Preservation of cerebrovascular tone and reactivity by sodium channel inhibition in experimental prolonged asphyxia in piglets
Preservation of cerebrovascular tone and reactivity by sodium channel inhibition in experimental prolonged asphyxia in piglets"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Sodium channels using cAMP as a second messenger play a role in the regulation of cerebral circulation and metabolism. Cerebrospinal fluid (CSF) cAMP levels have been shown to
correlate with the degree and duration of hypoxic injury and outcome and to be an indicator of cerebral vascular reactivity. We hypothesize that sodium channel inhibition either before or at
termination of experimental asphyxia will attenuate cerebrovascular alterations and maintain CSF cAMP levels. Three groups of piglets with closed cranial windows were studied: asphyxia or
group 1 (_n_ = 5) and two treatment groups. Pigs were treated with 50 mg/kg of sodium channel blocker before asphyxia (group 2, _n_ = 6) and after the termination of asphyxia and start of
reventilation (group 3, _n_ = 6). Asphyxia was sustained over 60 min by ventilating piglets with 10% O2 gas mixture and decreasing minute ventilation followed by 60 min of reventilation with
room air. Every 10 min, pial arterial diameters were measured, and CSF samples were collected for cAMP determination. Vascular reactivity to topically applied isoproterenol (10−4 M) was
evaluated 60 min after recovery. During asphyxia, cAMP levels in group 2 peaked and declined at a later time with mean values remaining significantly higher than those of groups 1 and 3.
During reventilation, CSF cAMP concentrations were highest in group 3 and lowest in group 1. Pial arteriolar dilation occurred during asphyxia in all three groups but to a lesser degree in
the pretreated group compared with groups 1 and 3. Pial arteriolar reactivity to isoproterenol postasphyxia was preserved in both groups 2 and 3. In summary, in newborn pigs, pretreatment
with sodium channel blocker resulted in higher CSF cAMP levels and a lesser degree of pial arteriolar dilation during prolonged asphyxia. Pretreatment or treatment at reventilation restored
vascular tone and reactivity. SIMILAR CONTENT BEING VIEWED BY OTHERS THE EFFECTS OF SODIUM BICARBONATE INFUSION ON CEREBROVASCULAR FUNCTION IN NEWBORN PIGS Article 03 December 2021
NEUROPROTECTIVE EFFECT OF INDOMETHACIN IN NORMAL PERFUSION PRESSURE BREAKTHROUGH PHENOMENON Article Open access 22 September 2020 DOSE-RELATED SYSTEMIC AND CEREBRAL HEMODYNAMIC EFFECTS OF
NOREPINEPHRINE IN NEWBORN PIGLETS WITH HYPOXIA-REOXYGENATION Article 03 April 2025 MAIN Considerable progress has been made in understanding the mechanisms causing brain damage. Medications
have been tried for neuroprotection in experimental models as well as in clinical settings, but still there is no effective drug capable of protecting brain tissue against hypoxia-ischemia
(1). This may be explained by the multifactorial mechanism of hypoxic-ischemic brain injury, difficulties in defining its onset, duration and severity, and the side effects reported in the
use of investigational drugs. Preservation of cerebral vascular reactivity and minimization of cerebral hemodynamic alterations caused by hypoxia-ischemia could be very important in cerebral
protection. Alteration of cerebral hemodynamics accompanies substantial brain damage in experimental and clinical settings (2–4) with associated disruption of autoregulation, the most
vulnerable regulatory mechanism of the cerebral circulation (3, 4). The level of cAMP detected in CSF correlates with the degree and the duration of asphyxia (5, 6) and has been suggested as
a possible indicator of altered cerebral vascular reactivity (6–8) and brain metabolic and energy state (9). Also CSF cAMP concentrations have been correlated with long-term
neurodevelopmental outcomes in neonates who had perinatal asphyxia (10). Various subtypes of sodium channels that use cAMP as second messenger have been detected in brain tissue and in
vascular cells (11). Sodium channels have a role in the regulation of cerebral function, hemodynamics, and metabolism (11, 12). However, pharmacologic modulation of voltage-gated sodium
channels for the purpose of neurovascular protection has received very little attention. In the present study, we hypothesized that sodium channel inhibition in severely asphyxiated newborn
piglets would maintain CSF cAMP levels and would attenuate alterations in cerebrovascular response and reactivity during asphyxia and recovery/reventilation. METHODS The surgical and
experimental procedures were reviewed and approved by the Animal Care and Use Committee of The University of Tennessee, Memphis. Piglets 2–5 d of age (1–2 kg) of either sex were anesthetized
and instrumented before the experiments. Piglets were initially anesthetized with intramuscular ketamine hydrochloride (33 mg/kg) and acepromazine (3.3 mg/kg) and maintained on intravenous
α-chloralose (50 mg/kg initially plus 3 mg·kg−1·h−1). The animals were ventilated with room air through a tracheotomy tube by using a Bournes BP200 infant ventilator. Catheters were inserted
into the femoral vein for maintenance of anesthesia and fluid administration (5 mL·kg−1·h−1 of 5% dextrose in water) and into the femoral arteries to record BP and heart rate for blood gas
sampling. Body temperature was maintained between 37 and 38°C. CLOSED CRANIAL WINDOW PLACEMENT FOR MEASUREMENTS OF PIAL ARTERIOLARDIAMETERS AND COLLECTION OF PERIARACHNOID CSF. Each piglet
had a closed cranial window inserted over the parietal cortex for measurements of pial arteriolar diameter, qualitative assessments of pial arteriolar flow, and collection of cortical
periarachnoid CSF for cAMP determination. The methods for insertion of a closed cranial window have been described previously (5, 6, 8). Pial arterioles were observed with a Wild dissecting
microscope, a video monitor, and a video microscaler. The images of the arterioles were displayed on the television monitor, and parallel lines projected by the microscaler bracketed the
sides of the vessels. As the arteriolar diameter changed, the lines were moved manually to correspond with the vessel walls. Precalibration of the distance between the lines allowed
determination of arteriolar diameter. PROTOCOL. Three groups of piglets were studied, group 1 or asphyxia-control group (_n_ = 5) and treatment groups 2 and 3 (_n_ = 12). Animals in group 2
(_n_ = 6) were treated with 50 mg/kg of novel nonselective sodium channel blocker HOE642 (cariporide mesilate, a Na+/H+ exchange blocker) before asphyxia, whereas animals in group 3 (_n_ =
6) were treated at the end of asphyxia, the beginning of reventilation. Na+ channel blocker HOE642 was provided by Hoeschst Marion Roussel, Chemical Research, G838, 65926 Frankfurt, Germany.
Group 1 animals or nontreated animals received an equal volume of normal saline infusion at the same rate. Thirty minutes after administration of normal saline or medication, asphyxia was
induced by ventilating the piglets with a gas mixture of 10% CO2, 10% O2, and 80% N2 and by decreasing the minute volume. Respiratory support and gas mixture were manipulated to maintain
systemic BP around 30 mm Hg and heart rate between 50 and 80 beats/min. At the end of 60 min of asphyxia, minute volume was increased, and piglets were ventilated with room air. Piglets were
monitored during 60 min of recovery. Arterial blood was collected every 10 min for blood gases and pH measurement using an Instrumentation Blood Gas Analyzer (Instrumentation Laboratory,
Lexington, MA). BP and heart rate were monitored continuously during the experiments. Cortical periarachnoid CSF was collected every 10 min for later measurement of cAMP. Diameters of two to
three randomly selected pial arterioles were recorded every 10 min during the experiments. Also, when diameter measurements were being made, it was possible to characterize qualitatively
the forward movement of RBC because of the thin arteriolar wall. RBC flow was described by one of the following: normal forward flow, increased forward flow, sluggish or decreased forward
flow, or no visible forward flow. Further, it was noted whether sludging or clumping of RBC was observed. Vascular reactivity to topically applied isoproterenol (10−4 M) was also evaluated
after 60 min of recovery. CAMP ASSAY. Cortical periarachnoid CSF (0.4 mL) collected from under the cranial window was mixed with EDTA (5 μL) and stored at −60°C until assayed. cAMP was
measured in CSF samples by using RIA procedures (6–8). STATISTICAL ANALYSIS. Values reported as mean ± SEM were compared within group and between groups over time by 3-way ANOVA with
repeated measures. Tukey's method was used for multiple comparisons. A _p_ value < 0.05 was considered significant. The association between pial arteriolar diameters and CSF cAMP
levels over time within group and between groups was analyzed using mixed linear models with random effects. The relationship between these variables within group was expressed as _y_ =_a_
+_b_ (log cAMP), wherein _y_ is the predicted pial arteriolar diameter, _a_ the intercept, and _b_ the slope. Statistics software used was S-PLUS (Mathsoft, Seattle, WA). RESULTS The groups
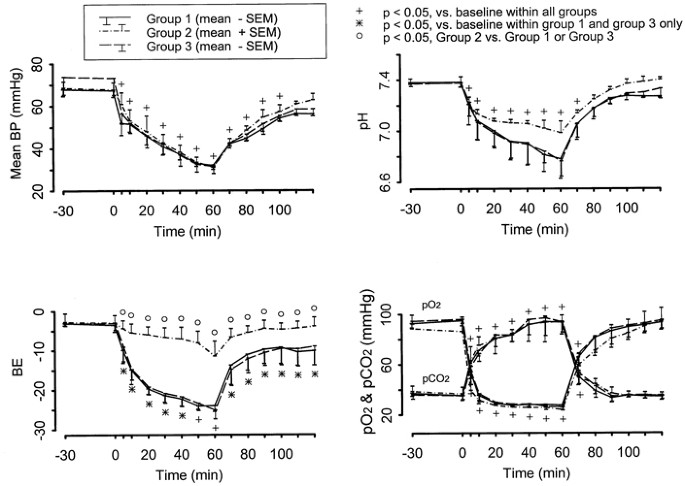
of piglets did not differ in weight, baseline normal BP, pH, and blood gas values (Fig. 1). With the onset of asphyxia, BP, pH, and PO2 quickly decreased and were maintained at critically
low levels by manipulating the degree of asphyxia with subsequent return to low normal level during recovery or reventilation. At each period after induction of asphyxia, BP, pH, PO2, and
PCO2 changed significantly from baseline within group, but these changes were not statistically different among groups. These are also illustrated in Figure 1. Piglets pretreated with sodium
channel blocker (group 2) tended to maintain slightly higher (although not statistically significant) pH values than in the other two groups throughout asphyxia. The base excess (BE) values
were significantly higher during asphyxia in group 2 compared with the other two groups (_p_ <.05); these differences were also observed during recovery/reventilation. During asphyxia,
CSF cAMP levels were significantly higher in the pretreated piglets compared with either group 1 or 3 (Fig. 2), with the peak CSF cAMP concentration occurring at 40 min of asphyxia. Levels
in group 2 decreased as asphyxia continued, but a higher level was maintained compared with either group 1 or 3. CSF cAMP levels increased during recovery/reventilation in all groups, with
levels higher in group 2 compared with group 1. CSF cAMP values in group 3 exceeded either those of group 1 or 2. The increase in pial arteriolar diameters with the onset of asphyxia (Fig.
3) was greater in groups 1 and 3 compared with group 2. Pial diameters in groups 1 and 3 decreased during the last one-half hour of asphyxia but remained greater than measurements in group 2
or values at baseline. During recovery, pial arteriolar diameters in groups 2 and 3 decreased toward baseline measurements, whereas diameters in group 1 piglets decreased but remained above
baseline. Analysis of the relationship between CSF cAMP and pial arteriolar diameter while controlling for time, using a linear mixed model with random effects, showed significant
correlations between these measurements within group 1 (_r_2 = 0.38, _p_ < 0.001) and group 2 (_r_2 = 0.30, _p_ < 0.001) as illustrated in Figure 4. No significant correlation was
noted between pial arteriolar diameters and CSF cAMP levels in group 3. In this group, the pial arteriolar diameter increased with increasing CSF cAMP during asphyxia, but, with treatment
upon initiation of reventilation, the significant increase in CSF cAMP was associated with a decrease in pial arteriolar diameter from a maximally dilated state toward baseline values. In
group 1 at onset of asphyxia, RBC movement within the pial arterioles qualitatively appeared to increase initially, but, as asphyxia continued, RBC movement decreased, and cells became
clumped together followed by complete cessation of RBC movement by the end of asphyxia. During recovery in group 1, initially some forward flow was noted but was transient, and ultimately
RBC forward movement ceased and sludging was observed. Similar findings were observed during asphyxia in group 3 but with subsequent acceleration of RBC movement during the recovery phase;
there was only minimal vessel engorgement. In group 2 piglets, however, persistent steady movement of RBC was observed throughout the whole period of asphyxia and recovery, with less
engorgement and no intravascular RBC clumping. After 60 min of recovery, determination of pial arteriolar response to isoproterenol showed significant increase in diameter in groups 2 and 3;
this increase in diameter was significantly greater compared with group 1 (Fig. 5). The associated increase in CSF cAMP concentrations with pial dilatory response to isoproterenol is also
shown in Figure 5. CSF cAMP concentrations increased significantly with isoproterenol administration in groups 2 and 3 but not in group 1. DISCUSSION We observed that compared with control
piglets, pretreatment with sodium channel blocker resulted in maintaining higher BE and higher CSF cAMP levels, attenuation of pial arteriolar dilation, and preservation of forward movement
of RBC during prolonged asphyxia. Treatment initiated at start of ventilation after asphyxia resulted in higher CSF cAMP concentrations during recovery compared with nontreated piglets.
Furthermore, we observed preservation of cerebrovascular reactivity during recovery from asphyxia when sodium channel blocker was administered either before asphyxia or at the start of
reventilation. Different subtypes of sodium channel have been detected in all types of brain tissue as well as in vascular, cardiac, and skeletal muscle cells and are important for normal
cellular function and metabolism (11, 12). A common mechanism of modulation of sodium channel activity on brain and vascular tissues is presumed to be cAMP-dependent phosphorylation (11, 13,
14). An increased level of cAMP reduces sodium influx in brain neurons, decreasing sodium channel number and activity and possibly attenuating excessive harmful sodium currents (15).
Down-modulation of voltage-gated sodium channels is probably an effective way of reducing energy demands by decreasing the amount of energy used for maintenance of ionic gradient across the
cell membrane (16, 17), and, therefore, it is possible that the metabolic requirement could be met by anaerobic metabolism (18, 19–22). However, if sodium channel stimulation is sustained
during prolonged asphyxia, excessive sodium influx would block anaerobic glycolysis, thus abolishing this only remaining source of ATP. It follows, then, that anaerobic glycolysis may be
maintained if sodium channel inhibitors are administered (23, 24). In pretreated piglets, we observed significantly less base deficit and a tendency for maintaining pH within an acceptable
range even with associated significant decrease in mean arterial BP and PaO2 and increase in PaCO2 during prolonged asphyxia. These findings possibly reflect a better preservation of
metabolism in general and in brain and vascular cell with administration of a nonselective sodium channel inhibitor. Perhaps with preservation of metabolism, there is also associated
preservation of response to the other vasoactive stimuli other than cAMP during asphyxia. This, in part, may explain the decreased correlation between cAMP and vascular diameter in our
pretreated animals (Fig. 4). The changes in CSF cAMP in our control animals are consistent with previous reports (5–8, 25). Levels of CSF cAMP, however, were modified by administration of
sodium channel blocker. Pretreatment with sodium channel blocker before asphyxia resulted in higher levels of CSF cAMP during prolonged asphyxia and during reventilation compared with
nontreated animals. Treatment with sodium channel blocker at the end of asphyxia or the beginning of reventilation also resulted in significant elevation of CSF cAMP during recovery.
Possible sources of cAMP in periarachnoid CSF are neurons, glial cells, and vascular endothelial and smooth muscle cells, and changes in cyclic nucleotide concentrations are likely
reflective of intracellular changes (6, 7, 25–27). Vigorous intracellular cAMP generation during interruption of O2 supply coupled with ATP depletion has been described (7, 26). Also, cAMP
is involved in the regulation of vasodilatory response to hypoxia-ischemia in newborn piglets (1, 8, 27). Cerebral circulation of newborn mammals has proven to be especially sensitive to the
vasorelaxant action of cAMP (6–8, 27, 28), and topical application of cAMP to the cerebral surface was shown to cause pial arterial vasodilation ranging from 10 to 25% (8). The role and
function of sodium channels during hypoxia-ischemia when there are associated alterations in vascular hemodynamics need elucidation (2–4). Cerebral vasodilation appears to be a consistently
observed phenomenon after hypoxia, hypercarbia, hypotension, and hypoxia-ischemia, both in experimental models and in human neonates (29–32). However, the physiologic significance of
cerebral vascular arteriolar dilation in response to hypoxia-ischemia is not known. In experimental prolonged partial asphyxia with hypotension, a “no-reflow” phenomenon was observed during
reventilation (5, 29, 33) despite cerebral dilation. Thus, cerebral vascular dilation does not necessarily indicate conservation of cerebral flow or autoregulation. Low cerebral blood flow
reported in term infants with hypoxic-ischemic encephalopathy during the first few days of life has been associated with poor outcome (34). However, in some studies, low cerebral blood flow
with minimal oxygen requirement and oxygen consumption, as estimated from positron emission scan and near-infrared studies, appeared to be compatible with completely normal long-term
neurologic outcome, as opposed to those infants who demonstrated increased oxygen consumption during the posthypoxic-ischemic period (19–22, 34–37). Perhaps limited or minimal cerebral
vascular dilation with some conservation of blood flow may provide protection against breakdown in the blood-brain barrier in the course of hypoxia-ischemia. Compared with control animals in
our study, pretreatment resulted in preservation of forward movement of RBC during prolonged asphyxia in the presence of minimal arteriolar dilation. Thus, there is a potential role for
sodium channel blockers in maintaining blood flow during prolonged hypoxia-ischemia. The marked pial arteriolar dilation during asphyxia and loss of vasoreactivity during recovery in our
control group probably reflect vascular paralysis as a result of hypoxia-ischemia. In contrast, sodium channel blocker administration either before asphyxia or in early reventilation was
associated with preservation of vascular reactivity. We can only speculate on the possible mechanisms by which sodium channel inhibition is associated with attenuation of cerebral vascular
alterations and elevation of cAMP during hypoxia-ischemia. At the beginning of asphyxia, with perfusion pressure and O2 supply relatively preserved, a quickly rising PCO2 or hypercapnic
response (27) results in CSF cAMP elevation with moderate physiologic cerebral vascular dilation. Early in asphyxia, this increase in cAMP also may lead to sodium channel down-regulation as
one of the mechanisms of local metabolic vascular motor control, limiting vessels from further dilation, vascular paralysis, and disruption of the blood-brain barrier. However, with longer
duration of hypoxia-ischemia, these adaptive mechanisms eventually fail, coinciding with a precipitous decrease in cAMP level in intracellular as well as extracellular fluids and associated
with cerebral vascular paralysis. These changes were evident in the nontreated piglets throughout the whole experiment and during asphyxia in piglets who were treated at initiation of
reventilation. Despite larger arteriolar lumen in the nontreated piglets, no reflow phenomenon was observed in our experiments as well as in other similar studies (5, 33, 4). Sodium channel
blocker administration resulting in a marked increase in CSF cAMP levels followed by a delay in its precipitous decrease maintained arterial vascular tone associated with conservation of
blood flow during prolonged asphyxia and preservation of physiologic response to other vasoactive stimuli during recovery or reventilation. Thus, from our findings in the first experiment of
sodium channel blocker administration in the newborn large mammal model of neonatal asphyxia, pharmacologic modulation of sodium channels may have a role in therapeutic or preventive
management in newborn hypoxic-ischemic injury. ABBREVIATIONS * CSF: cerebrospinal fluid * BP: blood pressure * RBC: red blood cell REFERENCES * duPlessis AJ, Johnston MV 1997
Hypoxic-ischemic brain injury in the newborn: cellular mechanisms and potential strategies for neuroprotection. _Clin Perinatol_ 24: 627–655 Article CAS Google Scholar * Pasternak JF 1993
Hypoxic-ischemic brain damage in the term infant. _Pediatr Clin North Am_ 40: 1061–1072 Article CAS Google Scholar * Lou HC 1998 Autoregulation of cerebral blood flow and brain lesions
in newborn infants. _Lancet_ 352: 1406 Article CAS Google Scholar * Paulson OB, Strandgaard S, Edvinsson L 1990 Cerebral autoregulation. _Cerebrovasc Brain Metab Rev_ 2: 161–193 CAS
PubMed Google Scholar * Pourcyrous M, Parfenova H, Bada HS, Korones SB, Leffler CW 1997 Changes in cerebral cyclic nucleotides and cerebral blood flow during prolonged asphyxia and
recovery in newborn pigs. _Pediatr Res_ 41: 617–623 Article CAS Google Scholar * Parfenova H, Shibata M, Zuckerman S, Mirro R, Leffler CW 1993 Cyclic nucleotides and cerebrovascular tone
in newborn pigs. _Am J Physiol_ 265: H1972–H1982 CAS PubMed Google Scholar * Parfenova H, Leffler CW 1996 Effects of hypercapnia on prostanoid and cAMP production of cerebral
microvascular cell cultures. _Am J Physiol_ 270: C1503–C1510 Article CAS Google Scholar * Parfenova H, Shibata M, Zuckerman S, Leffler CW 1994 CO2 and cerebral circulation in newborn
pigs: cyclic nucleotides and prostanoids in vascular regulation. _Am J Physiol_ 226: H1494–H1501 Google Scholar * Rudman D, Fleischer A, Kutner MH 1976 Concentration of 3′,5′-cyclic
adenosine monophosphate in ventricular cerebrospinal fluid of patients with prolonged coma after heart trauma or intracranial hemorrhage. _N Engl J Med_ 295: 635–638 Article CAS Google
Scholar * Pourcyrous M, Bada H, Yang W, Parfenova H, Wong SP, Korones SB, Leffler CW 1999 Prognostic significance of cerebrospinal fluid adenosine monophosphate in neonatal asphyxia. _J
Pediatr_ 134: 90–96 Article CAS Google Scholar * Urenjak J, Obrenovitch TP 1996 Pharmacological modulation of voltage-gated Na+ channels: a rational and effective strategy against
ischemic brain damage. _Pharmacol Rev_ 48: 21–67 CAS PubMed Google Scholar * Taylor CP, Meldrum BS 1995 Na+ channels as targets for neuroprotective drugs. _Trends Pharmacol Sci_ 16:
309–316 Article CAS Google Scholar * Costa MR, Catterall WA 1984 Cyclic AMP-dependent phosphorylation of the alpha subunit of the sodium channel in synaptic nerve ending particles. _J
Biol Chem_ 259: 8210–8218 CAS PubMed Google Scholar * Rossie S, Catterall WA 1984 AMP-dependent phosphorylation of voltage-sensitive sodium channel in primary cultures of red brain
neurons. _J Biol Chem_ 262: 12735–12744 Google Scholar * Lee M, Vest JW, Lai Y, Scheuer T, Catterall WA 1992 Functional modulation of brain sodium channels by cAMP-dependent
phosphorylation. _Neuron_ 8: 1151–1159 Article Google Scholar * Cummins TR, Xia Y, Haddad GG 1993 Human neocortical excitability is decreased during anoxia via sodium channel modulation.
_J Clin Invest_ 91: 608–615 Article CAS Google Scholar * Perez-Pinzon MA, Chan CY, Rosenthal M, Sick TJ 1992 Membrane and synaptic activity during anoxia in the isolated turtle
cerebellum. _Am J Physiol_ 263: R1057–R1063 Article CAS Google Scholar * Parer JT 1994 Fetal cerebral metabolism. _J Perinatol_ 15: 376–385 Google Scholar * Altman DL, Perlman JM, Volpe
JJ, Powers WJ 1993 Cerebral oxygen metabolism in newborns. _Pediatrics_ 92: 99–104 CAS PubMed Google Scholar * Powers WJ, Grubb RL, Darriet D, Raichle ME 1985 Cerebral blood flow and
cerebral metabolic rate of oxygen requirements for cerebral function and viability in humans. _J Cereb Blood Flow Metab_ 5: 600–608 Article CAS Google Scholar * Borch K, Greisen G 1998
Blood flow distribution in the normal human preterm brain. _Pediatr Res_ 43: 228–233 Article Google Scholar * Rosenbaum JL, Altman D, Cole FS, Yundt K, Levine V, Powers WJ 1996 Low
cerebral blood flow correlates with good neurological outcome among survival of neonatal hypoxic-ischemic encephalopathy. _Pediatr Res_ 39: 227Aabstr Article Google Scholar * Fellow LK,
Boutelle LK, Fillenz M 1993 Physiological stimulation increases nonoxidative glucose metabolism in the brain of the freely moving rat. _J Neurochem_ 59: 1258–1263 Article Google Scholar *
Taylor DL, Richards DA, Obrenovitch TP, Symon L 1994 Time course of changes in extracellular lactate evoked by transient K+-induced depolarization in the rat striatum. _J Neurochem_ 62:
23368–23374 Google Scholar * Leffler CW, Smith JS, Edrington JL, Zuckerman SL, Parfenova H 1997 Mechanisms of hypoxia-induced cerebrovascular dilation in the newborn pig. _Am J Physiol_
272: H1323–H1332 CAS PubMed Google Scholar * Kobayashi M, Lust WD, Passonneau JV 1997 Concentrations of energy metabolites and cyclic nucleotides during and after bilateral ischemia in
the gerbil cerebral cortex. _J Neurochem_ 29: 53–59 Article Google Scholar * Leffler CW 1997 Prostanoids: intrinsic modulators of cerebral circulation. _News Physiol Sci_ 12: 72–77 Google
Scholar * Rosenblum WI 1998 _In vivo_ evidence that an adenylate cyclase-cAMP system dilates cerebral arterioles in mice. _Stroke_ 19: 888–891 Article Google Scholar * Lou HC, Lassen NA,
Tweed WA, Johnson G, Jones M, Palahniuk RJ 1979 Pressure passive cerebral blood flow breakdown of the blood-brain barrier in experimental fetal asphyxia. _Acta Paediatr Scand_ 68: 57–62
Article CAS Google Scholar * Beller FK 1995 The cerebral palsy: a catastrophic misunderstanding in obstetrics. _Obstet Gynecol_ 50: 83 CAS Google Scholar * Brann AWA, Myers RE 1975
Central nervous system findings in the newborn monkey severe _in utero_ partial asphyxia. _Neurology_ 25: 327–338 Article Google Scholar * Myers RE 1972 Two patterns of perinatal brain
damage and their conditions of occurrence. _Am J Obstet Gynecol_ 112: 246–276 Article CAS Google Scholar * Ames A, Wright RL, Kowanda M, Thurston JM, Majino G 1968 Cerebral ischemia II.
_Am J Physiol_ 52: 437–453 Google Scholar * Van Bel F, Dorrepall CA, Benders MMNL, Zeeuwe PEM, Van de Bor M, Berger HM 1993 Changes in cerebral hemodynamics and oxygenation in the first 24
hours after birth asphyxia. _Pediatrics_ 92: 365–372 CAS PubMed Google Scholar * Sankaran K, Peters K, Finer N 1981 Estimated cerebral blood flow in term infants with hypoxic-ischemic
encephalopathy. _Pediatr Res_ 15: 1415–1418 Article CAS Google Scholar * Volpe JJ, Herscovitch P, Perlman JM, Kreusser KL, Raichle ME 1985 Positron emission tomography in the asphyxiated
term newborn: parasagittal impairment of cerebral blood flow. _Ann Neurol_ 17: 287–296 Article CAS Google Scholar * Greisen G, Trojaberg W 1987 Cerebral blood flow, Pa CO2 changes, and
visual evoked potential in mechanically ventilated preterm infants. _Acta Paediatr Scand_ 76: 394–400 Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Departments of Pediatrics, The University of Tennessee, Memphis, 38163, Tennessee, U.S.A. Vladimir Levine, Massroor Pourcyrous, Henrietta S Bada & Sheldon B Korones *
Departments of Obstetrics and Gynecology, The University of Tennessee, Memphis, 38163, Tennessee, U.S.A. Vladimir Levine, Massroor Pourcyrous, Henrietta S Bada & Sheldon B Korones *
Departments of Physiology, The University of Tennessee, Memphis, 38163, Tennessee, U.S.A. Massroor Pourcyrous, Helena Parfenova & Charles W Leffler * Department of Mathematical Science,
The University of Memphis, Tennessee, 38152, U.S.A. Wenjian Yang Authors * Vladimir Levine View author publications You can also search for this author inPubMed Google Scholar * Massroor
Pourcyrous View author publications You can also search for this author inPubMed Google Scholar * Henrietta S Bada View author publications You can also search for this author inPubMed
Google Scholar * Helena Parfenova View author publications You can also search for this author inPubMed Google Scholar * Wenjian Yang View author publications You can also search for this
author inPubMed Google Scholar * Sheldon B Korones View author publications You can also search for this author inPubMed Google Scholar * Charles W Leffler View author publications You can
also search for this author inPubMed Google Scholar ADDITIONAL INFORMATION Supported in part by UT Medical Group, Department of Obstetrics and Gynecology Educational Grant. RIGHTS AND
PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Levine, V., Pourcyrous, M., Bada, H. _et al._ Preservation of Cerebrovascular Tone and Reactivity by Sodium Channel
Inhibition in Experimental Prolonged Asphyxia in Piglets. _Pediatr Res_ 47, 376–380 (2000). https://doi.org/10.1203/00006450-200003000-00015 Download citation * Received: 08 April 1999 *
Accepted: 05 November 1999 * Issue Date: 01 March 2000 * DOI: https://doi.org/10.1203/00006450-200003000-00015 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
Trending News
Dolly in nashville: authenticity that makes room for rhinestonesWhen the renowned radio personality and Grand Ole Opry fixture Bill Cody walked onto the stage at the Ryman Auditorioum ...
Lunar influence on the east anglian herring fisheryABSTRACT FLUCTUATION in the yield from year to year, from month to month, and even from day to day, is one of the outsta...
With the grain | NatureAccess through your institution Buy or subscribe This is a preview of subscription content, access via your institution ...
Author correction: therapeutic potential of klf2-induced exosomal micrornas in pulmonary hypertensionCorrection to: _Nature Communications_https://doi.org/10.1038/s41467-020-14966-x, published online 4 March 2020. The ori...
Mathematical modelling: the cubic map in theory and practiceAccess through your institution Buy or subscribe This is a preview of subscription content, access via your institution ...
Latests News
Preservation of cerebrovascular tone and reactivity by sodium channel inhibition in experimental prolonged asphyxia in pigletsABSTRACT Sodium channels using cAMP as a second messenger play a role in the regulation of cerebral circulation and meta...
Histone deacetylase and cullin3–renkctd11 ubiquitin ligase interplay regulates hedgehog signalling through gli acetylationABSTRACT Hedgehog signalling is crucial for development and is deregulated in several tumours, including medulloblastoma...
Granite state challenge | super challenge | season 39 | episode 15This week on Granite State Challenge, the Falcons of Bow High School take on the Tomahawks of Merrimack High School. Onl...
Immune activation influences samhd1 expression and vpx-mediated samhd1 degradation during chronic hiv-1 infectionABSTRACT SAMHD1 restricts human immunodeficiency virus type 1 (HIV-1) replication in myeloid cells and CD4+ T cells, whi...
A Fourth State of Matter | NatureABSTRACT IN Mr. Crookes' communication on this subject (NATURE, vol. xxii. p. 153) occurs the sentence, “An isolate...