A recurrent copy number variation of the neb triplicate region: only revealed by the targeted nemaline myopathy cgh array
A recurrent copy number variation of the neb triplicate region: only revealed by the targeted nemaline myopathy cgh array"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Recently, new large variants have been identified in the nebulin gene (_NEB_) causing nemaline myopathy (NM). NM constitutes a heterogeneous group of disorders among the congenital
myopathies, and disease-causing variants in _NEB_ are a main cause of the recessively inherited form of NM. _NEB_ consists of 183 exons and it includes homologous sequences such as a 32-kb
triplicate region (TRI), where eight exons are repeated three times (exons 82–89, 90–97, 98–105). In human, the normal copy number of _NEB_ TRI is six (three copies in each allele).
Recently, we described a custom NM-CGH microarray designed to detect copy number variations (CNVs) in the known NM genes. The array has now been updated to include all the currently known 10
NM genes. The NM-CGH array is superior in detecting CNVs, especially of the _NEB_ TRI, that is not included in the exome capture kits. To date, we have studied 266 samples from 196 NM
families using the NM-CGH microarray, and identified a novel recurrent _NEB_ TRI variation in 13% (26/196) of the families and in 10% of the controls (6/60). An analysis of the breakpoints
revealed adjacent repeat elements, which are known to predispose for rearrangements such as CNVs. The control CNV samples deviate only one copy from the normal six copies, whereas the NM
samples include CNVs of up to four additional copies. Based on this study, _NEB_ seems to tolerate deviations of one TRI copy, whereas addition of two or more copies might be pathogenic.
SIMILAR CONTENT BEING VIEWED BY OTHERS COPY NUMBER VARIANTS FROM 4800 EXOMES CONTRIBUTE TO ~7% OF GENETIC DIAGNOSES IN MOVEMENT DISORDERS, MUSCLE DISORDERS AND NEUROPATHIES Article Open
access 13 February 2023 BIALLELIC STRUCTURAL VARIANTS IN THREE PATIENTS WITH ERCC8-RELATED COCKAYNE SYNDROME AND A POTENTIAL PITFALL OF COPY NUMBER VARIATION ANALYSIS Article Open access 26
August 2024 HAPLOTYPE INFORMATION OF LARGE NEUROMUSCULAR DISEASE GENES PROVIDED BY LINKED-READ SEQUENCING HAS A POTENTIAL TO INCREASE DIAGNOSTIC YIELD Article Open access 21 February 2024
INTRODUCTION Nemaline (rod) myopathy (NM; MIM IDs: NEM1 #609284, NEM2 #256030, NEM3 #161800, NEM4 #609285, NEM5 #605355, NEM6 #609273, NEM7 #610687, NEM8 #615348, NEM9 #615731, NEM10
#616165) is one of the most common congenital myopathies. Characteristically, NM presents with congenital proximal muscle weakness, but it is a very heterogeneous disorder including six
clinical categories, in which the severity and onset of the disease varies widely.1, 2 Typical histological findings include the presence of nemaline bodies (rods) in the muscle fibres.
Nemaline bodies are aggregates of Z-disc and thin-filament proteins of the muscle sarcomere.3 Hitherto, 10 different genes have been shown to cause MM (_ACTA1, NEB, TPM3, TPM2, TNNT1, CFL2,
KBTBD13, KLHL40, KLHL41_ and _LMOD3_). Variants in the nebulin gene (_NEB_) are the most common cause of recessively inherited NM (NEM2 #256030). _NEB_, is one of the largest genes in the
human genome, located in the chromosomal region 2q23.3 (152 341 850–152 591 001; GRCh37/hg19) and consisting of 249 kb of genomic sequence. _NEB_ contains 183 exons, but only one region, the
donor splice site of intron 32, may be considered a true mutational hotspot. The vast majority, 83% (132/159) of the families included in our recently published mutational update, were
heterozygous for two different pathogenic variants.2 The great variety of different _NEB_ variants makes mutation analysis cumbersome, and this is further accentuated by the complexity of
_NEB._ The gene includes regions with highly similar exons and intronic repeat elements such as _Alu_ and LINE. It also includes homologous sequences, such as the so-called triplicate region
(TRI) in the middle of the gene. TRI consists of eight exons that are repeated three times (exons 82–89, 90–97 and 98–105), all ~10 kb repeats showing high similarity (99%) with each
other.4 This type of homologous DNA segment is difficult to study, and it might even include variants that have been overlooked because of that. For example, the _NEB_ TRI is not included in
the typical exome sequencing capture kits because of the high similarity of the three copies. Therefore, developing alternative methods is essential for studying the region. Nebulin is a
gigantic protein of the muscle sarcomere, which acts as a molecular ruler regulating thin (actin) filament length, actin–myosin interactions and force generation.5, 6, 7 This highly
repetitive protein contains around two hundred 30- to 35-amino-acid-long simple repeats, each containing one actin-binding site. Moreover, the simple repeats are arranged into 22–30 super
repeats each containing a putative tropomyosin-binding site. Nebulin has several binding partners and it is an essential part of the properly functioning sarcomere of the striated muscle.7,
8, 9, 10 Currently, evidence is accumulating that large copy number variations (CNVs) in _NEB_ are more common than previously thought. In addition to the 2.5-kb Ashkenazi Jewish deletion of
_NEB_ exon 55,11, 12 other large deletions have recently been identified, including variations of different sizes, from part of one exon to more than half of the gene.2, 13 The normal copy
number of the _NEB_ TRI region has been estimated to be three copies in each allele.4 Therefore, the normal copy number of the _NEB_ TRI is six instead of the typical two copies. Mouse _Neb_
contains only one ~7.5 kb copy of the TRI region segments, implying that the triplication has occurred later during evolution. It has been suggested that the TRI region is the result of two
tandem duplications through _Alu_-mediated homologous recombination in an ancestor of human and chimpanzee.14 One of the most common mechanisms inducing CNVs is thought to be non-allelic
homologous recombination (NAHR) caused by misalignment and cross-over of non-allelic homologous DNA segments, such as low copy repeats. CNV breakpoints have also been shown to frequently
reside in regions of repeat elements. These include different SINEs (such as different _Alu_ repeats), LINEs, DNA repeat elements (such as MERs) and long-terminal repeats.15, 16 Repeat
elements are known to cause CNVs in the genome in general, but their role in the formation of TRI CNVs requires further investigations. In the current study, we describe the TRI CNVs that we
have identified in our study cohort of 196 families and 60 controls using our custom NM-CGH microarray, and hypothesize on the pathogenicity and possible mechanisms behind this recurrent
variation. MATERIALS AND METHODS SAMPLES The NM-CGH study included 266 DNA samples from 196 families with patients diagnosed with or suspected to have NM or a related myopathy, in whom one
or both pathogenic variants had remained unidentified. In addition, 60 normal control samples, 22 from Finland and 38 from CEPH (Centre d’Étude du Polymorphisme Humain), were studied. The
samples were received either as isolated DNA or as blood, cell lines, or muscle or skin biopsies from which DNA extraction was done using appropriate methods. MICROARRAY DESIGN, PROTOCOL AND
DATA ANALYSIS The NM-CGH 8x60k microarray (Oxford Gene Technology IP Limited, Oxford, UK) was designed (Human reference sequence, GRCh37/Hg19) as described in Kiiski _et al._13 The seven
genes causative for NM known at the time (_NEB, ACTA1, TPM3, TPM2, TNNT1, CFL2, KBTBD13_) were densely covered with a tiling approach (with one probe pair starting every 10 bp interval),
avoiding the most repetitive regions of these genes. In addition, this study includes samples that were run with the updated NM-CGH microarray version v2, also including the _KBTBD5_,
_KBTBD13_ and _LMOD3_ genes, and v3, also including one unpublished NM-associated gene. In v2 and v3, the control gene _TTN_ has been removed and the probe interval has been reduced from 10
to 20 bp in the intronic regions for every gene except for _NEB_. No other significant modifications were made in the NM-CGH array updates. The labelling, hybridization, scanning and data
analysis were done according to the manufacturer’s protocol (Oxford Gene Technology PI Ltd) as previously described in Kiiski _et al._13 The CytoSure Interpret Software v.4.2.5-4.6.85 (Hg19)
(Oxford Gene Technology Ltd) was used for graphic analysis of the data. The CBS algorithm was used and specific thresholds determined to allow for aberration calling. A setting for
‘multiple mappings’ was used in the analysis and in the graphic view, that is, each probe is shown in every genomic location where it can be located. Therefore, regarding the _NEB_ TRI, most
of the probes are shown three times, once for each homologue. The TRI variations were analyzed manually based on the logarithmic scale of the NM-CGH microarray results (Table 1). It was
possible to design ~180 unique probe pairs for this region, mostly based on small sequence differences in the TRI introns. EXOME SEQUENCING AND OTHER VARIANT ANALYSIS METHODS The variants
other than _NEB_ TRI CNVs were identified using dHPLC and Sanger sequencing as previously described.13, 17 In addition, exome sequencing was used. Exome capture and sequencing were done by
Oxford Gene Technology using the Agilent SureSelectXT All Exon 50 Mb target enrichment kit (protocol v1.2; Agilent Technologies, Santa Clara, CA, USA) on an Illumina HiSeq2000 platform using
TruSeq v3 chemistry (Illumina Inc, San Diego, CA, USA) and analysis was completed using the Oxford Gene Technology exome sequencing pipeline. A putative pre-mRNA splicing affecting variant
identified in family F2 was tested using a _NEB_ minigene construct as described previously.17 VARIANTS IN THE LOVD DATABASE The results have been submitted to the LOVD database
(http://www.LOVD.nl/NEB) with the submission ID numbers: NEB_00253-NEB_00261. The _NEB_ cDNA reference sequence NM_001271208.1 was used and the exon numbering is according to Donner _et
al._4 STATISTICAL ANALYSES Fisher’s exact test was used for determining the statistical significance of the results. BIOINFORMATICS METHODS The _NEB_ TRI region is highly repetitive and many
of the tiling array probes match multiple locations. To obtain an even coverage, the measurements for the ambiguous probes were randomly assigned to one of the possible locations. The data
were then analyzed using the GLAD package18 for the R environment19 and the breakpoints and copy numbers were inferred using the daglad function. Owing to the high probe density and variable
signal level across the target region, the default parameters of the function produced unrealistically high numbers of breakpoints. After experimenting with different parameter values, the
function was used with options _λ_=40 and _d_=10. The resulting breakpoints, along with the original signal intensities and the genome annotations for the target region, were visualized
using the GenomeGraphs20 and rtracklayer21 packages. RESULTS Using the NM-CGH array, we identified frequent CNVs of the _NEB_ TRI, some of the CNVs we interpret to be benign and other
pathogenic. _NEB_ TRI CNVs were identified in 13% of the studied families (26/196) with NM or NM-related myopathy (referred to as NM families from now on). In more detail, 5% (9/196) showed
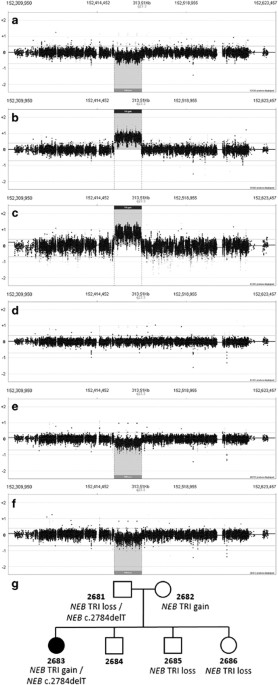
a loss and 8% (16/196) a gain of the _NEB_ TRI (Table 2). In addition, one family (F286) was found to include members with a gain, a loss or the normal copy number of the _NEB_ TRI (Figure 1
and Table 4). _NEB_ TRI variation was also identified in 10% of the control samples (6/60; Table 2). Based on this study, we suggest that one-copy losses or gains are benign and gains of
2–4 copies may be pathogenic. All the _NEB_ TRI deletions identified in the NM families and controls were losses of one TRI copy, that is,. 5/6 copies in total. One-copy losses were
identified in five control samples (5/60) and in nine NM families (9/196). In four of the NM families, the disease-causing variants, either in _NEB_ or in another NM-causing gene, had been
published previously. Novel disease-causing variants are published here for families F2 and F411. In family F2, the patients have inherited a splice site variant, c.508-7T>A, which was
shown on the RNA level to activate a cryptic acceptor splice site in intron 7, causing an insertion of five nucleotides leading to a frameshift p.Val170fs. In family F411, the patient is
compound heterozygous for two different frameshift variants, c.24372_24375del, in exon 172, and c.13134del in exon 86. In three families one or both disease-causing variants remain unknown
(Table 3). In the control sample cohort, a gain of one additional copy, that is, 7/6 copies, was identified in one sample (1/60; Table 2). However, among the NM families, there was a wider
spectrum of copy number gains (Tables 2, 3 and 4). Almost half (8/17) of the identified gains in the NM samples included two to four additional copies, that is, 8–10/6 copies in total
(Tables 2 and 4). This size category of CNVs was not identified in the control cohort. The patients in nine NM families carried one-copy gains. In one of these families, the disease-causing
variants had previously been identified in _LMOD3_ (Index Patient 3353, Table 3). Using exome sequencing, we found a frameshift variant c.24475_24479dupCACAA in exon 173 of _NEB_, in another
family (F407) segregating on the same allele as the TRI gain (Index Patient 4073, Table 3). The consanguineous healthy parents are both carriers of one TRI copy gain each, as well as a
_NEB_ frameshift variant, and the patient is homozygous for the frameshift variant and the TRI gain. In another family (F321), we identified, in addition to the _NEB_ TRI one-copy gain, a
novel complex deletion–insertion rearrangement concerning the _NEB_ exons 69–71 and intron 65 (Index Patient 3213, Table 3) using the NM-CGH array. The second variant remained unidentified
in this family. One or both disease-causing variants remain unknown altogether in seven families with a gain of one TRI copy. TRI copy gains of two or more copies were identified in patients
of eight NM families. In five of these families, another heterozygous pathogenic _NEB_ variant had been identified previously or is published here (Table 4). We had samples from all family
members in three of these families and these are described in more detail below. FAMILY 268 The index case 2683 (typical NM) has inherited a _NEB_ TRI four-copy gain from the mother and a
_NEB_ exon 28 frameshift variant (c.2784delT) from the father. The unaffected father has the frameshift variant (c.2784delT) on one allele and a TRI deletion (one-copy loss) on the other
allele, which suggests that one-copy deletions of TRI would not be pathogenic. We suggest that the TRI four-copy gain carried by the healthy mother, and inherited by the index patient, might
be disease causing (Figure 1). The pedigree of the family (Figure 1g) shows that the _NEB_ TRI gain segregates with the disorder, and only the combination of the _NEB_ TRI gain and the
frameshift variant result in the NM phenotype. FAMILY 7 The index case 573 (mild NM) has inherited a _NEB_ TRI three-copy gain from the father and a frameshift variant in _NEB_ exon 134
(c.20446delC) from the mother. We suggest that the three additional TRI copies might be pathogenic. The parents and sibling are unaffected mutation carriers. FAMILY 272 The index case 2723
(other form of NM) has inherited a _NEB_ TRI four-copy gain from the father and a frameshift variant in _NEB_ exon 81 (c.12048_12049delGA) from the mother. We suggest that the four
additional TRI copies might be pathogenic. The parents are unaffected mutation carriers. Exome sequencing of samples from the index patients in all three families (F268, F7 and F272) did not
reveal any variants with serious consequences for gene function in any of the other currently known NM-causing genes. In summary, the copy numbers deviated only by one copy in the control
samples, whereas deviations larger than that were only found in NM families. The difference is not, however, statistically significant (Fisher’s exact test; Table 2). Based on this study,
the _NEB_ TRI CNVs are the most common large recurrent _NEB_ variation characterized to date. BREAKPOINT ANALYSIS Breakpoint analyses were done for all samples that showed TRI CNVs. The
results achieved using the NM-CGH array indicate that the locations of the breakpoint regions are the same for both gains and losses (Figure 2). All variants start in intron 105, but the
exact breakpoint cannot be determined due to a probe gap within this intron because of repeat elements, across which no unique probes could be designed. The stop region seems to differ
slightly between different samples but it is always inside intron 81. Repeat elements, that is, LINEs (CR1 and L2) reside in introns 89, 97 and 105 and long-terminal repeats (ERVL) reside in
introns 82, 90 and 98. In addition, DNA transposon fossils (MER113A) are present in introns 88, 96 and 104. DISCUSSION In the current study, 13% (26/196) of the studied NM families showed a
_NEB_ TRI CNV. The identified losses were deletions of one copy, which did not seem to segregate with the disorder and were found in the control population (8%) more frequently than in the
NM families (5%). We estimate that the loss of one copy would cause a 1458 bp shortening of various transcripts and a 486-amino-acid shortening of the translated protein. One TRI copy
encodes 486 amino acids corresponding to two nebulin super repeats.4 The two remaining TRI copies would be enough for the allele to produce a functional protein. _NEB_ transcripts of many
different lengths are known to be produced in normal muscle, and therefore, this alteration might not cause a drastic effect on the thin actin filament structure of the sarcomere.22 In
family 268, the father showed a one-copy loss of TRI and a frameshift variant on the other allele, and he is healthy. In six families, the TRI deletion has also been found to be accompanied
by two identified pathogenic variants (Table 3). In addition, it is also frequent in controls (8%). Thus, one-copy deletions of _NEB_ TRI are unlikely to have a serious impact on protein
function. The identified gains have been categorized into two groups: gain of one TRI copy, which we interpret to be non-pathogenic, and gains of two to four TRI copies, which we interpret
to be pathogenic. In one family (Index Patient 3353, Table 3) with a one-copy gain, two other causative variants in another NM-causing gene, _LMOD3_, had previously been identified, and in
another family (Index Patient 4073, Table 3), we found a frameshift variant in _NEB_ exon 173 segregating with the one-copy gain. Also, one control sample was identified with one TRI copy
gain (1/60). Thus, it seems that deviations of one TRI copy in an allele, be it loss or gain, are tolerated. However, 4% of the NM families had patients with gains of more than one TRI copy
(8–10/6 copies present). Both disease-causing variants in _NEB_ had not previously been identified in these families.In those families with parental samples available for analysis, the TRI
CNV segregated with the disorder. Furthermore, this type of aberration was not encountered among the control samples. We speculate that gains of more than one copy may disrupt the stability
or secondary structure of the mRNA, and also the translation process. If so, no nebulin protein would be produced from this allele. If, on the other hand, RNA processing and translation were
to take place, the TRI gain would cause an increase in the length of the transcript and further of the nebulin protein. Each TRI gain would add 1458 bases to the mRNA, and 486 amino acids
to the protein. Therefore, a gain of four copies would add 5.8 kb to the mRNA, and 1944 amino acids or eight super repeats to the protein. The breakpoints of the TRI variations are difficult
to characterize further. Normally, the entire TRI region is ~32 kb in size and each one-copy gain would add ~10 kb to the gene. The identified losses of one TRI copy would set the region at
around 20 kb in size. The homology of the TRI region has proven to be very challenging especially for PCR studies. The last introns of each TRI repeat (introns 89 and 97 and 105) contain
repeat elements, such as _Alu_ and LINE repeats. These transposable elements are known to be capable of being involved in NAHR, thought to be the most common underlying mechanism for
recurrent CNVs.15, 16 The lack of further knowledge of these TRI variations makes it difficult to estimate the exact causative mechanism but NAHR seems plausible. It is thought that the
human _NEB_ TRI has emerged from two duplication events. This is based on the fact that the mouse _Neb_ gene only contains one copy of this region (exons 82–89) and is lacking the LINE-L2
elements as well as the exons corresponding to human _NEB_ exons 90–106.4 These repeat elements might explain the susceptibility of the _NEB_ TRI region to the recurrent copy number changes
discovered in this study. The NM-CGH microarray is an effective and easy method for revealing _NEB_ TRI CNVs. However, if there is a deletion in one allele and a duplication in the other,
the copy number would look normal and these cases would thus remain unidentified. Because the normal copy number of the _NEB_ TRI is six instead of two, it can be difficult to differentiate
between subtle differences in size, for example, between gains of three copies and gains of four copies, especially if the DNA quality is poor. Also, the NM-CGH array cannot determine which
allele harbours the CNV. Therefore, studying parental samples is necessary to verify the origin of the identified variant. Regarding duplications, the identification of the location or the
orientation of the duplicated region requires alternative methods. CONCLUSIONS AND FUTURE PROSPECTS The NM-CGH microarray has revealed a novel, recurrent CNV of the _NEB_ triplicate region,
apparently the most common large recurrent variant in _NEB_. The NM-CGH array is a very easy and effective method for detecting these CNVs. We will continue to use the NM-CGH array as a
first tier diagnostic method for new NM families, as well as analyzing samples from families and patients whose causative variants have remained unidentified. REFERENCES *
Wallgren-Pettersson C, Laing NG : Report of the 70th ENMC International Workshop: nemaline myopathy, 11-13 June 1999, Naarden, The Netherlands. _Neuromuscul Disord_ 2000; 10: 299–306.
Article CAS Google Scholar * Lehtokari VL, Kiiski K, Sandaradura SA _et al_: Mutation update: the spectra of nebulin variants and associated myopathies. _Hum Mutat_ 2014; 35: 1418–1426.
Article CAS Google Scholar * Wallgren-Pettersson C, Jasani B, Newman GR _et al_: Alpha-actinin in nemaline bodies in congenital nemaline myopathy: immunological confirmation by light and
electron microscopy. _Neuromuscul Disord_ 1995; 5: 93–104. Article CAS Google Scholar * Donner K, Sandbacka M, Lehtokari VL, Wallgren-Pettersson C, Pelin K : Complete genomic structure of
the human nebulin gene and identification of alternatively spliced transcripts. _Eur J Hum Genet_ 2004; 12: 744–751. Article CAS Google Scholar * Bang ML, Li X, Littlefield R _et al_:
Nebulin-deficient mice exhibit shorter thin filament lengths and reduced contractile function in skeletal muscle. _J Cell Biol_ 2006; 173: 905–916. Article CAS Google Scholar * Witt CC,
Burkart C, Labeit D _et al_: Nebulin regulates thin filament length, contractility, and Z-disk structure _in vivo_. _EMBO J_ 2006; 25: 3843–3855. Article CAS Google Scholar * Chandra M,
Mamidi R, Ford S _et al_: Nebulin alters cross-bridge cycling kinetics and increases thin filament activation: a novel mechanism for increasing tension and reducing tension cost. _J Biol
Chem_ 2009; 284: 30889–30896. Article CAS Google Scholar * Pfuhl M, Winder SJ, Castiglione Morelli MA, Labeit S, Pastore A : Correlation between conformational and binding properties of
nebulin repeats. _J Mol Biol_ 1996; 257: 367–384. Article CAS Google Scholar * Labeit S, Gibson T, Lakey A _et al_: Evidence that nebulin is a protein-ruler in muscle thin filaments.
_FEBS Lett_ 1991; 282: 313–316. Article CAS Google Scholar * Wang K : Titin/connectin and nebulin: giant protein rulers of muscle structure and function. _Adv Biophys_ 1996; 33: 123–134.
Article CAS Google Scholar * Anderson SL, Ekstein J, Donnelly MC _et al_: Nemaline myopathy in the Ashkenazi Jewish population is caused by a deletion in the nebulin gene. _Hum Genet_
2004; 115: 185–190. Article CAS Google Scholar * Lehtokari VL, Greenleaf RS, DeChene ET _et al_: The exon 55 deletion in the nebulin gene—one single founder mutation with world-wide
occurrence. _Neuromuscul Disord_ 2009; 19: 179–181. Article Google Scholar * Kiiski K, Laari L, Lehtokari VL _et al_: Targeted array comparative genomic hybridization—a new diagnostic tool
for the detection of large copy number variations in nemaline myopathy-causing genes. _Neuromuscul Disord_ 2013; 23: 56–65. Article CAS Google Scholar * Bjorklund AK, Light S, Sagit R,
Elofsson A : Nebulin: a study of protein repeat evolution. _J Mol Biol_ 2010; 402: 38–51. Article Google Scholar * Vissers LE, Bhatt SS, Janssen IM _et al_: Rare pathogenic microdeletions
and tandem duplications are microhomology-mediated and stimulated by local genomic architecture. _Hum Mol Genet_ 2009; 18: 3579–3593. Article CAS Google Scholar * Gu W, Zhang F, Lupski JR
: Mechanisms for human genomic rearrangements. _Pathogenetics_ 2008; 1: 4. Article Google Scholar * Lehtokari VL, Pelin K, Sandbacka M _et al_: Identification of 45 novel mutations in the
nebulin gene associated with autosomal recessive nemaline myopathy. _Hum Mutat_ 2006; 27: 946–956. Article CAS Google Scholar * Hupe P, Stransky N, Thiery JP, Radvanyi F, Barillot E :
Analysis of array CGH data: from signal ratio to gain and loss of DNA regions. _Bioinformatics_ 2004; 20: 3413–3422. Article CAS Google Scholar * R Core Team _R: A Language and
Environment for Statistical Computing_. R Foundation for Statistical Computing: : Vienna, Austria, 2013. * Durinck S, Bullard J, Spellman PT, Dudoit S : GenomeGraphs: integrated genomic data
visualization with R. _BMC Bioinformatics_ 2009; 10: 2. Article Google Scholar * Lawrence M, Gentleman R, Carey V : rtracklayer: an R package for interfacing with genome browsers.
_Bioinformatics_ 2009; 25: 1841–1842. Article CAS Google Scholar * Laitila J, Hanif M, Paetau A _et al_: Expression of multiple nebulin isoforms in human skeletal muscle and brain.
_Muscle Nerve_ 2012; 46: 730–737. Article CAS Google Scholar * Laing NG, Dye DE, Wallgren-Pettersson C _et al_: Mutations and polymorphisms of the skeletal muscle alpha-actin gene
(ACTA1). _Hum Mutat_ 2009; 30: 1267–1277. Article CAS Google Scholar * Yuen M, Sandaradura SA, Dowling JJ _et al_: Leiomodin-3 dysfunction results in thin filament disorganization and
nemaline myopathy. _J Clin Invest_ 2014; 124: 4693–4708. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Professor Dimitrios Zafeiriou and Dr Sascha Vermeer for
sending us samples from one nemaline myopathy family each, Professor Johan T den Dunnen for submitting variants into the LOVD database and help with the HVGS nomenclature, and Marilotta
Turunen for excellent technical assistance. This study was supported by grants from the Sigrid Jusélius Foundation, the Association Francaise contre les Myopathies, the Finska
Läkaresällskapet and the Medicinska understödsföreningen Liv och Hälsa. AUTHOR CONTRIBUTIONS KK designed the original as well as the updates of the NM-CGH microarray, set up the NM-CGH
method in the laboratory, ran half of the array hybridizations, carried out analysis and interpretation of the data, performed exome sequencing analyses and prepared the manuscript. V-LL
selected samples for the NM-CGH analyses, analyzed the dHPLC and Sanger sequencing results and wrote parts of the manuscript. AL did the bioinformatic analyses and wrote parts of the
manuscript. LA ran half of the NM-CGH hybridizations. JL performed the minigene experiment for family F2. CW-P was responsible for the project as PI. Her contribution included collection of
samples and clinical correlates, as well as writing parts of the manuscript. KP was responsible for the project as Co-PI. She participated in the design of the microarray, interpretation of
results and writing parts of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * The Folkhälsan Institute of Genetics and The Department of Medical and Clinical Genetics, Medicum,
University of Helsinki, Helsinki, Finland Kirsi Kiiski, Vilma-Lotta Lehtokari, Liina Ahlstén, Jenni Laitila, Carina Wallgren-Pettersson & Katarina Pelin * Institute of Biotechnology,
University of Helsinki, Helsinki, Finland Ari Löytynoja * Division of Genetics, Department of Biosciences, University of Helsinki, Helsinki, Finland Katarina Pelin Authors * Kirsi Kiiski
View author publications You can also search for this author inPubMed Google Scholar * Vilma-Lotta Lehtokari View author publications You can also search for this author inPubMed Google
Scholar * Ari Löytynoja View author publications You can also search for this author inPubMed Google Scholar * Liina Ahlstén View author publications You can also search for this author
inPubMed Google Scholar * Jenni Laitila View author publications You can also search for this author inPubMed Google Scholar * Carina Wallgren-Pettersson View author publications You can
also search for this author inPubMed Google Scholar * Katarina Pelin View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence
to Kirsi Kiiski. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no conflict of interest. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Kiiski, K., Lehtokari, VL., Löytynoja, A. _et al._ A recurrent copy number variation of the _NEB_ triplicate region: only revealed by the targeted nemaline myopathy CGH array. _Eur J Hum
Genet_ 24, 574–580 (2016). https://doi.org/10.1038/ejhg.2015.166 Download citation * Received: 19 December 2014 * Revised: 28 May 2015 * Accepted: 19 June 2015 * Published: 22 July 2015 *
Issue Date: April 2016 * DOI: https://doi.org/10.1038/ejhg.2015.166 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Dan Nigro on Working with Olivia Rodrigo and Chappell Roan | GRAMMYs 2024GRAMMYs 2024Dan Nigro on Working with Olivia Rodrigo and Chappell Roan | GRAMMYs 2024February 4, 2024 Dan Nigro talks wi...
Under cover: pulitzer prize novelist comes to newport library - newport beach newsThe Newport Beach Public Library has a long history of both recognizing, and sharing excellence through their myriad com...
Behave or your licence may get cancelled: assam transport department warns cab driversThe Assam transport department has issued a stern warning to the cab drivers operating in Guwahati city. The Assam trans...
Keith Elkins - Los Angeles TimesFounding Partner Elkins Kalt Weintraub Reuben Gartside LLP _Professional Services_ Keith Elkins is an attorney and found...
Major reshuffle of governors, kerala governor arif khan transferred to biharIn a significant reshuffle, President Droupadi Murmu has appointed new Governors for five states—Kerala, Bihar, Odisha, ...
Latests News
A recurrent copy number variation of the neb triplicate region: only revealed by the targeted nemaline myopathy cgh arrayABSTRACT Recently, new large variants have been identified in the nebulin gene (_NEB_) causing nemaline myopathy (NM). N...
Zooming in on a neutron-star merger jetObservations with a continent-wide array of radio telescopes show that the merger of two neutron stars, which produced g...
Queen news: delight for monarch as republican movement slumpsQUEEN MAKING LIFESTYLE CHANGES DUE TO AGE SAYS FITZWILLIAMS Australia has renewed its support to the Crown, with 40 perc...
Nufc boss eddie howe set to get freedom of newcastle in council vote next weekCITY COUNCIL LEADER KAREN KILGOUR WILL PRAISE EDDIE HOWE'S ACHIEVEMENTS SINCE TAKING CHARGE AS NEWCASTLE UNITED MAN...
Queen set hard-to-beat precedent with 'bafta-winning performance'The Queen's landmark speech on April 5, 2020, delivered as the country was plunged into the uncertainty of the firs...