Vaccination with klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity
Vaccination with klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The emergence of multidrug-resistant _Klebsiella pneumoniae_ highlights the need to develop preventive measures to ameliorate _Klebsiella_ infections. Bacteria-derived extracellular
vesicles (EVs) are spherical nanometer-sized proteolipids enriched with outer membrane proteins. Gram-negative bacteria-derived EVs have gained interest for use as nonliving complex
vaccines. In the present study, we evaluated whether _K. pneumoniae_-derived EVs confer protection against bacteria-induced lethality. _K. pneumoniae_-derived EVs isolated from _in vitro_
bacterial culture supernatants induced innate immunity, including the upregulation of co-stimulatory molecule expression and proinflammatory mediator production. EV vaccination via the
intraperitoneal route elicited EV-reactive antibodies and interferon-gamma-producing T-cell responses. Three vaccinations with the EVs prevented bacteria-induced lethality. As verified by
sera and splenocytes adoptive transfer, the protective effect of EV vaccination was dependent on both humoral and cellular immunity. Taken together, these findings suggest that _K.
pneumoniae_-derived EVs are a novel vaccine candidate against _K. pneumoniae_ infections. SIMILAR CONTENT BEING VIEWED BY OTHERS VACCINATION WITH A COMBINATION OF PLANKTONIC AND BIOFILM
VIRULENCE FACTORS CONFERS PROTECTION AGAINST CARBAPENEM-RESISTANT _ACINETOBACTER BAUMANNII_ STRAINS Article Open access 19 November 2022 _BURKHOLDERIA PSEUDOMALLEI_ OMVS DERIVED FROM
INFECTION MIMICKING CONDITIONS ELICIT SIMILAR PROTECTION TO A LIVE-ATTENUATED VACCINE Article Open access 29 January 2021 INSERTING OMP22 INTO THE FLAGELLIN PROTEIN, REPLACING ITS
HYPERVARIABLE REGION, RESULTS IN STRONGER PROTECTION AGAINST LETHAL _ACINETOBACTER BAUMANNII_ INFECTION Article Open access 12 November 2024 INTRODUCTION _Klebsiella pneumoniae_ is a
Gram-negative, encapsulated, facultative anaerobic bacterium, which is found in the normal flora of the mouth, skin and intestine.1 The most common infection caused by the _Klebsiella_
bacterium outside of hospitals is pneumonia, typically in the form of bronchopneumonia and bronchitis.2 _K. pneumoniae_ can cause destructive changes to the human lungs via inflammation and
hemorrhage, which can produce a thick, bloody, mucoid sputum.3 This bacterium gains access to the lower respiratory tract typically after a person aspirates microbes that colonize the
oropharyngeal mucosa. _Klebsiella_ infections are observed mostly in people with a weakened immune system and cause a high death rate of ~50%, even with antimicrobial therapy.4, 5 In
addition to pneumonia, _K. pneumoniae_ can also cause infections in the urinary tract, lower biliary tract and surgical wound sites.6, 7, 8 In recent years, _K. pneumoniae_ has become an
important pathogen in nosocomial infections. Intense antibiotic use is a factor that increases the risk of nosocomial infection with _Klebsiella_ bacteria, which are often resistant to
multiple antibiotics.9, 10 Fatal infections caused by multidrug-resistant _K. pneumoniae_ have created a pressing need to develop a preventive measure to ameliorate _Klebsiella_ infections.
Vaccination is the administration of antigenic material to produce protective immunity to a disease. Vaccination is regarded as the most cost-effective method for preventing infectious
diseases. This approach requires the induction of protective immunity, which is best achieved using an active immunization strategy with the ability to induce specific long-term protective
memory, the hallmark of adaptive immunity. The key to eradicate pathogenic bacterial infections is the activation of specific immune responses, such as humoral (or antibody-mediated) and
cellular (or T-cell-mediated) immunity. Gram-negative bacteria-derived extracellular vesicles (EVs), otherwise known as outer membrane vesicles, are spherical bilayered phospholipids 20–200
nm in diameter that harbor many proteins related to pathogenesis.11, 12, 13 Biochemical and proteomic studies have revealed that bacterial EVs are composed of outer membrane proteins,
lipopolysaccharide, outer membrane lipids, periplasmic proteins, DNA, RNA and other factors associated with virulence.14, 15, 16 Recent studies have revealed the potential of EVs derived
from Gram-negative bacteria, such as _Neisseria meningitidis_, _Acinetobacter baumannii_, _Porphyromonas gingivalis_, _Salmonella enterica serovar Typhimurium_, _Helicobacter pylori_ and
_Vibrio cholerae,_ in inducing protective immunity against bacterial infections in mice.17, 18, 19, 20, 21, 22, 23, 24, 25 _K. pneumoniae_ secretes outer membrane vesicles that can induce
the innate immune response.26 Various outer membrane proteins can be used as the candidate antigens for vaccine.27, 28, 29 In the present study, we evaluated whether _K. pneumoniae_-derived
EVs elicit a protective effect against _Klebsiella_ infections. This study showed that vaccination with _K. pneumoniae_-derived EVs effectively protected bacteria-induced lethality in a
murine sepsis model. In addition, this study clarified the underlying mechanisms of how EV vaccination provides protection against _Klebsiella_-induced sepsis. MATERIALS AND METHODS MICE AND
CELL CULTURES Six to seven-week-old female, wild-type C57BL/6 (genetic background from The Jackson Laboratory, Bar Harbor, ME, USA) were used in the present study. The experimental
protocols were approved by the Institutional Animal Care and Use Committee at Pohang University of Science and Technology, Pohang, Republic of Korea with approval number 2011-01-0017. A
total of 2 × 106 Raw 264.7 mouse macrophages (purchased from ATCC, Manassas, VA, USA) were cultured with Dulbecco's Modified Eagle's medium (DMEM) (Hyclone, Little Chalfont, UK)
containing 10% fetal bovine serum (FBS) and antibiotics (100 unit ml−1 penicillin and 100 μg ml−1 streptomycin) in 100 mm tissue culture dishes (TPP, Trasadingen, Swiss) at 37 °C until use
in an experiment. Extracted bone marrow-derived dendritic cells were cultured with Roswell Park Memorial Institute (RPMI) 1640 (Hyclone) containing 10% FBS, 50 μM 2-mercaptoethanol (ME) and
antibiotics (100 unit ml−1 penicillin and 100 μg ml−1 streptomycin). For the _ex vivo_ experiments, RPMI 1640 (Hyclone) containing 10% FBS, 50 μM 2-ME, 0.01 M
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid and antibiotics (100 unit ml−1 penicillin and 100 μg ml−1 streptomycin) was used to culture the splenocytes. BACTERIA STRAIN AND
PREPARATION OF _K. PNEUMONIAE_-DERIVED EVS _K. pneumoniae_ (ATCC 4208) was purchased from ATCC and was cultured in nutrient broth (Merck, Kenilworth, NJ, USA) at 37 °C shaking at 200 rpm to
an optical density of 1.5. The cultured bacteria were pelleted twice at 10 000 × _g_ for 20 min at 4 °C. The supernatants were filtered using a 0.45-μm bottle top vacuum filter (Corning,
Corning, NY, USA) and were concentrated with a 100-kDa hollow fiber membrane filter using the QuixStand Benchtop System (Amersham Biosciences, Little Chalfont, UK). The concentrated solution
was filtered using a 0.22-μm bottle top vacuum filter (Corning) to remove any remaining bacteria. The final filtered solution was ultracentrifuged at 150 000 × _g_ for 3 h at 4 °C in a type
45 Ti rotor (Beckman Instruments, Brea, CA, USA). Finally, the EV pellet was re-suspended in phosphate-buffered saline (PBS) and was quantitated using the Bradford assay (BIO-RAD, Hercules,
CA, USA). The prepared EVs were stored at −80 °C until use.30 All EV batch preparations were performed under the same conditions. TRANSMISSION ELECTRON MICROSCOPY The EVs were diluted with
PBS, and 10 μl of the suspension (50 μg ml−1) were dropped on 300-mesh copper grids (EMS, Hatfield, PA, USA). Uranyl acetate (2%) was then dropped onto the grids to stain the EVs. The images
were captured using a JEM1011 electron microscope (JEOL, Tokyo, Japan).31 DYNAMIC LIGHT SCATTERING The EVs were diluted with PBS to 500 ng ml−1, and the diameter size distribution was
measured by dynamic light scattering using a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK) and Dynamic V6 software.32 MACROPHAGE UPTAKE ASSAY The EVs were incubated with 2 μM
of DiI (Invitrogen, Waltham, MA, USA) for 1 h and were washed with PBS. The washed EVs were ultracentrifuged using a TLA 120.2 (Beckman Instruments) at 150 000 × _g_ at 4 °C for 3 h. The
pellet was re-suspended in PBS. Raw 264.7 mouse macrophages (2 × 105 cells per well) were incubated with DMEM (Hyclone) containing 10% FBS and antibiotics (100 unit ml−1 penicillin and 100
μg ml−1 streptomycin) on a gelatin-coated cover class in a 24-well cell culture plate for 24 h and were then treated 10 μg ml−1 of EVs for 1 and 6 h with DMEM containing antibiotics (100
unit ml−1 penicillin and 100 μg ml−1 streptomycin). After the stimulation, the cells were washed with PBS and fixed with 4% paraformaldehyde. For staining of the cell nucleus, the fixed
cells were treated with 20 μM Hoechst (Sigma-Aldrich, St Louis, MO, USA) and were mounted on a glass slide. A LSM 510 META laser scanning microscope (Carl Zeiss, Oberkochen, Germany) was
used to acquire the confocal images.33 _IN VITRO_ MACROPHAGE STUDIES Raw 264.7 mouse macrophages (5 × 105 cells per well) were incubated with DMEM containing 10% FBS and antibiotics (100
unit ml−1 penicillin and 100 μg ml−1 streptomycin) in a 24-well tissue culture plate at 37 °C for 24 h. The culture medium was removed and various doses of EVs (0.1–1000 ng/ml−1) in
serum-free media (DMEM) containing antibiotics (100 unit ml−1 penicillin and 100 μg ml−1 streptomycin) were added. After a 15 h stimulation, the culture medium was collected and the levels
of proinflammatory cytokines, such as interleukin (IL)-6 and tumor necrosis factor-alpha, were measured. EXPRESSION OF CELL SURFACE MOLECULES ON BONE MARROW-DERIVED DENDRITIC CELLS To obtain
mouse bone marrow-derived dendritic cells, mouse femurs were extracted and bone marrow was washed with cold PBS using a sterile syringe on a non-coated petri dish (SPL, Pocheon, Korea). At
day 0, the extracted bone marrow-derived dendritic cells (2 × 106 cells per plate) were cultured with 10 ml of RPMI 1640 (Hyclone) containing 20 ng ml−1 of granulocyte-macrophage
colony-stimulating factor (R&D Systems, Minneapolis, MN, USA), 10% FBS, 50 μM 2-ME and antibiotics (100 unit ml−1 penicillin and 100 μg ml−1 streptomycin). At day 3, 10 ml of the same
culture medium was added to the plate. At days 6 and 8, 10 ml of the culture medium were collected, and the cells were pelleted by centrifugation. Each pellet was re-suspended in 10 ml of
same medium and was added to the plate. At day 10, the cells were harvested by centrifugation. At day 11, the cells were transferred to a 24-well tissue culture plate for stimulation. The
prepared cells (5 × 105 cells per well in 24-well plate) were incubated with RPMI 1641 containing 10% FBS, 50 μM 2-ME and antibiotics (100 unit ml−1 penicillin and 100 μg ml−1 streptomycin).
After 24 h, the culture medium was removed, and then the various stimulators (lipopolysaccharide (Sigma-Aldrich) 10 100 ng ml−1; EVs 0.1 pg ml−1-1000 ng ml−1) in serum-free medium (RPMI
1641 containing 2-ME and antibiotics) were added to the cells. After 24 h of stimulation, the cells were stained with anti-CD11c-APC (BD Biosciences, Franklin Lakes, NJ, USA), anti-CD86-PE
(BD Biosciences), or anti- major histocompatibility complex class II- fluorescein isothiocyanate (BD Biosciences) antibodies. A FACSCalibur (BD Biosciences) instrument was used for
fluorescence-activated cell sorting analysis.13 LETHAL DOSE DETERMINATIONS OF _K. PNEUMONIAE_-INDUCED BACTERIAL SEPSIS For determining the lethal dose of _K. pneumonia_ infections, three
different doses (1 × 106, 1 × 107 and 1 × 108) of _K. pneumoniae_ strain in 100 μl of PBS were injected intraperitoneally into mice. The survival rate was observed every 12 h for 3 days.
EVALUATION OF THE PROTECTIVE EFFECT OF VACCINATION For confirming the immune response to EVs _in vivo_, the mice were injected intraperitoneally with 10, 100 and 1000 ng of _K.
pneumoniae_-derived EVs in 100 μl of PBS three times for 3 weeks at 1-week intervals. Seven days after the last immunization, the spleens and blood were extracted for analysis using
re-stimulation assay and to measure antibody titer, respectively. To confirm the protective effect of vaccination against bacterial infection, the mice were injected intraperitoneally with
0.5, 1 and 2 μg of EVs in 100 μl of PBS for the same period of time. Seven days after the last immunization, both the immunized and non-immunized (sham) mice were challenged with the lethal
dose of 1 × 108 colony forming units (CFU) of _K. pneumoniae_. The survival rate was observed every 12 h for 3 days. SPLENOCYTE RE-STIMULATION ASSAY The extracted spleens were mashed with
the plunger of 5-ml syringe onto a 100 μm cell strainer (BD Biosciences) with washing buffer (DMEM containing 2.5% FBS and 0.01 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid). The
separated spleen cells were treated with an ammonium chloride solution (STEM CELL, British Columbia, Canada) for 10 min at 4 °C to lyse the red blood cells. The splenocytes were washed with
washing buffer and were then filtered through a 40 μm cell strainer (BD Biosciences). Finally, the cells were incubated with RPMI 1640 containing 10% FBS, 50 μM 2-ME, 0.01 M
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid and antibiotics (100 unit ml−1 penicillin and 100 μg ml−1 streptomycin) in a 24-well plate (TPP) coated with 1 μg ml ml−1 of anti-CD3
(eBioscience, San Diego, CA, USA) and 1 μg ml−1 of anti-CD28 (eBioscience) antibodies for 12 h to re-stimulate the splenic T cells. MEASUREMENT OF THE EV ANTIBODY TITER EVs (100 ng) in 100
μl PBS were coated in a black 96-well plate (Greiner Bio-one, Monroe, NC, USA) for 24 h at 4 °C. The EV-coated plate was washed with PBS and blocked with the reagent diluent (PBS containing
1% BSA; Roche) for 1 h. After washing with PBS, the extracted naive (sham) and vaccinated mouse blood samples were centrifuged to separate the serum and cell fractions. The serum was diluted
with the reagent diluent (1:500) and was then incubated in the EV-coated plate for 2 h. After incubation, the plates were washed and treated with 200 ng ml−1 of horseradish
peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) antibody (Santa Cruz Biotechnology, CA, USA) for 2 h. After washing the plates, the enhanced chemiluminescence substrate (Thermo
Scientific, Kenilworth, NJ, USA) was added and analyzed using a Victor Wallac 1420 apparatus (PerkinElmer, Waltham, MA, USA). INTRACELLULAR CYTOKINE STAINING OF SPLENIC T CELLS For the
intracellular cytokine staining assay, the mice were immunized with three doses (10, 100 and 1000 ng) of _K. pneumoniae_ EVs using the above protocol. A week after the last immunization, the
spleens of both immunized and non-immunized mice were extracted for use in the re-stimulation assay. The separated splenocytes were incubated in 48-well plates coated with 1 μg ml−1 of
anti-CD3 (eBioscience) and anti-CD28 (eBioscience) antibodies at 37 °C for 12 h. The splenocytes were stained with anti-CD3-APC (BD Biosciences) and anti-CD4-FITC (BD Biosciences) antibodies
for 30 min at 4 °C followed by fixation for 10 min in 4% paraformaldehyde at room temperature. The splenocytes were then incubated with antibodies against the cytokines of interest
(anti-interferon (IFN)-γ-PE, anti-IL-17-PE and anti-IL-4-PE; BD Biosciences) for 30 min at room temperature followed by analysis using a FACSCalibur flow cytometer (BD Biosciences) with the
CELLQuest software.34 ADOPTIVE TRANSFER OF SERA AND SPLENOCYTES The mice were immunized intraperitoneally with 1 μg of EVs three times for 3 weeks. Seven days after the last immunization,
blood and spleens were extracted. The same method was used to acquire the serum and splenocytes. The extracted serum (100 μl per mouse) and splenocytes (1 × 108 cells per mouse) were
injected intraperitoneally into naive mice. At 24 h after the injection, 2.5 × 107 and 5 × 107 CFU of the bacteria administered intraperitoneally were used to challenge the mice transferred
with serum, splenocytes or sham (PBS). The survival rate was observed every 12 h for 3 days MEASUREMENT OF CYTOKINES The cytokine levels secreted from Raw 264.7 cells or mouse-derived
splenocytes were measured using a DuoSet ELISA according to the manufacturer’s protocol (R&D Systems). STATISTICAL ANALYSIS The survival curves were compared using the log-rank test. All
of the values are expressed as the mean±s.e.m., and the Student’s _t_-test was used to calculate _P-_ values based on comparisons with the appropriate control. RESULTS CHARACTERIZATION OF
_K. PNEUMONIAE_-DERIVED EVS To characterize EVs derived from _K. pneumoniae_, the EVs were extracted from the bacterial culture. Transmission electron microscopy analysis revealed that _K.
pneumoniae_ secreted EVs, which are spherical and contain a lipid-bilayer (Supplementary Figure 1a). Dynamic light scattering analysis revealed a mean diameter of 36 nm (Supplementary Figure
1b). _IN VITRO_ INNATE IMMUNE RESPONSE INDUCED BY _K. PNEUMONIAE_-DERIVED EVS Host defense relies on the concerted action of both innate immunity and antigen-specific adaptive immunity. We
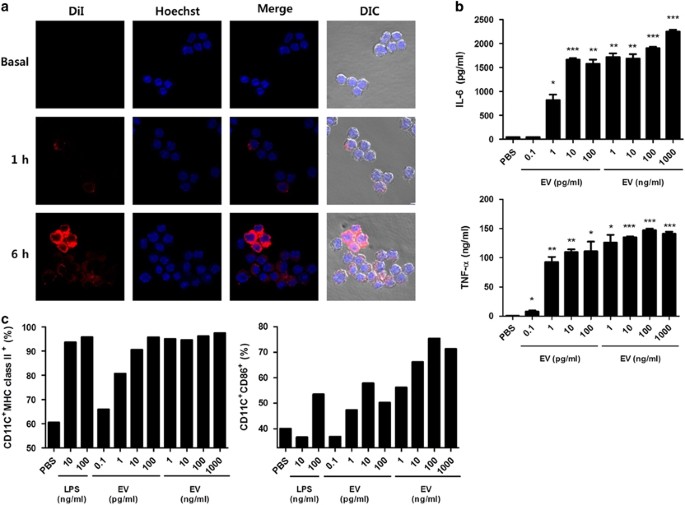
first evaluated innate immune responses induced by _K. pneumoniae_ EVs. When macrophages were treated with EVs for 1 and 6 h, the signals against EVs were observed in the cytoplasm of
macrophages, indicating that the EVs were internalized by antigen-presenting cells (Figure 1a). Moreover, the EV-treated macrophages efficiently induced the production of proinflammatory
cytokines, such as IL-6 and tumor necrosis factor-alpha (Figure 1b). The treatment of macrophages with EVs induced an increase in the expression of co-stimulatory major histocompatibility
complex class II and CD86 molecules (Figure 1c and Supplementary Figure 2). These results suggested that _K. pneumoniae_ EVs effectively activate innate immune responses by
antigen-presenting cells, which can prime T cells. _IN VIVO_ ANTIBODY PRODUCTION INDUCED BY VACCINATION WITH _K. PNEUMONIAE_ EVS To determine the effective dose of EVs for vaccination, we
assessed the effect of EV immunization on the induction of antigen-specific antibody and T-cell responses. During the immunization process, sera were obtained from EV- and sham-immunized
mice, and the EV-reactive IgG titer was also evaluated (Figure 2a). An increase in _K. pneumoniae_ EV-reactive IgG was detected after immunization with 10, 100 or 1000 ng of _K. pneumoniae_
EVs. However, _K. pneumoniae_ EV-reactive IgG was not detected in the sera from mice immunized with _Escherichia coli_ EVs, whereas _E. coli_ EV-reactive IgG increased in these mice (Figure
2b). These findings indicated that _K. pneumoniae_ EV vaccination induced the production of IgG antibody specific for _K. pneumoniae_ EVs. _IN VIVO_ T-CELL RESPONSES INDUCED BY _K.
PNEUMONIAE_ EV VACCINATION Next, to evaluate EV-specific T-cell responses, splenocytes were isolated from EV- and sham-immunized mice 1 week after the last immunization and were then
re-stimulated with anti-CD3 and anti-CD28 antibodies for 12 h. The EV-specific production of IFN-γ, the key cytokine produced by Th1 cells, was enhanced in EV-immunized mice in a
dose-dependent manner vs. the control (Figure 2c). However, EV-specific production of IL-17 and IL-4, the key cytokine of Th17 and Th2 cells, respectively, was not enhanced by vaccination
(Figure 2c). We also evaluated EV-specific T-cell responses using intracellular cytokine staining of splenocytes 12 h after the _in vitro_ stimulation with anti-CD3 and anti-CD28 antibodies,
as shown in Supplementary Figure 3a. This study showed that the expression of IFN-γ, IL-17 and IL-4 in CD4+ cells was enhanced by _K. pneumoniae_ EV immunization vs. sham immunization
(Figures 3a and b). Together, these findings suggest that immunization with _K. pneumonia_-derived EVs induces a strong Th1, IFN-γ-producing T-cell response in addition to Th17 and Th2
responses against EV antigens. EFFECT OF _K. PNEUMONIAE_-DERIVED EV VACCINATION ON PROTECTION AGAINST BACTERIA-INDUCED SEPSIS To test the efficacy of EV vaccination against _K.
pneumoniae_-induced sepsis, a mouse sepsis model was established by intraperitoneal injection with the different doses of _K. pneumoniae_ (Figure 4a, left panel). The lethal dose of _K.
pneumoniae_ in this model was 1 × 108 CFU (Figure 4a, right panel). To verify the ability of _K. pneumoniae_-derived EVs as an effective vaccine candidate, we examined the effect of EV
immunization on the development _K. pneumoniae_-induced sepsis (Figure 4b, left panel). Mice were injected intraperitoneally with different doses of the EV vaccine candidate every week for 3
weeks, were challenged with the lethal dose of _K. pneumoniae_ (1.0 × 108 CFU) 7 days after the final immunization, and the mice were then monitored every 12 h for 5 days for survival. All
of the mice immunized with sham died 36 h after the bacteria challenge; however, 80% of the mice survived after vaccination with 0.5 μg of the EVs and 100% of mice survived after vaccination
with 1 μg of the EVs. An increase in the EV quantity (2 μg) did not improve the vaccine efficacy (Figure 4b). Therefore, the 1 μg dose was chosen for the vaccine dosage. ADOPTIVE TRANSFER
OF SERA AND SPLENOCYTES FOR PROTECTION AGAINST _K. PNEUMONIA_-INDUCED LETHALITY Adoptive transfer is one alternative mean to induce immunization. To determine whether antibody-mediated
humoral or T-cell-mediated cellular immunity is important in conferring the EV-induced vaccination effect, we evaluated the protective effect of adoptive serum and splenocyte transfers as
shown in Supplementary Figure 3b. Naive mice that received splenocytes from EV-immunized mice effectively protected the bacteria with a 100% and 40% survival rate after 2.5 × 107 and 5 × 107
CFU of _K. pneumoniae_ challenge, respectively, whereas 40% and 0% of mice receiving splenocytes from sham-immunized mice survived the 2.5 × 107 and 5 × 107 CFU challenge, respectively
(Figures 5a and b). In addition, the naive mice that received sera from EV-immunized mice had an enhanced ability to survive after _K. pneumoniae_ challenge displaying 70% and 30% survival
rate after 2.5 × 107 and 5 × 107 CFU of _K. pneumoniae_ challenge, respectively, whereas 40% and 0% of mice receiving sera from the sham-immunized mice survived after the 2.5 × 107 and 5 ×
107 CFU challenge, respectively (Figures 5a and b). These results suggest that both humoral and cellular immunity confer the protective effect of _K. pneumoniae_ EV vaccination. DISCUSSION
Vaccination is considered to be the most effective and cost-effective method to prevent infectious diseases. In the present study, we aimed to evaluate the potential use of _K. pneumoniae_
EVs as a new vaccine material to prevent lethality by _K. pneumoniae_ infection. Vaccination with a non-lethal dose of _K. pneumoniae_ EVs effectively protected against lethal infection with
the same strain of _K. pneumoniae_ via both humoral and cellular immunity. These protective effects were accompanied by the involvement of innate and adaptive immune responses induced by
EVs, which included EV-reactive IgG and IFN-γ-mediated immune responses. These findings suggest that native EVs derived from _K. pneumoniae_ are a novel vaccine candidate for the prevention
of _K. pneumoniae_ infections. _K. pneumoniae_ is the most common cause of nosocomial respiratory tract and premature intensive care infections and the second most frequent cause of
Gram-negative bacteremia.35, 36, 37 Many studies have shown increasing resistance of this pathogen to antibiotics, resulting in an average rate of 1.63 outbreaks every year.35 A variety of
preventive measures have been attempted to reduce this incidence. However, passive immunization and immunoenhancers were shown to not be practical for the prevention _K. pneumoniae_
infections.38 During the last 40 years, the construction of effective vaccines against _K. pneumoniae_ has been attempted on numerous occasions. Among these efforts, capsular polysaccharide
was targeted as a vaccine antigen, but the variable capsular polysaccharide serotypes limited their use in vaccine production.39 The strategy of targeting non-capsular protein antigens
revealed that outer membrane proteins are protective antigens in the immune response evoked by _K. pneumoniae_.40 _K. pneumoniae_ EVs may be an ideal vaccine candidate because they harbor
many outer membrane proteins as well as immune stimulating pathogen-associated molecular patterns, including lipopolysaccharide. Indeed, the present study showed that _K. pneumoniae_ EVs
effectively protect against lethality induced by infection of the same strain of _K. pneumoniae_. Adjuvant affects the quality and magnitude of immunological responses. Various adjuvants,
including toll-like receptors 3, 4, 7 and 9 agonists, are used to enhance the immune response.41 The presence of lipopolysaccharide in EVs emphasizes the ability of EVs to act as a natural
adjuvant, thus allowing them to serve as potential adjuvant-free vaccine candidates for the delivery of various poorly immunogenic subunits.42 The present study also showed that _K.
pneumoniae_ EVs enhance the expression of co-stimulatory molecules in macrophages and the production of proinflammatory mediators, which indicates that _K. pneumoniae_ EVs have an adjuvant
capacity in T-cell priming. Antibody or T-cell responses have a key role in host defense toward _Klebsiella_ infections.43, 44 Capsular polysaccharide from _K. pneumoniae_ induces
thymus-independent humoral immunity.45 Animal experiments indicate that IFN-γ-deficient mice are more susceptible to bacterial infections compared with wild-type mice,46, 47 which suggests
that the Th1-cell response is important in the protection against bacterial infections. Furthermore, IL-17-producing Th17 cells are necessary for vaccine-induced protection against bacterial
infections by enhancing neutrophil recruitment to infection sites.48, 49 These data suggest that protection against bacterial infections requires both phagocyte recruitment and phagocytosis
mainly via Th17 cell and antibody-mediated immunity and also that the bactericidal activity of phagocytes is mediated mainly via Th1-cell immunity. Meanwhile, the present study showed that
_K. pneumoniae_ EV vaccination conferred protection against _K. pneumoniae_ infection, which was dependent on both humoral and cellular immunity. Note that _K. pneumoniae_ EV vaccination
induces the production of IFN-γ more than that of IL-4 and IL-17 from T cells. These findings suggest that _K. pneumoniae_ EV vaccination protects against bacterial infections mainly via
both antibody and IFN-γ-mediated immune responses. To the best of our knowledge, this is the first study to demonstrate _K. pneumoniae_ EVs as a vaccine candidate against _K. pneumoniae_
infections. Recently, we also found that _E. coli_ EVs effectively protect against bacteria-induced sepsis.13 Although the details of the mechanism underlying protection by EV vaccines still
needs to be unraveled, the present study provides new insight for a novel strategy of vaccine development using Gram-negative bacteria-derived EVs to protect against bacterial infections,
especially those caused by multi-drug-resistant bacteria. REFERENCES * Guo Y, Cen Z, Zou Y, Fang X, Li T, Wang J _et al_. Whole-genome sequence of Klebsiella pneumonia strain LCT-KP214. _J
Bacteriol_ 2012; 194: 3281. Article CAS PubMed PubMed Central Google Scholar * White RJ, Blainey AD, Harrison KJ, Clarke SK . Causes of pneumonia presenting to a district general
hospital. _Thorax_ 1981; 36: 566–570. Article CAS PubMed PubMed Central Google Scholar * Kawai T . Hypermucoviscosity: an extremely sticky phenotype of Klebsiella pneumoniae associated
with emerging destructive tissue abscess syndrome. _Clin Infect Dis_ 2006; 42: 1359–1361. Article CAS PubMed Google Scholar * Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N,
Nativ R _et al_. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. _Infect Control Hosp Epidemiol_ 2009; 30: 972–976. Article PubMed Google Scholar *
Gurntke S, Kohler C, Steinmetz I, Pfeifer Y, Eller C, Gastmeier P _et al_. Molecular epidemiology of extended-spectrum beta-lactamase (ESBL)-positive Klebsiella pneumoniae from bloodstream
infections and risk factors for mortality. _J Infect Chemother_ 2014; 20: 817–819. Article PubMed Google Scholar * Ipekci T, Seyman D, Berk H, Celik O . Clinical and bacteriological
efficacy of amikacin in the treatment of lower urinary tract infection caused by extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella pneumoniae. _J Infect Chemother_
2014; 20: 762–767. Article CAS PubMed Google Scholar * Ginawi I, Saleem M, Sigh M, Vaish AK, Ahmad I, Srivastava VK _et al_. Hospital acquired infections among patients admitted in the
medical and surgical wards of a non-teaching secondary care hospital in northern India. _J Clin Diagn Res_ 2014; 8: 81–83. CAS PubMed PubMed Central Google Scholar * Mpogoro FJ, Mshana
SE, Mirambo MM, Kidenya BR, Gumodoka B, Imirzalioglu C . Incidence and predictors of surgical site infections following caesarean sections at Bugando Medical Centre, Mwanza, Tanzania.
_Antimicrob Resist Infect Control_ 2014; 3: 25. Article PubMed PubMed Central Google Scholar * Podschun R, Ullmann U . Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy,
typing methods, and pathogenicity factors. _Clin Microbiol Rev_ 1998; 11: 589–603. Article CAS PubMed PubMed Central Google Scholar * Rodriguez EC, Saavedra SY, Leal AL, Alvarez C,
Olarte N, Valderrama A _et al_. [The spread of KPC-3 Klebsiella pneumoniae in hospitals in Bogota over a three-year period (2008-2010)]. _Biomedica_ 2014; 34: 224–231. Article PubMed
Google Scholar * Kuehn MJ, Kesty NC . Bacterial outer membrane vesicles and the host-pathogen interaction. _Genes Dev_ 2005; 19: 2645–2655. Article CAS PubMed Google Scholar *
Mashburn-Warren LM, Whiteley M . Special delivery: vesicle trafficking in prokaryotes. _Mol Microbiol_ 2006; 61: 839–846. Article CAS PubMed Google Scholar * Kim OY, Hong BS, Park KS,
Yoon YJ, Choi SJ, Lee WH _et al_. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. _J Immunol_ 2013; 190:
4092–4102. Article CAS PubMed Google Scholar * Horstman AL, Kuehn MJ . Bacterial surface association of heat-labile enterotoxin through lipopolysaccharide after secretion via the general
secretory pathway. _J Biol Chem_ 2002; 277: 32538–32545. Article CAS PubMed PubMed Central Google Scholar * Lee EY, Choi DS, Kim KP, Gho YS . Proteomics in gram-negative bacterial
outer membrane vesicles. _Mass Spectrom Rev_ 2008; 27: 535–555. Article CAS PubMed Google Scholar * Wai SN, Lindmark B, Soderblom T, Takade A, Westermark M, Oscarsson J _et al_.
Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. _Cell_ 2003; 115: 25–35. Article CAS Google Scholar * Verkaik NJ, de Vogel CP,
Boelens HA, Grumann D, Hoogenboezem T, Vink C _et al_. Anti-staphylococcal humoral immune response in persistent nasal carriers and noncarriers of Staphylococcus aureus. _J Infect Dis_ 2009;
199: 625–632. Article PubMed Google Scholar * Saunders NB, Shoemaker DR, Brandt BL, Moran EE, Larsen T, Zollinger WD . Immunogenicity of intranasally administered meningococcal native
outer membrane vesicles in mice. _Infect Immun_ 1999; 67: 113–119. CAS PubMed PubMed Central Google Scholar * Haneberg B, Dalseg R, Wedege E, Hoiby EA, Haugen IL, Oftung F _et al_.
Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans.
_Infect Immun_ 1998; 66: 1334–1341. CAS PubMed PubMed Central Google Scholar * Alaniz RC, Deatherage BL, Lara JC, Cookson BT . Membrane vesicles are immunogenic facsimiles of Salmonella
typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity _in vivo_. _J Immunol_ 2007; 179: 7692–7701. Article CAS PubMed Google
Scholar * McConnell MJ, Rumbo C, Bou G, Pachon J . Outer membrane vesicles as an acellular vaccine against _Acinetobacter baumannii_. _Vaccine_ 2011; 29: 5705–5710. Article CAS PubMed
Google Scholar * Dalseg R, Wedege E, Holst J, Haugen IL, Hoiby EA, Haneberg B . Outer membrane vesicles from group B meningococci are strongly immunogenic when given intranasally to mice.
_Vaccine_ 1999; 17: 2336–2345. Article CAS PubMed Google Scholar * Kesavalu L, Ebersole JL, Machen RL, Holt SC . Porphyromonas gingivalis virulence in mice: induction of immunity to
bacterial components. _Infect Immun_ 1992; 60: 1455–1464. CAS PubMed PubMed Central Google Scholar * Keenan J, Day T, Neal S, Cook B, Perez-Perez G, Allardyce R _et al_. A role for the
bacterial outer membrane in the pathogenesis of Helicobacter pylori infection. _FEMS Microbiol Lett_ 2000; 182: 259–264. Article CAS PubMed Google Scholar * Masignani V, Comanducci M,
Giuliani MM, Bambini S, Adu-Bobie J, Arico B _et al_. Vaccination against Neisseria meningitidis using three variants of the lipoprotein GNA1870. _J Exp Med_ 2003; 197: 789–799. Article CAS
PubMed PubMed Central Google Scholar * Lee JC, Lee EJ, Lee JH, Jun SH, Choi CW, Kim SI _et al_. Klebsiella pneumoniae secretes outer membrane vesicles that induce the innate immune
response. _FEMS Microbiol Lett_ 2012; 331: 17–24. Article CAS PubMed Google Scholar * Kurupati P, Teh BK, Kumarasinghe G, Poh CL . Identification of vaccine candidate antigens of an ESBL
producing Klebsiella pneumoniae clinical strain by immunoproteome analysis. _Proteomics_ 2006; 6: 836–844. Article CAS PubMed Google Scholar * Lundberg U, Senn BM, Schuler W, Meinke A,
Hanner M . Identification and characterization of antigens as vaccine candidates against Klebsiella pneumoniae. _Hum Vaccin Immunother_ 2013; 9: 497–505. Article CAS PubMed Google Scholar
* Hoppe S, Bier FF, von Nickisch-Rosenegk M . Identification of antigenic proteins of the nosocomial pathogen _Klebsiella pneumoniae_. _PLoS One_ 2014; 9: e110703. Article PubMed PubMed
Central Google Scholar * Lee EY, Bang JY, Park GW, Choi DS, Kang JS, Kim HJ _et al_. Global proteomic profiling of native outer membrane vesicles derived from Escherichia coli.
_Proteomics_ 2007; 7: 3143–3153. Article CAS Google Scholar * Park KS, Choi KH, Kim YS, Hong BS, Kim OY, Kim JH _et al_. Outer membrane vesicles derived from Escherichia coli induce
systemic inflammatory response syndrome. _PLoS One_ 2010; 5: e11334. Article PubMed PubMed Central Google Scholar * Hallett FR, Watton J, Krygsman P . Vesicle sizing: number
distributions by dynamic light scattering. _Biophys J_ 1991; 59: 357–362. Article CAS PubMed PubMed Central Google Scholar * Ellis TN, Leiman SA, Kuehn MJ . Naturally produced outer
membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. _Infect Immun_ 2010; 78:
3822–3831. Article CAS PubMed PubMed Central Google Scholar * Kim YS, Choi EJ, Lee WH, Choi SJ, Roh TY, Park J _et al_. Extracellular vesicles, especially derived from Gram-negative
bacteria, in indoor dust induce neutrophilic pulmonary inflammation associated with both Th1 and Th17 cell responses. _Clin Exp Allergy_ 2013; 43: 443–454. Article CAS PubMed Google
Scholar * Ahmad TA, El-Sayed LH, Haroun M, Hussein AA, El Ashry el SH . Development of immunization trials against _Klebsiella pneumoniae_. _Vaccine_ 2012; 30: 2411–2420. Article PubMed
Google Scholar * Lin WH, Tseng CC, Wu AB, Yang DC, Cheng SW, Wang MC _et al_. Clinical and microbiological characteristics of peritoneal dialysis-related peritonitis caused by _Klebsiella
pneumoniae_ in southern Taiwan. _J Microbiol Immunol Infect_ 2013; 48: 276–283. Article PubMed Google Scholar * Hawser S, Hoban DJ, Badal RE, Bouchillon SK, Biedenbach D, Hackel M _et
al_. Epidemiology and antimicrobial susceptibility of Gram-negative aerobic bacteria causing intra-abdominal infections during 2010-2011. _J Chemother_ 2014; 27: 67–73. Article PubMed
Google Scholar * Sun WS, Syu WJ, Ho WL, Lin CN, Tsai SF, Wang SH . SitA contributes to the virulence of _Klebsiella pneumoniae_ in a mouse infection model. _Microbes Infect_ 2014; 16:
161–170. Article CAS PubMed Google Scholar * Campbell WN, Hendrix E, Cryz S Jr, Cross AS . Immunogenicity of a 24-valent Klebsiella capsular polysaccharide vaccine and an eight-valent
Pseudomonas O-polysaccharide conjugate vaccine administered to victims of acute trauma. _Clin Infect Dis_ 1996; 23: 179–181. Article CAS PubMed Google Scholar * Bednarz-Misa I, Serek P,
Dudek B, Pawlak A, Bugla-Ploskonska G, Gamian A . Application of zwitterionic detergent to the solubilization of _Klebsiella pneumoniae_ outer membrane proteins for two-dimensional gel
electrophoresis. _J Microbiol Methods_ 2014; 107: 74–79. Article CAS PubMed Google Scholar * Fransen F, Boog CJ, van Putten JP, van der Ley P . Agonists of Toll-like receptors 3, 4, 7,
and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B. _Infect Immun_ 2007; 75: 5939–5946. Article CAS PubMed PubMed Central
Google Scholar * Chen DJ, Osterrieder N, Metzger SM, Buckles E, Doody AM, DeLisa MP _et al_. Delivery of foreign antigens by engineered outer membrane vesicle vaccines. _Proc Natl Acad Sci
USA_ 2010; 107: 3099–3104. Article CAS PubMed Google Scholar * Kuenen JD, van Dijke EE, Hol C, Bootsma HJ, Verhoef J, van Dijk H . Protective effects of orally administered,
Klebsiella-containing bacterial lysates in mice. _FEMS Immunol Med Microbiol_ 1994; 8: 69–75. Article CAS PubMed Google Scholar * Montgomery CP, Daniels M, Zhao F, Alegre ML, Chong AS,
Daum RS . Protective immunity against recurrent Staphylococcus aureus skin infection requires antibody and interleukin-17A. _Infect Immun_ 2014; 82: 2125–2134. Article PubMed PubMed
Central Google Scholar * Alcantar-Curiel MD, Martinez-Ramos A, Garcia-Latorre E . [Capsular polysaccharide of Klebsiella pneumoniae. II. Immunogenic properties]. _Rev Latinoam Microbiol_
1993; 35: 109–115. CAS PubMed Google Scholar * Murphy EA, Sathiyaseelan J, Parent MA, Zou B, Baldwin CL . Interferon-gamma is crucial for surviving a Brucella abortus infection in both
resistant C57BL/6 and susceptible BALB/c mice. _Immunology_ 2001; 103: 511–518. Article CAS PubMed PubMed Central Google Scholar * Roberts LM, Davies JS, Sempowski GD, Frelinger JA .
IFN-gamma, but not IL-17A, is required for survival during secondary pulmonary Francisella tularensis Live Vaccine Stain infection. _Vaccine_ 2014; 32: 3595–3603. Article CAS PubMed
PubMed Central Google Scholar * Ross PJ, Sutton CE, Higgins S, Allen AC, Walsh K, Misiak A _et al_. Relative contribution of Th1 and Th17 cells in adaptive immunity to Bordetella
pertussis: towards the rational design of an improved acellular pertussis vaccine. _PLoS Pathog_ 2013; 9: e1003264. Article CAS PubMed PubMed Central Google Scholar * Gopal R,
Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA _et al_. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. _Mucosal Immunol_
2013; 6: 972–984. Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank members of the animal facility in Pohang University of Science and
Technology Biotech Center (Pohang University of Science and Technology, Pohang, Republic of Korea) for their valuable technical assistance. This study was supported by grants from the Korea
Ministry of Health & Welfare, Republic of Korea (HI14C-2694-010014) and from KRIBB initiative programs. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Life Sciences, Pohang
University of Science and Technology (POSTECH), Pohang, Republic of Korea Won-Hee Lee, Hyun-Il Choi, Sung-Wook Hong & Yong Song Gho * Superbacteria Research Center, Korea Research
Institute of Bioscience and Biotechnology (KRIBB), Daejeon, Republic of Korea Kwang-sun Kim * Research Institute, AEON Medix, Inc, Pohang, Republic of Korea Seong Gyu Jeon Authors * Won-Hee
Lee View author publications You can also search for this author inPubMed Google Scholar * Hyun-Il Choi View author publications You can also search for this author inPubMed Google Scholar *
Sung-Wook Hong View author publications You can also search for this author inPubMed Google Scholar * Kwang-sun Kim View author publications You can also search for this author inPubMed
Google Scholar * Yong Song Gho View author publications You can also search for this author inPubMed Google Scholar * Seong Gyu Jeon View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Seong Gyu Jeon. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. ADDITIONAL
INFORMATION Supplementary Information accompanies the paper on Experimental & Molecular Medicine website SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 (JPG 168 KB) SUPPLEMENTARY
FIGURE 2 (JPG 157 KB) SUPPLEMENTARY FIGURE 3 (JPG 67 KB) RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not
included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-sa/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lee, WH., Choi, HI., Hong, SW. _et al._ Vaccination with _Klebsiella
pneumoniae_-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immunity. _Exp Mol Med_ 47, e183 (2015).
https://doi.org/10.1038/emm.2015.59 Download citation * Received: 25 April 2015 * Revised: 20 May 2015 * Accepted: 22 May 2015 * Published: 11 September 2015 * Issue Date: September 2015 *
DOI: https://doi.org/10.1038/emm.2015.59 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Drivers urged to pay car tax ahead of major ved changes next monthThe standard rate will increase by £10 for most cars which were first registered on or after April 1, 2017. For cars reg...
Wimbledon To Lose Familiar Face As Veteran BBC Presenter Sue Barker Calls Time After 30 YearsSue Barker at WimbledonBBC This year’s Wimbledon tennis tournament will see the end of an era. No, Roger Federer has not...
M&s trousers shoppers hail as 'perfect for smart casual' - and they're under £20IF YOU'RE LOOKING FOR SOME NEW TROUSERS THAT ARE SMART ENOUGH FOR THE OFFICE BUT ALSO COMFY ENOUGH FOR DAYTIME CHOR...
Kentucky life | rudy ayoroa; lincoln library and museum; mammoth cave | season 10 | episode 14Season 10 Episode 14 | 26m 30sVideo has Closed Captions | CC Meet Rudy Ayoroa and visit the Lincoln Library and Museum/M...
Spatio-temporal changes in the causal interactions among sustainable development goals in chinaABSTRACT Extensive efforts have been dedicated to deciphering the interactions associated with Sustainable Development G...
Latests News
Vaccination with klebsiella pneumoniae-derived extracellular vesicles protects against bacteria-induced lethality via both humoral and cellular immuniABSTRACT The emergence of multidrug-resistant _Klebsiella pneumoniae_ highlights the need to develop preventive measures...
Supersonic turbulent flow simulation using a scalable parallel modal discontinuous galerkin numerical methodABSTRACT The scalability and efficiency of numerical methods on parallel computer architectures is of prime importance a...
Wormlike micelles of polyoxyethylene dodecyl c12ej and heptaoxyethylene alkyl cie7 ethers. Hydrophobic and hydrophilic chain length dependence of theABSTRACT The micelles formed with pentaoxyethylene C12E5 and heptaoxyethylene C12E7 dodecyl ethers and heptaoxyethylene ...
Nonradioactive silver | NatureARTICLE PDF REFERENCES * Lindner, L., Brinkman, G. A., and Schimmel, A., _Nature_, 240, 463 (1972). Article ADS CAS G...
Identification of maspin and s100p as novel hypomethylation targets in pancreatic cancer using global gene expression profilingABSTRACT DNA hypomethylation is one of the major epigenetic alterations in human cancers. We have previously shown that ...