem. Soc. Rev._ 38, 3242–3272 (2009). Article CAS Google Scholar * Hayashi, T.,
Hayashizaki, K., Kiyoi, T. & Ito, Y. Asymmetric synthesis catalyzed by chiral ferrocenylphosphine-transition-metal complexes. 6 Practical asymmetric synthesis of 1,1′-binaphthyls via
asymmetric cross-coupling with a chiral [(alkoxyalkyl)ferrocenyl]monophosphine/nickel catalyst. _J. Am. Chem. Soc._ 110, 8153–8156 (1988). Article CAS Google Scholar * Shen, D., Xu, Y.
& Shi, S.-L. A bulky chiral N‑heterocyclic carbene palladium catalyst enables highly enantioselective Suzuki–Miyaura cross-coupling reactions for the synthesis of biaryl atropisomers.
_J. Am. Chem. Soc._ 141, 14938–14945 (2019). Article CAS Google Scholar * Qi, L.-W., Li, S., Xiang, S.-H., Wang, J. & Tan, B. Asymmetric construction of atropisomeric biaryls via a
redox neutral cross-coupling strategy. _Nat. Catal._ 2, 314–323 (2019). Article CAS Google Scholar * Link, A. & Sparr, C. Stereoselective arene formation. _Chem. Soc. Rev._ 47,
3804–3815 (2018). Article CAS Google Scholar * Zhao, K. et al. Enhanced reactivity by torsional strain of cyclic diaryliodonium in Cu-catalyzed enantioselective ring-opening reaction.
_Chem_ 4, 599–612 (2018). Article CAS Google Scholar * Wang, Y.-B. & Tan, B. Construction of axially chiral compounds via asymmetric organocatalysis. _Acc. Chem. Res._ 51, 534–547
(2018). Article CAS Google Scholar * Yamaguchi, K., Yamaguchi, J., Studer, A. & Itami, K. Hindered biaryls by C–H coupling: bisoxazoline-Pd catalysis leading to enantioselective C–H
coupling. _Chem. Sci._ 3, 2165–2169 (2012). Article CAS Google Scholar * Jia, Z.-J. et al. General enantioselective C–H activation with efficiently tunable cyclopentadienyl ligands.
_Angew. Chem. Int. Ed._ 56, 2429–2434 (2017). Article CAS Google Scholar * Jang, Y.-S., Woźniak, Ł., Pedroni, J. & Cramer, N. Access to _P_‐ and axially chiral biaryl phosphine oxides
by enantioselective Cp_x_ IrIII atalyzed C–H arylations. _Angew. Chem. Int. Ed._ 57, 12901–12905 (2018). Article CAS Google Scholar * Catellani, M., Frignani, F. & Rangoni, A. A
complex catalytic cycle leading to a regioselective synthesis of _o_,_o_′‐disubstituted vinylarenes. _Angew. Chem. Int. Ed._ 36, 119–122 (1997). Article CAS Google Scholar * Kim, D.-S.,
Park, W.-J. & Jun, C.-H. Metal–organic cooperative catalysis in C–H and C–C bond activation. _Chem. Rev._ 117, 8977–9015 (2017). Article CAS Google Scholar * Catellani, M., Motti, E.
& Ca’, N. D. Catalytic sequential reactions involving palladacycle-directed aryl coupling steps. _Acc. Chem. Res._ 41, 1512–1522 (2008). Article CAS Google Scholar * Ding, L., Sui, X.
& Gu, Z. Enantioselective synthesis of biaryl atropisomers via Pd/norbornene-catalyzed three-component cross-couplings. _ACS Catal._ 8, 5630–5635 (2018). Article CAS Google Scholar *
Zhao, Y.-B. et al. Exploiting the divergent reactivity of aryl–palladium intermediates for the rapid assembly of fluorene and phenanthrene derivatives. _Angew. Chem. Int. Ed._ 48, 1849–1852
(2009). Article CAS Google Scholar * Yang, L. et al. Palladium-catalyzed dynamic kinetic asymmetric transformation of racemic biaryls: axial-to-central chirality transfer. _J. Am. Chem.
Soc._ 137, 4876–4879 (2015). Article CAS Google Scholar * Shi, H., Herron, A. N., Shao, Y., Shao, Q. & Yu, J.-Q. Enantioselective remote _meta_-C–H arylation and alkylation via a
chiral transient mediator. _Nature_ 558, 581–585 (2018). Article CAS Google Scholar * Li, R., Liu, F. & Dong, G. Palladium-catalyzed asymmetric annulation between aryl iodides and
racemic epoxides using a chiral norbornene cocatalyst. _Org. Chem. Front._ 5, 3108–3112 (2018). Article CAS Google Scholar * Cao, Z. et al. Pd-catalyzed asymmetric allylic alkylation of
indoles and pyrroles by chiral alkene-phosphine ligands. _Org. Lett._ 13, 2164–2167 (2011). Article CAS Google Scholar * Shintani, R., Duan, W.-L., Okamoto, K. & Hayashi, T.
Palladium/chiral phosphine–olefin complexes: X-ray crystallographic analysis and the use in catalytic asymmetric allylic alkylation. _Tetrahedron Asymmetry_ 16, 3400–3405 (2005). Article
CAS Google Scholar * Liu, Z. & Du, H. Development of chiral terminal-alkene-phosphine hybrid ligands for palladium-catalyzed asymmetric allylic substitutions. _Org. Lett._ 12,
3054–3057 (2010). Article CAS Google Scholar * Lam, F. L. et al. Palladium–_(S_,_p__R)_-ferroNPS-catalyzed asymmetric allylic etherification: electronic effect of nonconjugated
substituents on benzylic alcohols on enantioselectivity. _Angew. Chem. Int. Ed._ 47, 1280–1283 (2008). Article CAS Google Scholar * Wen, W. et al. Chiral aldehyde catalysis for the
catalytic asymmetric activation of glycine esters. _J. Am. Chem. Soc._ 140, 9774–9780 (2018). Article CAS Google Scholar * Witzig, R. M., Fäseke, V. C., Häussinger, D. & Sparr, C.
Atroposelective synthesis of tetra-_ortho_-substituted biaryls by catalyst-controlled non-canonical polyketide cyclizations. _Nat. Catal._ 2, 925–930 (2019). Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS We are grateful to the National Natural Science Foundation of China (grant nos. 21871213 and 21801193), the China Postdoctoral Science Foundation (grant
nos. 2016M602339 and 2018M642894) and the start-up funding from WHU for financial support. We thank H. Cong and W. Yan (Wuhan University) for X-ray crystallographic analysis assistance. We
gratefully acknowledge P. Baran (TSRI), D. Ma and W. Tang (SIOC), H. Xu (Georgia State University), C. Wang and W.-B. Liu (Wuhan University), S. Yu (Nanjing University) and W. Xie (Northwest
A&F University) for helpful discussions, H. Xu (Georgia State University) and M. Yan (Fish & Richardson) for help with preparation of the manuscript. AUTHOR INFORMATION Author notes
* These authors contributed equally: Yu Hua and Qianwen Gao. AUTHORS AND AFFILIATIONS * Sauvage Center for Molecular Sciences, Engineering Research Center of Organosilicon Compounds &
Materials (Ministry of Education), College of Chemistry and Molecular Sciences and The Institute for Advanced Studies, Wuhan University, Wuhan, P. R. China Ze-Shui Liu, Yu Hua, Qianwen Gao,
Yuanyuan Ma, Hua Tang, Yong Shang, Hong-Gang Cheng & Qianghui Zhou Authors * Ze-Shui Liu View author publications You can also search for this author inPubMed Google Scholar * Yu Hua
View author publications You can also search for this author inPubMed Google Scholar * Qianwen Gao View author publications You can also search for this author inPubMed Google Scholar *
Yuanyuan Ma View author publications You can also search for this author inPubMed Google Scholar * Hua Tang View author publications You can also search for this author inPubMed Google
Scholar * Yong Shang View author publications You can also search for this author inPubMed Google Scholar * Hong-Gang Cheng View author publications You can also search for this author
inPubMed Google Scholar * Qianghui Zhou View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Q.Z. and Z.-S.L. conceived the idea. Q.Z. guided
the project and wrote the manuscript. Z.-S.L., Y.H., Q.G., Y.M., H.T. and Y.S. performed the experiments and analysed the data. Z.-S.L. and H.-G.C. participated in the preparation of the
manuscript. CORRESPONDING AUTHOR Correspondence to Qianghui Zhou. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary
Methods, Figs. 1–11, Tables 1–17 and references. SUPPLEMENTARY DATA 1 Crystallographic Data of compound 4da. SUPPLEMENTARY DATA 2 Crystallographic Data of compound 4g. SUPPLEMENTARY DATA 3
Crystallographic Data of compound 5j. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, ZS., Hua, Y., Gao, Q. _et al._ Construction of axial chirality
via palladium/chiral norbornene cooperative catalysis. _Nat Catal_ 3, 727–733 (2020). https://doi.org/10.1038/s41929-020-0494-1 Download citation * Received: 28 February 2020 * Accepted: 08
July 2020 * Published: 10 August 2020 * Issue Date: September 2020 * DOI: https://doi.org/10.1038/s41929-020-0494-1 SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
Trending News
Comments: week of march 1, 20211 The latest issue of _New York_ featured Chloé Zhao, Hollywood’s most-sought-after director, in her first major magazin...
Javascript support required...
Bold new ways to mix prints and patterns in outfitsFrom simple stripes to fabulous florals to pretty polka dots, a good print and pattern can elevate an outfit. But if you...
Cracking jokes: four rules for humourAlan Roberts is the author of The Philosophy of Humour, published by Palgrave in Spring 2019. (https://www.palgrave.com/...
Javascript support required...
Latests News
Construction of axial chirality via palladium/chiral norbornene cooperative catalysisABSTRACT Axially chiral biaryls are common structural motifs in functional materials, bioactive natural products, pharma...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
Wpp aunz enters scheme implementation deed with wpp plcWPP plc is set to complete its purchase of the remaining shares of WPP AUNZ before July following a Scheme Implementatio...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
Marie mcnair — scottish national partyCONTACT Scottish National Party Gordon Lamb House 3 Jackson's Entry Edinburgh, Scotland EH8 8PJ tel: 0800 633 5432 ...