Mirnas as biomarkers of nutritional therapy to achieve t2dm remission in patients with coronary heart disease: from the cordioprev study
Mirnas as biomarkers of nutritional therapy to achieve t2dm remission in patients with coronary heart disease: from the cordioprev study"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Type 2 diabetes mellitus (T2DM) is currently a major global public health problem. Although disease remission is possible, few biomarkers have been identified which can
help us select the diet that best promotes remission. Our aim was to study the potential of miRNAs as a tool to apply the Mediterranean diet or the low-fat diet in order to achieve T2DM
remission in patients with coronary heart disease. METHODS From the CORDIOPREV study (_n_ = 1002), a prospective, randomized, single-blind, controlled dietary intervention trial, all
patients newly diagnosed with T2DM (_n_ = 190) at baseline were included in the present study. Of these, after adhering to a low fat or Mediterranean diet for 60 months, 73 patients showed
T2DM remission (Responders) and 110 continued with the disease (Non-responders). Plasma levels of 56 miRNAs were determined by RT-PCR. Generalized linear model, ROC curves and COX regression
analyses were performed. RESULTS We observed that patients with low baseline plasma levels of _miR-let7b-3p_ showed a high probability of T2DM remission after the consumption of a low-fat
diet. In addition, patients with high levels of _miR-141-5p, miR-182_, and _miR-192_ at baseline showed a high probability of T2DM remission after following the Mediterranean diet. Scores
built using miRNAs and clinical variables showed that high levels of a low-fat diet score and a high Mediterranean diet score were associated with a high probability of T2DM remission.
CONCLUSION MiRNAs could be used as a tool for selecting the most efficient nutritional therapy (mediterranean or low-fat diet) to achieve T2DM remission in patients with coronary heart
disease. SIMILAR CONTENT BEING VIEWED BY OTHERS CIRCULATING MICRORNAS 34A, 122, AND 192 ARE LINKED TO OBESITY-ASSOCIATED INFLAMMATION AND METABOLIC DISEASE IN PEDIATRIC PATIENTS Article Open
access 13 May 2021 EFFECTS OF LUSEOGLIFLOZIN AND VOGLIBOSE ON HIGH-RISK LIPID PROFILES AND INFLAMMATORY MARKERS IN DIABETES PATIENTS WITH HEART FAILURE Article Open access 14 September 2022
LONG-TERM EFFICACY AND SAFETY OF EARLY ALOGLIPTIN INITIATION IN SUBJECTS WITH TYPE 2 DIABETES: AN EXTENSION OF THE SPEAD-A STUDY Article Open access 05 September 2023 INTRODUCTION Type 2
diabetes mellitus (T2DM) is currently one of the major problems for health services worldwide [1]. More than 48 million individuals in the European Union were estimated to have T2DM in 2019,
which represents 9.9% of the population. In addition, diabetes was the direct cause of 1.5 million deaths, and 48% of all deaths due to diabetes occurred before the age of 70 years [2].
Moreover, T2DM is also directly related to the increasing incidence of obesity, considered now as a pandemic [3]. Patients with T2DM undergo deterioration in their quality of life,
increasing the probability of death from cardiovascular disease, compared to healthy subjects [4]. In fact, subjects with a cardiovascular event and T2DM have a higher probability of a new
event than subjects without T2DM [5]. Although the disease involves several metabolic alterations, including dysregulation of glucose homeostasis and lipid metabolism, remission of the
disease is possible. Remission of T2DM is defined as reaching glucose levels below the range that is characteristic of diabetic patients, according to the ADA criteria, for 2 consecutive
years: glycosylated hemoglobin < 6.5%, fasting plasma glucose <126 mg/dL and 2 h plasma glucose after an oral glucose tolerance (OGTT) test <200 mg/dL, and the absence of drug
therapy or surgery [6]. However, the greatest likelihood of success in diabetes remission is due to bariatric surgery [7], and to a lesser extent to physical activity [8] or to diets
involving caloric restriction [9, 10]. In this context, adherence to heart-healthy diets has become a key non-invasive tool to achieve disease remission. However, given the genetic
differences between individuals, there is no universal dietary model applicable to the general population that facilitates T2DM remission. For this reason, genetic or epigenetic markers are
needed to help us choose the dietary model that most favors the remission of the disease. MicroRNAs (miRNAs) are short RNA sequences of 19–22 nucleotides that participate in the regulation
of gene expression, and they are, therefore, directly involved in the development and progression of different diseases [11]. In fact, previous studies by this research group have
demonstrated the potential of miRNAs to predict the development of T2DM [12] and even to select between two healthy diets, a low-fat diet and a Mediterranean model (Med diet), to benefit
patients with coronary heart disease (CHD) and help them remain free of T2DM [13]. In our previous study, we included all the non-T2DM at baseline of the CORDIPOREV study with the aim of
identifying a miRNA profile associated with T2DM according to the diet, in order to select the more suitable diet for preventing T2DM development. In contrast, we now hypothesize that a
miRNAs plasma profile may be associated to T2DM remission in a CORDIOPREV subset of patients with T2DM at baseline of the CORDIPOREV study. Based on these previous observations, our aim was
to study the potential of baseline miRNAs plasma levels as a tool to administer the Mediterranean diet or the low-fat diet to achieve T2DM remission after 5 years of follow-up in
newly-diagnosed T2DM patients with coronary heart disease, who were diagnosed with T2DM when recruited to the CORDIOPREV study. MATERIALS AND METHODS STUDY SUBJECTS This work was conducted
within the framework of the CORDIOPREV study. The rationale, methods, and baseline characteristics have been reported by Delgado-Lista et al. [14, 15] and provided in Clinicaltrials.gov
(NTC00924937). The CORDIOPREV study is an ongoing prospective, randomized, single-blind, controlled dietary intervention trial in 1002 patients with coronary heart disease, at high
cardiovascular risk. The inclusion/exclusion criteria have been described in detail elsewhere [15]. Briefly, the inclusion criteria were: patients with acute coronary syndrome (unstable
angina, acute myocardial infarction) and high-risk chronic CHD according to the following criteria: (A) acute myocardial infarction; (B) unstable angina; and (C) chronic high-risk ischemic
heart disease. The exclusion criteria were: (a) patients <20 years of age or >75 years old, or with a life expectancy <5 years; (b) severe heart failure, NYHA functional class III
or IV, with the exception of self-limited episodes of acute heart failure at the time of the acute ischemic event; (c) severe left ventricular systolic dysfunction (with ejection fraction
≤35%); (d) patients with restricted capacity to follow the protocol: those unable to follow the prescribed diet for whatever reason, due to personal or family circumstances; (e) risk factors
which are severe or difficult to control (such as hypertension and diabetes, where there is organ involvement that limits their survival, chronic renal failure and disabling clinical
manifestations of cerebral atherosclerosis); (f) chronic diseases unrelated to coronary risk; and (g) participants in other studies. The subjects were randomized into two different dietary
models (Mediterranean and low-fat diets) during a median follow-up of 7 years. Written consent was obtained from all the subjects before recruitment and the study protocol and all amendments
were approved by the Ethics Committee of Hospital Reina Sofia, following the Helsinki Declaration and good clinical practices. The present study (CORDIOPREV-DIRECT) included all the newly
diagnosed T2DM patients who had not been receiving glucose-lowering treatment at the beginning of the study (190 out of 1002 patients). Of these, 7 patients were excluded due to their
inability to perform the diagnostic test used in this work. T2DM remission was evaluated in the remaining 183 patients during the 5-year follow-up period. Moreover, 3 participants died
during the follow-up period without achieving diabetes remission. The 183 newly diagnosed T2DM patients were classified as Responders, patients who reverted from T2DM during a median of 60
months of the dietary intervention without the use of diabetes medication (_n_ = 73); or Non-Responders, who did not achieve diabetes remission at the end of the follow-up period (_n_ =
110). T2DM remission was defined as glycosylated hemoglobin <6.5%, fasting plasma glucose <126 mg/dL and 2 h plasma glucose after an oral glucose tolerance (OGTT) test <200 mg/dL,
for at least 2 consecutive years, and without the use of diabetes medication to lower blood glucose levels [16]. DIET, DIETARY ASSESSMENT, AND FOLLOW-UP VISITS The enrolled patients were
randomized in two different dietary patterns with well-known metabolic health effects: a Med diet rich in fat from olive oil, with 35% of the calories from fat (22% monounsaturated, 6%
polyunsaturated, <10% saturated), and a maximum of 50% carbohydrates and 15% protein; and the low-fat, high-complex carbohydrate diet (LFHCC) recommended by the National Cholesterol
Education Program and the American Heart Association, comprising <30% total fat (<10% saturated fat, 12–14% MUFA fat, and 6–8% PUFA fat), 15% protein, and a minimum of 55%
carbohydrates. The follow-up visits and dietary adherence in the CORDIOPREV study have been reported previously by Quintana-Navarro et al. [17]. During the study, dieticians administered
personalized individual face-to-face interviews at inclusion and every 6 months (Food Frequency Questionnaire (FFQ), Mediterranean Diet Adherence Screener (MEDAS) [18] to assess Med diet
adherence, or used a 9-item dietary screener for the low-fat diet to assess low-fat diet adherence [19], among other variables). Additionally, 2-h group sessions were held every 3–4 months
to reinforce the dietary recommendations and to deliver the resource materials. Finally, a bimonthly telephone follow-up was conducted in order to monitor compliance with the assigned diet,
negotiate nutrition goals, and reinforce the dietary recommendations. Full study diets, dietary assessments, and follow-up visits have been reported previously [15]. BIOCHEMICAL MEASUREMENTS
OF METABOLIC PARAMETERS Venous blood from the participants was collected in tubes containing Ethylenediaminetetraacetic acid (EDTA) as an anticoagulant after a 12-h overnight fast. Lipid
variables (HDL-c, LDL-c, and triglycerides), glucose homeostasis variables (glucose, insulin, and glycosylated hemoglobin (HbA1c)), and inflammatory variables (hs-CRP) were determined, as
previously reported [12]. ORAL GLUCOSE TOLERANCE TEST The oral glucose tolerance test (OGTT) has been reported previously [12, 20]. In summary, patients underwent a standard Matsuda test at
baseline and year-to-year during the follow-up period. After an overnight fast, blood was sampled from a vein before the oral glucose intake (0 min) and again after a 75 g flavored glucose
load (75 g dextrose monohydrate in 250 mL water, NUTER. TEC GLUCOSA 50). Blood samples were taken at 30, 60, 90, and 120 min to determine glucose and insulin concentrations [21]. ISOLATION
OF CIRCULATING MIRNAS FROM PLASMA SAMPLES Venous blood from the 183 newly-diagnosed T2DM patients who had not been receiving glucose-lowering treatment at the beginning of the study was
collected at baseline (day 0 before dietary intervention) in tubes containing EDTA, and centrifuged at 2000×_g_ for 10 min to separate the plasma from the blood cells. RNA isolation was
carried out from plasma samples, as previously described by Jimenez-Lucena et al. [12]. CDNA SYNTHESIS AND CIRCULATING MIRNAS LEVELS USING REAL-TIME PCR The cDNA synthesis was carried out
using the TaqMan MicroRNA Reverse Transcription Kit (Life Technologies—Thermofisher Scientific, Carlsbad, CA, USA), following the manufacturer’s instructions, as previously described in our
group by Jimenez-Lucena et al. [12]. The circulating miRNAs study was carried out on 56 miRNAs, of which our group had previously studied 28 in a population of non-diabetic patients [12,
22]. The remaining 28 miRNAs were selected based on a bibliographic search according to their association with insulin sensitivity, insulin secretion, inflammation, and the growth and
proliferation of beta-cells (Supplementary Table 1). We measured the levels of miRNAs at the baseline of the CORDIOPREV study using the OpenArray® platform (Life Technologies—Thermofisher
Scientific, Carlsbad, CA, USA), following the manufacturer’s instructions. The relative expression data were analyzed using OpenArray® Real-Time qPCR Analysis Software (Life
Technologies—Thermofisher Scientific, Carlsbad, CA, USA) and the normalization method has been described previously [16]. STATISTICS The differences between Responders and Non-Responders in
the study population baseline characteristics were assessed using One-way ANOVA analysis. To analyze the interaction between diet and groups, an analysis of the baseline characteristics of
the participants was carried out using a univariate general linear model. In addition, the 183 patients included in our study were classified according to the tertiles (T1 = low levels, T2 =
intermediate levels and T3 = high levels) of circulating levels of 45 miRNAs and then COX regression analyses were performed for each miRNA separately for each diet, where T1 was considered
as the reference. We also assessed the hazard ratio (HR) between T3 and T1, with 95% CI. To evaluate the differences in circulating levels of miRNAs between Responders and Non-Responders
according to the diet consumed by the patients, a One-Way ANOVA analysis was performed separately by diet. _p_ Values < 0.05 were considered statistically significant. These analyses were
performed using SPSS software (now PASW Statistic for Windows, version 21) (IBM, Chicago, IL, USA). In addition, clinical variables (BMI, Age, HDL-c, triglycerides) and miRNA data were
transformed (centering and scaling) using the preProcess function from the _caret_ package through the free statistical software “R” version 3.6.1. This function uses two methods for the
normalization and transformation of the variables included in the models. Thus, the method = "center" subtracts the mean of each variable from each absolute value of the variable,
while the method = "scale" divides each value of the variable by its standard deviation. Next, ROC curve analyses were carried out in two steps, first through the glm (General
lineal model) function, which is used to fit generalized linear models and gives a symbolic description of the linear predictor and a description of the error distribution [23]. Next, we
performed the ROC curve using the pROC library. pROC is a tool for visualizing, smoothing, and comparing ROC curves. The AUC can be compared with a statistical test based on U statistics or
bootstrap analysis. After the analysis, we evaluated the AUC, sensitivity, specificity, accuracy and threshold values for each model built. The most significant variables were identified by
Pr(>|_z_|) values < 0.05, registered in the summary of each glm analysis. T2DM SCORES BASED ON MIRNAS ADDED TO CLINICAL VARIABLES In the present study, based on miRNAs and clinical
variables, T2DM remission scores were calculated with the aim of selecting a dietary model (Med or low-fat diets) that promotes disease remission. To this end, we calculated the score in
three steps. First, we performed a glm analysis using the R software, including four miRNAs and six clinical variables, and we assessed the _z_ value of each variable in the summary analysis
separately for both the Med and low-fat diets (Supplementary Tables 2 and 3). Next, we multiplied the _z_ value of each variable by the absolute values of the variables in all the patients
included in the study. Third, the miRNAs and clinical variables were added together to obtain a single score per subject. To evaluate the probability of T2DM remission based on scores, the
183 patients included in our study were classified according to the tertiles of the T2DM remission score for both the Med and low-fat diets (T1, low score; T2, intermediate score; T3, high
score). Using the SPSS software (now PASW Statistic for Windows, version 21) (IBM, Chicago, IL, USA), we performed a Cox regression analysis for each diet assessing the hazard ratio (HR),
with T1 as the reference. OPTIMAL CUT-OFF POINTS FOR T2DM REMISSION SCORE BY DIET To identify the cut-off points that allow us to select a dietary model that promotes remission using the
scores, a ROC analysis was initially carried out. Next, we obtained the coordinates of the curve for each score (T2DM remission score for the low-fat diet and T2DM remission score for the
Mediterranean diet). After that, the Youden index was calculated according to the equation (sensitivity + specificity) − 1 [24]. The Youden index was then plotted against the score values
for both the Med and low-fat diets. We identified the maximum value of the Youden index with its respective score value, which was considered as the cut-off point. RESULTS BASELINE
CHARACTERISTICS OF PATIENTS ACCORDING TO THE DIET The baseline characteristics of the study population have been previously published [16, 25]. In summary, patients who did not achieve
diabetes remission at the end of the follow-up period (Non-Responders) showed higher weight, BMI, waist circumference, HbA1c, fasting glucose, insulin and HOMA-IR than patients who reverted
from T2DM during a median of 60 months of dietary intervention (Responders) (Supplementary Table 4). According to diet, we observed significant differences between the two diets in relation
to systolic blood pressure (_p_ diet vs group = 0.007). No significant differences were observed in the diet _vs_ group interaction in the other baseline characteristics (Table 1). COX
REGRESSION ANALYSIS Of the 56 miRNAs selected for the study, 9 did not amplify in at least 80% of the samples and 2 (_hsa-miR-143_ and _hsa-miR-144_) were used for data normalization, as
previously described by our group [12, 22]. In order to test the potential relationship between miRNA levels and T2DM remission associated with the consumption of a specific diet, we
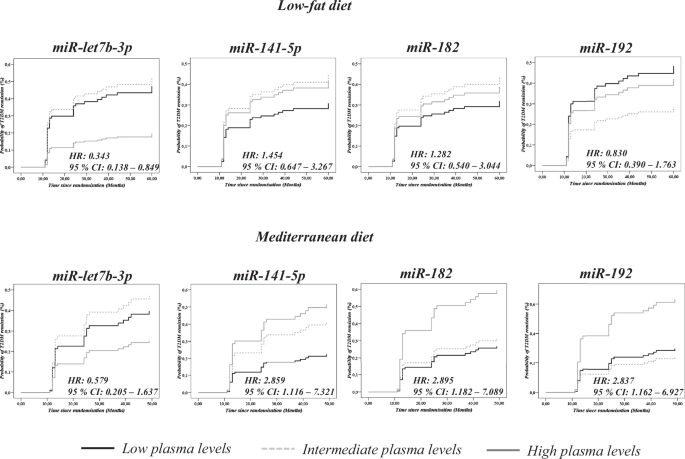
performed a Cox regression analysis for each miRNA of the remaining 45 included in the panel. We found that low baseline plasma levels of _miR-let7b-3p_ were associated with a higher
probability of T2DM remission when the low-fat diet was consumed, but no association between _miR-let7b-3p_ plasma levels and T2DM remission was found when patients consumed the Med diet. In
addition, high baseline plasma levels of _miR-141-5p_, _miR-182_, and _miR-192_ were linked to a higher probability of T2DM remission when the Med diet was consumed, while no association
was observed when patients consumed the low-fat diet (Fig. 1) (Supplementary Table 5). RECEIVER OPERATING CHARACTERISTIC CURVE ANALYSIS To evaluate the potential of miRNAs plasma levels as a
tool to select the most suitable dietary pattern for inducing T2DM remission, ROC curve analyses were performed separately by diet. A model was built including patients who followed a
low-fat diet with the clinical parameters (age, gender, HDL-c, triglycerides, BMI and intensity of statins treatment), showing an AUC = 0.695 (95% CI = 0.592–0.798) (Fig. 2A), which
increased to AUC = 0.767 (95% CI = 0.673-0.861) by adding the plasma levels of _miR-let7b-3p_ (Fig. 2B) (Supplementary Table 6). In addition, a model built with the clinical parameters
including patients who followed the Med diet showed an AUC = 0.750 (95% CI = 0.641–0.859) (Fig. 2C), which increased to an AUC = 0.803 (95% CI = 0.707–0.899) by adding the miRNAs
_miR-141-5p_, _miR-182_, _miR-192_ to the clinical parameters (Fig. 2D) (Supplementary Table 6). T2DM REMISSION SCORES A score built with the clinical parameters (age, gender, BMI, HDL-c,
triglycerides, intensity of statin treatment) and _miR-let7b-3p_ was associated with T2DM remission after the consumption of the low-fat diet. Thus, patients who consumed the low-fat diet
were categorized by tertiles of the T2DM remission score and a COX regression analysis was performed using tertile 1 (T1) as the reference. We observed that patients with high scores showed
a higher probability of T2DM remission when consuming the low-fat diet (HR low vs high = 3.90 (95% CI: 1.562–9.725)) (Fig. 3A). In contrast, no statistically significant associations were
observed when this score was applied to patients who consumed the Med diet (Supplementary Fig. 1). Furthermore, a score including the clinical parameters and _miR-141-5p_, _miR-182_ and
_miR-192_ was associated by COX regression analysis to T2DM remission in the patients who consumed the Med diet. We observed that patients with high scores showed a higher probability of
T2DM remission when consuming the Med diet (HR low vs high: 11.84 (95% CI: 2.728–51.392)) (Fig. 3B). In contrast, no statistically significant associations were observed when this score was
applied to patients who consumed the low-fat diet (Supplementary Fig. 1). OPTIMAL CUT-OFF POINTS FOR T2DM REMISSION SCORES To identify the cut-off points for T2DM remission scores, we first
calculated the Youden index for the ROC curves obtained with both the Med and low-fat scores. We then plotted the values of each score against the values of the Youden index, selecting the
highest value of the index with the corresponding score value. Thus, the optimal cut-off point for the T2DM remission score was 4.22 for the low-fat diet, and −5.04 for the Med diet (Fig.
4). DISCUSSION Our study suggests the potential of circulating levels of specific miRNAs for selecting a specific dietary pattern to achieve type 2 diabetes mellitus (T2DM) remission in
patients with coronary heart disease. In the study, we showed that a low baseline plasma level of _miR-let7b-3p_ was associated with T2DM remission when a low-fat diet was consumed.
Conversely, high baseline plasma levels of _miR-141-5p_, _miR-182_, and _miR-192_ were associated with T2DM remission when consuming the Med diet. The increasing incidence of obesity has led
to an increase in the incidence of metabolic diseases such as T2DM, making it one of the leading causes of death in the world [26]. Moreover, subjects with a cardiovascular event and T2DM
have a higher probability of a new event than those subjects without T2DM [5]. For this reason, efforts have been focused on achieving T2DM disease remission, which is defined as achieving
normal glucose levels without pharmacological treatment [6]. However, to date, this goal has been achieved mainly with invasive methods such as bariatric surgery [27], physical activity [8],
or dietary interventions focused on caloric restriction [9, 10]. In line with this, diabetes remission can also be achieved by the consumption of healthy dietary models such as the
Mediterranean diet or the low-fat diet [28, 29]. However, given the genetic and epigenetic differences of the population, these dietary models should not be generalized to the whole
population and it is important to identify markers to select the dietary model which the patient will benefit most from in order to achieve T2DM remission [30, 31]. Currently, miRNAs are
recognized as important regulators of gene expression and central players in the control of several biological and pathological processes associated with diseases such as T2DM [32].
Additionally, previous studies have demonstrated the association between the dietary components, such as polyphenols, micronutrients, macronutrients, phytochemicals and the expression of
miRNAs [33]. Other studies have demonstrated differences in miRNA levels at different timepoints after bariatric surgery. Indeed, a meta-analysis identified previous studies in humans with
post-surgery times ranging from 3 to 24 months, in which deregulation of miRNA diversity was demonstrated [34], suggesting a dynamic change between diabetes remission and miRNA plasma
levels. In our study, we explored the capacity of miRNAs to predict diabetes remission according to the diet consumed by analyzing baseline plasma levels. We found that ROC curve analyses
showed an AUC = 0.767 when _miR-let7b-3p_ are added to the clinical parameters for the low-fat diet, whereas an AUC = 0.803 was observed after the addition of _miR-141-5p_, _miR-182_,
_miR-192_ to the clinical parameters for the Med diet. Previous studies have demonstrated the differential expression of _miR-let7b-5p_ among healthy people, subjects with T2DM, and T2DM and
diabetic retinopathy [35]. It has also been demonstrated in animal models that _miR-let-7b-5p_ not only upregulated the mt-Cytb gene in mitochondria, but also downregulated insulin receptor
substrate 1 in cytosol in db/db mice [36]. However, few studies have addressed the mechanistic role of _miR-let7b-3p_ in the development or remission of T2DM. In relation to _miR-141-5p_, a
previous study showed that elevated levels of this miRNA were associated with increased beta-cell apoptosis [37]. In turn, previous studies in animal models showed that _miR-182_
participates in glucose homeostasis by regulating the _FOXO1_ and _PDK4_ genes, decreasing blood glucose levels [38]. Finally, _miR-192_ has been reported as being upregulated in diabetic
patients and patients with diabetic nephropathy [39]. However, the overall information about gene expression and/or modulation by diet of these miRNAs is scarce, and even more so in the
context of T2DM. In addition, future studies are needed to investigate the interaction of dietary components and gene expression, which may determine the effect of a specific diet on T2DM
remission, or on global health. Furthermore, several scores have been developed to predict T2DM development, including the PREDIMED-Clinical score (in patients with high cardiovascular
disease) [40], the discrimination of Finnish Diabetes Risk Score (FINDRISC) [41], and the German Diabetes Risk Score (GDRS) [42], in addition to the DiaREM score for T2DM remission [43].
However, such scores do not evaluate remission or help to select the most suitable diet to prevent diabetes development. A previous study performed by our group demonstrated that miRNAs
could be used to select the most suitable dietary pattern to prevent T2DM development [13]. This previous study focused exclusively on the non-T2DM patients at the beginning of the
CORDIOPREV study, of whom some developed T2DM during the follow-up period. In other words, we studied T2DM incidence, but we did not build an epigenetic score based on miRNAs and clinical
variables as we have done in the current work for T2DM remission. Along these lines, in another study, we demonstrated the use of a clinical-epigenetic score to predict disease remission
[16]. In the current work, we have demonstrated that miRNA plasma levels could be used to predict the most suitable diet to induce T2DM remission by building specific clinical-epigenetic
scores for the low-fat and a Med diet. Our study proposes, for the first time, the use of diet-specific clinic-epigenetic scores (Low-Fat score and Med score) to select the dietary pattern
that patients with CHD and T2DM should follow to achieve T2DM remission more efficiently. Nevertheless, our study has some limitations; firstly, we conducted a targeted study on 28 miRNAs
with previously published knowledge, which means that other miRNAs that might be of interest but are not published were not included. Secondly, our study was conducted in patients with
cardiovascular disease, which limits the extrapolation of the results to a healthy population. In conclusion, our results indicate that miRNAs could be applied as a clinical tool to select
the most efficient nutritional therapy (Mediterranean or low-fat diet) to achieve T2DM remission in patients with coronary heart disease. DATA AVAILABILITY Collaborations with the CORDIOPREV
Study are open to Biomedical Institutions, always after an accepted proposal for a scientific work. Depending on the nature of the collaboration, electronic data, hard copy data, or
biological samples should be provided. All collaborations will be made after a collaboration agreement. Terms of the collaboration agreement will be specific for each collaboration, and the
extent of the shared documentation (i.e., deidentified participant data, data dictionary, biological samples, hard copy, or other specified data sets) will be also specifically set on the
light of each work. REFERENCES * Ginter E, Simko V. Type 2 diabetes mellitus, pandemic in 21st century. Adv Exp Med Biol. 2012;771:42–50. Article PubMed Google Scholar * Evaluation IfHMa.
Global Burden of Disease Collaborative Network, Global Burden of Disease Study 2019 (GBD 2019) Results (Institute for Health Metrics and Evaluation—IHME) 2022 [cited 18 October 2022].
https://vizhub.healthdata.org/gbd-results/. * Bluher M. Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol. 2019;15:288–98. Article PubMed Google Scholar * Gu K, Cowie CC,
Harris MI. Diabetes and decline in heart disease mortality in US adults. J Am Med Assoc. 1999;281:1291–7. Article CAS Google Scholar * Martin-Timon I, Sevillano-Collantes C,
Segura-Galindo A, Del Canizo-Gomez FJ. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diab. 2014;5:444–70. Article Google Scholar * Tangelloju
S, Little BB, Esterhay RJ, Brock G, LaJoie AS. Type 2 diabetes mellitus (T2DM) “Remission” in non-bariatric patients 65 years and older. Front Public Health. 2019;7:82. Article PubMed
PubMed Central Google Scholar * Sista F, Abruzzese V, Clementi M, Guadagni S, Montana L, Carandina S. Resolution of type 2 diabetes after sleeve gastrectomy: a 2-step hypothesis. Surg Obes
Relat Dis. 2018;14:284–90. Article PubMed Google Scholar * Ades PA, Savage PD, Marney AM, Harvey J, Evans KA. Remission of recently diagnosed type 2 diabetes mellitus with weight loss
and exercise. J Cardiopulm Rehabilit Prev. 2015;35:193–7. Article Google Scholar * Leslie WS, Ford I, Sattar N, Hollingsworth KG, Adamson A, Sniehotta FF, et al. The Diabetes Remission
Clinical Trial (DiRECT): protocol for a cluster randomised trial. BMC Fam Pract. 2016;17:20. Article PubMed PubMed Central Google Scholar * Taylor R, Al-Mrabeh A, Zhyzhneuskaya S, Peters
C, Barnes AC, Aribisala BS, et al. Remission of human type 2 diabetes requires decrease in liver and pancreas fat content but is dependent upon capacity for beta cell recovery. Cell Metab.
2018;28:667. Article CAS PubMed Google Scholar * Li Y, Kowdley KV. MicroRNAs in common human diseases. Genomics Proteom Bioinforma. 2012;10:246–53. Article CAS Google Scholar *
Jimenez-Lucena R, Rangel-Zuniga OA, Alcala-Diaz JF, Lopez-Moreno J, Roncero-Ramos I, Molina-Abril H, et al. Circulating miRNAs as predictive biomarkers of type 2 diabetes mellitus
development in coronary heart disease patients from the CORDIOPREV study. Mol Ther Nucleic Acids. 2018;12:146–57. Article PubMed PubMed Central Google Scholar * Jimenez-Lucena R,
Alcala-Diaz JF, Roncero-Ramos I, Lopez-Moreno J, Camargo A, Gomez-Delgado F, et al. MiRNAs profile as biomarkers of nutritional therapy for the prevention of type 2 diabetes mellitus: from
the CORDIOPREV study. Clin Nutr. 2021;40:1028–38. Article CAS PubMed Google Scholar * Delgado-Lista J, Alcala-Diaz JF, Torres-Pena JD, Quintana-Navarro GM, Fuentes F, Garcia-Rios A, et
al. Long-term secondary prevention of cardiovascular disease with a Mediterranean diet and a low-fat diet (CORDIOPREV): a randomised controlled trial. Lancet. 2022;399:1876–85. *
Delgado-Lista J, Perez-Martinez P, Garcia-Rios A, Alcala-Diaz JF, Perez-Caballero AI, Gomez-Delgado F, et al. CORonary Diet Intervention with Olive oil and cardiovascular PREVention study
(the CORDIOPREV study): Rationale, methods, and baseline characteristics: a clinical trial comparing the efficacy of a Mediterranean diet rich in olive oil versus a low-fat diet on
cardiovascular disease in coronary patients. Am Heart J. 2016;177:42–50. Article CAS PubMed PubMed Central Google Scholar * Rangel-Zuniga OA, Vals-Delgado C, Alcala-Diaz JF,
Quintana-Navarro GM, Krylova Y, Leon-Acuna A, et al. A set of miRNAs predicts T2DM remission in patients with coronary heart disease: from the CORDIOPREV study. Mol Ther Nucleic Acids.
2021;23:255–63. Article CAS PubMed Google Scholar * Quintana-Navarro GM, Alcala-Diaz JF, Lopez-Moreno J, Perez-Corral I, Leon-Acuna A, Torres-Pena JD, et al. Long-term dietary adherence
and changes in dietary intake in coronary patients after intervention with a Mediterranean diet or a low-fat diet: the CORDIOPREV randomized trial. Eur J Nutr. 2020;59:2099–110. Article
PubMed Google Scholar * Martinez-Gonzalez MA, Garcia-Arellano A, Toledo E, Salas-Salvado J, Buil-Cosiales P, Corella D, et al. A 14-item Mediterranean diet assessment tool and obesity
indexes among high-risk subjects: the PREDIMED trial. PloS ONE. 2012;7:e43134. Article CAS PubMed PubMed Central Google Scholar * Estruch R, Ros E, Salas-Salvado J, Covas MI, Corella D,
Aros F, et al. Retraction and republication: primary prevention of cardiovascular disease with a mediterranean diet. N Engl J Med 2013;368:1279–90. N. Engl J Med. 2018;378:2441–2. Article
PubMed Google Scholar * Roncero-Ramos I, Jimenez-Lucena R, Alcala-Diaz JF, Vals-Delgado C, Arenas-Larriva AP, Rangel-Zuniga OA, et al. Alpha cell function interacts with diet to modulate
prediabetes and Type 2 diabetes. J Nutr. Biochem. 2018;62:247–56. Article CAS PubMed Google Scholar * Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose
tolerance testing: comparison with the euglycemic insulin clamp. Diab Care. 1999;22:1462–70. Article CAS Google Scholar * Jimenez-Lucena R, Camargo A, Alcala-Diaz JF, Romero-Baldonado C,
Luque RM, van Ommen B, et al. A plasma circulating miRNAs profile predicts type 2 diabetes mellitus and prediabetes: from the CORDIOPREV study. Exp Mol Med. 2018;50:1–12. Article PubMed
Google Scholar * Marschner IC. glm2: Fitting Generalized Linear Models with Convergence Problems. R J. 2011;3:12–5. Article Google Scholar * Fluss R, Faraggi D, Reiser B. Estimation of
the Youden Index and its associated cutoff point. Biometr J. 2005;47:458–72. Article Google Scholar * Gutierrez-Mariscal FM, Cardelo MP, de la Cruz S, Alcala-Diaz JF, Roncero-Ramos I,
Guler I, et al. Reduction in circulating advanced glycation end products by mediterranean diet is associated with increased likelihood of type 2 diabetes remission in patients with Coronary
Heart Disease: from the Cordioprev study. Mol Nutr Food Res. 2021;65:e1901290. Article PubMed Google Scholar * Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW,
et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diab Res Clin Pract. 2018;138:271–81. Article CAS Google Scholar * Sjostrom L,
Peltonen M, Jacobson P, Ahlin S, Andersson-Assarsson J, Anveden A, et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and
macrovascular complications. J Am Med Assoc. 2014;311:2297–304. Article Google Scholar * Brown A, McArdle P, Taplin J, Unwin D, Unwin J, Deakin T, et al. Dietary strategies for remission
of type 2 diabetes: a narrative review. J Hum Nutr Diet. 2022;35:165–78. Article PubMed Google Scholar * Esposito K, Maiorino MI, Petrizzo M, Bellastella G, Giugliano D. The effects of a
Mediterranean diet on the need for diabetes drugs and remission of newly diagnosed type 2 diabetes: follow-up of a randomized trial. Diab Care. 2014;37:1824–30. Article CAS Google Scholar
* Gao M, Jebb SA, Aveyard P, Ambrosini GL, Perez-Cornago A, Carter J, et al. Associations between dietary patterns and the incidence of total and fatal cardiovascular disease and all-cause
mortality in 116,806 individuals from the UK Biobank: a prospective cohort study. BMC Med. 2021;19:83. Article CAS PubMed PubMed Central Google Scholar * Neuhouser ML. The importance
of healthy dietary patterns in chronic disease prevention. Nutr Res. 2019;70:3–6. Article CAS PubMed Google Scholar * Flynt AS, Lai EC. Biological principles of microRNA-mediated
regulation: shared themes amid diversity. Nat Rev Genet. 2008;9:831–42. Article CAS PubMed PubMed Central Google Scholar * Garcia-Segura L, Perez-Andrade M, Miranda-Rios J. The emerging
role of MicroRNAs in the regulation of gene expression by nutrients. J Nutrigenetics Nutrigenomics. 2013;6:16–31. CAS Google Scholar * Langi G, Szczerbinski L, Kretowski A. Meta-analysis
of differential miRNA expression after bariatric surgery. J Clin Med. 2019;15:8. * Mastropasqua R, D’Aloisio R, Costantini E, Porreca A, Ferro G, Libertini D, et al. Serum microRNA Levels in
Diabetes Mellitus. Diagnostics (Basel). 2021;11:11. Google Scholar * Li H, Dai B, Fan J, Chen C, Nie X, Yin Z, et al. The different roles of miRNA-92a-2-5p and let-7b-5p in mitochondrial
translation in db/db Mice. Mol Ther Nucleic Acids. 2019;17:424–35. Article CAS PubMed PubMed Central Google Scholar * Belgardt BF, Ahmed K, Spranger M, Latreille M, Denzler R,
Kondratiuk N, et al. The microRNA-200 family regulates pancreatic beta cell survival in type 2 diabetes. Nat Med. 2015;21:619–27. Article CAS PubMed Google Scholar * Zhang D, Li Y, Yao
X, Wang H, Zhao L, Jiang H, et al. miR-182 Cle. Cell Rep. 2016;16:757–68. Article PubMed Google Scholar * Saadi G, El Meligi A, El-Ansary M, Alkemary A, Ahmed G. Evaluation of
microRNA-192 in patients with diabetic nephropathy. Egypt J Intern Med. 2019;31:122–8. Article Google Scholar * Guasch-Ferre M, Bullo M, Costa B, Martinez-Gonzalez MA, Ibarrola-Jurado N,
Estruch R, et al. A risk score to predict type 2 diabetes mellitus in an elderly Spanish Mediterranean population at high cardiovascular risk. PloS ONE. 2012;7:e33437. Article CAS PubMed
PubMed Central Google Scholar * Brodovicz KG, Dekker JM, Rijkelijkhuizen JM, Rhodes T, Mari A, Alssema M, et al. The Finnish Diabetes Risk Score is associated with insulin resistance but
not reduced beta-cell function, by classical and model-based estimates. Diabet Med. 2011;28:1078–81. Article CAS PubMed Google Scholar * Schulze MB, Boeing H, Haring HU, Fritsche A,
Joost HG. Validation of the German Diabetes Risk Score with metabolic risk factors for type 2 diabetes. Dtsch Med Wochenschr. 2008;133:878–83. Article CAS PubMed Google Scholar * Craig
Wood G, Horwitz D, Still CD, Mirshahi T, Benotti P, Parikh M, et al. Performance of the DiaRem score for predicting diabetes remission in two health systems following bariatric surgery
procedures in Hispanic and non-Hispanic white patients. Obes Surg. 2018;28:61–8. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We would like to express our
gratitude to all the subjects who participated in the study, and the Córdoba branch of the Biobank of the Sistema Sanitario Público de Andalucía (Andalusia, Spain) for providing the human
biological samples. We would also like to thank the EASP (Escuela Andaluza de Salud Publica), Granada, Spain, which performed the randomization process for this study. The CORDIOPREV study
is supported by the Fundación Patrimonio Comunal Olivarero, Junta de Andalucía (Consejería de Salud, Consejería de Agricultura y Pesca, Consejería de Innovación, Ciencia y Empresa),
Diputaciones de Jaén y Córdoba, Centro de Excelencia en Investigación sobre Aceite de Oliva y Salud and Ministerio de Medio Ambiente, Medio Rural y Marino, Gobierno de España; Ministerio de
Economía y Competitividad (AGL2012/39615, PIE14/00005, and PIE 14/00031 to JL-M; AGL2015-67896-P to JL-M and AC); Consejería de Innovación, Ciencia y Empresa, Proyectos de Investigación de
Excelencia, Junta de Andalucía (CVI-7450 to JL-M); and by the Fondo Europeo de Desarrollo Regional (FEDER), JPI HDHL-NutriCog (PCIN-2016-084 to JL-M). The CIBEROBN is an initiative of the
Instituto de Salud Carlos III, Madrid, Spain. Oriol Alberto Rangel Zúñiga and Antonio Camargo are supported by an ISCIII research contract (Programa Miguel-Servet CP19/00142 and CP14/00114,
respectively, funded by Instituto de Salud Carlos III, and co-funded by the Fondo Social Europeo “_El FSE invierte en tu futuro_”). JMO is supported by the US Department of Agriculture,
under agreement No. 8050-51000-098-00D. Thanks also go to Jose Andres Morales Martinez and Rosario Carreras for providing technical assistance. AUTHOR INFORMATION Author notes * These
authors contributed equally: Juan Francisco Alcala-Diaz, Antonio Camargo. * These authors jointly supervised this work: Oriol Alberto Rangel-Zuñiga, Jose Lopez-Miranda. AUTHORS AND
AFFILIATIONS * Lipids and Atherosclerosis Unit, Internal Medicine Unit, Reina Sofia University Hospital, 14004, Córdoba, Spain Juan Francisco Alcala-Diaz, Antonio Camargo, Cristina
Vals-Delgado, Ana Leon-Acuña, Helena Garcia-Fernandez, Antonio P. Arenas-de Larriva, Magdalena Perez-Cardelo, Marina Mora-Ortiz, Pablo Perez-Martinez, Javier Delgado-Lista, Oriol Alberto
Rangel-Zuñiga & Jose Lopez-Miranda * Department of Medical and Surgical Sciences, University of Córdoba, 14004, Córdoba, Spain Juan Francisco Alcala-Diaz, Antonio Camargo, Cristina
Vals-Delgado, Ana Leon-Acuña, Helena Garcia-Fernandez, Antonio P. Arenas-de Larriva, Magdalena Perez-Cardelo, Marina Mora-Ortiz, Pablo Perez-Martinez, Javier Delgado-Lista, Oriol Alberto
Rangel-Zuñiga & Jose Lopez-Miranda * Maimónides Biomedical Research Institute of Córdoba (IMIBIC), Córdoba, Spain Juan Francisco Alcala-Diaz, Antonio Camargo, Cristina Vals-Delgado, Ana
Leon-Acuña, Helena Garcia-Fernandez, Antonio P. Arenas-de Larriva, Magdalena Perez-Cardelo, Marina Mora-Ortiz, Pablo Perez-Martinez, Javier Delgado-Lista, Maria Del Mar Malagon, Oriol
Alberto Rangel-Zuñiga & Jose Lopez-Miranda * CIBER Fisiopatologia de la Obesidad y la Nutricion (CIBEROBN), Instituto de Salud Carlos III, 28029, Madrid, Spain Juan Francisco
Alcala-Diaz, Antonio Camargo, Cristina Vals-Delgado, Ana Leon-Acuña, Antonio P. Arenas-de Larriva, Pablo Perez-Martinez, Javier Delgado-Lista, Maria Del Mar Malagon, Jose M. Ordovas, Oriol
Alberto Rangel-Zuñiga & Jose Lopez-Miranda * Department of Cell Biology, Physiology and Immunology, University of Córdoba, 14004, Córdoba, Spain Maria Del Mar Malagon * Nutrition and
Genomics Laboratory, Jean Mayer-US Department of Agriculture Human Nutrition Research Center on Aging, Tufts University, Boston, MA, 0211, USA Jose M. Ordovas * Centro Nacional de
Investigaciones Cardiovasculares, 28029, Madrid, Spain Jose M. Ordovas * IMDEA Food Institute, CEI UAM + CSIC, 28049, Madrid, Spain Jose M. Ordovas Authors * Juan Francisco Alcala-Diaz View
author publications You can also search for this author inPubMed Google Scholar * Antonio Camargo View author publications You can also search for this author inPubMed Google Scholar *
Cristina Vals-Delgado View author publications You can also search for this author inPubMed Google Scholar * Ana Leon-Acuña View author publications You can also search for this author
inPubMed Google Scholar * Helena Garcia-Fernandez View author publications You can also search for this author inPubMed Google Scholar * Antonio P. Arenas-de Larriva View author publications
You can also search for this author inPubMed Google Scholar * Magdalena Perez-Cardelo View author publications You can also search for this author inPubMed Google Scholar * Marina
Mora-Ortiz View author publications You can also search for this author inPubMed Google Scholar * Pablo Perez-Martinez View author publications You can also search for this author inPubMed
Google Scholar * Javier Delgado-Lista View author publications You can also search for this author inPubMed Google Scholar * Maria Del Mar Malagon View author publications You can also
search for this author inPubMed Google Scholar * Jose M. Ordovas View author publications You can also search for this author inPubMed Google Scholar * Oriol Alberto Rangel-Zuñiga View
author publications You can also search for this author inPubMed Google Scholar * Jose Lopez-Miranda View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS Conception and design of the experiments: JFA-D, AC, CV-D, OAR-Z, and JL-M; recruitment and nutritional control of the volunteers: JFA-D, AL-A, APA-L, PP-M, JD-L, and JL-M;
experiments and data collection: JFA-D, AC, CV-D, HG-F and OAR-Z; data analysis and interpretation: AC, CV-D, MP-C, MM-O, OAR-Z, and JL-M; writing the paper: AC, OAR-Z and JL-M; study
conception and design: PP-M, JD-L and JL-M; critical revision of the paper for important intellectual content: MDM, JMO and JL-M; integrity of the data and the accuracy of the data analysis:
JFA-D, AC, PP-M, JD-L, OAR-Z and JL-M. All the authors were involved in writing the paper and gave their final approval to the submitted and published versions. CORRESPONDING AUTHOR
Correspondence to Jose Lopez-Miranda. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains
neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction
in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the
licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article
are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Alcala-Diaz, J.F., Camargo, A., Vals-Delgado, C. _et al._
MiRNAs as biomarkers of nutritional therapy to achieve T2DM remission in patients with coronary heart disease: from the CORDIOPREV study. _Nutr. Diabetes_ 15, 7 (2025).
https://doi.org/10.1038/s41387-025-00362-1 Download citation * Received: 28 August 2023 * Revised: 22 January 2025 * Accepted: 31 January 2025 * Published: 22 February 2025 * DOI:
https://doi.org/10.1038/s41387-025-00362-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
1735 influence of maturation and hyperoxia on surfactant phospholipids and pulmonary compliance in the premature and term rabbitABSTRACT Iowa College of Medicine, Iowa City, IA 52242. Premature (29 days gestation) or term (31 days) rabbits were del...
Reading che guevara in his own wordsChe Guevara was killed on 9 October 1967, but new books by him are still appearing. He was a prolific writer and the Cen...
A divalent sirna chemical scaffold for potent and sustained modulation of gene expression throughout the central nervous systemABSTRACT Sustained silencing of gene expression throughout the brain using small interfering RNAs (siRNAs) has not been ...
Harman measurements for thermoelectric materials and modules under non-adiabatic conditionsABSTRACT Accuracy of the Harman measurement largely depends on the heat transfer between the sample and its surroundings...
1189 use of biostator glucose controller (closed-loop) to estimate insulin needs on a portable pump (open-loop): effect of previous metabolic controlABSTRACT Fourteen insulinopenic diabetics were treated with a closed-loop Biostator Glucose Controller for 24-48 hours f...
Latests News
Mirnas as biomarkers of nutritional therapy to achieve t2dm remission in patients with coronary heart disease: from the cordioprev studyABSTRACT BACKGROUND Type 2 diabetes mellitus (T2DM) is currently a major global public health problem. Although disease ...
A lifetime of service: josie’s dedication to helping kidsAt a time when many of her friends are living a quiet life, Josephine “Josie” Watson’s days can get busy and loud. She l...
A more general role for wr-2721 in cancer therapyAccess through your institution Buy or subscribe This is a preview of subscription content, access via your institution ...
About va form 26-6381 | veterans affairs* Form name: Application for Assumption Approval and/or Release from Personal Liability to the Government on a Home Loan...
Epicardial cells derived from human embryonic stem cells augment cardiomyocyte-driven heart regenerationABSTRACT The epicardium and its derivatives provide trophic and structural support for the developing and adult heart. H...