Recommendations from the european commission initiative on breast cancer for multigene testing to guide the use of adjuvant chemotherapy in patients with early breast cancer, hormone receptor positive, her-2 negative
Recommendations from the european commission initiative on breast cancer for multigene testing to guide the use of adjuvant chemotherapy in patients with early breast cancer, hormone receptor positive, her-2 negative"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Predicting the risk of recurrence and response to chemotherapy in women with early breast cancer is crucial to optimise adjuvant treatment. Despite the common practice of
using multigene tests to predict recurrence, existing recommendations are inconsistent. Our aim was to formulate healthcare recommendations for the question _“Should multigene tests be used
in women who have early invasive breast cancer, hormone receptor-positive, HER2-negative, to guide the use of adjuvant chemotherapy?”_ METHODS The European Commission Initiative on Breast
Cancer (ECIBC) Guidelines Development Group (GDG), a multidisciplinary guideline panel including experts and three patients, developed recommendations informed by systematic reviews of the
evidence. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Evidence to Decision frameworks were used. Four multigene tests were evaluated: the 21-gene recurrence
score (21-RS), the 70-gene signature (70-GS), the PAM50 risk of recurrence score (PAM50-RORS), and the 12-gene molecular score (12-MS). RESULTS Five studies (2 marker-based design RCTs, two
treatment interaction design RCTs and 1 pooled individual data analysis from observational studies) were included; no eligible studies on PAM50-RORS or 12-MS were identified and the GDG did
not formulate recommendations for these tests. CONCLUSIONS The ECIBC GDG suggests the use of the 21-RS for lymph node-negative women (conditional recommendation, very low certainty of
evidence), recognising that benefits are probably larger in women at high risk of recurrence based on clinical characteristics. The ECIBC GDG suggests the use of the 70-GS for women at high
clinical risk (conditional recommendation, low certainty of evidence), and recommends not using 70-GS in women at low clinical risk (strong recommendation, low certainty of evidence).
SIMILAR CONTENT BEING VIEWED BY OTHERS PROCURE EUROPEAN CONSENSUS ON BREAST CANCER MULTIGENE SIGNATURES IN EARLY BREAST CANCER MANAGEMENT Article Open access 24 February 2023 CONCORDANCE
BETWEEN RESULTS OF INEXPENSIVE STATISTICAL MODELS AND MULTIGENE SIGNATURES IN PATIENTS WITH ER+/HER2− EARLY BREAST CANCER Article 08 February 2021 MULTIGENE PROFILES TO GUIDE THE USE OF
NEOADJUVANT CHEMOTHERAPY FOR BREAST CANCER: A COPENHAGEN BREAST CANCER GENOMICS STUDY Article Open access 31 May 2023 BACKGROUND Breast cancer is the most frequently diagnosed cancer among
women.1 In the European Union, including UK, 404,920 women were diagnosed with breast cancer and 98,755 died because of this disease in 2018.2 Hormone receptor (HoR)-positive (i.e. oestrogen
receptor (ER)- and/or progesterone receptor (PR)-positive), human epidermal growth factor receptor 2 (HER2)-negative breast cancer represents about 70% of breast cancer diagnosed in western
countries.3 At the time of diagnosis, around 60% of this type of cancer has not spread to lymph nodes,3 and approximately 15% of these women will develop a recurrence within 10 years if
treated with adjuvant endocrine therapy alone.4,5 The risk of recurrence could be reduced by the addition of chemotherapy.5 However, given the relatively low risk of recurrence and the
partial effectiveness of chemotherapy in these women, most would be over-treated if all received chemotherapy. The same rationale would apply to women with HoR-positive, HER2-negative
invasive breast cancer with 1–3 positive lymph nodes.6 Several prognostic factors, including clinical-pathological features such as age, tumour size, percentage of ER- and PR-positive cells
as well as Ki67-index,7,8,9 predict the risk of recurrence and can help identify women who would benefit the most from chemotherapy. Although these factors have been shown to discriminate
different prognostic groups, they showed no or minimal predictive value on the response to chemotherapy.10 In the last 15 years, different tests have been developed to stratify patients with
early breast cancer into different risk of recurrence groups by analysing the activity of various genes. Although these multigene tests use diverse techniques (RT-PCR, microarray, and
others) and diverse target gene combinations, they all focus on genes involved in cell proliferation. The tests provide recurrence risk profiles categorised in different ways. Some tests
have been explicitly proposed to provide additional information to clinical-pathological features, as the 12-MS and the PAM50-RORS. Based on the results of the MINDACT trial,6 the
application of the 70-GS also takes into account clinical prognostic characteristics, while the 21-RS has been proposed to substitute clinical risk-based treatment decisions. The European
Commission Initiative on Breast Cancer (ECIBC) aims to provide evidence-based recommendations for screening and diagnosis of breast cancer.11 The Guidelines Development Group (GDG) of the
ECIBC prioritised a clinical question on the use of multigene tests to guide the use of adjuvant chemotherapy in HoR-positive, HER2-negative and lymph node-negative or up to 3 lymph
nodes-positive invasive breast cancer. Four multigene tests, used to stratify women with breast cancer into different groups according to recurrence risk,12,13,14,15,16,17 are included in
the clinical question (Supplementary Table 1): 21-RS (Oncotype DX, Genomic Health Inc), 12-MS (EndoPredict, Myriad Genetics Inc), PAM50-RORS (Prosigna test, NanoString Technologies Inc.),
and 70-GS (MammaPrint; Agendia Inc). Direct comparison between different tests is beyond the scope of these recommendations. METHODS STRUCTURED QUESTION AND OUTCOME PRIORITISATION The
clinical question “_Should multigene tests be used in patients who have HoR-positive, HER-2 negative, lymph node-negative or up to 3 lymph nodes-positive invasive breast cancer to guide the
use of adjuvant chemotherapy_” was structured following the Population, Intervention, Comparison and Outcomes (PICO) format (Table 1). The outcomes were also prioritised by the GDG using a
nine-point scale (7 to 9 critical; 4 to 6 important; 1 to 3 of limited importance), as suggested by the Grading of Recommendations Assessment, Development and Evaluation (GRADE)
approach.11,18 The GDG decided not to attempt any head-to-head comparisons between the different tests. SYSTEMATIC REVIEW DATA SOURCES AND SEARCHES MEDLINE (May 2018), EMBASE (May 2018) and
CENTRAL (May 2018) databases were searched, using pre-defined algorithms, for both systematic reviews and individual studies (Supplementary Table 2); this original search was continuously
run up to October 2018. Lists of references of the included studies were reviewed and members of the GDG were requested to provide additional studies. STUDY SELECTION Randomised controlled
trials (RCT) and cohort studies (including pooled analyses of studies), either from prospective or retrospective analysis, of stored specimen samples were included as long as they applied
any of the four tests as predictive markers for guiding the use of adjuvant chemotherapy (Supplementary Fig. 1) A predictive marker identifies the differential benefit of a treatment based
on the marker status. Thus, we included the following assessment approaches: (a) _Marker-based strategy design_: patients are assigned to a treatment arm depending on whether they received
treatment (i.e. endocrine therapy or endocrine plus chemotherapy) according to the test results or according to usual clinical practice. The predictive value is assessed by comparing the
outcomes from the testing-based arm versus the non-testing arm; (b) _Treatment interaction design_: patients are divided into groups based on the marker status (i.e. high and low marker
status). Then they are allocated to receive endocrine therapy or endocrine treatment plus chemotherapy. The predictive value is assessed by observing the relative efficacy of treatment
differences between marker status and treatment assignments. Studies that only reported prognosis data based on marker status (without considering differential treatment effect), individual
observational studies, abstracts or conference communications not published as full text articles, and articles published in a language other than English were excluded. With respect to
economic evidence, cost-utility, cost-benefit, and cost-consequences, analyses were included if conducted within clinical trials, as well as observational and modelling studies, published in
English during the last decade (Supplementary Table 3). After a calibration process, each reviewer (CCA and KP) assessed titles and abstracts for eligibility. Subsequently, two reviewers
(CCA and KP), independently, reviewed the full text of all the pre-selected references. Discrepancies were solved either by consensus or with the help of a third reviewer (DR) (Supplementary
Fig. 2 and Supplementary Table 4). DATA EXTRACTION AND RISK OF BIAS ASSESSMENT Two reviewers (CCA and KP) independently assessed risk of bias and extracted the following information from
each study: first author, year of publication, country, study design, inclusion and exclusion criteria, number of patients, age, participants’ characteristics and prioritised outcomes. The
risk of bias of the included RCTs was assessed using the Cochrane Risk of Bias tool for randomised trials.19 Cohort studies were assessed with the “Risk Of Bias In Non-randomised Studies -
of Interventions-I” (ROBINS-I) tool.20 For economic evaluations, one reviewer (MP) screened the search results and used the NICE methodology checklist to assess applicability and
methodological limitations.21 Studies with poor applicability and/or high risk of bias were excluded (Supplementary Fig. 2). DATA ANALYSIS Descriptive statistics were used to summarise the
characteristics of the included patients across studies. The effect measures for prioritised outcomes and their corresponding 95% confidence intervals (CIs) were reported as presented in
individual studies. CERTAINTY OF THE EVIDENCE The certainty of evidence per outcome and overall certainty was rated using the GRADE approach. For each recommendation, the GDG received a
Summary of Findings (SoF) table and a first draft of an evidence to decision framework (EtD).22 COMPARISON SCENARIOS AND MODELLING A simple _deterministic decision tree model_ without
discounting was built by PGR with input from the rest of the GDG to estimate the downstream consequences of testing patients with the multigene tests versus different scenarios of usual care
(Supplementary Fig. 3). For the 21-RS, the general model assumptions were: the population of eligible women was divided into the three risk groups, as reported in the TAILORx trial at
recruitment until 2008 (14% low risk of recurrence, 68% intermediate, 18% high).23 Rate of events, observed in the RCTs, were applied to the simulated usual care arms. Clinical risk of
recurrence was classified as low and high according to the modified AdjuvantOnline!Score.24,25 Results are based on a fixed observation time of 10 years. Two strategies for implementing the
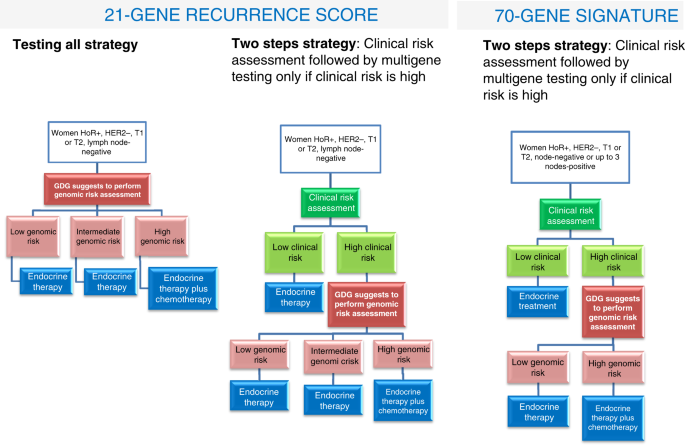
multigene test to guide the use of adjuvant chemotherapy were considered as interventions (Supplementary Fig. 3a): * 1. All women would undergo multigene testing and adjuvant chemotherapy
would be given accordingly (only to those classified in the high genomic risk group, i.e. with a score ≥26). * 2. Only women with high clinical risk would undergo multigene testing, and only
those with high genomic risk would receive chemotherapy. Women with low clinical risk would not receive it. Two scenarios were considered as usual care comparators (“C” of the PICO
framework) (Supplementary Fig. 3b): * 1. All women would be referred to adjuvant chemotherapy (assuming 18.4% would not comply, i.e. the proportion of women not receiving chemotherapy among
those assigned to the treatment arm in TAILORx).23 * 2. Women would receive adjuvant chemotherapy only if the clinical risk is high. The model assumes that women with low clinical and high
genomic risk, as well as those with low clinical and low genomic risk, do not benefit from adjuvant chemotherapy. For sensitivity analyses, in women of low clinical and high genomic risk,
two different assumptions were used to estimate the benefits: (a) The advantage of receiving adjuvant chemotherapy is equivalent to that observed in the MINDACT trial6 at five years, and the
effect is maintained at 10 years; (b) the advantage from adjuvant chemotherapy is equivalent to that observed by Paik and colleagues26 for all women at high genomic risk, independent of
their clinical risk. Distributions of the clinical risk within the multigene risk strata are those reported in the TAILORx trial.23 For the 70-GS, we focused only on comparing strategy 2
with scenario 2 in which women at high clinical risk would be tested and/or treated, because the evidence from the MINDACT trial indicates a very small benefit, if any, from adjuvant
chemotherapy in women with low clinical risk, independent of their genomic risk.6 EVIDENCE TO DECISION AND RECOMMENDATION FORMULATION The process the ECIBC GDG used to formulate
recommendations has been described in a dedicated article published elsewhere.11 In brief, a subgroup of GDG members including experts on the topic and an informed patient (the so-called
PICO responsible unit), took primary responsibility for the review and completion of the first draft of the SoF tables and the EtD frameworks, conducted initially by the systematic review
team. The frameworks were used in the meetings to help the complete GDG formulate the recommendations. Subsequently they were reviewed by a technical team from the Joint Research Centre, the
PICO responsible unit and the systematic review team. Finally, the recommendations and frameworks were approved by the GDG. RESULTS INCLUDED STUDIES We included five studies (Supplementary
Fig. 1): two RCTs,6,23 two secondary analyses of stored tissue blocks collected from former parent clinical trials26,27 and one pooled analysis of observational studies12 from four
previously reported validation studies, including unpublished data (Supplementary Table 5).28,29,30 21 GENE RECURRENCE SCORE TREATMENT INTERACTION DESIGN STUDIES Paik and colleagues26
provided estimates for distant recurrence free survival in patients with lymph node-negative breast cancer stratified into three levels of the 21-RS risk groups. Adding chemotherapy to
endocrine therapy, compared to endocrine therapy alone, may have a different effect on recurrence across groups, i.e. a larger effect in women with higher 21-RS, but the evidence is very
uncertain: hazard ratio (HR) of 1.31 (95% CI 0.46–3.78), 0.61 (95% CI 0.24–1.59) and 0.26 (95% CI 0.13–0.53) in low, intermediate and high risk groups, respectively (Supplementary Table 6).
Albain and colleagues27 included stored tumour specimens for genomic testing of postmenopausal women with HoR-positive, node-positive breast cancer. They performed an analysis adjusted by
the number of positive nodes that suggests no benefit for chemotherapy on disease free survival (DFS) in the low genomic risk group (HR = 1.02; 95% CI 0.54–1.93) and a potential advantage in
the high genomic risk (HR = 0.59, 95% CI 0.35–1.01) (Supplementary Table 6). The authors refer similar results for overall survival (OS). MARKER-BASED STRATEGY Sparano and colleagues23
provided results for several disease-free survival (DFS)-related outcomes among women with an intermediate genomic risk group (11 to 26 risk score) allocated to either endocrine therapy
alone or chemotherapy plus endocrine therapy. The as-treated results suggest little to no difference in the risk of recurrence with chemotherapy plus endocrine therapy for invasive DFS (HR
1.14; 95% CI 0.99–1.31). For distant metastases, local recurrence and OS, similar results were observed (Supplementary Table 6). 70-GS TREATMENT INTERACTION DESIGN STUDIES Knauer and
colleagues12 described results from a pooled database analysis with a median follow-up time of 7.1 years. Patients with a low and high genomic risk who received chemotherapy may have a lower
risk of recurrence than those with endocrine therapy alone, but the evidence is very uncertain (HR 0.26; 95% CI 0.03–2.02 and HR 0.35; 95% CI 0.17–0.71, respectively). The results for
mortality were consistent with the observed pattern of the risk of recurrence but the evidence was also very uncertain (HR 0.58; 95% CI 0.07–4.98; HR 0.21; 95%CI 0.07–0.59, respectively)
(Supplementary Table 7). MARKER-BASED STRATEGY Cardoso and colleagues6 reported DFS and OS among patients in the clinical/genomic discordant-risk groups which were allocated to receive
either chemotherapy in addition to endocrine therapy or endocrine therapy alone. Women with high clinical risk and low genomic risk may have an increase of DFS (HR 0.64; 95% CI 0.43–0.95),
of distant metastases free survival (HR 0.65; 95% CI 0.38–1.10) and of OS (HR 0.63; 95% CI 0.29–1.37) (Supplementary Table 7). The group of low clinical risk and high genomic risk showed
imprecise effects and uncertain evidence for DFS (HR 0.74; 95% CI 0.40–1.39), distant metastases free survival (HR 0.90; 95% CI 0.40–2.01) and OS (HR 0.72; 95% CI 0.23–2.24).6 MODELLING FOR
PREDICTING IMPACT OF TESTING ON PATIENT’S OUTCOMES Depending on the different treatment scenarios (all women are referred to chemotherapy or only women with high clinical risk are treated
with chemotherapy) and genetic testing strategies (genetic testing carried out in all women or testing only those with high clinical risk), the number of women who avoid chemotherapy by
using the 21-RS would change from more than 600 to about 200. Survival outcomes did not change substantially (Table 2) for the 21-RS based on the benefits from adding chemotherapy in the
MINDACT trial.6 However, on the assumption that all women with high genomic score would obtain the same benefits from adding chemotherapy as observed by Paik and colleagues,26 independently
from their clinical risk, the intervention could potentially prevent 37 distant metastases compared to a scenario in which only women with high clinical risk would be treated with
chemotherapy. On the other hand, we considered for the 70-GS a two-step strategy according to the results of the MINDACT trial, testing only women with high clinical risk.6 Consequently, the
only scenario considered was one in which only high-risk women would receive chemotherapy (Table 3). The use of the 70-GS would result in an avoidance of chemotherapy in about 230 women out
of 1000 associated with small increase of recurrences. RESULTS FROM THE SYSTEMATIC REVIEW OF ECONOMIC EVIDENCE From the primary literature search and from the two identified systematic
reviews,31,32 12 cost-effectiveness evaluations were identified (Supplementary Fig. 2 and Supplementary Table 4).33,34,35,36,37,38,39,40,41,42,43,44 The GDG agreed that these economic
evaluations used models that were not directly applicable to the clinical question of interest. Therefore, cost-effectiveness was evaluated considering the benefits and harms estimated using
the GDG’s ad-hoc model described above (Supplementary Fig. 3) and the costs reported by the studies included in the literature review. Eight studies reported costs for the use of the 21-RS
in women with negative lymph nodes,33,34,35,36,37,38,39,40,41 whereas three reported costs for women with up to 3 positive lymph nodes.42,43,44 The reported costs of the 21-RS were EUR 3180
per patient in five out of the 11 studies included. These costs did not show significant differences between countries or over time. For the 70-GS, two studies reported costs of EUR 3153 per
patient for the use of this assay in women with negative lymph nodes.40,41 CERTAINTY OF EVIDENCE The overall certainty of the evidence was rated as low to very low. The main concerns across
studies were risk of bias, indirectness of trial populations and imprecision (Supplementary Tables 6 and 7). The evidence was also downgraded for indirectness due to the assumptions used
for the model that implied the use of evidence from one population in another population and from different duration of follow-up across studies. EVIDENCE TO DECISION FRAMEWORKS 21-RS The
GDG judged the anticipated desirable effects (i.e. the avoided chemotherapy treatments) of using the test to guide chemotherapy to be large and the undesirable effects (i.e. increase in
recurrence) trivial, with very low certainty of the evidence. The costs were considered large, though no cost-effectiveness study was included. A negative impact on equity was considered a
potential concern (Table 4). For women with HoR-positive, HER2-negative, node-negative invasive breast cancer, the ECIBC GDG suggests the use of the 21-RS to guide the use of chemotherapy
(conditional recommendation, very low certainty of the evidence, Table 5). The recommendation is conditional because the certainty of evidence was very low and the downstream consequences of
avoiding chemotherapy were not quantified, thus making the balance of benefits and harms difficult to determine, together with the large resource (costs) requirements (Table 4, see also
https://healthcare-quality.jrc.ec.europa.eu/sites/default/files/Guidelines/EtDs/Updated/ECIBC_GLs_EtD_21_gene_recurrence_score.pdf). The GDG did not consider women with node-positive
invasive breast cancer in this recommendation, because they were not included in the TAILORx trial,23 the main source for model parameters. The GDG also stated that sub-populations with high
clinical risk (defined according to AdjuvantOnline!)24,25 may experience larger net desirable consequences and provide a more favourable cost-effectiveness profile (Fig. 1). On the other
hand, women with low clinical risk may experience smaller or no net desirable consequences. Indirect evidence from the MINDACT trial using the 70-GS supports that conclusion. In fact, in
this trial there are very small, if any, benefits from chemotherapy in low clinical risk women, independently of the genomic risk.6 New relevant results have been published on the 21-RS
since the systematic review used for this recommendation was conducted.45,46 Recent data from the TAILORx trial stratified by age and clinical risk have been published.45 The authors suggest
that women below 50 with an intermediate genomic risk score could have a benefit from adding chemotherapy to endocrine therapy if their clinical risk is high but the absence of a dose
response and very imprecise effect estimates suggest that chance could play a major role. Furthermore, the reported analysis suggests that data for evaluating the potentially most efficient
two-step testing strategy is missing.47,48 Mariotto and colleagues49 showed that application of the 21-RS risk to decide whether or not to provide chemotherapy would produce savings in the
actual US real clinical practice. Another analysis using the same data indicates that savings would be much larger if testing would be performed according to a two-step strategy.50 The GDG,
for the moment, judged that the new evidence is consistent with the recommendation. 70-GS In light of the results from the MINDACT trial,6 the GDG decided to split the recommendation
according to clinical risk of the population under study (at low and high clinical risk, Table 5 and Fig. 1). In the low clinical risk group the GDG recommends against using the 70-GS
testing to guide the use of chemotherapy (strong recommendation, very low certainty of the evidence) as there are no apparent benefits and there are very large costs (EUR 3153 per patient)
(https://healthcare-quality.jrc.ec.europa.eu/sites/default/files/Guidelines/EtDs/Updated/ECIBC_GLs_EtD_70_gene_testing_low_risk.pdf). For women with HoR-positive, HER2-negative,
node-negative or up to 3 lymph nodes-positive invasive breast cancer at high clinical risk, the ECIBC GDG suggests using the 70-GS test to guide the use of chemotherapy. The judgments
favoured the intervention in the high clinical risk population due to the moderate desirable effect, a balance that probably favours the use of 70-GS testing, and the large savings (Table
4). The recommendation is conditional mainly because of the low certainty of the evidence about the effects. The GDG also stated that the proportion of women with 2 or 3 positive lymph nodes
was small, therefore making the results less clear in this subgroup
(https://healthcare-quality.jrc.ec.europa.eu/sites/default/files/Guidelines/EtDs/Updated/ECIBC_GLs_EtD_70_gene_testing_high_risk.pdf). DISCUSSION STATEMENT OF PRINCIPAL FINDINGS The ECIBC
GDG suggests the use of 21-RS in lymph node-negative women, recognising that benefits are probably larger in women at high clinical risk and suggests the use of the 70-GS only for women at
high clinical risk. STRENGTHS AND WEAKNESSES OF THE STUDY The strength of the recommendations includes the ECIBC’s adherence to the requirements for trustworthy development of
guidelines.51,52,53 Previously, we described some limitations of our guidelines.11,54 The weakness of the deterministic decision tree model used is that it is, to some extent, a simplistic
approach and some assumptions are questionable (i.e. negligible effects in low clinical risk, same effects in studies with different duration of follow-up). Furthermore, we did not actually
quantify the side effects of chemotherapy, considering that avoiding any unnecessary chemotherapy was a desirable effect. RELATION TO OTHER GUIDELINES NICE recently published guidance on
multigene testing.55 The panel decided to evaluate the evidence for the four commercially available multigene tests included in the ECBIC question, and for the IHC4 + C test.56,57 The NICE
guidelines did not follow the GRADE methodology and also had a different goal, i.e. deciding which tests should be funded by the UK National Health Service. The NICE 21-RS recommendation is
similar to the ECIBC recommendation, a conditional recommendation limited to those patients in which the risk of distant recurrence is intermediate, using a validated tool such as PREDICT58
or the Nottingham Prognostic Index (Table 6). This approach is based on the assumption that in some patients the clinical and pathological prognostic parameters are consistent (low or high
risk), and that multigene testing does not provide additional information. In particular, the NICE panel recommended against the use of the 70-GS, based on cost-effectiveness considerations
(Table 6). Unfortunately, the NICE guideline does not allow comparison of our estimates of desirable and undesirable health effects. Some methodological differences might explain the
divergent results: since real practice varies across Europe, we preferred to use theoretical scenarios as comparators and interventions not accounting for non-compliance, while the NICE
model used real practice as comparator (the prevalence of chemotherapy used in this group of women in the UK), and as intervention (a change in the probability of receiving chemotherapy
given the test result). Furthermore, the model used by NICE assumed that the tests are only prognostically relevant but are not predictive of the response to treatment, i.e. they calculated
a constant HR of 0.77 for chemotherapy in addition to endocrine therapy compared to endocrine therapy alone in all risk groups. In contrast, the ECIBC GDG judged the benefits to be trivial
or small in the low clinical risk group. Finally, for the 21-RS a commercial-in-confidence discounted test cost was used to model cost-effectiveness, while for the 70-GS the regular market
price was used. It is worth noting that despite NICE stating that the major benefit of the genetic testing strategies would be a reduction of chemotherapy, the cost models predict health
benefits only if chemotherapy is increased. The American Society of Clinical Oncology (ASCO) also provided recommendations on the use of multigene tests.59,60,61 Despite a different
methodological approach, the direction of the recommendations is the same, but the strength is not (Table 6). There are differences in the grading of certainty of the evidence, considered as
high by the ASCO panellists, while the GDG valued the evidence as very low for the 21-RS and low for the 70-GS. Unfortunately, we were unable to deduce the details of the ASCO evidence
rating approach and also the criteria and judgments that were used to determine the strength of the recommendations. Unlike us, ASCO and NICE made recommendations on the 12-MS and the
PAM50-RORS to guide treatment decisions,55,62 mainly because they did not exclude studies based on prognostic results only. MEANING OF THE STUDY The implications of our recommendations are
context dependent. The criteria used for making decisions on the provision or not of adjuvant treatment differ between countries. Therefore, the cost-benefit profile of introducing one of
the multigene tests might also vary across countries. Decreasing costs for the tests would support a more widespread use. For these considerations, the GDG decided not to establish a
threshold of recurrence risk to recommend genomic risk assessment, or a threshold for adding adjuvant chemotherapy, since these thresholds are context specific. In conclusion, the ECIBC GDG
recommendations for or against the use of 21-RS and 70-RS are justified based on the judgments made. The transparency of our approach allows understanding the rationale for making different
recommendations for the two tests and risk groups. UNANSWERED QUESTIONS AND FUTURE RESEARCH Data protection issues may be of relevance for the 21-RS because processing of samples is
centralised in one US lab and requires shipping samples abroad. Furthermore, transparent information on test results is not available and reproducibility has been questioned.63 The GDG
recommends research on exploring in what subgroups the use of 21-RS would have larger anticipated benefits as well as carrying out longer follow-up studies for 70-GS. The recommendations
will be updated according to the ECIBC monitoring strategy in place (https://healthcare-quality.jrc.ec.europa.eu/discover-ecibc/methodologies/guidelines-updating). Furthermore, the GDG is
exploring the possibility of evaluating biomarkers that may assist decision making regarding the administration of adjuvant chemotherapy on the basis of their ability to identify women with
a sufficiently low risk of relapse that would allow them to be spared from chemotherapy. In contrast to the presented evidence evaluation, this would enable evidence-based and transparent
recommendations based on prognostic cohort studies, randomised or not, that predict the recurrence risk of different subgroups. We are currently working on a healthcare question on the
significance of Ki67 using this strategy. In a next step, such an approach might also make it possible to evaluate multigene tests in the assessment for which we found no usable evidence in
the predictive search strategy used here, but for which data on the prognostic value are available.13,14,15,16,17,64 REFERENCES * Ferlay, J. E. M., Lam, F., Colombet, M., Mery, L., Piñeros,
M., Znaor, A. et al. _Global Cancer Observatory: Cancer Today_ (International Agency for Research on Cancer, Lyon, France, 2018). Google Scholar * ECIS. European Cancer Information System
From https://ecis.jrc.ec.europa.eu (2019). * Howlader, N., Altekruse, S. F., Li, C. I., Chen, V. W., Clarke, C. A., Ries, L. A. G. et al. US incidence of breast cancer subtypes defined by
joint hormone receptor and HER2 status. _J. Natl Cancer Inst._ 28, 106 (2014). Google Scholar * Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal
therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. _Lancet_ 365, 1687–1717 (2005). Google Scholar * Early Breast Cancer Trialists’
Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. _Lancet_ 378,
771–784 (2011). Google Scholar * Cardoso, F., van’t Veer, L. J., Bogaerts, J., Slaets, L., Viale, G., Delaloge, S. et al. 70-gene signature as an aid to treatment decisions in early-stage
breast cancer. _N. Engl. J. Med._ 375, 717–729 (2016). CAS PubMed Google Scholar * Goldhirsch, A., Wood, W. C., Coates, A. S., Gelber, R. D., Thurlimann, B., Senn, H. J. et al. Strategies
for subtypes–dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. _Ann. Oncol._ 22,
1736–1747 (2011). CAS PubMed PubMed Central Google Scholar * Coates, A. S., Winer, E. P., Goldhirsch, A., Gelbert, R. D., Gnant, M., Piccart-Gebhart, M. et al. Tailoring
therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. _Ann. Oncol._ 26, 1533–1546 (2015).. *
Petrelli, F., Viale, G., Cabiddu, M. & Barni, S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: a systematic review and meta-analysis of 64,196 patients.
_Breast Cancer Res. Treat._ 153, 477–491 (2015). PubMed Google Scholar * Early Breast Cancer Trialists’ Collaborative, Group. Comparisons between different polychemotherapy regimens for
early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. _Lancet_ 379, 432–444 (2012). Google Scholar * Schünemann, H. J., Lerda, D., Dimitrova,
N., Alonso-Coello, P., Gräwingholt, A., Quinn, C. et al. Methods for development of the European Commission Initiative on Breast Cancer Guidelines: Recommendations in the Era of Guideline
Transparency. _Ann. Intern. Med._ https://doi.org/10.7326/M18-3445 (2019). * Knauer, M., Mook, S., Rutgers, E. J., Bender, R. A., Hauptmann, M., van de Vijver, M. J. et al. The predictive
value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. _Breast Cancer Res. Treat._ 120, 655–661 (2010). CAS PubMed Google Scholar * Dowsett, M., Sestak, I.,
Lopez-Knowles, E., Sidhu, K., Dunbier, A. K., Cowens, J. W. et al. Comparison of PAM50 risk of recurrence score with oncotype DX and IHC4 for predicting risk of distant recurrence after
endocrine therapy. _J Clin Oncol._ 31, 2783–90. (2013). PubMed Google Scholar * Gnant, M., Sestak, I., Filipits, M., Dowsett, M., Balic, M., Lopez-Knowles, E. et al. Identifying clinically
relevant prognostic subgroups of postmenopausal women with node-positive hormone receptor-positive early-stage breast cancer treated with endocrine therapy: a combined analysis of ABCSG-8
and ATAC using the PAM50 risk of recurrence score and intrinsic subtype. _Ann. Oncol._ 26, 1685–1691 (2015). CAS PubMed Google Scholar * Sestak, I., Cuzick, J., Dowsett, M.,
Lopez-Knowles, E., Filipits, M., Dubsky, P. et al. Prediction of late distant recurrence after 5 years of endocrine treatment: a combined analysis of patients from the Austrian breast and
colorectal cancer study group 8 and arimidex, tamoxifen alone or in combination randomized trials using the PAM50 risk of recurrence score. _J. Clin. Oncol._ 33, 916–922 (2015). CAS PubMed
Google Scholar * Buus, R., Sestak, I., Kronenwett, R., Denkert, C., Dubsky, P., Krappmann, K. et al. Comparison of EndoPredict and EPclin with oncotype DX recurrence score for prediction
of risk of distant recurrence after endocrine therapy. _J. Natl Cancer Inst._ 108, djw149 (2016). * Sestak, I., Buus, R., Cuzick, J., Dubsky, P., Kronenwett, R., Denkert, C. et al.
Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. _JAMA Oncol._ 4, 545–553 (2018).
PubMed PubMed Central Google Scholar * Guyatt, G. H., Oxman, A. D., Kunz, R., Atkins, D., Brozek, J., Vist, G. et al. GRADE guidelines: 2. Framing the question and deciding on important
outcomes. _J. Clin. Epidemiol._ 64, 395–400 (2011). PubMed Google Scholar * Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D. et al. The cochrane
collaboration’s tool for assessing risk of bias inrandomised trials. _BMJ_ 343, d5928 (2011). PubMed PubMed Central Google Scholar * Sterne, J. A., Hernán, M. A., Reeves, B. C., Savovic,
J., Berkman, N. D., Viswanathan, M. et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. _BMJ_ 355, i4919 (2016). PubMed PubMed Central Google
Scholar * NICE (National Institute for Health and Care Excellence). The guidelines manual: appendix G. NICE methodology checklist for economic evaluations.
https://www.nice.org.uk/process/pmg6/resources/the-guidelines-manual-appendices-bi-2549703709/chapter/appendix-g-methodology-checklist-economic-evaluations (2012). * Alonso-Coello, P.,
Oxman, A. D., Moberg, J., Brignardello-Petersen, R., Akl, E. A., Davoli, M. et al. GRADE evidence to decision (EtD) frameworks: a systematic and transparent approach to making well informed
healthcare choices. 2: clinical practice guidelines. _BMJ_ 353, i2089 (2016). PubMed Google Scholar * Sparano, J. A., Gray, R. J., Makower, D. F., Pritchard, K. I., Albain, K. S., Hayes,
D. F. et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. _N. Engl. J. Med._ 379, 111–121 (2018). CAS PubMed PubMed Central Google Scholar * Ravdin, P.
M., Siminoff, L. A., Davis, G. J., Mercer, M. B., Hewlett, J., Gerson, N. et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. _J.
Clin. Oncol._ 19, 980–991 (2001). CAS PubMed Google Scholar * Olivotto, I. A., Bajdik, C. D., Ravdin, P. M., Speers, C. H., Coldman, A. J., Norris, B. D. et al. Population-based
validation of the prognostic model ADJUVANT! for early breast cancer. _J. Clin. Oncol._ 23, 2716–2725 (2005). PubMed Google Scholar * Paik, S., Tang, G., Shak, S., Kim, C., Baker, J., Kim,
W. et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. _J. Clin. Oncol._ 24, 3726–3734 (2006). CAS PubMed Google
Scholar * Albain, K. S., Barlow, W. E., Shak, S., Hortobagyi, G. N., Livingston, R. B., Yeh, I. T. et al. Breast cancer intergroup of north america. prognostic and predictive value of the
21-gene recurrence score assay in a randomized trial of chemotherapy for postmenopausal, node-positive, estrogen receptor-positive breast cancer. _Lancet Oncol._ 11, 55–65 (2010). CAS
PubMed Google Scholar * van de Vijver, M. J., He, Y. D., van’t Veer, L. J., Dai, H., Hart, A. A. M., Voskuil, D. W. et al. A gene-expression signature as a predictor of survival in breast
cancer. _N. Engl. J. Med._ 347, 1999–2009 (2002). PubMed Google Scholar * Bueno-de-Mesquita, J. M., Linn, S. C., Keijzer, R., Wesseling, J., Nuyten, D. S. A., van Krimpen, C. et al.
Validation of 70-gene prognosis signature in node-negative breast cancer. _Breast Cancer Res. Treat._ 117, 483–495 (2009). CAS PubMed Google Scholar * Mook, S., Schmidt, M. K., Viale, G.,
Pruneri, G., Eekhout, I., Floore, A. et al. The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1–3 positive lymph nodes in an independent validation
study. _Breast Cancer Res. Treat._ 116, 295–302 (2009). CAS PubMed Google Scholar * Blok, E. J., Bastiaanet, E., van den Hout, W. B., Liefers, G. J., Smit, V. T. H. B. M., Kroep, J. R. et
al. Systematic review of the clinical and economic value of gene expression profiles for invasive early breast cancer available in Europe. _Cancer Treat. Rev._ 62, 74–90 (2018). CAS PubMed
Google Scholar * Wang, S. Y., Dang, W., Richman, I., Mougalian, S. S., Evans, S. B. & Gross, C. P. Cost-effectiveness analyses of the 21-gene assay in breast cancer: systematic review
and critical appraisal. _J. Clin. Oncol._ 36, 1619–1627 (2018). PubMed PubMed Central Google Scholar * Davidson, J. A., Cromwell, I., Ellard, S. L., Lohrisch, C., Gelmon, K. A.,
Shenkier, T. et al. A prospective clinical utility and pharmacoeconomic study of the impact of the 21-gene Recurrence Score(R) assay in oestrogen receptor positive node negative breast
cancer. _Eur. J. Cancer_ 49, 2469–2475 (2013). CAS PubMed Google Scholar * Jahn, B., Rochau, U., Kurzthaler, C., Hubalek, M., Miksad, R., Sroczynski, G. et al. Personalized treatment of
women with early breast cancer: a risk-group specific cost-effectiveness analysis of adjuvant chemotherapy accounting for companion prognostic tests OncotypeDX and Adjuvant!Online. _BMC
Cancer_ 17, 685 (2017). PubMed PubMed Central Google Scholar * Katz, G., Romano, O., Foa, C., Vataire, A. L., Chantelard, J. V., Hervé, R. et al. Economic impact of gene expression
profiling in patients with early-stage breast cancer in France. _PLoS ONE_ 10, e0128880 (2018). Google Scholar * Lyman, G. H., Cosler, L. E., Kuderer, N. M. & Homberger, J. Impact of a
21-gene RT-PCR assay on treatment decisions in early-stage breast cancer: An economic analysis based on prognostic and predictive validation studies. _Cancer_ 109, 1011–1018 (2007). PubMed
Google Scholar * Ontario Health Technology Advisory Committee, Medical Advisory Secretariat. Gene expression profiling for guiding adjuvant chemotherapy decisions in women with early breast
cancer: an evidence- based and economic analysis. _Ont. Health Technol. Assess Ser_ 10, 1–57 (2010). Google Scholar * Paulden, M., Franek, J., Pham, B., Bedard, P. L., Trudeau, M. &
Krahn, M. Cost-effectiveness of the 21-gene assay for guiding adjuvant chemotherapy decisions in early breast cancer. _Value Health_ 16, 729–739 (2013). PubMed Google Scholar * Vataire, A.
L., Laas, E., Aballea, S., Gligorov, J., Rouzier, R. & Chéreau, E. Cost-effectiveness of a chemotherapy predictive test. _Bull Cancer_ 99, 907–914 (2012). PubMed Google Scholar *
Kondo, M., Hoshi, S. L., Ishiguro, H. & Toi, M. Economic evaluation of the 70-gene prognosis-signature (MammaPrint) in hormone receptor-positive, lymph node-negative, human epidermal
growth factor receptor type 2-negative early stage breast cancer in Japan. _Breast Cancer Res. Treat._ 133, 759–68. (2012). PubMed Google Scholar * Ward, S., Scope, A., Rafia, R., Pandor,
A., Harnan, S., Evans, P. et al. Gene expression profiling and expanded immunohistochemistry tests to guide the use of adjuvant chemotherapy in breast cancer management: a systematic review
and cost-effectiveness analysis. _Health Technol. Assess_ 17, 1–302 (2013). CAS PubMed PubMed Central Google Scholar * Blohmer, J. U., Rezai, M., Kummel, S., Kühn, T., Warm, M.,
Friedrich, K. et al. Using the 21-gene assay to guide adjuvant chemotherapy decision-making in early- stage breast cancer: a cost-effectiveness evaluation in the German setting. _J. Med.
Econ._ 16, 30–40 (2013). CAS PubMed Google Scholar * Nerich, V., Curtit, E., Bazan, F., Montcuquet, P., Villanueva, C., Chaigneau, L. et al. Economic assessment of the routine use of
Oncotype DX assay for early breast cancer in Franche-Comte region. _Bull Cancer_ 101, 681–689 (2014). PubMed Google Scholar * Vanderlaan, B. F., Broder, M. S., Chang, E. Y., Oratz, R.
& Bentley, T. G. K. Cost-effectiveness of 21-gene assay in node-positive, early-stage breast cancer. _Am. J. Manag. Care_ 17, 455–464 (2011). PubMed Google Scholar * Sparano, J. A.,
Gray, R. J., Makower, D. F., Albain, K. S., Saphner, T. J., Badve, S. S. et al. Clinical outcomes in early breast cancer with a high 21-gene recurrence score of 26 to 100 assigned to
adjuvant chemotherapy plus endocrine therapy: a secondary analysis of the TAILORx randomized clinical trial. _JAMA Oncol._ https://doi.org/10.1001/jamaoncol.2019.4794 (in press). * Sparano,
J. A., Gray, R. J., Ravdin, P. M., Makower, D. F., Pritchard, K. I., Albain, K. S. et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. _N. Engl. J. Med._
380, 2395–2405 (2019). CAS PubMed PubMed Central Google Scholar * Giorgi Rossi, P., Lebeau, A. & Schünemann, H. J. Clinical and genomic risk in adjuvant therapy for breast cancer.
_N. Engl. J. Med._ 381, 1289–1290 (2019). PubMed Google Scholar * Dowsett, M. & Turner, N. Estimating risk of recurrence for early breast cancer: integrating clinical and genomic risk.
_J. Clin. Oncol._ 37, 689–692 (2019). CAS PubMed Google Scholar * Mariotto, A., Jayasekerea, J., Petkov, V., Schechter, C. B., Enewold, L., Helzlsouer, K. J. et al. Expected monetary
impact of oncotype DX score-concordant systemic breast cancer therapy based on the TAILORx trial. _J. Natl Cancer Inst._ 112, 154–160 (2020). PubMed Google Scholar * Giorgi Rossi, P. &
Paci, E. Re: expected monetary impact of oncotype DX score-concordant systemic breast cancer therapy based on the TAILORx trial. _J. Natl Cancer Inst._ https://doi.org/10.1093/jnci/djz125,
in press (2021). * Oxman, A. D., Fretheim, A. & Schünemann, H. J. SURE. Improving the use of research evidence in guideline development: introduction. _Health Res. Policy Syst._ 4, 12
(2006). PubMed PubMed Central Google Scholar * Schünemann, H. J., Wiercioch, W., Etxeandia, I., Falavigna, M., Santesso, N., Mustafa, R. et al. Guidelines 2.0: systematic development of a
comprehensive checklist for a successful guideline enterprise. _CMAJ_ 186, E123–E142 (2014). PubMed Central Google Scholar * Woolf, S., Schünemann, H. J., Eccles, M. P., Grimshaw, J. M.
& Shekelle, P. Developing clinical practice guidelines: types of evidence and outcomes; values and economics, synthesis, grading, and presentation and deriving recommendations.
_Implement Sci._ 7, 61 (2012). PubMed PubMed Central Google Scholar * Schünemann, H. J., Lerda, D., Quinn, C., Follmann, M., Alonso-Coello, P., Giorgi Rossi P. et al. Breast cancer
screening and diagnosis: a synopsis of the European breast guidelines. _Ann. Intern. Med._ https://doi.org/10.7326/M19-2125, in press (2021). * NICE National Institute for Health Care and
Excellence (2018). Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer. Diagnostic Guidance DG34.
https://www.nice.org.uk/guidance/dg34/resources/tumour-profiling-tests-to-guide-adjuvant-chemotherapy-decisions-in-early-breast-cancer-pdf-1053750722245 (2020). * Cuzick, J., Dowsett, M.,
Pineda, S., Wale, C., Salter, J., Quinn, E. et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2
immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. _J. Clin. Oncol._ 29, 4273–4278 (2011). PubMed Google Scholar * Barton, S.,
Zabaglo, L., A’Hern, R., Turner, N., Ferguson, T., O´Neill, S. et al. Assessment of the contribution of the IHC4 + C score to decision making in clinical practice in early breast cancer.
_Br. J. Cancer_ 106, 1760–1765 (2012). CAS PubMed PubMed Central Google Scholar * Candido Dos Reis, F. J., Wishart, G. C., Dicks, E. M., Greenberg, D., Rashbass, J., Schmidt, M. K. et
al. An updated PREDICT breast cancer prognostication and treatment benefit prediction model with independent validation. _Breast Cancer Res._ 19, 58 (2017). PubMed PubMed Central Google
Scholar * Harris, L. N., Ismaila, N., McShane, L. M., Andre, F., Collyar, D. E., Gonzalez-Angulo, A. M. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women
with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. _J. Clin. Oncol._ 34, 1134–1150 (2016). CAS PubMed PubMed Central Google
Scholar * Krop, I., Ismaila, N., Andre, F., Bast, R. C., Barlow, W., Collyar, D. E. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage
invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. _J. Clin. Oncol._ 35, 2838–2847 (2017). PubMed Google Scholar * Andre, F.,
Ismaila, N., Henry, N. L., Somerfield, M. R., Bast, R. C., Barlow, W. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast
cancer: ASCO Clinical Practice Guideline Update-Integration of Results From TAILORx. _J. Clin. Oncol._ 37, 1956–1964 (2019). CAS PubMed Google Scholar * Harnan, S., Tappenden, P., Cooper,
K., Stevens, J., Bessey, A., Rafia, R. et al. Tumour profiling tests to guide adjuvant chemotherapy decisions in early breast cancer: a systematic review and economic analysis. _Health
Technol Assess_ 23, 1–328 (2019). PubMed PubMed Central Google Scholar * Schildgen, V., Warm, M., Brockmann, M. & Schildgen, O. Oncotype DX breast cancer recurrence score resists
inter-assay reproducibility with RT(2)-profiler multiplex RT-PCR. _Sci. Rep._ 9, 20266 (2019). CAS PubMed PubMed Central Google Scholar * Sestak, I., Martín, M., Dubsky, P., Kronenwett,
R., Rojo, F., Cuzick, J. et al. Prediction of chemotherapy benefit by EndoPredict in patients with breast cancer who received adjuvant endocrine therapy plus chemotherapy or endocrine
therapy alone. _Breast Cancer Res Treat._ 176, 377–386 (2019). CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS To Kevin Pacheco (K.P.) for his support in
the systematic review of effectiveness of the multigene tests described in this publication. ECIBC CONTRIBUTOR GROUP Mariangela Autelitano18, Bettina Borisch19, Xavier Castells20, Edoardo
Colzani21, Jan Daneš22, Patricia Fitzpatrick23, Livia Giordano24, Solveig Hofvind25, Lydia Ioannidou-Mouzaka26, Susan Knox27, Lennarth Nystrom28, Elena Parmelli29, Elsa Perez30, Alberto
Torresin31, Ruben Van Engen32, Cary Van Landsveld-Verhoeven32, Ken Young33 AUTHOR INFORMATION Author notes * These authors contributed equally: Paolo Giorgi Rossi, Annette Lebeau AUTHORS AND
AFFILIATIONS * Azienda Unità Sanitaria Locale—IRCCS di Reggio Emilia, Reggio Emilia, Italy Paolo Giorgi Rossi * Department of Pathology, University Medical Center Hamburg-Eppendorf,
Hamburg, Germany Annette Lebeau * Iberoamerican Cochrane Center, Biomedical Research Institute (IIB Sant Pau-CIBERESP), Barcelona, Spain Carlos Canelo-Aybar, David Rigau, Pablo Alonso-Coello
& Margarita Posso * Department of Paediatrics, Obstetrics and Gynaecology, Preventive Medicine, and Public Health, PhD Programme in Methodology of Biomedical Research and Public Health,
Universitat Autònoma de Barcelona, Bellaterra, Spain Carlos Canelo-Aybar * European Commission, Joint Research Centre (JRC), Ispra, Italy Zuleika Saz-Parkinson * Instituto de Salud Carlos
III, Health Technology Assessment Agency, Avenida Monforte de Lemos 5, Madrid, Spain Zuleika Saz-Parkinson * St. Vincent’s University Hospital, Dublin, Ireland Cecily Quinn * Department of
Clinical Epidemiology, Biostatistics and Bioinformatics, Amsterdam UMC, University of Amsterdam, Amsterdam Public Health Institute, Amsterdam, The Netherlands Miranda Langendam * Cardiff and
Vale UHB - General Surgery, Cardiff, UK Helen Mcgarrigle * Havyatt Lodge, Havyatt Road, Langford, North Somerset, UK Sue Warman * Department for Health Evidence, Radboud University Medical
Center, Nijmegen, the Netherlands Mireille Broeders * Dutch Expert Centre for Screening, Nijmegen, the Netherlands Mireille Broeders * Radiologie am Theater, Paderborn, NRW, Germany Axel
Graewingholt * Department of Epidemiology and Evaluation, IMIM (Hospital del Mar Medical Research Institute), Barcelona, Spain Margarita Posso * Research Network on Health Services in
Chronic Diseases (REDISSEC), Barcelona, Spain Margarita Posso * Centre for Cancer Prevention, Queen Mary University of London, Charterhouse Square, London, UK Stephen Duffy * Michael G.
DeGroote Cochrane Canada and McGRADE Centres; Department of Health Research Methods, Evidence and Impact, McMaster University Health Sciences Centre, Hamilton, Ontario, Canada Holger J.
Schünemann * Cancer Registry of Milan, Milan, Italy Mariangela Autelitano * Institute of Global Health. University of Geneva, Geneva, Switzerland Bettina Borisch * IMIM, Barcelona, Spain
Xavier Castells * European Centre for Disease Control and prevention (ECDC), Solna, Sweden Edoardo Colzani * Charles University in Prague, Prague, Czech Republic Jan Daneš * National
Screening Service, Dublin, Ireland Patricia Fitzpatrick * CPO-Piedmont - AOU Citta` della Salute e della Scienza, Torino, Italy Livia Giordano * Cancer Registry of Norway, Oslo, Norway
Solveig Hofvind * University of Athens Medical School, Athens, Greece Lydia Ioannidou-Mouzaka * Europa Donna, Milan, Italy Susan Knox * Umea University, Umea, Sweden Lennarth Nystrom *
European Commission, Joint Research Centre, Ispra, Italy Elena Parmelli * University Hospital Dr. Josep Trueta, Girona, Spain Elsa Perez * Ospedale Niguarda Ca’ Granda, Milan, Italy Alberto
Torresin * Dutch Reference Centre for Screening, Nijmegen, The Netherlands Ruben Van Engen & Cary Van Landsveld-Verhoeven * National Coordinating Centre for the Physics of Mammography,
Guildford, UK Ken Young Authors * Paolo Giorgi Rossi View author publications You can also search for this author inPubMed Google Scholar * Annette Lebeau View author publications You can
also search for this author inPubMed Google Scholar * Carlos Canelo-Aybar View author publications You can also search for this author inPubMed Google Scholar * Zuleika Saz-Parkinson View
author publications You can also search for this author inPubMed Google Scholar * Cecily Quinn View author publications You can also search for this author inPubMed Google Scholar * Miranda
Langendam View author publications You can also search for this author inPubMed Google Scholar * Helen Mcgarrigle View author publications You can also search for this author inPubMed Google
Scholar * Sue Warman View author publications You can also search for this author inPubMed Google Scholar * David Rigau View author publications You can also search for this author inPubMed
Google Scholar * Pablo Alonso-Coello View author publications You can also search for this author inPubMed Google Scholar * Mireille Broeders View author publications You can also search
for this author inPubMed Google Scholar * Axel Graewingholt View author publications You can also search for this author inPubMed Google Scholar * Margarita Posso View author publications
You can also search for this author inPubMed Google Scholar * Stephen Duffy View author publications You can also search for this author inPubMed Google Scholar * Holger J. Schünemann View
author publications You can also search for this author inPubMed Google Scholar CONSORTIA THE ECIBC CONTRIBUTOR GROUP * Mariangela Autelitano * , Bettina Borisch * , Xavier Castells * ,
Edoardo Colzani * , Jan Daneš * , Patricia Fitzpatrick * , Livia Giordano * , Solveig Hofvind * , Lydia Ioannidou-Mouzaka * , Susan Knox * , Lennarth Nystrom * , Elena Parmelli * , Elsa
Perez * , Alberto Torresin * , Ruben Van Engen * , Cary Van Landsveld-Verhoeven * & Ken Young CONTRIBUTIONS All authors contributed to the definition of the research protocol. C.C.A.,
D.R., P.A.C. and M.P. were responsible for conducting the systematic review. P.G.R. and A.L. prepared the first draft of the article. All authors contributed to the interpretation and
reporting of the results. All authors revised the manuscript and provided comments on subsequent versions of the article. All authors read and approved the final manuscript prior to
submission and are accountable for all aspects of the work. CORRESPONDING AUTHOR Correspondence to Zuleika Saz-Parkinson. ETHICS DECLARATIONS ETHICS APPROVAL AND CONSENT TO PARTICIPATE Not
applicable. CONSENT TO PUBLISH Not applicable. DATA AVAILABILITY All data sources used during this study are described in this published article and its additional information files. The
datasets analysed are available from the corresponding author on reasonable request. COMPETING INTERESTS Members of the Guideline Development Group (GDG) do not receive financial
compensation for their work but are reimbursed by the E.C. for travel-related expenses for the meetings organised by the JRC. Dr. Giorgi Rossi as former-PI of an independent study on
HPV-based cervical cancer screening, funded by the Italian Ministry of Health, data owner, conducted negotiations with Roche diagnostics, Hologic-Genprobe, Becton-Dickinson to obtain
reagents at reduced price or for free; the reagents obtained were not used in his institution. Dr. Lebeau reports grants and reimbursement for travel-related expenses related to consultancy
from Roche Pharma AG, reimbursement for travel-related expenses related to consultancy from Novartis Oncology, and grants from BioNTech Diagnostics GmbH outside the submitted work. Dr.
Saz-Parkinson was employed by the European Commission, coordinating the ECIBC Guidelines Development Group. Dr. Quinn is Chair of the European Working Group for Breast Screening Pathology
(EWGBSP). Various companies have provided some sponsorship to the EWGBSP for group meetings. Dr. Gräwingholt is the responsible radiologist for screening unit Paderborn, Germany, consultant
radiologist for screening programs in Switzerland, and consultant radiologist for Hellenic School of Senology. Dr. Canelo-Aybar, Dr. Rigau, Dr. Posso Rivera, and Dr. Alonso-Coello reports
that his institution received payments from the European Commission to develop the systematic reviews informing the recommendations. Authors not named here have disclosed no conflicts of
interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M18-3445. FUNDING INFORMATION This work was supported by the European Commission.
The E.C. did not have any role in the study design, collection, analysis and interpretation of the data. The researchers were independent of the funders and all authors, external and
internal, had full access to all of the data (including statistical reports and tables) in the study and take responsibility for the integrity of the data and the accuracy of the data
analysis. DISSEMINATION DECLARATION Results will be disseminated through the ECIBC webpage (https://healthcare-quality.jrc.ec.europa.eu/european-breast-cancer-guidelines) and specific women
versions of these recommendations will also be made available to facilitate access to lay people. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. Members of the ECIBC Contributor Group are listed above Acknowledgements. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Giorgi Rossi, P., Lebeau, A., Canelo-Aybar, C. _et al._ Recommendations from the European Commission Initiative on Breast Cancer for multigene testing to guide the use of adjuvant
chemotherapy in patients with early breast cancer, hormone receptor positive, HER-2 negative. _Br J Cancer_ 124, 1503–1512 (2021). https://doi.org/10.1038/s41416-020-01247-z Download
citation * Received: 01 October 2020 * Revised: 10 December 2020 * Accepted: 17 December 2020 * Published: 18 February 2021 * Issue Date: 27 April 2021 * DOI:
https://doi.org/10.1038/s41416-020-01247-z SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Page not found - Behind The NewsSorry, page not found This might be because:The page you were looking for was removed, had its name changed, or is tempo...
Phagosome maturation: going through the acid testKEY POINTS * Eukaryotic cells engulf a variety of particles during their lifetime, including potentially pathogenic micr...
environment by Jerry Handgraaf from netherlandsenvironment by Jerry Handgraaf from netherlandsdesigner's own words:For the fancy butcher and car fitter. Crystal circle...
Stocks decline into bear market territoryThe longest bull market in history ended Wednesday, as the Dow Jones industrial average closed at 23,553.22, down 1,464....
Prenatal tobacco exposure modulated the association of genetic variants with diagnosed adhd and its symptom domain in children: a community based caseABSTRACT The purpose of our study was to test the hypothesis that prenatal tobacco smoking exposure (PSE) could modulate...
Latests News
Recommendations from the european commission initiative on breast cancer for multigene testing to guide the use of adjuvant chemotherapy in patients wABSTRACT BACKGROUND Predicting the risk of recurrence and response to chemotherapy in women with early breast cancer is ...
Bioluminescence imaging reveals a significant role for complement in liver transduction following intravenous delivery of adenovirusABSTRACT The effect of complement on transgene expression was evaluated _in vivo_ and _in vitro_ using mice lacking comp...
Krishnanagar election result 2024 live updates: tmcs' mahua moitra has won this lok sabha seatThe polling for Krishnanagar was held in Phase 4 on 13 May. The key candidates for the 2024 General Elections in Krishna...
Invention and Discovery | NatureAccess through your institution Buy or subscribe This is a preview of subscription content, access via your institution ...
Bbc strictly fans 'screaming' as unexpected star appears on live showAN UNEXPECTED CELEBRITY WAS SPOTTED IN THE AUDIENCE OF STRICTLY'S SATURDAY NIGHT LIVE SHOW, WITH EAGLE-EYED VIEWERS...