Glutathione s-transferase p1 (gstp1) directly influences platinum drug chemosensitivity in ovarian tumour cell lines
Glutathione s-transferase p1 (gstp1) directly influences platinum drug chemosensitivity in ovarian tumour cell lines"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND: Chemotherapy response in ovarian cancer patients is frequently compromised by drug resistance, possibly due to altered drug metabolism. Platinum drugs are metabolised by
glutathione _S_-transferase P1 (_GSTP1)_, which is abundantly, but variably expressed in ovarian tumours. We have created novel ovarian tumour cell line models to investigate the extent to
which differential _GSTP1_ expression influences chemosensitivity. METHODS: Glutathione _S_-transferase P1 was stably deleted in A2780 and expression significantly reduced in
cisplatin-resistant A2780DPP cells using Mission shRNA constructs, and MTT assays used to compare chemosensitivity to chemotherapy drugs used to treat ovarian cancer. Differentially
expressed genes in _GSTP1_ knockdown cells were identified by Illumina HT-12 expression arrays and qRT–PCR analysis, and altered pathways predicted by MetaCore (GeneGo) analysis. Cell cycle
changes were assessed by FACS analysis of PI-labelled cells and invasion and migration compared in quantitative Boyden chamber-based assays. RESULTS: Glutathione _S_-transferase P1 knockdown
selectively influenced cisplatin and carboplatin chemosensitivity (2.3- and 4.83-fold change in IC50, respectively). Cell cycle progression was unaffected, but cell invasion and migration
was significantly reduced. We identified several novel _GSTP1_ target genes and candidate platinum chemotherapy response biomarkers. CONCLUSIONS: Glutathione _S_-transferase P1 has an
important role in cisplatin and carboplatin metabolism in ovarian cancer cells. Inter-tumour differences in _GSTP1_ expression may therefore influence response to platinum-based chemotherapy
in ovarian cancer patients. SIMILAR CONTENT BEING VIEWED BY OTHERS TARGETING MRE11 OVERCOMES PLATINUM RESISTANCE AND INDUCES SYNTHETIC LETHALITY IN XRCC1 DEFICIENT EPITHELIAL OVARIAN
CANCERS Article Open access 19 July 2022 OVERLAPPING GENE DEPENDENCIES FOR PARP INHIBITORS AND CARBOPLATIN RESPONSE IDENTIFIED BY FUNCTIONAL CRISPR-_CAS9_ SCREENING IN OVARIAN CANCER Article
Open access 28 October 2022 TTK INHIBITION INCREASES CISPLATIN SENSITIVITY IN HIGH-GRADE SEROUS OVARIAN CARCINOMA THROUGH THE MTOR/AUTOPHAGY PATHWAY Article Open access 07 December 2021
MAIN Ovarian cancer often presents at an advanced stage where treatment is rarely curative (Kristensen and Trope, 1997). Chemotherapy with platinum-based drug regimens (combining cisplatin
or carboplatin with paclitaxel) can be initially effective, but longer-term treatment is frequently compromised by the development of drug-resistant disease (Vaughan et al, 2011). Platinum
drugs are thought to act by promoting the formation of intra-strand DNA crosslinks, thus inhibiting DNA translation and replication (Eastman, 1987). Although several candidate drug
resistance mechanisms, including impaired DNA repair, decreased drug uptake, increased drug efflux and detoxification, have been proposed (reviewed by Galluzzi et al, 2012), we are still
some way from understanding the molecular basis of drug resistance, and from developing biomarkers to predict the onset and monitor the development of drug-resistant disease in cancer
patients. In recent work, we used qRT–PCR analysis to quantify inter-tumour differences in the expression of multiple candidate genes associated with disease progression and chemotherapy
response in ovarian tumours (Smith et al, 2012). One of the most abundantly expressed genes in ovarian tumours and tumour cell lines was glutathione _S_-transferase P1 (_GSTP1_, EC
2.5.1.18), a polymorphic phase II drug-metabolising enzyme, which conjugates the antioxidant tri-peptide glutathione with many toxic hydrophobic and electrophilic xenobiotics to facilitate
elimination (reviewed by Hayes and Pulford, 1995). Increased _GSTP1_ expression has been reported in pre-neoplastic lesions in chemically induced animal cancer models (Satoh et al, 1985) and
in many human tumours, relative to surrounding normal tissues (Shea et al, 1988). Although a useful neoplasia biomarker, it is not known whether increased _GSTP1_ expression directly
influences carcinogenesis, or is simply a bystander effect, where expression of this highly inducible gene is increased as part of the adaptive response mounted by the neoplastic cell. In
support of a direct role in carcinogenesis, however, _GSTP1_ inhibits the stress-inducible c-Jun N-terminal kinase (_JNK_) _in vitro_, and _JNK_ activity is reduced in _GSTP1_ null mice
(Adler et al, 1999; Yin et al, 2000; Elsby et al, 2003). Additional roles for _GSTP1_ in the regulation of genes including _TRAF2_ (Wu et al, 2006), _CDK5_ (Sun et al, 2011) and the _FAS_
death receptor (Anathy et al, 2012) have also been described. Purified _GSTP1_ conjugates cisplatin _in vitro_ (Hagrman et al, 2004), and _GSTP1_ expression is increased in human tumour cell
lines either inherently or made resistant to chemotherapy drugs including cisplatin and various alkylating agents (Black and Wolf, 1991; McLellan and Wolf, 1999), although a direct
association linking differential glutathione conjugation with platinum drug resistance in human ovarian tumours has not been convincingly demonstrated. In support of a functional
association, heterologous _GSTP1_ expression in _Saccharomyces cerevisiae_ (Black et al, 1990) or of various _GSTP1_ alleles in _Escherichia Coli_ influenced sensitivity to platinum and
additional chemotherapy drugs (Ishimoto and Ali-Osman, 2002). In contrast, similar experiments in breast cancer MCF7 cells revealed only modest effects on platinum sensitivity (Peklak-Scott
et al, 2008), while partial _GSTP1_ knockdown in the adriamycin-resistant human colorectal cancer cell line M7609 increased sensitivity not only to the selection agent, but to cisplatin,
melphalan and etoposide (Ban et al, 1996). We therefore created novel ovarian tumour cell line models in which _GSTP1_ expression is stably deleted, to investigate whether differential
_GSTP1_ expression directly influenced chemosensitivity to platinum drugs, and to other drugs routinely used in the treatment of ovarian cancer patients. We have used whole-genome
transcriptional profiling analysis and various quantitative phenotypic assays to identify gene expression and associated phenotypic differences in parental and _GSTP1_ knockdown cell lines.
MATERIALS AND METHODS OVARIAN TUMOUR CELL LINES A2780 and cisplatin-resistant derivative A2780DPP cells were obtained from ATCC (LGC Standards, Teddington, UK), via Cancer Research UK Cell
Services. A2780 cells were maintained in RPMI-1640 media supplemented with 10% FBS, and A2780DPP cells (derived _in vitro_ following long-term cisplatin selection; Behrens et al, 1987) in
RPMI-1640 media supplemented with 15% FBS and 1 _μ_ M cisplatin. Both cell lines were maintained in 37 °C incubators, supplemented with 5% CO2. CREATION AND CHARACTERISATION OF STABLE
_GSTP1_ KNOCKDOWN CELL LINES Glutathione _S_-transferase P1 expression was stably knocked-down in A2780 and expression significantly reduced in A2780DPP cells by RNA interference using
Mission shRNA plasmids (Sigma-Aldrich, Gillingham, UK). Five unique _GSTP1_-specific shRNA plasmids (clones TRCN0000083773, TRCN0000083774, TRCN0000083775, TRCN0000083776 and TRCN0000083777)
and a negative control plasmid (empty vector control, SHC001) were purchased as glycerol stocks and plasmid DNA extracted using plasmid DNA maxi prep kits (Qiagen, Manchester, UK) according
to the manufacturer’s instructions. A2780 and A2780DPP cells (2.5 × 105 cells per well in six-well plates) were transfected with each plasmid using lipofectamine (Life Technologies,
Paisley, UK), and shRNA-containing cells selected with puromycin. Individual cell colonies were picked using cloning cylinders, harvested for mRNA analysis (A2780DPP cells) or expanded to 75
cm2 tissue culture flasks and harvested for mRNA and protein analysis (A2780 cells). _GSTP1_ knockdown in A2780 cells was confirmed by qRT–PCR analysis and western blotting, and by qRT–PCR
analysis in A2780DPP cells. Cell growth rates were compared by plating 1 × 105 cells from each cell line in individual wells of six-well dishes (day 0). Cells were harvested daily by
trypsinisation (days 2–10) and counted using a haemocytometer. RNA EXTRACTION AND QRT–PCR ANALYSIS Cells were grown to 80% confluency in 75 cm2 flasks, harvested by trypsinisation, counted
using a haemocytometer, and 1 × 107 cells used for RNA extraction using a Qiagen RNeasy mini kit (Qiagen), following the manufacturer’s protocol for mammalian cells, with additional on
column DNAse digestion (RNAse free DNAse kit, Qiagen). RNA yield and integrity were initially assessed from absorbance readings at 260 and 280 nm using a Nanodrop 1000 spectrophotometer
(Thermo Fisher Scientific, Loughborough, UK), and confirmed using an Agilent Bioanalyzer 2100 and RNA 6000 Nano LabChip Kit (Agilent Technologies, Wokingham, UK) according to the
manufacturer’s guidelines. RNA was reverse transcribed into cDNA (50 ng RNA per 20 _μ_l RT reaction) using High Capacity RNA to cDNA kits (Life Technologies) according to the manufacturer’s
instructions, and the expression of _GSTP1_ (Taqman probe ID Hs00168310_m1) and the loading control _18S ribosomal RNA_ (Hs03003631_g1) assessed by qRT–PCR analysis, as previously described
(Smith et al, 2012), where 20 _μ_l individual reaction mixes (per well) contained 10 _μ_l Taqman Universal PCR Master Mix (Life Technologies), 1 _μ_l gene-specific Taqman probe, 1 _μ_l cDNA
and 8 _μ_l sterile water. Each reaction was performed in triplicate and run on the Standard Real-Time PCR program on a 7900 Taqman real-time PCR system (Applied Biosystems, Warrington, UK)
using pre-defined thermal cycling conditions (50 °C for 2 min, 94.5 °C for 10 min, 40 cycles of (97 °C for 30 s and 59 °C for 1 min)). Similarly, the expression of _ALX1_ (Hs00232518_m1),
_CDH2_ (Hs00983056_m1), _FOXC1_ (Hs00559473_s1), _LAYN_ (Hs00379511_m1), _TM4SF_ (Hs00371661_m1) and _VCAN_ (Hs00171642_m1) was additionally investigated by qRT–PCR analysis in A2780 and
A2780/GSTP1 cells. Analysis was performed using SDS 2.3 software (Applied Biosystems); optimal experimental baselines and thresholds were chosen for each gene, and gene expression
quantitated by cycle threshold (Ct) values. Relative expression values were determined by comparing the expression of each target gene with the invariant ‘loading control’ _18S ribosomal
RNA_, as previously described (Smith et al, 2012). All samples were analysed in triplicate and gene expression calculated relative to 18S ribosomal RNA±compound error ((s.d. target
gene)2+(s.d. 18S ribosomal RNA)2)½, where s.d.=standard deviation of the mean of triplicate replicates. WHOLE-GENOME MICROARRAY MRNA ANALYSIS IN A2780 AND A2780/_GSTP1_ CELLS RNA was
prepared from A2780 and A2780/_GSTP1_ cells and integrity assessed as described above. Each RNA sample was converted to biotinylated amplified cRNA using an Illumina TotalPrep RNA
Amplification kit (Life Technologies) according to the manufacturer’s guidelines, and cRNA quality and concentration confirmed on the Agilent Bioanalyzer 2100, as described above. cRNA
samples were hybridised in triplicate on Illumina Human HT-12 BeadChip Arrays (Illumina, Little Chesterford, UK) using standard protocols optimised by the Genetics Core, Wellcome Trust
Clinical Research Facility, University of Edinburgh. BIOINFORMATICS ANALYSIS Gene expression data were analysed using Bioconductor 2.7 (http://bioconductor.org), running on R 2.12.1.
Normalised probeset expression measures were calculated using log2 transformation and quantile normalisation using the ‘Lumi’ package. To identify significant differences in gene expression
in A2780 and A2780/GSTP1 cells, moderated Student's _t_-tests were performed using empirical Bayes statistics in the ‘Limma’ package, and resulting _P_-values adjusted for multiple
testing using the false discovery rate (FDR) Benjamini and Hochberg method (Smyth, 2004); probe sets with adjusted _P_-value FDR _q_<0.05 were called differentially expressed.
Differentially expressed probes were also subjected to Metacore Pathway analysis (Thomson Reuters, London, UK) to identify enrichment of pathways and processes, using hyper-geometric
distributions to determine the most enriched gene sets (FDR _q_<0.05). PROTEIN EXPRESSION AND WESTERN BLOTTING Cells for protein extraction were plated in six-well dishes, cultured until
confluent, growth media removed and washed with ice-cold PBS before lysis in 0.5 ml RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 5 mM EDTA, 0.1% SDS, 0.5% sodium deoxycholate, 1% Nonidet P-40)
supplemented with 2% protease inhibitor cocktail (Sigma-Aldrich). Lysed cells were centrifuged (13 000 r.p.m; 10 min) to pellet cell debris, and protein concentrations of the resulting cell
supernatants determined by Bradford Assay (Bio-Rad, Hemel Hempstead, UK), relative to a standard curve prepared from serial dilutions of bovine serum albumin (0–1 mg ml–1), with absorbance
readings at 595 nm. Glutathione _S_-transferase P1 expression was analysed in protein extracts from each cell line by western blotting, following SDS-PAGE. Each protein sample (40 _μ_g) was
diluted in equal volumes of 5 × sample buffer (10% SDS, 250 mM Tris-HCl (pH 6.8), 1 mg ml–1 bromophenol blue, 0.5 M DTT, 50% glycerol), denatured and separated by SDS-PAGE using 12%
Mini-PROTEAN 3 polyacrylamide gels (Bio-Rad) in Tris-glycine buffer (25 mM Tris pH 8.3, 250 mM glycine, 0.1% SDS). Following electrophoresis, proteins were transferred to nitrocellulose
membranes in Tris-glycine-methanol buffer (48 mM Tris pH 8.3, 39 mM glycine, 0.037% SDS, 10% methanol) and nonspecific antibody binding blocked by incubation for 2 h in TBST (25 mM Tris-HCl
pH 7.6, 150 mM NaCl, 0.05% Tween-20) containing 5% milk powder. Membranes were then incubated overnight with a rabbit polyclonal _GSTP1_ primary antibody ((Henderson et al, 1998), diluted 1
: 1000) or a mouse monoclonal _β_-_actin_ antibody (sc-47778, Santa Cruz Biotechnology Inc., Heidelberg, Germany, diluted 1 : 1000), washed in PBST (PBS supplemented with 0.05% Tween-20) and
incubated for 1 h with a swine anti-rabbit polyclonal secondary antibody (Dako PO399, diluted 1 : 1000, _GSTP1_, Dako, Ely, UK) or goat anti-mouse polyclonal secondary antibody (Dako PO447,
diluted 1 : 1000, _β-_actin). Immunoblots were developed using an ECL-chemiluminescence kit (Merck Millipore, Watford, UK) according to the manufacturer’s instructions. ANALYSIS OF CELLULAR
GLUTATHIONE LEVELS Total cellular glutathione (GSSG+GSH) levels were compared in A2780 and A2780/GSTP1 cells using a Glutathione Assay kit (Sigma-Aldrich) according to the manufacturer’s
guidelines. Cells were harvested, washed in PBS, counted using a haemocytometer and re-suspended to a final concentration of 1 × 108 cells ml–1 in PBS. Cells were then pelleted,
de-proteinised with 5% 5-sulfosalicyclic acid and glutathione levels assessed using a kinetic assay in which catalytically active glutathione reduces 5,5-dithiobis(2-nitrobenzoic acid)
(DTNB) to 5-dithiobis(2-nitrobenzoic acid) (TNB). TNB production was measured spectrophotometrically at 412 nm, with kinetic reads at 1-min intervals for 5 min, and glutathione levels
extrapolated from a standard curve of serial dilutions of reduced glutathione (positive values for both GSH and GSSG are obtained in the reaction). OVARIAN CELL LINE CHEMOSENSITIVITY ASSAYS
MTT assays (Mosmann, 1983) were used to compare chemosensitivity of A2780 and A2780/_GSTP1_ cells to the _GSTP1_ model substrate ethacrynic acid and chemotherapy drugs cisplatin,
carboplatin, paclitaxel, doxorubicin, topotecan and gemcitabine. Each cell line was plated in a 96-well plate (5000 cells per well) and treated in triplicate with serial dilutions of each
drug. Ethacrynic acid was used at concentrations from 2.5 to 80 _μ_ M, and chemotherapy drugs at concentrations selected to mimic typical peak plasma levels in ovarian cancer patients (range
0–200% (peak plasma); cisplatin 0–25 _μ_ M, carboplatin 0–85 _μ_ M, paclitaxel 0–32 _μ_ M, doxorubicin 0–6 _μ_ M, gemcitabine 0–190 _μ_ M and topotecan 0–56 _μ_ M; Konecny et al, 2000).
Cells were drug treated for 72 h, media removed and 100 _μ_l of a 0.5 mg ml–1 MTT solution (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide in phenol red-free DMEM) added and
cells incubated at 37 °C for 3 h. The resulting formazan crystals were solubilised in DMSO, quantitated spectrophotometrically at 570 nm and the percentage of live cells remaining following
each drug treatment calculated (assigning a value of 100% to vehicle-treated cells). IC50 values were calculated from log dose-response curves using Prism 6 software (GraphPad Software Inc.,
La Jolla, CA, USA). CELL INVASION AND MIGRATION ASSAYS Cell invasion and migration was assessed using 24-well InnoCyte Cell Invasion and Migration Assays (Merck Millipore) according to the
manufacturer’s guidelines. Cell invasion was assessed by plating cells in serum-free medium in invasion chambers (8 _μ_m membranes) coated with basement membrane matrix, which prevents
non-invasive cells from passing through the membrane pores. Similarly, cell migration was compared in A2780 and A2780/GSTP1 cells by plating each cell line in serum-free medium in migration
chambers. In both assays, serum-containing medium was added to each well to induce cell migration, assessed by staining cells attached to the lower side of the membrane with the fluorescent
cell permeable dye Calcein-AM, and measuring fluorescence at 485 nm (excitation) and 520 nm (emission). Negative control A2780 cells were additionally treated with the anti-migratory drug
Latrunculin A. FACS ANALYSIS Cell cycle parameters were compared in A2780 and A2780/GSTP1 cells by flow cytometry following propidium iodide labelling of cell line DNA. Cells were untreated,
or treated with 12.66 _μ_ M cisplatin (to represent typical 100% patient peak plasma levels) for 1, 4 or 24 h then fixed in ice-cold 70% ethanol overnight at −20 °C, re-suspended in PBS and
stained by incubation with propidium iodide (40 _μ_g ml–1, Sigma-Aldrich) and RNAse A (100 _μ_g ml–1, Sigma-Aldrich) for 30 min at 37 °C in the dark. Cell cycle parameters (10 000 cells per
sample) were analysed using a FACScan flow cytometer (Becton Dickinson, Oxford, UK) and Cellquest Pro software to determine cell cycle phases and cells in sub-Go/G1. STATISTICAL ANALYSIS
All statistical tests were performed using the SPSS statistics package version 20.0 (IBM, New York, NY, USA). Independent sample _t_-tests were used to assess differences in gene expression
identified by qRT–PCR analysis and invasion and migration changes in A2780 and A2780/GSTP1 cells. RESULTS We previously showed that _GSTP1_ is abundantly expressed in human ovarian tumours,
and highlighted marked inter-tumour differences in _GSTP1_ expression (Smith et al, 2012). To investigate whether individuality in _GSTP1_ expression influences chemotherapy response, we
used shRNA-mediated gene silencing to stably knockdown _GSTP1_ in the ovarian tumour cell line A2780, created from a chemotherapy-naive ovarian cancer patient (Behrens et al, 1987). A2780
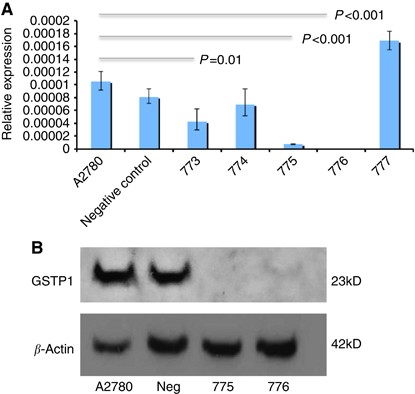
cells were transfected with each of five _GSTP1_-specific Mission shRNA pLKO.1-puro plasmids (773, 774, 775, 776 and 777) and a negative control plasmid as described in Materials and Methods
section. Following lipofectamine selection, multiple antibiotic-resistant colonies were screened for _GSTP1_ knockdown by qRT–PCR analysis, and gene knockdown confirmed in cells transfected
with plasmids 773, 775 and 776 (Figure 1A), with maximum reduction in shRNA construct 775 and 776-transfected cells. _GSTP1_ protein expression was evaluated by western blotting, with
complete loss of _GSTP1_ expression confirmed in shRNA construct 775-transfected cells and >95% reduction in protein expression in construct 776-transfected cells (Figure 1B). GSTP1-775
knockdown cells (A2780/GSTP1) were therefore selected for use in additional experiments, following additional confirmation of increased total cellular glutathione levels (A2780 83.16±1.67
_μ_ M and A2780/GSTP1 116.85±4.67 _μ_ M), consistent with reduced _GSTP1_ catalytic activity. MTT assays were then used to compare chemosensitivity of A2780 and A2780/GSTP1 cells to the
diuretic and well-characterised _GSTP1_ substrate ethacrynic acid. _GSTP1_ conjugates and detoxifies ethacrynic acid (Ahokas et al, 1984), which was significantly more toxic to _GSTP1_ null
cells (1.3-fold increase in IC50, _P_=0.004, Figure 2A). Sensitivity to platinum-based chemotherapy drugs was then compared in A2780 and A2780/GSTP1 cells and significant differences in
chemosensitivity observed following treatment with cisplatin (2.28-fold decrease in IC50, _P_=0.03, Figure 2B) and carboplatin (4.83-fold decrease in IC50, _P_=0.007, Figure 2C), and a less
pronounced decrease in sensitivity to paclitaxel (1.58-fold increase in IC50, _P_=0.02, Figure 2D). Chemosensitivity to additional drugs used to treat ovarian cancer was also compared but no
significant differences in IC50 values identified (doxorubicin 0.13 _vs_ 0.11 _μ_ M, gemcitabine 3.32 _vs_ 3.34 _μ_ M and topotecan 1.00 _vs_ 1.12 _μ_ M in A2780 and A2780/GSTP1 cells,
respectively (all _P_-values >0.05)). To confirm our findings, MTT assays were repeated in GSTP1-776 cells and very similar changes in cisplatin, carboplatin and paclitaxel
chemosensitivity identified (data not shown). As _GSTP1_ knockdown significantly increased cisplatin and carboplatin chemosensitivity in A2780 cells, we further investigated whether _GSTP1_
knockdown in the related platinum-resistant A2780DPP cell line (Behrens et al, 1987) could re-sensitise these drug-resistant cells to platinum-based chemotherapy. A2780DPP cells were
therefore transfected with shRNA constructs 775 and 776, optimised for _GSTP1_ knockdown in the experiments described above. In contrast to drug-sensitive A2780 cells, however, although
puromycin-resistant colonies were initially formed in A2780DPP cells in multiple replicate experiments, the colonies formed were relatively small (10–20 cells per colony) and were viable for
only 48 h (Figure 3A). As our control transfections resulted in viable colony formation in both A2780 and A2780DPP cells, we hypothesised that _GSTP1_ knockdown may be lethal in A2780DPP
cells, which are grown in medium containing 1 _μ_ M cisplatin to maintain the drug-resistant phenotype. To confirm _GSTP1_ knockdown in A2780DPP cells, we isolated individual
puromycin-resistant colonies 24 h after colony formation using cloning cylinders, and extracted sufficient RNA to confirm _GSTP1_ knockdown by qRT–PCR analysis (Figure 3B). Unfortunately, we
were unable to harvest sufficient A2780DPP/GSTP1 knockdown cells to perform western blotting, cytotoxicity assays or more detailed phenotypic characterisation, but our confirmation of
_GSTP1_ knockdown, together with selective platinum toxicity in puromycin-resistant clones is consistent with an essential role for _GSTP1_ in glutathione conjugation and cisplatin
detoxification. We further attempted to confirm this hypothesis by knocking down _GSTP1_ in A2780DPP cells grown in the absence of cisplatin selection, but found that control untransfected
A2780DPP cells did not retain a stable drug-resistant phenotype over the 6-week experimental period in the absence of cisplatin selection (data not shown). To investigate the cellular
phenotypes resulting from _GSTP1_ knockdown in A2780 cells, Illumina HT-12 Expression BeadChip Arrays were used to compare gene expression in A2780 and A2780/GSTP1 cells. Each HT-12 array
contains >47 000 unique probe sets, corresponding to >28 000 coding transcripts; 2671 probes were significantly more highly expressed, and 2717 probes less highly expressed in
A2780/GSTP1 cells. Of these, ⩾2-fold differences in gene expression were identified for 336 gene transcripts—the most significantly differentially expressed genes are summarised in
Supplementary Table 1 (Supplementary Information), and changes in gene expression predicted by BeadChip Array analysis confirmed by qRT–PCR analysis for selected up- and downregulated genes
(Figure 4). It was of particular interest to note that no compensatory changes in additional GST isoforms were identified in A2780/GSTP1 cells. Pathway and process enrichment analysis was
then used to identify common differentially regulated pathways or processes in A2780 and A2780/GSTP1 cells (Supplementary Table 2). Consistent with these predictions, the most significant
differences in gene expression were seen for genes (e.g., N-cadherin (CDH2), versican (VCAN), L6 cell surface antigen (TM4SF1) and layilin (LAYN)) associated with cell invasion, migration,
metastasis and the epithelial–mesenchymal transition. Consistent with known functions of the GSTs, several differentially regulated pathways and processes were associated with lipid
metabolism and with the oxidative stress response, while additional interesting associations suggested altered cell cycle regulation and differences in apoptosis, cell invasion and
migration. We therefore used quantitative cell growth, invasion and migration assays and FACS analysis to compare A2780 and A2780/GSTP1 cell phenotypes. We did not observe significant
differences in cell growth rates, where A2780 and A2780/GSTP1 cells had doubling times of 0.91 and 1.03 days, respectively (_P_=0.462), or in cell cycle parameters, assessed by FACS analysis
of propidium iodide-labelled untreated cells and stressed cells acutely treated with cisplatin (Figure 5A). In contrast, consistent with the gene expression and pathway/process differences
described above, we found that both cell invasion (Figure 5B) and migration (Figure 5C) was significantly reduced in A2780/GSTP1 cells. DISCUSSION Conjugation of glutathione with
platinum-based chemotherapy drugs is an important detoxification mechanism, which promotes drug clearance, limits the formation of DNA crosslinks and reduces toxicity (Peklak-Scott et al,
2008). It is therefore logical that the expression of glutathione-conjugating enzymes including _GSTP1_ is increased as an adaptive response in drug-resistant tumour cells (Black and Wolf,
1991; McLellan and Wolf, 1999) and that reduced _GSTP1_ activity may influence chemosensitivity. Several studies, however, have described increased _GSTP1_ expression in drug-resistant cell
lines following exposure to drugs which are not detoxified by glutathione conjugation (Wang et al, 1989), or which are not _GSTP1_ substrates (Tew, 1994)—a direct role for _GSTP1_ in
platinum chemosensitivity therefore remains to be unequivocally established. We have shown for the first time that stable deletion of _GSTP1_ in A2780 ovarian tumour cells significantly and
selectively increases sensitivity to cisplatin and carboplatin, drugs routinely used to treat ovarian cancer, a notoriously drug-resistant and clinically intractable disease. Importantly,
loss of _GSTP1_ expression in the cisplatin-resistant A2780 subline A2780DPP was toxic to the cells in the presence of cisplatin, consistent with an important catalytic role for _GSTP1_ in
glutathione conjugation in the detoxification pathway of platinum drugs. Consistent with this hypothesis, we found significant differences in intracellular glutathione levels and in
sensitivity to the GST substrate ethacrynic acid in _GSTP1_ null cells. Although the role of _GSTP1_ in ovarian tumour chemosensitivity has not been studied, transient siRNA-mediated _GSTP1_
knockdown in a diverse panel of leukaemia and lymphoma cell lines has recently also been reported to influence cisplatin sensitivity (Chen et al, 2013). Glutathione _S_-transferase P1 has
also been proposed to have a non-catalytic role in promoting cell proliferation by binding to and inhibiting _JNK_ (Adler et al, 1999)—_JNK_ activity is increased when _GSTP1_ activity is
reduced, either by small molecule _GSTP1_ inhibitors or in _GSTP1_ null mice (Henderson et al, 1998). It is logical to propose, therefore, that stable _GSTP1_ deletion in A2780 ovarian
tumour cells may result in _JNK_ activation. Consistent with this hypothesis, interrogation of our gene expression microarray data sets revealed increased expression of several _JNK_
regulatory genes including the toxicity and _JNK_ inducer _TAJ_ (_TNFRSF19_, 8.33-fold, adjusted _P_-value 1.25 × 10−18, Supplementary Table 1), and _JNK_ target genes including the
p53-inducible proteins _TP53I3_ (_PIG3_, 5.02-fold, adjusted _P_-value 1.73 × 10−16) and _CDKN1A_ (_p21_, 4.87-fold, adjusted _P_-value 9.62 × 10−15) in _GSTP1_ knockdown cells. Although
many of the genes up- and downregulated by _GSTP1_ deletion are associated with the _RAS/MAPK_ pathway (Supplementary Table 1), we did not detect a significant difference in _RAS/MAPK_
pathway activation, using a quantitative _RAF-1-_binding/Ras GTPase ELISA to assess pathway activation in _GSTP1_ knockdown cells (data not shown). In contrast, several differentially
expressed genes (e.g., _CDH2_) and associated pathways and processes suggested that _GSTP1_ knockdown may significantly inhibit cell invasion and migration—these predictions were confirmed
experimentally, and are consistent with the hypothesis that increased _GSTP1_ expression promotes neoplastic transformation and the development of drug resistance. We were therefore
surprised to find that _GSTP1_ knockdown did not significantly influence cell growth rate or alter cell cycle progression in A2780 cells, although similar findings have been reported in a
series of ovarian cancer cell lines made resistant to cisplatin, carboplatin and paclitaxel (Li et al, 2004), suggesting limitations of the use of immortalised cancer cell line models.
Similarly, and consistent with a previous report of comparable _GSTP1_ expression in A2780 cells and the cisplatin-resistant derivatives C70 and C200 (Townsend et al, 2002), we did not
detect a significant increase in _GSTP1_ expression in platinum-resistant A2780DPP cells. In contrast, increased _GSTP1_ expression has been described in many drug-resistant cell lines
(Kuroda et al, 1991; Kotoh et al, 1997; Tozawa et al, 2008; Yang et al, 2009), including ovarian tumour lines resistant to platinum drugs (Lewis et al, 1988; Parekh and Simpkins, 1996;
Yanagie et al, 2009). Recent data from our own laboratory, where we see increased _GSTP1_ expression in a novel drug-resistant A2780 subline immediately following _de novo_ platinum
selection, further support the hypothesis that increased _GSTP1_ expression may not be maintained in long-term culture of immortalised tumour cells. In light of these concerns, we are
currently extending our analysis to additional immortalised cell lines, with different histologies and genetic backgrounds and to primary cell lines derived from ascites from drug-sensitive
and drug-resistant ovarian cancer patients. Glutathione _S_-transferase P1 expression in cancer patients may also be influenced by the presence of allelic variants—_GSTP1_ Ile105Val (rs1695)
and _GSTP1_ Ala114Val (rs1138272), with homozygote rare allele frequencies of approximately 12% and 2% in the Caucasian population, respectively (Zimniak et al, 1994; Ali-Osman et al, 1997;
Harries et al, 1997; Sachse et al, 2002). Unlike _GSTM1_ and _GSTT1_, however, where common polymorphisms result in complete gene deletions (Board, 1981), variant _GSTP1_ alleles differ
from the consensus reference sequence by single amino-acid substitutions, resulting in less pronounced, less frequent and less well-characterised phenotypes (Peklak-Scott et al, 2008). In
contrast, however, our recent gene expression profiling experiments in human ovarian tumours describe marked (>70-fold) inter-tumour differences in _GSTP1_ expression, suggesting that
individuality in glutathione-conjugating activity, although not genetically determined, could significantly influence response to platinum-based chemotherapy in ovarian cancer patients.
Studying the influence of inter-individual differences in _GSTP1_ expression on disease progression or chemotherapy response in ovarian cancer patients is challenging—the disease is
frequently diagnosed at an advanced stage, limiting the availability of matched normal and tumour samples, and serial matched drug-sensitive and drug-resistant tumour biopsies are rarely
available. Increased _GSTP1_ expression in ovarian tumours (_n_=41), relative to unmatched healthy ovarian samples (_n_=12) and benign tumours (_n_=25) has been described in a small Chinese
study (Cheng et al, 2000) and, consistent with our findings, _GSTP1_ expression in ovarian cyst fluid has been correlated with higher relapse rates following platinum-based chemotherapy
(Boss et al, 2001). In similar studies, _GSTP1_ expression in ovarian cyst fluid correlated both with serum CA125 levels and reduced patient survival (Kolwijck et al, 2009), while in a small
series (_n_=30) of matched first and second-look laparotomies, increased _GSTP1_ expression was associated with disease progression, assessed by both more frequent relapses and reduced
survival (Surowiak et al, 2005). Confirmation of a direct role for _GSTP1_ in chemotherapy response is important, not only in the prediction of response to platinum-based chemotherapy, but
as a candidate response biomarker for new generation chemotherapy drugs, designed to exploit increased _GSTP1_ expression in tumours relative to surrounding normal tissues. For example,
TLK-286 (Telcyta, canfosfamide) was identified as the lead candidate in a rationally designed series of selectively toxic glutathione analogues (Lyttle et al, 1994), which continues to be
evaluated both as a single agent and in combination chemotherapy in phase II and III clinical trials in ovarian cancer and other solid tumours. TLK-286 is metabolically activated by _GSTP1_
and cytotoxicity has been correlated with _GSTP1_ expression (Rosario et al, 2000; Dourado et al, 2013). Increased _GSTP1_ expression in tumours and in drug-resistant cells is also the
target of a new class of GST suicide inhibitors, including 7-nitro-2,1,3-benzoxadiazole (NBDHEX) (Ricci et al, 2005), which acts to induce apoptosis by promoting dissociation of the
_GSTP1/JNK1_ complex in leukaemia cell lines (Turella et al, 2005). Similar effects were recently observed in mesothelioma cell lines, where NBDHEX synergised with cisplatin (De Luca et al,
2013). We have described marked inter-tumour differences in _GSTP1_ expression but, unlike fibroblast growth factor (_FGF_) family genes, pre-treatment tumour _GSTP1_ expression was not
influenced by tumour histology or associated with altered survival (Smith et al, 2012). Our study did not have sufficient power to perform a meaningful assessment of the potential role of
_GSTP1_ in chemotherapy response, although we believe that lack of routine access to comparable clinical biopsy or tumour samples from ovarian cancer patients pre- and post-treatment may
significantly limit tumour biomarker utility. It is interesting to note, however, that post-chemotherapy _GSTP1_ expression was associated with progression-free survival in a small study
(_n_=41 patients) where pre- and post-chemotherapy biopsies were available (Saip et al, 2005). We are therefore currently recruiting ovarian cancer patients to clinical studies in which we
are collecting serial serum and ascites samples from matched drug-sensitive and drug-resistant patients for quantitative biomarker profiling. _GSTP1_ expression has previously been
investigated by immunostaining in ovarian ascites samples, and shown to correlate with both primary tumour expression and cisplatin chemosensitivity (Kase et al, 1998). Our microarray data
sets provide numerous examples of _GSTP1_-dependent gene expression changes, and may therefore allow us to identify additional biomarkers, which correlate with _GSTP1_ activity. Additional
candidate _GSTP1_ biomarkers have recently identified in studies describing an inverse association between _GSTP1_ expression, chemosensitivity and expression of the regulatory microRNA
_miR-513a-3p_ in cisplatin-resistant A549 lung cancer cells (Zhang et al, 2012). Similarly, interleukin-6 (_IL-6_) production has been shown to be elevated in both serum and ascites samples
from ovarian cancer patients and to be inversely associated with platinum chemosensitivity and survival (Scambia et al, 1995). Of obvious relevance to our findings, _GSTP1_ has recently been
shown to be an _IL-6_ target gene (Wang et al, 2010), while inhibition of _GSTP1_ activity by an _IL-6_ or IL-6 receptor mAb correlates with increased platinum (and paclitaxel) sensitivity
in renal cancer cells (Mizutani et al, 1995). It will therefore be of particular interest to compare GSTP1 and IL-6 levels in serum and ascites samples from drug-sensitive and drug-resistant
ovarian cancer patients. In summary, and consistent with the findings of a recent similar study in mesothelioma (Chen et al, 2014), we have shown that _GSTP1_ selectively influences
sensitivity to cisplatin and carboplatin in ovarian tumour cells. Additional studies to evaluate the role of _GSTP1_ and co-regulated genes as clinical response biomarkers of disease
progression and platinum chemosensitivity in ovarian cancer patients are underway in our laboratory. CHANGE HISTORY * _ 09 SEPTEMBER 2014 This paper was modified 12 months after initial
publication to switch to Creative Commons licence terms, as noted at publication _ REFERENCES * Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ,
Wolf CR, Davis RJ, Ronai Z (1999) Regulation of JNK signaling by GSTp. _EMBO J_ 18 (5): 1321–1334. Article CAS PubMed PubMed Central Google Scholar * Ahokas JT, Davies C, Ravenscroft
PJ, Emmerson BT (1984) Inhibition of soluble glutathione S-transferase by diuretic drugs. _Biochem Pharmacol_ 33 (12): 1929–1932. Article CAS PubMed Google Scholar * Ali-Osman F, Akande
O, Antoun G, Mao JX, Buolamwini J (1997) Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene
variants. Evidence for differential catalytic activity of the encoded proteins. _J Biol Chem_ 272 (15): 10004–10012. Article CAS PubMed Google Scholar * Anathy V, Roberson E, Cunniff B,
Nolin JD, Hoffman S, Spiess P, Guala AS, Lahue KG, Goldman D, Flemer S, van der Vliet A, Heintz NH, Budd RC, Tew KD, Janssen-Heininger YM (2012) Oxidative processing of latent Fas in the
endoplasmic reticulum controls the strength of apoptosis. _Mol Cell Biol_ 32 (17): 3464–3478. Article CAS PubMed PubMed Central Google Scholar * Ban N, Takahashi Y, Takayama T, Kura T,
Katahira T, Sakamaki S, Niitsu Y (1996) Transfection of glutathione S-transferase (GST)-pi antisense complementary DNA increases the sensitivity of a colon cancer cell line to adriamycin,
cisplatin, melphalan, and etoposide. _Cancer Res_ 56 (15): 3577–3582. CAS PubMed Google Scholar * Behrens BC, Hamilton TC, Masuda H, Grotzinger KR, Whang-Peng J, Louie KG, Knutsen T,
McKoy WM, Young RC, Ozols RF (1987) Characterization of a cis-diamminedichloroplatinum(II)-resistant human ovarian cancer cell line and its use in evaluation of platinum analogues. _Cancer
Res_ 47 (2): 414–418. CAS PubMed Google Scholar * Black SM, Beggs JD, Hayes JD, Bartoszek A, Muramatsu M, Sakai M, Wolf CR (1990) Expression of human glutathione S-transferases in
Saccharomyces cerevisiae confers resistance to the anticancer drugs adriamycin and chlorambucil. _Biochem J_ 268 (2): 309–315. Article CAS PubMed PubMed Central Google Scholar * Black
SM, Wolf CR (1991) The role of glutathione-dependent enzymes in drug resistance. _Pharmacol Ther_ 51 (1): 139–154. Article CAS PubMed Google Scholar * Board PG (1981) Biochemical
genetics of glutathione-S-transferase in man. _Am J Hum Genet_ 33 (1): 36–43. CAS PubMed PubMed Central Google Scholar * Boss EA, Peters WH, Roelofs HM, Boonstra H, Steegers EA, Massuger
LF (2001) Glutathione S-transferases P1-1 and A1-1 in ovarian cyst fluids. _Eur J Gynaecol Oncol_ 22 (6): 427–432. CAS PubMed Google Scholar * Chen J, Hurford M, Mekan S, Simpkins H
(2013) Downregulation of glutathione transferase pi sensitizes lymphoma/leukaemia cells to platinum-based chemotherapy. _Br J Haematol_ 162 (1): 135–137. Article CAS PubMed PubMed Central
Google Scholar * Chen J, Solomides C, Simpkins H (2014) Sensitization of mesothelioma cells to platinum-based chemotherapy by GSTpi knockdown. _Biochem Biophys Res Commun_ 447 (1): 77–82.
Article CAS PubMed Google Scholar * Cheng G, Zhu H, Sun L (2000) [The expression of multiple drug resistance associated genes in ovarian cancer]. _Zhonghua fu chan ke za zhi_ 35 (2):
87–90. CAS PubMed Google Scholar * De Luca A, Pellizzari Tregno F, Sau A, Pastore A, Palumbo C, Alama A, Cicconi R, Federici G, Caccuri AM (2013) Glutathione S-transferase P1-1 as a
target for mesothelioma treatment. _Cancer Sci_ 104 (2): 223–230. Article CAS PubMed Google Scholar * Dourado DF, Fernandes PA, Ramos MJ, Mannervik B (2013) Mechanism of glutathione
transferase P1-1-catalyzed activation of the prodrug canfosfamide (TLK286, TELCYTA). _Biochemistry_ 52 (45): 8069–8078. Article CAS PubMed Google Scholar * Eastman A (1987) The
formation, isolation and characterization of DNA adducts produced by anticancer platinum complexes. _Pharmacol Ther_ 34 (2): 155–166. Article CAS PubMed Google Scholar * Elsby R,
Kitteringham NR, Goldring CE, Lovatt CA, Chamberlain M, Henderson CJ, Wolf CR, Park BK (2003) Increased constitutive c-Jun N-terminal kinase signaling in mice lacking glutathione
S-transferase Pi. _J Biol Chem_ 278 (25): 22243–22249. Article CAS PubMed Google Scholar * Galluzzi L, Senovilla L, Vitale I, Michels J, Martins I, Kepp O, Castedo M, Kroemer G (2012)
Molecular mechanisms of cisplatin resistance. _Oncogene_ 31 (15): 1869–1883. Article CAS PubMed Google Scholar * Hagrman D, Goodisman J, Souid AK (2004) Kinetic study on the reactions of
platinum drugs with glutathione. _J Pharmacol Exp Ther_ 308 (2): 658–666. Article CAS PubMed Google Scholar * Harries LW, Stubbins MJ, Forman D, Howard GC, Wolf CR (1997) Identification
of genetic polymorphisms at the glutathione S-transferase Pi locus and association with susceptibility to bladder, testicular and prostate cancer. _Carcinogenesis_ 18 (4): 641–644. Article
CAS PubMed Google Scholar * Hayes JD, Pulford DJ (1995) The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer
chemoprotection and drug resistance. _Crit Rev Biochem Mol Biol_ 30 (6): 445–600. Article CAS PubMed Google Scholar * Henderson CJ, Smith AG, Ure J, Brown K, Bacon EJ, Wolf CR (1998)
Increased skin tumorigenesis in mice lacking pi class glutathione S-transferases. _Proc Natl Acad Sci USA_ 95 (9): 5275–5280. Article CAS PubMed PubMed Central Google Scholar * Ishimoto
TM, Ali-Osman F (2002) Allelic variants of the human glutathione S-transferase P1 gene confer differential cytoprotection against anticancer agents in Escherichia coli. _Pharmacogenetics_
12 (7): 543–553. Article CAS PubMed Google Scholar * Kase H, Kodama S, Nagai E, Tanaka K (1998) Glutathione S-transferase pi immunostaining of cisplatin-resistant ovarian cancer cells in
ascites. _Acta Cytologica_ 42 (6): 1397–1402. Article CAS PubMed Google Scholar * Kolwijck E, Zusterzeel PL, Roelofs HM, Hendriks JC, Peters WH, Massuger LF (2009) GSTP1-1 in ovarian
cyst fluid and disease outcome of patients with ovarian cancer. _Cancer Epidemiol Biomarkers Prev_ 18 (8): 2176–2181. Article CAS PubMed Google Scholar * Konecny G, Crohns C, Pegram M,
Felber M, Lude S, Kurbacher C, Cree IA, Hepp H, Untch M (2000) Correlation of drug response with the ATP tumorchemosensitivity assay in primary FIGO stage III ovarian cancer. _Gynecol Oncol_
77 (2): 258–263. Article CAS PubMed Google Scholar * Kotoh S, Naito S, Yokomizo A, Kohno K, Kuwano M, Kumazawa J (1997) Enhanced expression of gamma-glutamylcysteine synthetase and
glutathione S-transferase genes in cisplatin-resistant bladder cancer cells with multidrug resistance phenotype. _J Urol_ 157 (3): 1054–1058. Article CAS PubMed Google Scholar *
Kristensen GB, Trope C (1997) Epithelial ovarian carcinoma. _Lancet_ 349 (9045): 113–117. Article CAS PubMed Google Scholar * Kuroda H, Sugimoto T, Ueda K, Tsuchida S, Horii Y, Inazawa
J, Sato K, Sawada T (1991) Different drug sensitivity in two neuroblastoma cell lines established from the same patient before and after chemotherapy. _Int J Cancer_ 47 (5): 732–737. Article
CAS PubMed Google Scholar * Lewis AD, Hayes JD, Wolf CR (1988) Glutathione and glutathione-dependent enzymes in ovarian adenocarcinoma cell lines derived from a patient before and after
the onset of drug resistance: intrinsic differences and cell cycle effects. _Carcinogenesis_ 9 (7): 1283–1287. Article CAS PubMed Google Scholar * Li L, Luan Y, Wang G, Tang B, Li D,
Zhang W, Li X, Zhao J, Ding H, Reed E, Li QQ (2004) Development and characterization of five cell models for chemoresistance studies of human ovarian carcinoma. _Int J Mol Med_ 14 (2):
257–264. CAS PubMed Google Scholar * Lyttle MH, Satyam A, Hocker MD, Bauer KE, Caldwell CG, Hui HC, Morgan AS, Mergia A, Kauvar LM (1994) Glutathione-S-transferase activates novel
alkylating agents. _J Med Chem_ 37 (10): 1501–1507. Article CAS PubMed Google Scholar * McLellan LI, Wolf CR (1999) Glutathione and glutathione-dependent enzymes in cancer drug
resistance. _Drug Resist Updates_ 2 (3): 153–164. Article CAS Google Scholar * Mizutani Y, Bonavida B, Koishihara Y, Akamatsu K, Ohsugi Y, Yoshida O (1995) Sensitization of human renal
cell carcinoma cells to cis-diamminedichloroplatinum(II) by anti-interleukin 6 monoclonal antibody or anti-interleukin 6 receptor monoclonal antibody. _Cancer Res_ 55 (3): 590–596. CAS
PubMed Google Scholar * Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. _J Immunol Methods_ 65 (1-2):
55–63. Article CAS PubMed Google Scholar * Parekh H, Simpkins H (1996) Cross-resistance and collateral sensitivity to natural product drugs in cisplatin-sensitive and -resistant rat
lymphoma and human ovarian carcinoma cells. _Cancer Chemother Pharmacol_ 37 (5): 457–462. Article CAS PubMed Google Scholar * Peklak-Scott C, Smitherman PK, Townsend AJ, Morrow CS (2008)
Role of glutathione S-transferase P1-1 in the cellular detoxification of cisplatin. _Mol Cancer Ther_ 7 (10): 3247–3255. Article CAS PubMed PubMed Central Google Scholar * Ricci G, De
Maria F, Antonini G, Turella P, Bullo A, Stella L, Filomeni G, Federici G, Caccuri AM (2005) 7-Nitro-2,1,3-benzoxadiazole derivatives, a new class of suicide inhibitors for glutathione
S-transferases. Mechanism of action of potential anticancer drugs. _J Biol Chem_ 280 (28): 26397–26405. Article CAS PubMed Google Scholar * Rosario LA, O'Brien ML, Henderson CJ,
Wolf CR, Tew KD (2000) Cellular response to a glutathione S-transferase P1-1 activated prodrug. _Mol Pharmacol_ 58 (1): 167–174. Article CAS PubMed Google Scholar * Sachse C, Smith G,
Wilkie MJ, Barrett JH, Waxman R, Sullivan F, Forman D, Bishop DT, Wolf CR Colorectal Cancer Study G (2002) A pharmacogenetic study to investigate the role of dietary carcinogens in the
etiology of colorectal cancer. _Carcinogenesis_ 23 (11): 1839–1849. Article CAS PubMed Google Scholar * Saip P, Tuzlali S, Demir K, Sakar B, Yavuz E, Berkman S, Bengisu E, Topuz E (2005)
Value of glutathion-S transferase pi as a prognostic factor in epithelial ovarian carcinoma. _Eur J Gynaecol Oncol_ 26 (1): 90–94. CAS PubMed Google Scholar * Satoh K, Kitahara A, Soma
Y, Inaba Y, Hatayama I, Sato K (1985) Purification, induction, and distribution of placental glutathione transferase: a new marker enzyme for preneoplastic cells in the rat chemical
hepatocarcinogenesis. _Proc Natl Acad Sci USA_ 82 (12): 3964–3968. Article CAS PubMed PubMed Central Google Scholar * Scambia G, Testa U, Benedetti Panici P, Foti E, Martucci R,
Gadducci A, Perillo A, Facchini V, Peschle C, Mancuso S (1995) Prognostic significance of interleukin 6 serum levels in patients with ovarian cancer. _Br J Cancer_ 71 (2): 354–356. Article
CAS PubMed PubMed Central Google Scholar * Shea TC, Kelley SL, Henner WD (1988) Identification of an anionic form of glutathione transferase present in many human tumors and human tumor
cell lines. _Cancer Res_ 48 (3): 527–533. CAS PubMed Google Scholar * Smith G, Ng MT, Shepherd L, Herrington CS, Gourley C, Ferguson MJ, Wolf CR (2012) Individuality in FGF1 expression
significantly influences platinum resistance and progression-free survival in ovarian cancer. _Br J Cancer_ 107 (8): 1327–1336. Article CAS PubMed PubMed Central Google Scholar * Smyth
GK (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. _Stat Appl Genet Mol Biol_ 3: Article3. Article PubMed Google Scholar
* Sun KH, Chang KH, Clawson S, Ghosh S, Mirzaei H, Regnier F, Shah K (2011) Glutathione-S-transferase P1 is a critical regulator of Cdk5 kinase activity. _J Neurochem_ 118 (5): 902–914.
Article CAS PubMed Google Scholar * Surowiak P, Materna V, Kaplenko I, Spaczynski M, Dietel M, Lage H, Zabel M (2005) Augmented expression of metallothionein and glutathione
S-transferase pi as unfavourable prognostic factors in cisplatin-treated ovarian cancer patients. _Virchows Arch_ 447 (3): 626–633. Article CAS PubMed Google Scholar * Tew KD (1994)
Glutathione-associated enzymes in anticancer drug resistance. _Cancer Res_ 54 (16): 4313–4320. CAS PubMed Google Scholar * Townsend DM, Shen H, Staros AL, Gate L, Tew KD (2002) Efficacy
of a glutathione S-transferase pi-activated prodrug in platinum-resistant ovarian cancer cells. _Mol Cancer Ther_ 1 (12): 1089–1095. CAS PubMed PubMed Central Google Scholar * Tozawa K,
Oshima T, Kobayashi T, Yamamoto N, Hayashi C, Matsumoto T, Miwa H (2008) Oxaliplatin in treatment of the cisplatin-resistant MKN45 cell line of gastric cancer. _Anticancer Res_ 28 (4B):
2087–2092. CAS PubMed Google Scholar * Turella P, Cerella C, Filomeni G, Bullo A, De Maria F, Ghibelli L, Ciriolo MR, Cianfriglia M, Mattei M, Federici G, Ricci G, Caccuri AM (2005)
Proapoptotic activity of new glutathione S-transferase inhibitors. _Cancer Res_ 65 (9): 3751–3761. Article CAS PubMed Google Scholar * Vaughan S, Coward JI, Bast RC, Berchuck A, Berek
JS, Brenton JD, Coukos G, Crum CC, Drapkin R, Etemadmoghadam D, Friedlander M, Gabra H, Kaye SB, Lord CJ, Lengyel E, Levine DA, McNeish IA, Menon U, Mills GB, Nephew KP, Oza AM, Sood AK,
Stronach EA, Walczak H, Bowtell DD, Balkwill FR (2011) Rethinking ovarian cancer: recommendations for improving outcomes. _Nat Rev Cancer_ 11 (10): 719–725. Article CAS PubMed PubMed
Central Google Scholar * Wang Y, Niu XL, Qu Y, Wu J, Zhu YQ, Sun WJ, Li LZ (2010) Autocrine production of interleukin-6 confers cisplatin and paclitaxel resistance in ovarian cancer cells.
_Cancer Lett_ 295 (1): 110–123. Article CAS PubMed Google Scholar * Wang YY, Teicher BA, Shea TC, Holden SA, Rosbe KW, al-Achi A, Henner WD (1989) Cross-resistance and
glutathione-S-transferase-pi levels among four human melanoma cell lines selected for alkylating agent resistance. _Cancer Res_ 49 (22): 6185–6192. CAS PubMed Google Scholar * Wu Y, Fan
Y, Xue B, Luo L, Shen J, Zhang S, Jiang Y, Yin Z (2006) Human glutathione S-transferase P1-1 interacts with TRAF2 and regulates TRAF2-ASK1 signals. _Oncogene_ 25 (42): 5787–5800. Article
CAS PubMed Google Scholar * Yanagie H, Hisa T, Ogata A, Miyazaki A, Nonaka Y, Nishihira T, Osada I, Sairennji T, Sugiyama H, Furuya Y, Kidani Y, Takamoto S, Takahashi H, Eriguchi M (2009)
Improvement of sensitivity to platinum compound with siRNA knockdown of upregulated genes in platinum complex-resistant ovarian cancer cells _in vitro_. _Biomed Pharmacother_ 63 (8):
553–560. Article CAS PubMed Google Scholar * Yang JX, Luo Y, Qiu HM, Tang WX (2009) Characterization and resistance mechanisms of cisplatin-resistant human hepatocellular carcinoma cell
line. _Saudi Med J_ 30 (1): 35–40. PubMed Google Scholar * Yin Z, Ivanov VN, Habelhah H, Tew K, Ronai Z (2000) Glutathione S-transferase p elicits protection against H2O2-induced cell
death via coordinated regulation of stress kinases. _Cancer Res_ 60 (15): 4053–4057. CAS PubMed Google Scholar * Zhang X, Zhu J, Xing R, Tie Y, Fu H, Zheng X, Yu B (2012) miR-513a-3p
sensitizes human lung adenocarcinoma cells to chemotherapy by targeting GSTP1. _Lung Cancer_ 77 (3): 488–494. Article PubMed Google Scholar * Zimniak P, Nanduri B, Pikula S,
Bandorowicz-Pikula J, Singhal SS, Srivastava SK, Awasthi S, Awasthi YC (1994) Naturally occurring human glutathione S-transferase GSTP1-1 isoforms with isoleucine and valine in position 104
differ in enzymic properties. _Eur J Biochem_ 224 (3): 893–899. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to the Genetics Core, Wellcome
Trust Clinical Research Facility, University of Edinburgh for help with microarray analysis and to Dr Michael Boylan, Flow Cytometry Core Facility, University Dundee for FACS advice. We
gratefully acknowledge funding from a Scottish Funding Council Strategic Research Development Grant, from Cancer Research UK (C4639/A10822) and from the Melville Trust for the Care and Cure
of Cancer. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Division of Cancer Research, Medical Research Institute, University of Dundee, Ninewells Hospital and Medical School, Dundee, DD1
9SY, UK L Sawers, B R Ihrig, H C Young, C R Wolf & G Smith * Dundee Cancer Centre, NHS Tayside, Ninewells Hospital and Medical School, 9SY, Dundee DD1, UK M J Ferguson * Bioinformatics
and Biostatistics Service, Cancer Research UK, 44 Lincolns Inn Fields, London WC2A 3PX, London, UK P Chakravarty * Division of Cancer Research, Cancer Research UK Molecular Pharmacology
Unit, Medical Research Institute, Ninewells Hospital and Medical School, Dundee, DD1 9SY, UK C R Wolf Authors * L Sawers View author publications You can also search for this author inPubMed
Google Scholar * M J Ferguson View author publications You can also search for this author inPubMed Google Scholar * B R Ihrig View author publications You can also search for this author
inPubMed Google Scholar * H C Young View author publications You can also search for this author inPubMed Google Scholar * P Chakravarty View author publications You can also search for this
author inPubMed Google Scholar * C R Wolf View author publications You can also search for this author inPubMed Google Scholar * G Smith View author publications You can also search for
this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to G Smith. ADDITIONAL INFORMATION This work is published under the standard license to publish agreement. After 12
months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. Supplementary Information
accompanies this paper on British Journal of Cancer website SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES (DOC 192 KB) RIGHTS AND PERMISSIONS From twelve months after its original
publication, this work is licensed under the Creative Commons Attribution-NonCommercial-Share Alike 3.0 Unported License. To view a copy of this license, visit
http://creativecommons.org/licenses/by-nc-sa/3.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sawers, L., Ferguson, M., Ihrig, B. _et al._ Glutathione _S_-transferase P1
(_GSTP1_) directly influences platinum drug chemosensitivity in ovarian tumour cell lines. _Br J Cancer_ 111, 1150–1158 (2014). https://doi.org/10.1038/bjc.2014.386 Download citation *
Received: 07 May 2014 * Revised: 11 June 2014 * Accepted: 18 June 2014 * Published: 10 July 2014 * Issue Date: 09 September 2014 * DOI: https://doi.org/10.1038/bjc.2014.386 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * _GSTP1_ * glutathione * platinum * chemosensitivity * drug resistance * personalised medicine
Trending News
Do I Get Social Security Survivor Benefits?0:44 Videos de AARP Do I Get Social Security Survivor Benefits? Facebook Twitter LinkedIn The requirements for Social Se...
J balvin's new album 'vibras' hits the storesLos Angeles, May 25 (IANS) "Mi Gente" hitmaker J Balvin has unveiled his new album titled "Vibras". ...
Some states tax your social security benefitsMINNESOTA For 2024, Minnesotans with AGIs of up to $82,190 for an individual and up to $105,380 for a couple filing join...
The moral imperative | British Dental JournalIt never ceases to amuse me how defensive people can be about the National Health Service, regarding it as a deified rel...
Search for girl moves to mountain cabinRIVERSIDE — Detectives on Friday searched a mountain cabin belonging to relatives of a San Bernardino police officer who...
Latests News
Glutathione s-transferase p1 (gstp1) directly influences platinum drug chemosensitivity in ovarian tumour cell linesABSTRACT BACKGROUND: Chemotherapy response in ovarian cancer patients is frequently compromised by drug resistance, poss...
Mechanical metamaterials made of freestanding quasi-bcc nanolattices of gold and copper with ultra-high energy absorption capacityABSTRACT Nanolattices exhibit attractive mechanical properties such as high strength, high specific strength, and high e...
Kcet 2022: application window reopens on may 29, here's how to fill registration formKCET Application 2022: The Karnataka Examinations Authority is scheduled to reopen Karnataka Common Entrance Test 2022 r...
Inequality in high-cost borrowing and unemployment insurance generosity in us states during the covid-19 pandemicABSTRACT US consumers may turn to the private market for credit when income and government benefits fall short. The most...
Dwp wrongly chased mum and aspiring nurse for money – now they are deadTWO WOMEN IN GREATER MANCHESTER WERE HOUNDED BY THE DWP OVER SUPPOSED DEBTS WHICH LED TO THEM LOSING THEIR HOUSING BENEF...