Impressive near-infrared brightness and singlet oxygen generation from strategic lanthanide–porphyrin double-decker complexes in aqueous solution
Impressive near-infrared brightness and singlet oxygen generation from strategic lanthanide–porphyrin double-decker complexes in aqueous solution"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Although lanthanide double-decker complexes with hetero-macrocyclic ligands as functional luminescent and magnetic materials have promising properties, their inferior water
solubility has negated their biomedical applications. Herein, four water-soluble homoleptic lanthanide (LN = GD, ER, YB and LA) sandwiches with diethylene-glycol-disubstituted porphyrins
(DD) are reported, with their structures proven by both quantum chemical calculations and scanning tunneling microscopy. Our findings demonstrate that the near-infrared emission intensity
and singlet oxygen (1O2) quantum yields of YBDD and GDDD in aqueous media are higher than those of the reported capped lanthanide monoporphyrinato analogues, YBN and GDN; the brightness and
luminescence lifetime in water of YBDD are greater than those of YBN. This work provides a new dimension for the future design and development of molecular theranostics-based water-soluble
double-decker lanthanide bisporphyrinates. SIMILAR CONTENT BEING VIEWED BY OTHERS THERMALLY-ASSISTED PHOTOSENSITIZED EMISSION IN A TRIVALENT TERBIUM COMPLEX Article Open access 22 June 2023
UNTYPICAL TUNEABLE EMISSION ACTIVATED BY NIR RADIATION OBSERVED IN GADOLINIUM BORATE NANOMATERIALS DOPED WITH YB3+, HO3+ AND CE3+ IONS Article Open access 11 November 2024 DESIGN OF AN
OPEN-SHELL NITROGEN-CENTERED DIRADICALOID WITH TUNABLE STIMULI-RESPONSIVE ELECTRONIC PROPERTIES Article Open access 14 October 2022 INTRODUCTION Near-infrared (NIR) luminescent lanthanide
materials have been widely utilized and increasingly researched in telecommunications engineering, laser technology, and biomedical science by virtue of their extraordinary photophysical
properties1,2,3,4,5. However, challenges remain that lanthanides are intrinsically constrained by the Laporte-forbidden 4_f_–4_f_ transitions that render their direct excitation rather
inefficient6,7. To circumvent this issue, _π_-conjugated hetero-macrocycles, such as porphyrins, possessing (i) high-absorption cross-sections, (ii) triplet states resonating well with
lanthanide absorption bands, and (iii) four “hard” nitrogen donor atoms matching “hard” lanthanides, have become promising antenna in use for optimal energy sensitization and protective
coordination8,9,10,11,12,13,14. Sandwich-type lanthanide–porphyrin complexes can afford more preferable or even surprising emission results, given that double-decker lanthanide complexes
have recently spanned the fields of electrochromic/optoelectronic devices, photovoltaic cells, single-molecule magnets, and even molecular rotors—though with few bio-related
counterparts15,16,17,18. Despite their well-characterized, long-lived NIR emission, and 1O2 generation, most lanthanide–macrocycle complexes suffer from inferior water solubility that
considerably hampers their further development in biomedical fields17,18. Tarakanova et al. performed the first comprehensive study on double-decker lanthanide complexes and investigated
their interaction with water. Unfortunately, only one incorporated water molecule was considered and only intramolecular hydrogen bonding was examined, without the description of an aqueous
solution19. Recently, water-soluble gadolinium–porphyrin complexes were reported by Zang et al.20. Their complexes have two porphyrin rings but do not form a sandwich structure. Therefore,
their recorded molar extinction coefficients and singlet oxygen quantum yield in water were much lower than those of our Gd-analogues, although these two series of complexes both have
similar two porphyrin rings as antenna chromophores. The porphyrin structure is rigid and its excited energy can be nonradiatively transferred to an acceptor21. Thus, we recently focused on
and have already reported three water-soluble (up to 1 μM), polyethylene glycol (PEG) chain-conjugated, capped lanthanide monoporphyrinates of (i) organelle specificity, YBRHB22, (ii) tumor
selectivity, YBN23, and (iii) photodynamic therapy, GDN24. Herein, we introduce four water-soluble porphyrin-based lanthanide double-decker complexes (LNDD, where Ln = La, Er, Gd and Yb,
Fig. 1a) with remarkable NIR photophysical properties in aqueous solution. Upon the strategic installation of two optimally short hydrophilic methylated diethylene glycol (DEG) chains on the
tailor porphyrin POR(2DEG) for sandwich lanthanide complexation, YBDD exhibited improved NIR luminescence quantum yield and lifetime in water and outperformed previously reported YBN. The
singlet oxygen generation efficiency in terms of quantum yield (ΦO2) of GDDD was measured to be slightly higher than that of GDN. Our findings substantiate the hypotheses that the
double-decker complexation between porphyrins can (i) facilitate better lanthanide sensitization in the presence of two antenna chromophores rather than one and (ii) minimize the innersphere
quenching effect by lowering the number of bound water molecules under the macrocyclic sandwich design. This work provides unique results for the photophysical data of LNDD in aqueous media
and more importantly, a new dimension for the future design and development of molecular theranostics-based water-soluble double-decker lanthanide bisporphyrinates. Structural elucidation
in this study was performed using various techniques, as described herein, since it is difficult to prepare single crystals suitable for X-ray analysis. RESULTS STRUCTURAL CHARACTERIZATION
BY CALCULATION The porphyrin dianion unit is planar with perpendicular aromatic rings (Supplementary Fig. S31). The structures of lanthanide double-decker porphyrins have been previously
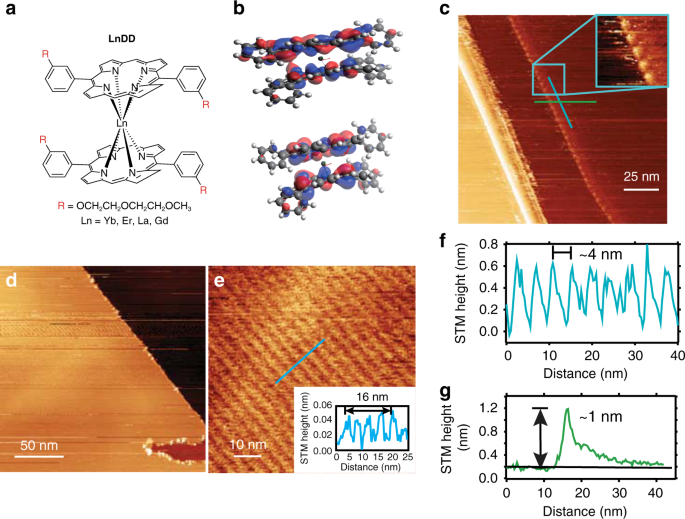
discussed in the literature25,26. The schematic structure of the double-decker complexes is depicted in Fig. 1a. The skeleton structure, without DEG sidechains and with or without a negative
charge, was optimized by calculation (refer to Section 5, SI) because the total charge depends upon the pH and solvent. The structure optimization of YBDD and [YBDD]− using MOPAC27 in the
LUMPAC 1.3.028,29 suite of programs is shown in Supplementary Fig. S32a, b, and is similar to that using the ORCA30 program (Supplementary Fig. S33) and Firefly QC31 package (Supplementary
Figs. S35 and S37), which is partially based upon the GAMESS (US)32 source code. The porphyrin ring system is no longer planar due to (i) the cation-π attractive forces and (ii) π–π
repulsive forces. The N–N distance from the bottom to the top of the double decker is comparable with the distances within each sandwich layer. The structure was also optimized for the AlDD
system (Supplementary Fig. S36) and shows six short Al–N bonds and two longer bonds. These bonds give rise to a distorted structure. The calculated highest occupied molecular orbital/lowest
unoccupied molecular orbital (HOMO/LUMO) are also given for LNDD in Fig. 1b and Supplementary Fig. S34 (DEG chains are omitted for clarity). STRUCTURAL CHARACTERIZATION BY SCANNING TUNNELING
MICROSCOPY Scanning tunneling microscopy (STM) is an advanced technique that can be used for probing molecular assemblies on an individual molecule basis. The study of porphyrins assembly
and structure at the vacuum and liquid interface on surfaces is relatively advanced33. In particular, several studies have been undertaken on double-decker structured molecules34,35. YBDD
was deposited on a clean highly oriented pyrolytic graphite (HOPG) (0001) surface by placing a drop of dilute solution and evaporating at room temperature. The molecules formed
self-assembled motifs without further treatment through surface adsorption and diffusion33. The STM topographic image in Fig. 1c shows a high-magnification image of a region of a drop-cast
surface with additional features decorating the step edges. As shown in the zoom inset, these form a ~4 nm periodic row of separation, and an apparent height of ~1 nm (line profile Fig. 1g)
is present. This height, which was recorded at −1.5 V filled state, is strongly influenced by the electronic effects of both the tip apex and molecular surface junctions36,37. In a different
trial with YBDD, a close-packed arrangement was observed and is shown in Fig. 1d, e. This ordered arrangement is long-ranged ~100 nm and aligned parallel to the HOPG step edge direction.
The features also show a separation of ~4 nm in the direction perpendicular to the step direction (parallel unresolved) as indicated by the height line profile in Fig. 1e inset. Therefore, a
templating effect originating at the step is suggested. The overall behavior of the drop-cast double-decker YBDD on HOPG is in line with previous studies of double-decker motifs and
porphyrin ligands, with a favorable interaction and ability to spontaneously form a periodic assembly. The large ~4 nm spacing between resolvable features is consistent with literature
accounts of similarly structured molecules with the spacing correlated to alkyl chain length34,38 with individual molecules packing face-on with the oxy-alkyl chain R groups having a
favorable arrangement on the HOPG surface, leading to the observed spacing. We attribute the observed features, rows and protrusions (Fig. 1c, e) to single molecules with further work
underway to resolve the exact inner-molecular structure. STRUCTURAL CHARACTERIZATION BY NUCLEAR MAGNETIC RESONANCE The synthesis and characterization of the double-decker porphyrinate
lanthanide complexes with Ln = La, Er, Gd, and Yb trivalent ions are shown in Supplementary Scheme S1, Supplementary Figs. S1–S10 and Supplementary Table S1. Due to the paramagnetic
properties of the latter three lanthanide ion complexes, LADD was synthesized as the analogue for nuclear magnetic resonance (NMR) analysis. Upon the addition of hydrazine hydrate, a
well-resolved LADD NMR spectrum could be obtained (Fig. 2) because hydrazine hydrate served as a reducing agent and assisted the formation of monoanionic diamagnetic complexes9. The protons
of the single ligand POR(2DEG) can be categorized into peripheral and internal. The peripheral aromatic protons are typically located approximately at 6.5–10.0 ppm, while the DEG sidechain
aliphatic protons normally lie within the range of 1.5–4.0 ppm. The peak of the hydrazine hydrate mixed with DMSO-_d_6 is observed at 2.6 ppm. The ring current effect strongly shifts the two
internal protons on the porphyrin upfield to −3.2 ppm. The disappearance of internal N–H peaks and the proton shifting can then serve as an indication of metallization with the lanthanide
ion. No signal is observed in the negative range (equated to internal N–H protons) in the spectrum of LADD, while all peaks are subjected to upfield shifting due to the anisotropy of the
_f_-metal ion as well as the impact of lanthanide-induced shifts10. It is noted that the theoretically most possible supramolecular trimers or even multiple aggregate structures can also
give rise to similar NMR spectra, but the high-resolution mass spectra (HRMS) and STM images corroborate the double-decker structure of LADD (and thus the LNDD series) unambiguously
(Supplementary Fig. S6). PHOTOPHYSICAL STUDIES AND BRIGHTNESS Photophysical properties of LnDD (Ln = Yb, Er, Gd, La) have been measured (Supplementary Figs. S20–S24) and summarized in
Supplementary Table S3. Upon photoexcitation at 425 nm (representing the strongest absorption band, the B or Soret band, Fig. 3a, black experimental spectrum), YBDD showcases superior
photophysical performance compared with the monoporphyrinato counterpart/analogue YBN (serving as the control) under various solvent systems (Supplementary Figs. S11–14 and Supplementary
Table S2). The NIR quantum yields of YBDD were measured by comparison with the standard YBTPP(TP), which was reported as 3.2% in dichloromethane with the same excitation wavelength of 425
nm39. The NIR emission quantum yields of YBDD in toluene (water) were recorded as 3.5% (2.8%), while those of YBN in these solvents were 2.8% (2.7%), shown in Table 1. To explain these
results, firstly, YBDD, which has two antenna ligand groups, should transcend YBN, which has only one. As shown in Fig. 3b, the emission spectrum of YBDD comprises several parts: the
porphyrin ligand visible-NIR emission and the Yb3+ (2F5/2 → 2F7/2) NIR emission, which has an equal peak height in this figure. The peaks at 647 and 699 nm represent the porphyrin
fluorescence from the Q-band singlet nominally labeled S1. The S2 singlet (B-band) emission is also observed at a much weaker intensity and at shorter wavelengths (not shown). From the
comparison with the low temperature 77 K emission spectrum of YBDD (Supplementary Fig. S19), the hot emission bands 1, 2, and 3 in Fig. 3b may correspond to the transitions from the three
excited states of 2F5/2, and the energy intervals between band 3 (975 nm; 10,260 cm−1) and bands 4–6 in Supplementary Fig. S19 can identify the three levels above the ground state energy of
2F7/2. The energy transfer from the porphyrin ligands to the Yb3+ ion is observed to be efficient because the metal ion is not excited by 425 nm radiation in the absence of an antenna.
However, the presence of both ligand fluorescence and lanthanide emission at room temperature suggests that the energy transfer rate from the porphyrin to Yb3+ is similar to the nanosecond
regime. The lower emission quantum yield of YBDD in water than that in toluene is attributable to the quenching by high-frequency O–H vibrations. The trivalent lanthanide ions belong to the
hard Lewis acid category with the coordination number of up to 8–12 so that under saturation of the lanthanides’ inner coordination sphere by ligands offers vacancies for solvent molecule
coordination35. The YBN system was confirmed to have unsaturated seven-coordinated Yb3+: four N from the porphyrin ring and three O from the Kläui [(η5-C5H5) Co{(MeO)2P = O}3]− anion capped
oxygen atoms. To shield the Yb3+ ion in an aqueous environment and suppress luminescence quenching, the double-decker complexation strategy in YBDD fulfills the eight-coordination number
requirement. Brightness is the product of quantum yield and molar attenuation coefficient40,41, and demonstrates the radiant energy emitted per frequency interval unit area per solid angle.
For bioimaging purposes where low dosage is preferred because of adverse effects, the brightness is a more superior indicator of applicability than quantum yield, since, with higher
brightness, low-abundance fluorescent compounds are detected more easily. The brightness of YBDD exceeds that of YBN by a factor of 1.37 (Table 1). The NIR 2F5/2 → 2F7/2 emission lifetimes
of YBDD and YBN were determined to be 23.6 μs in water (Fig. 3c and Supplementary Fig. S17, S18) and 28.2 μs in toluene, which are both higher than the values for YBN (Table 1). YBDD shows a
longer 2F5/2 → 2F7/2 lifetime, which mainly results from its higher symmetry than YBN. With a more symmetric structure, the _f-f_ transition mechanism is of less forced electric dipole
character and more vibronic, and the lifetime for a specific transition is longer42. This trend is consistent with the measured NIR emission quantum yields in water and toluene. It is worth
noting that most porphyrin-based NIR dyes for biological applications have little emission in the NIR-II biological window because no metal ion is coordinated. Furthermore, the maximal
absorption peaks of these dyes are usually located only from 650 to 800 nm43. Both of these reasons limit the application prospects. One commercially available NIR-II dye (NIR-II dye
#900883, Sigma-Aldrich) has a similar emission peak located at 1050 nm, which is the same as that of YBDD, but its NIR emission quantum yield is ~2%, which is lower than that of YBDD. The
impressive NIR emission quantum yields and long NIR emission lifetime of YBDD in aqueous solution, together with its hydrophilic property, hold tremendous promise as a (NIR) bioimaging
probe. SINGLET-OXYGEN GENERATION As a cross-system validation, the singlet oxygen quantum yield of GDDD was also examined in chloroform by comparison with the spectrum of the reference
compound H2TPP (_Φ_Δ = 55% in CHCl3). A new-generation anticancer agent GDN, which consists of only one porphyrin ring, with high-singlet oxygen quantum yield was selected to serve as a
comparison. The near-infrared 1O2 phosphorescence spectra of GDDD, GDN and the reference are shown in Fig. 4a. From these spectra, the singlet oxygen quantum yields of GDDD and GDN were
measured at 66% and 51%, respectively. The singlet-oxygen quantum yield was also evaluated in aqueous solution with a PBS buffer using rose bengal (RB) as the standard by absorption changes
of the decomposition of 9,10-anthracenediyl-bis (methylene) dimalonic acid (ABDA) at 402 nm (Supplementary Figs. S15 and S16). The values of _Φ_Δ were determined as 46% for GDDD and 42% for
GDN. Hence GDDD displayed superior singlet oxygen generation in both organic and aqueous media. The comparison with two U.S. Food & Drug Administration approved PDT agents, porfimer
sodium (Photofrin®) and 5-aminolevulinic acid (Levulan®) was made. Although GDDD shows lower-singlet oxygen quantum yield (46% in aqueous solution, Photofrin®: 89%; Levulan®: 56%), it has a
much higher maximal absorptivity (GDDD: 223,872 M−1 cm−1 @412 nm, and 52480 M−1 cm−1 @580 nm) than these two commercial photosensitizers (Photofrin®: 3000 M−1 cm−1 @632 nm; Levulan®: 5000
M−1 cm−1 @632 nm)44. With a double-decker porphyrinato structure and the resulting high molar extinction coefficient values, GDDD shows great applicability in photodynamic effects, which is
also consistent with the high brightness of YBDD. In vitro experiments have also been performed to practically compare photodynamic therapeutic efficiency in different cell lines, which also
suggest GDDD as a potential PDT agent (Supplementary Figs. S25–S30 and Supplementary Table S4). The energy gap between the antenna donor state and the lanthanide ion plays a crucial role in
the energy transfer efficiency. The lowest triplet state of the lanthanide double-decker complex was determined experimentally from phosphorescence. The 77 K phosphorescence spectra of GDDD
and GDN are shown in Fig. 4b. The zero-phonon lines are at a very similar wavelength ( ~ 745 nm: 13405 cm−1), and the prominent vibrational progression in the ring carbon–nitrogen
stretching mode of 1410 cm−1 is at a lower energy. The triplet energy level is therefore located at 2610 cm−1 above the highest 2F5/2 level of Yb3+ in YBDD. The optimum energy gap has been
given as between 2000 and 5000 cm−1 to eradicate back energy transfer45,46. The weak features marked 1 and 2 in Fig. 4b correspond to the singlet fluorescence bands S1(0,0) and S1(0,1), as
in Fig. 3b for YBDD at 298 K. The triplet state lifetimes of GDDD and GDN at 77 K were measured as 0.21 ± 0.03 and 0.14 ± 0.02 ms, respectively. ABSORPTION SPECTRUM AND TRANSIENT ABSORPTION
SPECTROSCOPY Previous calculations of the energy levels of double-decker complexes have shown poor agreement with experiments47,48. Herein, the absorption spectrum was modeled from the
optimized structure by two programs. First, an excited states calculation was performed using the RM1 semiempirical quantum chemistry method using the LUMPAC suite of programs28,29, and the
calculated result is shown as the dashed blue line in Fig. 3a. The strong singlet-singlet transition is located at 346 nm. In the alternative calculation using ORCA30, this feature is
shifted to lower energy at 530 nm (red dashed line, Fig. 3a). Transient absorption (TA) spectroscopy, as two-dimensional spectroscopy, was used to investigate both the spectral and temporal
properties of the samples. The femtosecond (fs) TA spectra at different delay times for YBDD in chloroform at low laser fluence are displayed in Fig. 5a. The S0 → S2 Soret absorption band is
shown in orange color, and its stimulated emission band has a small red shift with respect to the ground-state bleach and gives a negative signal49. The triplet–triplet (T1 → Tn) absorption
bands are observed at longer wavelengths (440–530 nm)50, with maximum intensity at 451 nm, corresponding to the terminal state energy of 35,578 cm−1. The lifetimes of the bleach and the
excited state transients for YBDD were determined by monitoring at wavelengths of 424 and 451 nm, respectively. (Fig. 5b). The two results are effectively the same and are in the picosecond
scale, denoting a rapid singlet-to-triplet intersystem crossing. The femtosecond TA absorption spectra were also obtained using a higher pump fluence (Fig. 5c). The pulsed laser with high
fluence produces a thermal effect of the YBDD, which causes distortions of the porphyrin structures and results in significant redshifts in the electronic absorption spectra51. In contrast
to the lower fluence, a redshift of the Soret band (Δ_λ_ = + 36 nm) and the T1 → Tn absorption bands were observed. It is worth noting that the structural change was detected instantly by
ultrafast TA spectroscopy: the peak at 501 nm started to shift to 527 nm after 100 ps, and the whole conformation changing process was completed in nanoseconds (Fig. 1d). Furthermore, the
formation of the triplet state from the singlet excited state is clearly observed from the kinetics at 527 nm in Fig. 5d. In contrast to the 527 nm, the excited singlet state at 501 nm
de-excited exponentially to the ground states. However, when using nanosecond (ns) TA spectroscopy, the detailed kinetics of the triplet–triplet absorption peak at approximately 526 nm could
not be resolved since the conformation was changing too quickly (Fig. 5e). The ground state bleach recovery lifetime was extracted from the nanosecond TA spectra (0.69 μs), which is
consistent with the decay lifetime by monitoring deactivation of the triplet state signal at 526 nm (0.69 μs) (Fig. 5f). Isosbestic points were found in all TA spectra (Fig. 5a, c, e), which
suggest that only one single photoexcited species was formed in each case. DISCUSSION The porphyrin moiety acts as a viable antenna under excitation at 425 nm for the ytterbium ion.
Absorption by the Soret band is followed by an internal conversion cascade to lower singlets and intersystem crossing to T1. Calculation shows that there is considerably more than one
singlet and one triplet state involved in this cascade. The energy transfer from T1 lifts the Yb3+ ion from the 2F7/2 ground state to 2F5/2. This change of Δ_J_ = 1 is consistent with the
first order selection rules for exchange or quadrupole interaction. On the other hand, the donor T1 → S0 nonradiative transition is dipole forbidden. Considering the separation between Yb3+
and the porphyrin ring of less than 2 Å, the dominant energy transfer mechanism is most likely to be the exchange mechanism. We report here the first water-soluble lanthanide–porphyrin
double-decker complex with structural characterization using NMR, HRMS, STM, and computational chemistry techniques. NIR imaging and cytotoxic 1O2 generation of the complexes have also been
developed. In our work, the major improvement for bioapplications—considering the low-quantum yield of Yb3+ in the NIR region—comes from the enhancement of the brightness of the potential
bioimaging probe YBDD. This property, brightness, has not yet been widely recognized as the yardstick for applicability, compared with quantum yield, but it shows higher practical
significance for bioimaging purposes. MATERIALS AND METHODS GENERAL SYNTHESIS Dichloromethane (DCM), methanol (MeOH) and n-hexanol were dried by refluxing with calcium hydride (CaH2) before
setting up a reaction. All the chemicals and reagents were of high quality and could be used directly. Reaction processes were monitored by thin-layer chromatography, and further monitored
using a UV lamp. Silica gel or Al2O3 were used for purification in most cases. High-performance liquid chromatography (HPLC) methods were used for final products with high polarity. NMR
spectra were recorded by either a 400 (1H: 400 MHz, 13C: 100 MHz) or a 500 (1H: 500 MHz, 13C: 1250 MHz) spectrometer. High-resolution mass spectra were recorded on a Bruker Autoflex II
(Bruker Dalton GmBH) MALDI-TOF mass spectrometer (characterized by _m/z_). PROCEDURES FOR THE PREPARATION OF 5,15-BIS(3-(2-(2-METHOXYETHOXY)ETHOXY)PHENYL)PORPHYRIN (POR-2DEG)
Di(1H-pyrrol-2-yl)methane (788.84 mg, 5.4 mmol) was dissolved in 1 L dry DCM in a round flask, and 3-(2-(2-methoxyethoxy)ethoxy)benzaldehyde (1.21 g, 5.4 mmol) was added to the solution
which was stirred for 30 min under a nitrogen atmosphere to remove oxygen. Next, trifluoroacetic acid (0.24 mL, 3.24 mmol) was added slowly. The mixture was stirred at room temperature for 3
h under a nitrogen atmosphere. After this time, 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) (1.47 g, 6.48 mmol) was added, and the mixture was stirred for an additional 1 h. Then, 2 mL
of triethylamine was added to quench the unreacted TFA. The mixture was stirred for 10 min, and the solvent was removed. The product was purified through silica gel with the solvent gradient
DCM: MeOH (100:1). GENERAL PROCEDURES FOR THE PREPARATION OF LNDD (LN = YB, ER, GD AND LA) 5,15-bis(3-(2-(2-methoxyethoxy)ethoxy)phenyl)porphyrin (80.0 mg, 0.12 mmol), Ln(acac)3._x_H2O
(0.48 mmol), and 1,8-diazabicyclo(5.4.0)undec-7-ene (DBU, 114 μL, 0.79 mmol) were dissolved in 10 mL dry hexanol. The mixture was bubbled with nitrogen for 20 min at room temperature and
then refluxed for 12 h under a nitrogen atmosphere. After reaction completion, the contents were cooled down to room temperature and mixed with 30 mL hexane. The precipitate was dissolved in
DCM and transferred to an Al2O3 column for purification. (DCM: MeOH 20:1). HPLC was then used for further purification with a preparative column (C18, 10.0 × 250 mm, 5 μm particle size).
The final product was confirmed by MALDI-TOF mass spectral analysis operating in the positive-ion mode using the α-cyano-4-hydroxycinnamic acid matrix. SCANNING TUNNELING MICROSCOPY An HOPG
sample (10 × 10 mm) (SPI) was mounted on a Ta plate. The surface was exfoliated with scotch tape and the surface was verified in a UHV Omicron VT-STM at 10–9 mbar. The STM tip (VT-STM
Omicron) was Pt/Ir and was prepared by degassing at 100 °C for 10 h and then further cleaned by electron bombardment using a tip preparation tool (Omicron), 2 A, 2 mA, and 950 V for 2 s.
In-plane _x_–_y_ calibration was performed by measuring the atomically resolved HOPG surface lattice parameters. To prepare the monolayer film samples, a droplet (5–25 μL) (1 mmol) of a
solution in chloroform or toluene was placed in the centre of a clean surface of HOPG and allowed to evaporate at room temperature. The films were dried under roughing vacuum at 10−2 mbar
for 2 h then transferred to the UHV system for STM imaging. GENERAL SPECTROSCOPIC CHARACTERIZATIONS The absorption spectra of the final products were measured in aqueous solution in the
range 200–800 nm using an HP Agilent UV-8453 Spectrophotometer. The emission spectra from 400 to 1600 nm were obtained by the Fluorolog-3 TCSPC (Horiba) combined fluorescence lifetime and
steady-state spectrometer. The spectrometer was equipped with an NL-C2 Pulsed Diode Controller NanoLED, which produces picosecond and nanosecond optical pulses at a wide range of wavelengths
from the ultraviolet to NIR. TA SPECTROSCOPY Helios spectrometers (Ultrafast systems, FL, USA) were used to perform femtosecond transient absorption spectroscopy. The detailed experimental
setup of the fs-TA is given in the literature52. Briefly, a white-light continuum probe pulse was generated in a 2-mm-thick sapphire plate utilizing a small fraction of the fundamental
output of a Ti:sapphire femtosecond regenerative amplifier that was operating at 800 nm with 35 fs pulses and a repetition rate of 1 kHz. Pump pulses at 395 nm were formed in an optical
parametric amplifier (Newport Spectra-Physics). In a 2-mm-thick cuvette cell containing the sample solutions, the pump and probe pulses were overlapped temporally and spatially. The probe
light transmitted from the sample was gathered and focused on a broadband UV–visible detector to observe the change in absorbance (Δ_A_). The nanosecond TA spectroscopic measurements were
also performed at 395 nm following laser pulse excitation. The ns-TA spectra were recorded using the pump-probe EOS setup (Ultrafast systems, FL, USA), in which a standard probe beam was
split into two: one travels through the sample, and the other one is sent directly to the reference spectrometer, which monitors the fluctuations in the probe beam intensity. The detailed
experimental setup of the EOS can be found elsewhere53. REFERENCES * Kuriki, K., Koike, Y. & Okamoto, Y. Plastic optical fiber lasers and amplifiers containing lanthanide complexes.
_Chem. Rev._ 102, 2347–2356 (2002). Article Google Scholar * Eliseeva, S. V. & Bünzli, J. C. G. Lanthanide luminescence for functional materials and bio-sciences. _Chem. Soc. Rev._ 39,
189–227 (2010). Article Google Scholar * Bünzli, J. C. G. & Eliseeva, S. V. Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. _J. Rare
Earths_ 28, 824–842 (2010). Article Google Scholar * Ye, H. Q. et al. Organo-erbium systems for optical amplification at telecommunications wavelengths. _Nat. Mater._ 13, 382–386 (2014).
Article ADS Google Scholar * Bünzli, J. C. G. Lanthanide luminescence for biomedical analyses and imaging. _Chem. Rev._ 110, 2729–2755 (2010). Article Google Scholar * Montgomery, C. P.
et al. Cell-penetrating metal complex optical probes: targeted and responsive systems based on lanthanide luminescence. _Acc. Chem. Res._ 42, 925–937 (2009). Article Google Scholar *
Armelao, L. et al. Design of luminescent lanthanide complexes: from molecules to highly efficient photo-emitting materials. _Coord. Chem. Rev._ 254, 487–505 (2010). Article Google Scholar
* Beeby, A. et al. Porphyrin sensitization of circularly polarised near-IR lanthanide luminescence: enhanced emission with nucleic acid binding. _Chem. Commun._ 0, 1183–1184 (2000). Article
Google Scholar * Weiss, R. & Fischer, J. Lanthanide phthalocyanine complexes. In (eds Kadish, K. M., Smith, K. M. & Guilard, R.) _The Porphyrin Handbook._ Ch. 105 (San Diego:
Academic Press, 2003). * Wong, W. K. et al. Synthesis, structure, reactivity and photoluminescence of lanthanide(III) monoporphyrinate complexes. _Coord. Chem. Rev._ 251, 2386–2399 (2007).
Article Google Scholar * Lu, G. F. et al. Dysprosium heteroleptic corrole-phthalocyanine triple-decker complexes: synthesis, crystal structure, and electrochemical and magnetic properties.
_Inorg. Chem._ 56, 11503–11512 (2017). Article Google Scholar * Zhao, H. M., Zang, L. X. & Guo, C. S. Influence of lanthanide ion energy levels on luminescence of corresponding
metalloporphyrins. _Phys. Chem. Chem. Phys._ 19, 7728–7732 (2017). Article Google Scholar * Amokrane, A. et al. Role of π-radicals in the spin connectivity of clusters and networks of Tb
double-decker single molecule magnets. _ACS Nano_ 11, 10750–10760 (2017). Article Google Scholar * Serrano, G. et al. Bilayer of terbium double-decker single-molecule magnets. _J. Phys.
Chem. C_ 120, 13581–13586 (2016). Article Google Scholar * He, H. S. Near-infrared emitting lanthanide complexes of porphyrin and BODIPY dyes. _Coord. Chem. Rev._ 273-274, 87–99 (2014).
Article Google Scholar * Bulach, V., Sguerra, F. & Hosseini, M. W. Porphyrin lanthanide complexes for NIR emission. _Coord. Chem. Rev._ 256, 1468–1478 (2012). Article Google Scholar
* Montalban, A. G. et al. Lanthanide porphyrazine sandwich complexes: synthetic, structural and spectroscopic investigations. _J. Chem. Soc. Dalton Trans._ 0, 3269–3273 (2001). Article
Google Scholar * Birin, K. P., Gorbunova, Y. G. & Tsivadze, A. Y. Selective one-step synthesis of triple-decker (porphyrinato)(phthalocyaninato) early lanthanides: the balance of
concurrent processes. _Dalton Trans._ 40, 11539–11549 (2011). Article Google Scholar * Tarakanova, E. N. et al. Double-decker bis(tetradiazepinoporphyrazinato) rare earth complexes:
crucial role of intramolecular hydrogen bonding. _Dalton Trans._ 45, 12041–12052 (2016). Article Google Scholar * Zang, L. X. et al. Water-soluble gadolinium porphyrin as a multifunctional
theranostic agent: Phosphorescence-based oxygen sensing and photosensitivity. _Dyes Pigments_ 142, 465–471 (2017). Article Google Scholar * Peter, H. Inorganic, organometallic and
coordination chemistry. In (eds Kadish, K. M., Smith, K. M. & Guilard, R.) _The Porphyrin Handbook._ Ch. 18 (San Diego: Academic Press, 2003). * Zhang, T. et al. Water-soluble
mitochondria-specific ytterbium complex with impressive NIR emission. _J. Am. Chem. Soc._ 133, 20120–20122 (2011). Article Google Scholar * Zhang, T. et al. Porphyrin-based ytterbium
complexes targeting anionic phospholipid membranes as selective biomarkers for cancer cell imaging. _Chem. Commun._ 49, 7252–7254 (2013). Article ADS Google Scholar * Zhang, T. et al. In
vivo selective cancer-tracking gadolinium eradicator as new-generation photodynamic therapy agent. _Proc. Natl. Acad. Sci. USA_ 111, E5492–E5497 (2014). Article Google Scholar * Ng, D. K.
P. & Jiang, J. Z. Sandwich-type heteroleptic phthalocyaninato and porphyrinato metal complexes. _Chem. Soc. Rev._ 29, 433–442 (1997). Article Google Scholar * Spyroulias, G. A. &
Coutsolelos, A. G. Evidence of protonated and deprotonated forms of symmetrical and asymmetrical lutetium(III) porphyrin double-deckers by 1H-NMR spectroscopy. _Inorg. Chem._ 35, 1382–1385
(1996). Article Google Scholar * Stewart, J. MOPAC2016. http://OpenMOPAC.net. (2016). * Filho, M. A. M. et al. Parameters for the RM1 quantum chemical calculation of complexes of the
trications of thulium, ytterbium and lutetium. _PLoS ONE_ 11, e0154500 (2016). Article Google Scholar * Dutra, J. D. L., Bispo, T. D. & Freire, R. O. LUMPAC lanthanide luminescence
software: efficient and user friendly. _J. Comput. Chem._ 35, 772–775 (2014). Article Google Scholar * Neese, F. The ORCA program system. _Wiley Interdiscip. Rev._ 2, 73–78 (2012). Google
Scholar * Granovsky, A. A. Firefly version 8.2.0. http://classic.chem.msu.su/gran/firefly/index.html. (2013). * Schmidt, M. W. et al. General atomic and molecular electronic structure
system. _J. Comput. Chem._ 14, 1347–1363 (1993). Article Google Scholar * Otsuki, J. STM studies on porphyrins. _Coord. Chem. Rev._ 254, 2311–2341 (2010). Article Google Scholar *
Otsuki, J. et al. Arrays of double-decker porphyrins on highly oriented pyrolytic graphite. _Langmuir_ 22, 5708–5715 (2006). Article Google Scholar * Inose, T. et al. Switching of
single‐molecule magnetic properties of TbIII–porphyrin double‐decker complexes and observation of their supramolecular structures on a carbon surface. _Chemistry_ 20, 11237 (2014). Article
Google Scholar * Sautet, P. Images of adsorbates with the scanning tunneling microscope: theoretical approaches to the contrast mechanism. _Chem. Rev._ 97, 1097–1116 (1997). Article Google
Scholar * Palmer, R. E. & Guo, Q. Imaging thin films of organic molecules with the scanning tunnelling microscope. _Phys. Chem. Chem. Phys._ 4, 4275–4284 (2002). Article Google
Scholar * Wang, H. N. et al. Chain‐length‐adjusted assembly of substituted porphyrins on graphite. _Surf. Interface Anal._ 32, 266–270 (2001). Article Google Scholar * Foley, T. J. et al.
Facile preparation and photophysics of near-infrared luminescent lanthanide(III) monoporphyrinate complexes. _Inorg. Chem._ 42, 5023–5032 (2003). Article Google Scholar * Tanner, P. A. et
al. Misconceptions in electronic energy transfer: bridging the gap between chemistry and physics. _Chem. Soc. Rev._ 47, 5234–5265 (2018). Article Google Scholar * Liu, Z. H. et al.
Brightness calibrates particle size in single particle fluorescence imaging. _Opt. Lett._ 40, 1242–1245 (2015). Article ADS Google Scholar * Shavaleev, N. M. et al. Influence of symmetry
on the luminescence and radiative lifetime of nine-coordinate europium complexes. _Inorg. Chem._ 54, 9166–9173 (2015). Article Google Scholar * Luo, S. L. et al. A review of NIR dyes in
cancer targeting and imaging. _Biomaterials_ 32, 7127–7138 (2011). Article Google Scholar * Ormond, A. B. & Freeman, H. S. Dye sensitizers for photodynamic therapy. _Materials_ 6,
817–840 (2013). Article ADS Google Scholar * Malta, O. L. Mechanisms of non-radiative energy transfer involving lanthanide ions revisited. _J. Noncryst. Solids_ 354, 4770–4776 (2008).
Article ADS Google Scholar * Werts, M. H. V., Jukes, R. T. F. & Verhoeven, J. W. The emission spectrum and the radiative lifetime of Eu3+ in luminescent lanthanide complexes. _Phys.
Chem. Chem. Phys._ 4, 1542–1548 (2002). Article Google Scholar * Langlois, A. et al. Metal dependence on the bidirectionality and reversibility of the singlet energy transfer in artificial
special pair-containing dyads. _Inorg. Chem._ 56, 2506–2517 (2017). Article Google Scholar * Liao, M. S., Watts, J. D. & Huang, M. J. DFT/TDDFT study of lanthanideIII mono- and
bisporphyrin complexes. _J. Phys. Chem. A_ 110, 13089–13098 (2006). Article Google Scholar * Berera, R., Van Grondelle, R. & Kennis, J. T. M. Ultrafast transient absorption
spectroscopy: principles and application to photosynthetic systems. _Photosynth. Res._ 101, 105–118 (2009). Article Google Scholar * Masih, D. et al. Photoinduced triplet-state electron
transfer of platinum porphyrin: a one-step direct method for sensing iodide with an unprecedented detection limit. _J. Mater. Chem. A_ 3, 6733–6738 (2015). Article Google Scholar *
Parusel, A. B. J., Wondimagegn, T. & Ghosh, A. Do nonplanar porphyrins have red-shifted electronic spectra? A DFT/SCI study and reinvestigation of a recent proposal. _J. Am. Chem. Soc._
122, 6371–6374 (2000). Article Google Scholar * Bose, R. et al. Direct femtosecond observation of charge carrier recombination in ternary semiconductor nanocrystals: the effect of
composition and shelling. _J. Phys. Chem. C_ 119, 3439–3446 (2015). Article Google Scholar * El-Ballouli, A. O. et al. Quantum confinement-tunable ultrafast charge transfer at the PbS
quantum dot and phenyl-C61-butyric acid methyl ester interface. _J. Am. Chem. Soc._ 136, 6952–6959 (2014). Article Google Scholar Download references ACKNOWLEDGEMENTS This work was
supported by the Hong Kong Baptist University (HKBU), Hong Kong Research Grants Council (HKBU 12300117), and the HKBU-HKPolyU Joint Research Program (RC-ICRS/16-17/02). We thank Dr. Zhenyu
Liu for his technical support in spectroscopic measurements and Professor Alex Granovsky for communication concerning the use of Firefly. AUTHORS CONTRIBUTIONS K.-L.W., W.-K.W. and P.A.T.
conceived and supervised the project. P.A.T. performed the calculations. J.-X.Z., W.-L.C. and Y.Z. synthesized the ligands and the complexes. C.X. measured the spectroscopic properties.
H.-F.C. performed biological tests. P.M., G.T.H., A.A. and O.F.M. conducted the STM and TAS experiments. AUTHOR INFORMATION Author notes * These authors contributed equally: Jing-Xiang
Zhang, Wai-Lun Chan, Chen Xie AUTHORS AND AFFILIATIONS * Department of Chemistry, Hong Kong Baptist University, Kowloon Tong, Hong Kong S.A.R., China Jing-Xiang Zhang, Wai-Lun Chan, Chen
Xie, Yan Zhou, Peter A. Tanner, Wai-Kwok Wong & Ka-Leung Wong * Hanshan Normal University, Chaozhou, Guangdong Province, China Jing-Xiang Zhang * Department of Biology, Hong Kong Baptist
University, Kowloon Tong, Hong Kong S.A.R., China Ho-Fai Chau * KAUST Solar Center, Division of Physical Science and Engineering, King Abdullah University of Science and Technology (KAUST),
Thuwal, 23955-6900, Saudi Arabia Partha Maity, George T. Harrison, Aram Amassian & Omar F. Mohammed Authors * Jing-Xiang Zhang View author publications You can also search for this
author inPubMed Google Scholar * Wai-Lun Chan View author publications You can also search for this author inPubMed Google Scholar * Chen Xie View author publications You can also search for
this author inPubMed Google Scholar * Yan Zhou View author publications You can also search for this author inPubMed Google Scholar * Ho-Fai Chau View author publications You can also
search for this author inPubMed Google Scholar * Partha Maity View author publications You can also search for this author inPubMed Google Scholar * George T. Harrison View author
publications You can also search for this author inPubMed Google Scholar * Aram Amassian View author publications You can also search for this author inPubMed Google Scholar * Omar F.
Mohammed View author publications You can also search for this author inPubMed Google Scholar * Peter A. Tanner View author publications You can also search for this author inPubMed Google
Scholar * Wai-Kwok Wong View author publications You can also search for this author inPubMed Google Scholar * Ka-Leung Wong View author publications You can also search for this author
inPubMed Google Scholar CORRESPONDING AUTHORS Correspondence to Peter A. Tanner, Wai-Kwok Wong or Ka-Leung Wong. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have
no conflict of interest. SUPPLEMENTARY INFORMATION 41377_2019_155_MOESM1_ESM.DOCX SUPPLEMENTAL INFORMATION for Impressive Near-Infrared Brightness and Singlet Oxygen Generation from
Strategic Lanthanide–Porphyrin Double–Decker Complexes in Aqueous Solution RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, JX., Chan, WL., Xie, C. _et al._ Impressive near-infrared brightness and
singlet oxygen generation from strategic lanthanide–porphyrin double-decker complexes in aqueous solution. _Light Sci Appl_ 8, 46 (2019). https://doi.org/10.1038/s41377-019-0155-9 Download
citation * Received: 27 December 2018 * Revised: 11 April 2019 * Accepted: 19 April 2019 * Published: 22 May 2019 * DOI: https://doi.org/10.1038/s41377-019-0155-9 SHARE THIS ARTICLE Anyone
you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative
Trending News
Robert tombs — a reply to george magnus | thearticleOne can be sure of getting a sharp response to opinion pieces in the _Financial Times_, especially if connected with Bre...
Saportareport - valued voices share insights about atlanta and beyond.LATEST NEWS The flair and rhythm of the Atlanta Caribbean Carnival Parade will takeover downtown streets on Saturday fro...
Metabolic make-up of nash: from fat and sugar to amino acidsNAFLD is regarded unquestionably as one of the components of the metabolic syndrome. Hence, metabolic perturbations occu...
Another darn breach? How to protect your personal dataMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
Operating status | va puget sound health care | veterans affairsVA Puget Sound health care facility operating statuses and emergency information. FACILITY OPERATING STATUSES * Normal s...
Latests News
Impressive near-infrared brightness and singlet oxygen generation from strategic lanthanide–porphyrin double-decker complexes in aqueous solutionABSTRACT Although lanthanide double-decker complexes with hetero-macrocyclic ligands as functional luminescent and magne...
Effects of propranolol during pregnancy and development of rats. I. Adverse effects during pregnancyABSTRACT Summary: Pregnant rats, treated with high doses of propranolol, gave birth to small for dates neonates. Litter ...
From neural 'is' to moral 'ought': what are the moral implications of neuroscientific moral psychology?ABSTRACT Many moral philosophers regard scientific research as irrelevant to their work because science deals with what ...
Mhc class i-independent activation of virtual memory cd8 t cells induced by chemotherapeutic agent-treated cancer cellsABSTRACT Cancer cells can evade immune recognition by losing major histocompatibility complex (MHC) class I. Hence, MHC ...
N-acetylglucosamine-6-sulfate sulfatase in man: deficiency of the enzyme in a new mucopolysaccharidosisABSTRACT Summary: The study of a 5-year old patient with a mucopolysaccharidosis different from those already known has ...