Aggressive nk-cell leukemia: clinical subtypes, molecular features, and treatment outcomes
Aggressive nk-cell leukemia: clinical subtypes, molecular features, and treatment outcomes"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Aggressive NK-cell leukemia (ANKL) is a rare form of NK cell neoplasm sporadically affecting people from Asia and Central and South America. The median overall survival (OS) is less than 2
months, irrespective of treatments. Epstein-Barr virus (EBV) is mostly detected in the leukemia cells and is proposed to contribute to the pathogenesis of ANKL1,2,3. ANKL represents a
distinct disease entity within the continuous spectrum of EBV-associated T/NK-cell lymphoproliferative diseases (EBV-T/NK-LPDs). Patients with ANKL usually manifest a fulminant and extremely
aggressive clinical course. However, some clinicopathologic features may be shared with different types of EBV-T/NK-LPDs, which leads to the occurrence of a few cases with features
intermediate between two similar disorders4,5,6. The diagnosis of ANKL largely relies on the identification of morphologically and immunophenotypically aberrant leukemia cells7. Chromosomal
gains and losses, activating _STAT3_ and _STAT5_ mutations, and _HACE1_ hypermethylation have only been sporadically detected8,9,10. Moreover, optimal therapy of ANKL has not yet been
established11. To date, less than 350 cases of ANKL have been described in English literature worldwide. Because of the rarity of ANKL, the clinical features, potential pathogenesis,
therapeutic strategies, and prognostic factors still lack in understanding. A multicenter study is critically needed for better understanding of this disease. Here we conducted a 13-year
retrospective study with 113 confirmed ANKL patients enrolled in 10 clinical centers located in different geographic regions across China. All cases were centrally reviewed by three
hematopathologists and three hematologists. Study design, enrolled clinical centers, and data collection were described in the Supplementary Methods. This study was approved by the
institutional review board of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. Informed consent was obtained from each individual in accordance with
the principles expressed in the Declaration of Helsinki. From October 2003 to July 2016, a total of 161 suspected cases were collected, and 113 cases with eligibility consensuses after
central review were finally enrolled. All the patients were of the Han nationality living in the mainland China and had no history of chronic active EBV disease (CAEBV), severe mosquito bite
allergy, hydroa vacciniforme, or other T/NK-LPDs. Patient eligibility and general characteristics, including immunophenotyping and EBV detection of leukemia cells, were summarized in
Supplementary Results and Supplementary Tables S1 and S2. The distribution of onset age was illustrated in Supplementary Fig. S1A showing an incidence peak in patients between 21 and 30
years old (29.20%, 33/113), with a male to female ratio of nearly 2:1 in this decade. The median OS was only 55 days (Supplementary Tables S2) and 1-year survival rate was only 4.42% (5/113;
Supplementary Fig. S1B), which indicated a dismal outcome of ANKL. Intriguingly, a subacute clinical course was demonstrated in 18 ANKL patients (15.93%, 18/113). They manifested infectious
mononucleosis (IM)-like symptoms (including fever, lymphocytosis or mononucleosis, lymphadenopathy, and hepatosplenomegaly) for more than 90 days (median: 115 days, range: 90–450 days),
prior to the fulminant onset (Table 1). Female predominance (_P_ = 0.007) was revealed in these patients. Alleviated hyperferritinemia (_P_ < 0.001), transaminitis (ALT, _P_ = 0.009), and
hypofibrinogenemia (_P_ = 0.038), suggesting alleviated hemophagocytic lymphohistiocytosis (HLH), liver impairment, and coagulopathy, were also noted at diagnosis in these patients (Table 1
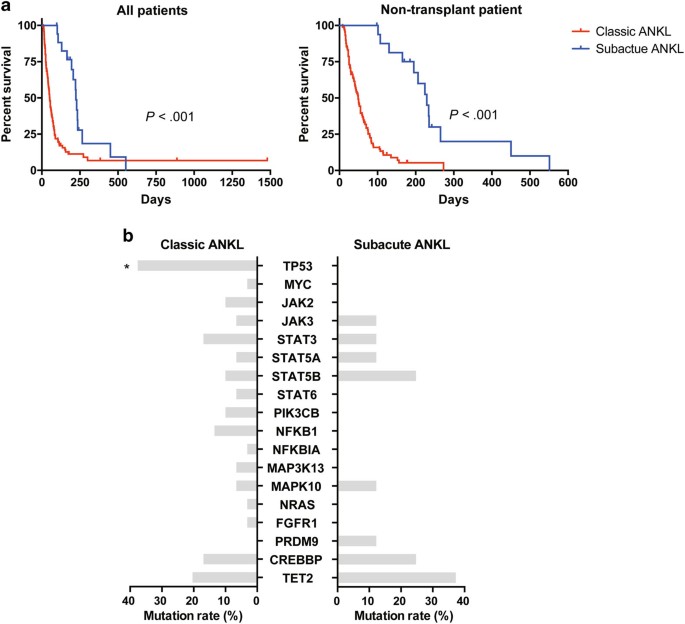
and Supplementary Table S3). A marked survival advantage (_P_ < 0.001) was revealed, irrespective of whether or not the patients who received allogeneic hematopoietic stem cell
transplantation (allo-HSCT) were excluded (Table 1 and Fig. 1a). After all, allo-HSCT is currently the only treatment modality that can independently improve the survival of ANKL
patients11,12,13. Moreover, even if the prolonged prodromal phases were not included in the estimation of the OS of these patients, this marked survival advantage could still be observed in
non-transplant patients (_P_ = 0.042; Supplementary Fig. S2). All these data suggested that patients with prolonged prodromal phases, whom we define here as “subacute ANKL”, may represent a
clinical subtype of ANKL which differed from the others, whom we define here as “classic ANKL”. To clarify the underlying pathogenesis of the two clinical subtypes, genes of interest were
screened by Ion Torrent AmpliSeq™ using a custom sequencing panel in 37 ANKL patients, including 8 subacute ANKL patients and 29 classic ANKL patients. The panel contained 18 candidate genes
(Fig. 1b) identified in our previous whole-genome sequencing analysis of eight ANKL patients, including transcriptional factors, JAK-STAT pathway genes, other signaling pathway genes, and
epigenetic regulators. The sequencing depth of these samples was more than 2000×. Our results showed that the _TP53_ gene had a significant lower mutation rate in subacute ANKL than that in
classic ANKL (_P_ = 0.038; Fig. 1b and Supplementary Table S4). Except for that, the other gene mutation patterns including genes in the JAK-STAT pathway were similar between the two groups,
suggesting that the key driving mechanism is still similar between the two subtypes of ANKL. Notably, _TP53_ mutations were not found in patients of subacute ANKL subtype (Table 1 and Fig.
1b), while enriched in 11 classic ANKL patients (37.93%, 11/29; Supplementary Fig. S3). This result was consistent with the relatively moderate clinical course and improved survival for
subacute ANKL patients. The treatment decision for each patient was made at each clinical center after careful assessment. Since there is no standardized initial treatment for ANKL,
chemotherapeutic regimens varied. CHOP-like (containing anthracycline and vincristine), L-ASPA-based (SMILE, AspaMetDex, L-GemOx, and L-ASPA plus dexamethasone)14, 15, and HLH-04 regimens
(containing dexamethasone and etoposide) were conducted in this study. Seven patients were subjected to allo-HSCT with myeloablative conditioning regimen when they achieved CR after
chemotherapy (CHOP-like, _n_ = 1; L-ASPA-based, _n_ = 6). The median time from diagnosis to allo-HSCT was 73 days (range: 38–128 days). The clinical characteristics of patients in each
subgroup were summarized in Supplementary Table S5, and no differences between each subgroups were revealed. The median follow-up time was 55 days (range: 8–1480 days) for the entire cohort
and 887 days (range: 384–1480 days) for 3 survivors. Patients receiving allo-HSCT exhibited significantly superior survivals when compared to the others without allo-HSCT (_P_ < 0.001).
The median OS was 300 days (range: 174–1480 days) and 2-year OS rate was 42.86% (3/7; Supplementary Fig. S4A). Further subgroup analysis for patients receiving chemotherapy alone revealed
significant OS benefit achieved only in patients treated with L-ASPA-based chemotherapy (_n_ = 19, _P_ = 0.008; Supplementary Fig. S4B). Of the 19 patients with L-ASPA-based chemotherapy
alone, 13 patients received a median of two (range: 1–4) cycles of AspaMetDex. The median OS was 115 days (range: 37–450 days). As induction therapy, the CR and overall response rates (ORR)
of AspaMetDex in newly diagnosed patients was 30.77% (4/13) and 76.92% (10/13), respectively. Reduction of plasma EBV DNA copies and ferritin levels were observed in nine (69.23%) patients,
which was in accordance with the ORR. Grade 3 and Grade 4 hematologic adverse events were common and were recorded in 7 (53.84%) patients. Five of them had severe infection. Meanwhile,
damage of liver function was rare (7.69%, 1/13) and no patient died of regimen-related side effects. We evaluated a variety of clinical features and therapeutic strategies as possible
factors that of prognostic significance. Univariate analysis revealed the following clinical factors to be significantly associated with shorter survival: thrombocytopenia (<30 × 109/l),
elevated serum LDH level (>800 IU/l), hypoalbuminemia (<35 g/l), hyperferritinemia (>1500 IU/l), classic ANKL, treatment without L-asparagine-based chemotherapy, or allo-HSCT
(Supplementary Table S6). Multivariate analysis rendered elevated serum LDH level (>800 IU/l), clinical subtype, treatment (administration of L-asparagine-based chemotherapy, and
allo-HSCT) to be valuable predictors of survival (Supplementary Table S7). In the present study, we systemically explored the features of this deadly type of NK-cell neoplasms. Especially, a
subtype of ANKL with clinical and molecular characteristics was depicted. According to the clinicopathological evolution of EBV-T/NK-LPDs5, we propose to define these patients as “subacute
ANKL”. Although this subtype resembles CAEBV-transformed ANKL (EBV-T/NK- LPDs category A3)6 in some aspects of clinical presentations, they are different in onset age and clinical course4,
5. CAEBV is an indolent disease primarily affecting children and young adults4. While ANKL usually presents a relatively acute disease mainly occurs in the middle-aged population, even
though a subacute clinical course may manifest in a subset of patients. By identifying the group of subacute ANKL patients, clinicians should be alert to those ANKL patients presenting only
IM-like symptoms during their prolonged prodromal phases, since early diagnosis and timely treatment before they evolve into aggressive phases is critical for survival. In addition,
AspaMetDex was revealed to be an effective and well-tolerated initial treatment in ANKL patients. Multivariate analysis in our study showed that the administration of allo-HSCT was one of
the major factors affecting survival (HR = 0.022, 95% CI: 0.005–0.097). Accordingly, the administration of AspaMetDex as induction therapy, SMILE as post-remission consolidation therapy13
and allo-HSCT as finally curative therapy12 could be a promising treatment strategy for ANKL patients. The presence of bias can hardly be avoided, due to the retrospective nature of our
study plus the rarity of ANKL. However, these data depicted the clinical features and molecular characteristics of ANKL, and furthermore provided insights into the treatments and outcomes of
this deadly type of leukemia. REFERENCES * Swerdlow, S. H. et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. _Blood._ 127, 2375–2390 (2016).
Article CAS PubMed PubMed Central Google Scholar * Kwong, Y. L. et al. Management of T-cell and natural-killer-cell neoplasms in Asia: consensus statement from the Asian Oncology Summit
2009. _Lancet Oncol._ 10, 1093–1101 (2009). Article PubMed Google Scholar * Suzuki, R. et al. Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of
cytotoxic NK cells. _Leukemia_ 18, 763–770 (2004). Article CAS PubMed Google Scholar * Kimura, H. et al. EBV-associated T/NK-cell lymphoproliferative diseases in nonimmunocompromised
hosts: prospective analysis of 108 cases. _Blood_ 119, 673–686 (2012). Article CAS PubMed Google Scholar * Takahashi, E. et al. Clinicopathological analysis of the age-related
differences in patients with Epstein-Barr virus (EBV)-associated extranasal natural killer (NK)/T-cell lymphoma with reference to the relationship with aggressive NK cell leukaemia and
chronic active EBV infection-associated lymphoproliferative disorders. _Histopathology_ 59, 660–671 (2011). Article PubMed Google Scholar * Ohshima, K. et al. Proposed categorization of
pathological states of EBV-associated T/natural killer-cell lymphoproliferative disorder (LPD) in children and young adults: overlap with chronic active EBV infection and infantile fulminant
EBV T-LPD. _Pathol. Int._ 58, 209–217 (2008). Article CAS PubMed Google Scholar * Li, C. et al. Abnormal immunophenotype provides a key diagnostic marker: a report of 29 cases of de
novo aggressive natural killer cell leukemia. _Transl. Res._ 163, 565–577 (2014). Article CAS PubMed Google Scholar * Gao, L. M. et al. Clinicopathologic characterization of aggressive
natural killer cell leukemia involving different tissue sites. _Am. J. Surg. Pathol._ 40, 836–846 (2016). Article PubMed Google Scholar * Nicolae, A. et al. EBV-negative aggressive
NK-cell leukemia/lymphoma: clinical, pathologic, and genetic features. _Am. J. Surg. Pathol._ 41, 67–74 (2017). Article PubMed Google Scholar * Nakashima, Y. et al. Genome-wide
array-based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK-cell leukemia and extranodal Nk/T-cell
lymphoma, nasal type. _Genes Chromosomes Cancer_ 44, 247–255 (2005). Article CAS PubMed Google Scholar * Ishida, F. et al. Aggressive natural killer cell leukemia: therapeutic potential
of L-asparaginase and allogeneic hematopoietic stem cell transplantation. _Cancer Sci._ 103, 1079–1083 (2012). Article CAS PubMed Google Scholar * Hamadani, M. et al. Allogeneic
hematopoietic cell transplantation for aggressive NK cell leukemia. A Center for International Blood and Marrow Transplant Research Analysis. _Biol. Blood Marrow Transplant._ 23, 853–856
(2017). Article PubMed Google Scholar * Jung, K. S. et al. L-asparaginase-based regimens followed by allogeneic hematopoietic stem cell transplantation improve outcomes in aggressive
natural killer cell leukemia. _J. Hematol. Oncol._ 9, 41 (2016). Article PubMed PubMed Central Google Scholar * Jaccard, A. et al. Efficacy of L-asparaginase with methotrexate and
dexamethasone (AspaMetDex regimen) in patients with refractory or relapsing extranodal NK/T-cell lymphoma, a phase 2 study. _Blood_ 117, 1834–1839 (2011). Article CAS PubMed Google
Scholar * Yamaguchi, M. et al. Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the
NK-Cell Tumor Study Group study. _J. Clin. Oncol._ 29, 4410–4416 (2011). Article CAS PubMed Google Scholar Download references ACKNOWLEDGMENTS We thank all the faculty and staff in the
Clinical and Laboratory Unit of the Department of Hematology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology for their clinical and technical support.
This work is supported by the funding from the National Natural Science Foundation of China (81570196 to J.-F.Z.; 81670152 to L.H.; 81600120 to N.W.; and 81300410 to D.W.), the Key Program
of the National Natural Science Foundation of China (81230052 to J.-F.Z.), and the National High Technology Research and Development Program of China (863 program 2012AA02A507 to J.-F.Z. and
No.2014AA020532 to L.H.). AUTHOR INFORMATION Author notes * Y.-T. Tang, D. Wang, and H. Luo contributed equally to this work. AUTHORS AND AFFILIATIONS * Department of Hematology, Tongji
Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China Y-T Tang, D Wang, H Luo, M Xiao, N Wang, X-L Hu, Y Luo, X Mao, J Huang, W Zhang, L-S Sheng, L-J
Zhu, Z Shang, L-L Gao, P-L Zhang, M Zhou, K-G Zhou, J-F Zhou & L Huang * Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, China H-S Zhou & Q-F Liu
* Key Laboratory of Genomic and Precision Medicine, Collaborative Innovation Center of Genetics and Development, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing, China D
Liu, S-P Ling & Q-F Wang * Department of Pathology, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China Q-L Ao * Institute of Hematology
and Blood Diseases Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Tianjing, China L-G Qiu, Q-F Wang & J-F Zhou * Department of Hematology, Peking
University Shenzhen Hospital, Shenzhen, China H-Y Zhang * Department of Hematology, the First Affiliated Hospital of Nanjing Medical University and Jiangsu Province Hospital, Nanjing, China
J-Y Li * Department of Hematology, the First Affiliated Hospital, Zhejiang University College of Medicine, Hangzhou, China J Jin * Department of Hematology, Beijing Friendship Hospital,
Capital Medical University, Beijing, China L Fu * Shanghai Institute of Hematology, State Key Laboratory of Medical Genomics, Shanghai Rui Jin Hospital, Shanghai Jiao Tong University School
of Medicine, Shanghai, China W-L Zhao * Department of Hematology, Southwest Hospital, Third Military Medical University, Chongqing, China J-P Chen * Department of Hematology, Guangdong
General Hospital and Guangdong Academy of Medical Sciences, Guangzhou, China X Du * Division of Experimental Hematology and Cancer Biology, Cincinnati Children’s Hospital Medical Center,
Cincinnati, OH, USA G Huang * Division of Pathology, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH, USA G Huang * University of Chinese Academy of Sciences, Beijing, China
Q-F Wang * Cancer Biology Research Center, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China J-F Zhou & L Huang Authors * Y-T Tang View
author publications You can also search for this author inPubMed Google Scholar * D Wang View author publications You can also search for this author inPubMed Google Scholar * H Luo View
author publications You can also search for this author inPubMed Google Scholar * M Xiao View author publications You can also search for this author inPubMed Google Scholar * H-S Zhou View
author publications You can also search for this author inPubMed Google Scholar * D Liu View author publications You can also search for this author inPubMed Google Scholar * S-P Ling View
author publications You can also search for this author inPubMed Google Scholar * N Wang View author publications You can also search for this author inPubMed Google Scholar * X-L Hu View
author publications You can also search for this author inPubMed Google Scholar * Y Luo View author publications You can also search for this author inPubMed Google Scholar * X Mao View
author publications You can also search for this author inPubMed Google Scholar * Q-L Ao View author publications You can also search for this author inPubMed Google Scholar * J Huang View
author publications You can also search for this author inPubMed Google Scholar * W Zhang View author publications You can also search for this author inPubMed Google Scholar * L-S Sheng
View author publications You can also search for this author inPubMed Google Scholar * L-J Zhu View author publications You can also search for this author inPubMed Google Scholar * Z Shang
View author publications You can also search for this author inPubMed Google Scholar * L-L Gao View author publications You can also search for this author inPubMed Google Scholar * P-L
Zhang View author publications You can also search for this author inPubMed Google Scholar * M Zhou View author publications You can also search for this author inPubMed Google Scholar * K-G
Zhou View author publications You can also search for this author inPubMed Google Scholar * L-G Qiu View author publications You can also search for this author inPubMed Google Scholar *
Q-F Liu View author publications You can also search for this author inPubMed Google Scholar * H-Y Zhang View author publications You can also search for this author inPubMed Google Scholar
* J-Y Li View author publications You can also search for this author inPubMed Google Scholar * J Jin View author publications You can also search for this author inPubMed Google Scholar * L
Fu View author publications You can also search for this author inPubMed Google Scholar * W-L Zhao View author publications You can also search for this author inPubMed Google Scholar * J-P
Chen View author publications You can also search for this author inPubMed Google Scholar * X Du View author publications You can also search for this author inPubMed Google Scholar * G
Huang View author publications You can also search for this author inPubMed Google Scholar * Q-F Wang View author publications You can also search for this author inPubMed Google Scholar *
J-F Zhou View author publications You can also search for this author inPubMed Google Scholar * L Huang View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS J.-F.Z. and L.H. designed and supervised this study; L.H., D.W., Y.-T.T., and Y.L. wrote and revised the manuscript; J.-F.Z., L.H., D.W., M.X., X.M., and Q.-L.A. were
responsible for diagnosis and reviewing pathology; L.H., D.W., Y.-T.T., H.L., and X.-L.H. mainly analyzed the clinical data; D.L., S.-P.L., M.X., W.Z., and J.H. performed AmpliSeq and
sequencing data analyses; D.W., Y.-T.T., H.L., N.W., L.-S.S., L.-J.Z., Z.S., L.-L.G., P.-L.Z., M.Z., and K.-G.Z. collected clinical data. H.-S.Z., L.-G.Q., Q.-F.L., H.-Y.Z., J.-Y.L., J.J.,
L.F., W.-L.Z., J.-P.C., and X.D. provided clinical data of enrolled patients. H.-S.Z., G.H., and Q-.F.W. provided support in the project. CORRESPONDING AUTHOR Correspondence to L Huang.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative
Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the
original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in
the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended
use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tang, YT., Wang, D., Luo, H. _et al._ Aggressive NK-cell leukemia: clinical
subtypes, molecular features, and treatment outcomes. _Blood Cancer Journal_ 7, 660 (2017). https://doi.org/10.1038/s41408-017-0021-z Download citation * Received: 11 August 2017 * Revised:
01 October 2017 * Accepted: 10 October 2017 * Published: 21 December 2017 * DOI: https://doi.org/10.1038/s41408-017-0021-z SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
Trending News
The best (nontoxic) pesticides and insecticides, according to gardenersEven though it’s easier than ever to buy plants online, keeping them alive is still a challenge. So we’re digging up eve...
Baba vanga 2019 predictions: shock claims of cataclysmVILLAGERS VISIT BULGARIAN MYSTIC BABA VANGA The Bulgarian mystic, dubbed by her followers the Balkan Nostradamus, is sa...
The aarp minute: july 22, 2020Memorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
How to recognise french road signs for recommended, not obligatory, behaviourTHE SIGNS ARE BECOMING INCREASINGLY USED IN SOME AREAS Some road signs in France signal ‘recommended’ behaviour for moto...
Bandra boy kidnappers who took `5 lakh held1978 Blockbuster _Inqaar_ reportedly inspired a kidnapping 23 years later, with four accused forcing a minor victim’s fa...
Latests News
Aggressive nk-cell leukemia: clinical subtypes, molecular features, and treatment outcomesAggressive NK-cell leukemia (ANKL) is a rare form of NK cell neoplasm sporadically affecting people from Asia and Centra...
Wrapup 1-u. N. , trump denounce north korea, but no sign of any actionThe U. N. Security Council denounced North Korea's weekend missile launch, urging members to "redouble efforts...
Naep gains are elusive in key areasThe Bush administration said last week that newly released 2005 results from “the nation’s report card” confirm that the...
Post-9/11 transition and case management (tcm) | veterans affairsVA LIAISONS FOR SERVICE MEMBERS SEPARATING/RETIRING FROM THE MILITARY VA Liaisons for Healthcare are there to help you a...
'returnships' are internships for older workersIf you saw the Robert De Niro-Anne Hathaway film _The Intern_, you might be forgiven if you think that such an internshi...