Subliminal stimuli modulate somatosensory perception rhythmically and provide evidence for discrete perception
Subliminal stimuli modulate somatosensory perception rhythmically and provide evidence for discrete perception"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Despite being experienced as continuous, there is an ongoing debate if perception is an intrinsically discrete process, with incoming sensory information treated as a succession of
single perceptual cycles. Here, we provide causal evidence that somatosensory perception is composed of discrete perceptual cycles. We used in humans an electrotactile temporal
discrimination task preceded by a subliminal (i.e., below perceptual threshold) stimulus. Although not consciously perceived, subliminal stimuli are known to elicit neuronal activity in
early sensory areas and modulate the phase of ongoing neuronal oscillations. We hypothesized that the subliminal stimulus indirectly, but systematically modulates the ongoing oscillatory
phase in S1, thereby rhythmically shaping perception. The present results confirm that, without being consciously perceived, the subliminal stimulus critically influenced perception in the
discrimination task. Importantly, perception was modulated rhythmically, in cycles corresponding to the beta-band (13–18 Hz). This can be compellingly explained by a model of discrete
perceptual cycles. SIMILAR CONTENT BEING VIEWED BY OTHERS DYNAMIC CHANGES IN SOMATOSENSORY AND CEREBELLAR ACTIVITY MEDIATE TEMPORAL RECALIBRATION OF SELF-TOUCH Article Open access 03 May
2024 PERCEPTUAL EXPERIENCE IN SOMATOSENSORY TEMPORAL DISCRIMINATION IS INDEXED BY A MID-LATENCY FRONTO-CENTRAL ERP DIFFERENCE Article Open access 05 March 2025 A CONTINUUM OF INVARIANT
SENSORY AND BEHAVIORAL-CONTEXT PERCEPTUAL CODING IN SECONDARY SOMATOSENSORY CORTEX Article Open access 31 March 2021 INTRODUCTION Although perception appears smooth and continuous in our
subjective experience, it has been discussed whether the nature of sensory information processing is intrinsically discrete. Within such a framework, incoming sensory information would be
grouped in consecutive separated perceptual cycles or snapshots1,2,3. A snapshot or perceptual cycle, thus, forms the temporal unit of perceptual experience, leading to rhythmic or cyclic
perception4. While the ongoing debate whether perception is continuous or discrete has been put forward at least a century ago5,6, the hypothesis of discrete perception has only recently
regained new support from neuroimaging studies. These studies have shown that the periodic modulation of subjects’ perception was related to the phase of ongoing neuronal oscillations in the
alpha and beta band located in the parieto-occipital or primary somatosensory cortex (S1)7,8,9. Said neuronal oscillations might thus form the neurophysiological basis of periodic
modulations of perception, suggesting that neuronal oscillations in specific frequencies define perceptual cycles. However, current experimental evidence for discrete perception and its
putative underlying neuronal mechanisms is mostly of correlative nature, while causal evidence remains scarce10,11. Consequently, the theory of discrete perception remains controversially
discussed12,13. To advance this discussion, it would be necessary to causally modulate the rhythmic patterns of perception (i.e., the perceptual cycles). Here, we use the term causal to
define a process in which an independent variable (e.g., the onset of a putative perceptual cycle on behavioral level or the phase of neuronal oscillations on neurophysiological level) is
experimentally and systematically modulated while measuring the corresponding changes on the dependent variable (i.e., rhythmic perception). This causal approach stands in contrast to the
simultaneous measurement of both variables without systematic variation, which would result in correlative evidence. The causal approach would allow for the possibility to gather
experimental evidence for or against the theory of discrete perception and shed light on the patterns of perceptual cycles. We assessed this relationship by using an electrotactile temporal
discrimination task which was preceded by a subliminal (i.e., below perceptual threshold) stimulus. Operationally, the use of subliminal stimuli is advantageous compared to the use of
suprathreshold stimuli. Because subliminal stimuli intensities are insufficient to initiate global network activity14,15 and these stimuli are not consciously perceived, the risk of
perceptually confusing preceding subliminal stimuli with the subsequent target stimuli of the temporal discrimination task or masking the target stimuli is minimized. In addition,
suprathreshold stimuli might attract exogenous (i.e., task-independent) and conscious attention. Conscious attention has been utilized in previous studies to induce a reset event. These
studies have shown that conscious attention can trigger rhythmical patterns of behavior and neuronal activity7,16,17, which might interfere with the proposed cycles of perception. While we
cannot exclude that a subliminal stimulus also triggers (unconscious) attentional mechanisms, the subliminal stimulus enables us to exclude conscious attention mechanisms and investigate how
an unconsciously perceived event modulates perception. Despite not being consciously perceived, subliminal stimuli trigger neuronal activity in early sensory areas15,18,19. Other studies
report subliminal stimuli to elicit weak evoked responses in somatosensory areas19,20 and fMRI BOLD decreases related to functional inhibition19,21. Albeit not being consciously perceived,
subliminal stimuli have been shown to affect the perception of subsequently presented stimuli22. This effect on perception is presumably mediated by the modulation of phase of ongoing
neuronal oscillations in sensory areas (e.g., refs 23, 24). This process of phase resetting is well documented within and across sensory modalities for suprathreshold stimuli25,26,27,28,
whereas reports on subthreshold stimuli remain scarce20. We hypothesized that by presenting the subliminal stimulus at systematically varying time points relative to the discrimination task,
the phase of ongoing neuronal oscillations and consequently the starting point of a perceptual cycle would be modulated systematically (though indirectly; see ref. 24 for a similar paradigm
in the visual domain). Accordingly, perception in the discrimination task should vary rhythmically. Such results would provide valuable evidence for discrete perception which would go
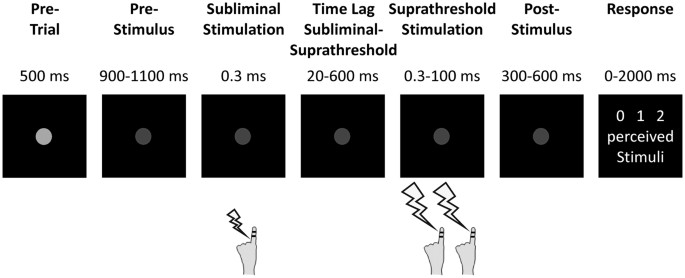
beyond studies that report correlative evidence for perceptual cycles. RESULTS Subjects performed a temporal perceptual discrimination task (see Materials and Methods section for details) in
which they received either zero, one or two suprathreshold electrotactile target stimuli separated by specific stimulus onset asynchronies (SOAs; Fig. 1)9,29. Crucially, these target
stimuli were preceded by a subliminal electrotactile stimulus. The time lag between the subliminal stimulus and the first target stimulus was systematically varied (20–600 ms). After
presentation of the target stimuli, subjects had to report the amount of perceived electrotactile stimuli (i.e., zero, one, or two stimuli). When no target stimuli were presented, but only
the subliminal stimulus (i.e., the control condition), subjects on average perceived 0.03 ± 0.03 stimuli [mean ± SD] (Fig. 2A), demonstrating that the subliminal stimuli were not perceived
as target stimuli. After presentation of one target stimulus, subjects perceived 1.04 ± 0.08 stimuli, averaged across all time lags between subliminal and target stimuli. When two target
stimuli were presented, subjects’ responses increased monotonically with increasing SOA between the two stimuli (Fig. 2A; see Materials and Methods section for details). A repeated measures
ANOVA revealed highly significantly different responses across conditions (F(5,95) = 666.5, p < 0.01). Post-hoc pairwise t-tests revealed highly significant differences between all SOAs
(p < 0.01 for all comparisons). Next, we investigated potential periodic relationships between subjects’ response rates and the time lag between subliminal stimulus and target stimuli by
applying Fourier transformation on perceptual response rates (see Fig. 2B,C for exemplary single subject data, see Supplementary Figure 1 for an overview of all single subject data; see Fig.
2D for the group-level average data). The spectra showed a highly significant peak between 13–18 Hz (p < 0.01) and a second peak between 1–2 Hz which, however, did not reach statistical
significance (p = 0.11; Fig. 2D). To assess whether the rhythmic modulation of perception was phase-locked, i.e., whether the subliminal stimulus induced a phase resetting, we computed the
average phase angle across the entire interval between subliminal stimulus and the first target stimulus for each subject. For each subject, we selected the phase angle of the specific
frequency within the beta-band (13–24 Hz) showing the highest amplitude. The selected phase angles (−1.92 ± 1.03 radians [mean ± SD]) significantly differed from a uniform distribution
(Rayleigh test for non-uniformity across subjects; z = 4.485, p < 0.01). In addition, phase consistency computed for each frequency separately (i.e., without a-priori selection of the
individual frequency) across subjects showed a peak of phase consistency between ~12–16 Hz, which however did not differ significantly from a uniform distribution. DISCUSSION We investigated
if perception in an electrotactile temporal discrimination task is influenced by a preceding subliminal stimulus. Despite not being perceived consciously, the subliminal stimulus modulated
perception rhythmically with a periodicity of 13–18 Hz. Furthermore, phase angles within the beta-band across subjects significantly differed from a uniform distribution, indicating
consistent phase across subjects. We propose an explanation of the results based on our recent findings in an MEG study9. Here, subjects received two electrotactile stimuli (similar to the
present study, but without any subliminal stimuli). Subjects’ perception varied between one or two perceived stimuli from trial to trial. Perceptual variability depended on the phase of
ongoing neuronal oscillations in the alpha and lower beta frequency band (8–20 Hz) in S1. We have proposed a model stating that if the two electrotactile stimuli fall within one cycle of the
8–20 Hz oscillations, they are perceived as a single stimulus, but if they fall within separate cycles, they are perceived as two distinct stimuli (Fig. 3). Accordingly, our model states
that cycles of neuronal oscillations in the alpha-/beta-band define discrete perceptual cycles in the somatosensory domain. We propose that this model explains the present results: In
ongoing neuronal oscillations, the phases - and thus the perceptual cycles - are randomly distributed with respect to the to-be-perceived target stimuli. Consequently, also subjects’
perception varies randomly from trial to trial (provided that the SOA is smaller than the cycle length). In the present study, the subliminal stimulus presumably resets the phase of ongoing
neuronal oscillations20, which is supported by the finding of consistent phase across subjects within the beta-band. Depending on the time-lag between subliminal stimulus and the first
target stimulus, this phase reset determines if the two target stimuli fall within one or two cycles, leading to the perception of one or two stimuli, respectively (Fig. 3). Accordingly, the
frequency of the rhythmic variation in perception is determined by the cycle length of those neuronal oscillations that define the discrete perceptual cycles. Based on the MEG data, we
proposed that the perceptual cycles are defined by neuronal oscillations in the 8–20 Hz frequency band9. This proposition is confirmed by the 13–18 Hz fluctuation of perception induced by
the subliminal stimulus (Fig. 2D). However, since the present effect is located at the lower end of the classical beta band and in between the “classical” centers of somatosensory alpha (~10
Hz) and beta (~20 Hz) oscillations (or mu-rhythm30,31), a clear distinction from the alpha frequency band remains difficult. Accordingly, in our previous MEG study, we found a significant
phase difference between perceiving “2” and “1” stimuli in the frequency range 8–20 Hz, thus encompassing the classical alpha- as well as the lower beta-band (albeit more strongly pronounced
in the beta-band9). Although the behavioral effects in our present study do not show a peak in the classical alpha-band (8–12 Hz), it still remains not fully clear which frequency band(s)
the effect might be assigned to. Future MEG/EEG studies investigating the neurophysiological basis of a phase resetting, might clarify this question. Most previous studies providing evidence
for a causal influence of neuronal oscillations on perception modulated neuronal oscillations by inducing an external rhythm to the brain10,11,25. In contrast, we do not induce an external
rhythm to the brain nor does our single subliminal stimulus contain a temporal structure. Thus, any rhythmicity in the data cannot be explained by an externally induced rhythm but is
putatively due to reset of ongoing neuronal oscillations16. Recent studies reported rhythmic modulations of behavioral performance following within-modality or crossmodal reset stimuli16,26.
While these studies investigated visual perception, our results provide novel evidence for rhythmic patterns of somatosensory perception. Furthermore, these studies often found low
frequency rhythms in the delta to alpha range (<1 to 12 Hz) and assigned the rhythmic pattern to rhythmic fluctuations of visual attention16. In contrast, we find the significant rhythmic
fluctuations in the beta-band in the somatosensory domain. Most importantly, these studies did not address the question of whether perception is a continuous or discrete process. In
addition to the few studies providing evidence for discrete perceptual cycles in the visual and somatosensory domain, our results critically extend these studies by demonstrating that
perception can be systematically modulated as predicted by a model of perceptual cycles9. Subliminal stimulation intensities were selected for the preceding stimulus in order to guarantee
that performance in the temporal discrimination task relied solely on the suprathreshold target stimuli (i.e., that the preceding stimulus would not be perceptually confused with the target
stimuli). One might expect that a suprathreshold preceding stimulus would likewise, or even more likely, elicit a phase reset and thus lead to similar results. A suprathreshold stimulus
might, however, additionally trigger conscious attentional sampling mechanisms7,16,17. Such conscious attentional sampling mechanisms might interfere with our proposed perceptual cycles.
While we cannot exclude that the subliminal stimulus also triggers (unconscious) attentional mechanisms, we were able to study a phase reset that is unnoticed by subjects. In addition, a
preceding suprathreshold stimulus could perceptually mask the subsequent target stimuli. This would affect the perception of the target stimuli and could even render the target stimuli near
invisible for short intervals between preceding and target stimuli (see ref. 16 for a similar effect). Thus, by using a subliminal stimulus, the results are less likely confounded by other,
unintentional processes. In addition, we believe that our results are even more intriguing due to the fact that subliminal stimulation can modulate perception. In line with studies
demonstrating phase resets in response to suprathreshold stimuli (e.g., refs 26, 27, 28), there is evidence that subliminal tactile stimuli can induce oscillatory phase resets in the
somatosensory cortex20 and the finding of consistent phase across subjects likewise suggests such a phase reset. Nonetheless, it should be noted that our behavioral approach provides no
direct measure of oscillatory phase. Thus, although the theory of phase resets has been brought forward by other studies (e.g., refs 23, 24) and is compelling and offers an elegant
explanation for our results, future MEG/EEG studies should aim to confirm the present hypothesis of a phase reset of neuronal oscillations as the underlying process of our results. To
conclude, our findings demonstrate a rhythmic modulation in the beta-band (13–18 Hz) of perception by subliminal, i.e., not consciously perceived stimuli. The findings support a model of
perceptual cycles in the somatosensory domain9. The results provide novel causal evidence for discrete and cyclic perception. MATERIALS AND METHODS SUBJECTS Twenty-five healthy subjects
participated in the study after providing written informed consent in accordance with the Declaration of Helsinki. The study and methods were approved by the Ethical Committee of the Medical
Faculty, Heinrich-Heine-University Düsseldorf, and in line with the guidelines of the Declaration of Helsinki. All subjects had normal or corrected-to-normal vision and reported no sensory
impairments, known history of neurological disorders or use of neuro-modulatory medication. Two subjects had to be excluded because they perceived the subliminal stimulation even at minimal
stimulation amplitude. Three subjects (#4, #9, #22) were excluded from further analysis because they either perceived subliminal stimulation or because they showed a bottom or ceiling
effects in their response distribution (see Analysis section for a detailed explanation of the exclusion criteria). Thus, twenty subjects (13 females, age: 27.6 ± 5.6 years [mean ± SD])
remained for further analysis. STIMULI AND PROCEDURE Subjects were seated in a dimmed and sound-attenuated room. Visual instructions were projected on a translucent screen (60 Hz refresh
rate), which was centrally positioned 57 cm in front of the subjects. Each trial started with the presentation of a light grey dot in the middle of the screen for 500 ms (Fig. 1). Next, the
light grey dot decreased in luminance, signaling the start of the stimulation period. After a jittered time period of 900–1100 ms in which only the fixation dot was present, subjects
received electrotactile stimuli on their left index finger. First, subjects were stimulated with one subliminal stimulus (i.e., stimulation with subthreshold amplitude levels) followed by
zero, one, or two suprathreshold target stimuli (see below for details on stimulation parameters). The subliminal stimulus was applied by means of an electrode pair located at the base of
the left index finger. Current amplitudes of the subliminal stimulation (1.2 ± 0.3 mA [mean ± SD]) were determined individually for each subject prior to the experiment and set to 85% of the
individual perceptual threshold, so that subjects did not consciously perceive this stimulus. Target stimuli were applied by means of an electrode pair located at the tip of the left index
finger. Target stimuli amplitudes (2.5 ± 0.5 mA) were individually set to a level where subjects could clearly perceive stimulation, but below pain threshold. The time lag between the
subliminal stimulus and the first target stimulus were pseudo-randomly varied from 20 to 600 ms in steps of 20 ms. All electrotactile stimuli were applied for 0.3 ms and generated by a
Stimulus Current Generator (DeMeTec GmbH, Langgöns, Germany). After stimulation, the fixation dot was present for a jittered time period between 300–600 ms before written instructions were
presented. Subjects had to report their perception of the target stimuli, i.e., if they perceived either zero, one single or two temporally separate stimuli. If subjects did not respond
within 2 seconds or responded before the presentation of the instructions, a warning was presented visually and the trial was repeated at the end of the block. Responses were given by button
press with the index, middle and ring finger of the right hand. Button configurations for reporting one or two stimuli were randomized from trial to trial between the right index and middle
finger. The perception of zero stimuli was always reported by a button press with the right ring finger. No further feedback was given. Prior to each experiment, we presented to each
subject suprathreshold target stimuli (without subliminal stimuli) with varying stimulus onset asynchronies (SOA). This way, we determined in a staircase procedure the individual SOA for
which the respective subject perceived stimulation with two suprathreshold electrical stimuli as two separate stimuli in 50% of all trials and as one stimulus in the other 50% of trials
(subsequently labeled intermediate SOA; 31.9 ± 15.7 ms; average difference 8.2 ± 15.1 ms (mean ± SD) across blocks). In the following main experiment, subjects were stimulated with two
target stimuli separated by this intermediate SOA in 300 trials. In addition, subjects were stimulated with two target stimuli separated by an SOA with ±50% length of the intermediate SOA in
90 trials, respectively. Furthermore, trials with a predetermined SOA of 0 ms (i.e., only one stimulus was presented) and trials with long SOA (+120% intermediate SOA length) were presented
in 60 trials, respectively. Finally, in on average 60 trials no target stimuli were presented. This condition served as a control condition (subsequently labeled subliminal control) to
guarantee that the subjects did not perceive the subliminal stimulation. In summary, subjects received 660 trials presented in randomized order. The experiment consisted of two identical
blocks. Each block began with the staircase procedure in order to determine the individual intermediate SOA, followed by the main experiment containing 660 trials as described above. After
200 trials, subjects had the possibility to take self-paced breaks. In addition, subjects were offered a break between the two blocks. Each block had a duration of ~20 min. Stimulus
presentation was controlled by means of Presentation software (Neurobehavioral Systems, Albany, NY, USA). Before beginning the experiment, each subject received instructions of the
experimental task but remained naïve to the purpose of the experiment. ANALYSIS Behavioral data were first analyzed with regard to perceptual response rates (i.e., perceived zero, one or two
stimuli) for each condition (subliminal control, 0 ms SOA, intermediate SOA, ± 50% intermediate SOA, and 120% intermediate SOA), pooled across all time lags between subliminal and target
stimuli. Perceptual response rates were averaged across both blocks and across subjects and compared across conditions by means of a repeated measures ANOVA and post-hoc paired t-tests. For
the analysis of perceptual response rates as a function of the time lag between subliminal stimulation and the first target stimulus, only trials with intermediate SOA were analyzed. All
other conditions (subliminal control, 0 ms SOA, ±50% intermediate SOA, and 120% intermediate SOA,) served only as control conditions and/or to mask the main condition in order to minimize
learning effects or perceptual biases. Subliminal control trials (i.e., trials in which only the subliminal stimulus was presented, without target stimuli) were used as a control condition
to guarantee that subjects did not perceive the subliminal stimulation. Blocks in which subjects reported to perceive >10% of subliminal control trials were discarded from further
analysis (6 blocks rejected). Blocks in which response rates showed bottom or ceiling effects (mean perception of either “1” or “2” in two or more adjacent time lags in trials with
intermediate SOA) were discarded from analysis, because these bottom or ceiling effects would have affected the spectral decomposition (6 blocks rejected). For all trials with intermediate
SOA, we computed for each block mean response rates for each subject as a function of time lag between subliminal and target stimuli. To this end, individual mean response rates were
computed for each 20 ms shift of the subliminal stimulus relative to the target stimuli (i.e., subliminal stimulus presented 600 ms vs. 580 ms vs. 560 ms … vs. 20 ms before the first target
stimulus), resulting in a temporal resolution of 20 ms (i.e., 50 Hz, resulting in a Nyquist frequency of 25 Hz). To investigate potential periodic relationships between perceptual response
rates and the time lag between subliminal stimulation and the first target stimulus, we computed a Fourier transformation on the perceptual response rates within each block. Perceptual
reports were zero padded (1000 ms trial length) and multiplied with a single Hanning taper before Fourier transformation. Spectral analysis was performed for frequencies between 1 and 24 Hz
(i.e., below the Nyquist frequency) in steps of 1 Hz. Subsequently, we averaged for each subject the results of the two Fourier transformations (one per block). Statistical analysis of the
spectral amplitudes was performed using a nonparametric randomization approach32. The null hypothesis states that perceptual reports are independent of the time lag between subliminal
stimulation and target stimuli. Since regarding to the null hypothesis, there is no periodicity or other temporal structure in the perceptual performance, time points are exchangeable. Thus,
we randomly exchanged time points 1000 times to generate a randomization distribution against which observed data were compared16. These randomizations were performed for each subject
individually (i.e., for each subject and for each block separately). For each randomization, we performed the same analysis as for the observed data as described above. This procedure
resulted in 1000 spectra for each subject and block, which constituted the null distribution per subject and block. Then, we combined per subject the null distributions of the two blocks to
achieve one null distribution per subject for further analysis. Next, we statistically tested for each frequency independently the observed data against the null distribution across subjects
by means of a nonparametric permutation approach16,32. First, we took the median of the null distribution and computed t-values between observed data and the median value by means of an
independent t-test. This approach resulted in t-values (not corrected for multiple comparisons) for each frequency. Secondly, we applied a non-parametric cluster-based permutation approach
to correct for multiple comparisons32. To this end, we thresholded the t-values at t = 1.96 (p < 0.05). This resulted in clusters of adjacent frequencies. Cluster-level test statistics
were calculated by taking the sum of the t-values within a cluster. Next, we computed a cluster-level null distribution by re-computing the frequency t-maps after randomly permuting the data
(under the null hypothesis of no difference, and thus exchangeability, between observed data and shuffled data). This process of random permutation was repeated 1000 times. For each
repetition, we re-computed the cluster-level statistics as described above, which served as the cluster-level null distribution. The proportion of elements in the null distribution exceeding
the observed cluster-level test statistic was used to estimate a p-value for each cluster. This statistical approach effectively controls for multiple comparisons across time points and
channels (see ref. 32 for a detailed discussion on cluster-based nonparametric tests) and has been used for statistical control of similar behavioral data (e.g., refs 16, 17). This analysis
corresponds to a random effects analysis16. Analysis of phase was based on the complex output of the Fourier transformation of the perceptual response rates per block. Fourier transformation
parameters were equal to the spectral analysis (see above). For each block of each subject, phase angles were computed for each frequency (1–24 Hz), then normalized by their amplitude and
averaged over blocks. For each subject, we determined the frequency showing the highest amplitude within the beta-band range (13–24 Hz) based on the across-block averaged Fourier
transformations. Average phase angles for this individual frequency were selected for each subject, respectively, and statistically compared against a uniform distribution by means of a
Rayleigh test. We also computed phase consistency across subjects for all frequencies (i.e., without a-priori selection of the individual frequency). To this end, we computed the complex
output of the fast Fourier transformation (FFT) of the perceptual response rates per block (see Material and Methods for parameters of the FFT above). For each block of each subject, phase
angles were computed for each frequency (1–24 Hz), then normalized by their amplitude and averaged over blocks. Finally, we averaged the phase angles per frequency across subjects. All data
analysis was performed using Matlab (Mathworks inc., Natick, MA, USA) and the FieldTrip toolbox33 (www.fieldtriptoolbox.org). Circular data analysis was performed using the CircStat
toolbox34. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Baumgarten, T. J. _et al_. Subliminal stimuli modulate somatosensory perception rhythmically and provide evidence for discrete
perception. _Sci. Rep._ 7, 43937; doi: 10.1038/srep43937 (2017). PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. REFERENCES * Stroud, J. M. In _Information theory in psychology: problems and methods_(Free Press, New York, NY, US), pp. 174–207 (1956). * Harter, M. R.
Excitability cycles and cortical scanning: A review of two hypotheses of central intermittency in perception. _Psychol Bull._ 68, 47–58 (1967). Article CAS Google Scholar * VanRullen, R.
& Koch, C. Is perception discrete or continuous? _Trends Cogn Sci._ 7, 207–213 (2003). Article Google Scholar * VanRullen, R. Perceptual Cycles. _Trends Cogn Sci._ 20, 723–735 (2016).
Article Google Scholar * von Baer, K. E. In _Aus baltischer Geistesarbeit. Reden und Aufsätze_. Vol. 1, edited by Alexander, Keyserling (Jonck and Poliewsky, Riga, 1908). * Bergson, H.
_Creative evolution_(Henry Holt, New York, NY, US, 1911). * Busch, N. A. & VanRullen, R. Spontaneous EEG oscillations reveal periodic sampling of visual attention. _Proc Natl Acad Sci
USA_ 107, 16048–16053 (2010). Article CAS ADS Google Scholar * Chakravarthi, R. & VanRullen, R. Conscious updating is a rhythmic process. _Proc Natl Acad Sci USA_ 109, 10599–10604
(2012). Article CAS ADS Google Scholar * Baumgarten, T. J., Schnitzler, A. & Lange, J. Beta oscillations define discrete perceptual cycles in the somatosensory domain. _Proc Natl
Acad Sci USA_ 112, 12187–12192 (2015). Article CAS ADS Google Scholar * Cecere, R., Rees, G. & Romei, V. Individual differences in alpha frequency drive crossmodal illusory
perception. _Curr Biol._ 25, 231–235 (2015). Article CAS Google Scholar * Gundlach, C., Müller, M. M., Nierhaus, T., Villringer, A. & Sehm, B. Phasic modulation of human somatosensory
perception by transcranially applied oscillating currents. _Brain Stimul._(2016). * Holcombe, A. O., Clifford, C. W., Eagleman, D. M. & Pakarian, P. Illusory motion reversal in tune
with motion detectors. _Trends Cogn Sci._ 9, 559–560 (2005). Article Google Scholar * Kline, K. A. & Eagleman, D. M. Evidence against the temporal subsampling account of illusory
motion reversal. _J Vis._ 8, 13 (2008). Article Google Scholar * Dehaene, S., Changeux, J.-P., Naccache, L., Sackur, J. & Sergent, C. Conscious, preconscious, and subliminal
processing: a testable taxonomy. _Trends Cogn Sci._ 10, 204–211 (2006). Article Google Scholar * Weisz, N. et al. Prestimulus oscillatory power and connectivity patterns predispose
conscious somatosensory perception. _Proc Natl Acad Sci USA_ 111, E417–E425 (2014). Article CAS ADS Google Scholar * Landau, A. N. & Fries, P. Attention samples stimuli rhythmically.
_Curr Biol._ 22, 1000–1004 (2012). Article CAS Google Scholar * Landau, A. N., Schreyer, H. M., van Pelt, S. & Fries, P. Distributed attention is implemented through theta-rhythmic
gamma modulation. _Curr Biol._ 25, 2332–2337 (2015). Article CAS Google Scholar * Ress, D. & Heeger, D. J. Neuronal correlates of perception in early visual cortex. _Nat Neurosci._ 6,
414–420 (2003). Article CAS Google Scholar * Nierhaus, T. et al. Imperceptible somatosensory stimulation alters sensorimotor background rhythm and connectivity. _J Neurosci._ 35,
5917–5925 (2015). Article CAS Google Scholar * Palva, S., Linkenkaer-Hansen, K., Näätänen, R. & Palva, J. M. Early neural correlates of conscious somatosensory perception. _J
Neurosci._ 25, 5248–5258 (2005). Article CAS Google Scholar * Blankenburg, F. et al. Imperceptible stimuli and sensory processing impediment. _Science_ 299, 1864 (2003). Article CAS
Google Scholar * Bauer, F., Cheadle, S. W., Parton, A., Müller, H. J. & Usher, M. Gamma flicker triggers attentional selection without awareness. _Proc Natl Acad Sci USA_ 106, 1666–1671
(2009). Article CAS ADS Google Scholar * Diederich, A., Schomburg, A., Colonius, H. & Hamed, S. B. Saccadic reaction times to audiovisual stimuli show effects of oscillatory phase
reset. _PLoS One_ 7, e44910 (2012). Article CAS ADS Google Scholar * Drewes, J., Zhu, W., Wutz, A. & Melcher, D. Dense sampling reveals behavioral oscillations in rapid visual
categorization. _Sci Rep_. 5, 16290 (2015). Article CAS ADS Google Scholar * Lakatos, P., Karmos, G., Mehta, A. D., Ulbert, I. & Schroeder, C. E. Entrainment of neuronal oscillations
as a mechanism of attentional selection. _Science_ 320, 110–113 (2008). Article CAS ADS Google Scholar * Romei, V., Gross, J. & Thut, G. Sounds reset rhythms of visual cortex and
corresponding human visual perception. _Curr Biol._ 22, 807–813 (2012). Article CAS Google Scholar * Fiebelkorn, I. C., Saalmann, Y. B. & Kastner, S. Rhythmic sampling within and
between objects despite sustained attention at a cued location. _Curr Biol.: CB_ 23, 10.1016/j.cub.2013.10.063 (2013). * Mercier, M. R. et al. Neuro-oscillatory phase alignment drives
speeded multisensory response times: an electro-corticographic investigation. _J Neurosci._ 35, 8546–8557 (2015). Article CAS Google Scholar * Baumgarten, T. J., Schnitzler, A. &
Lange, J. Prestimulus alpha power influences tactile temporal perceptual discrimination and confidence in decisions. _Cereb Cor_. 26, 891–903 (2016). Article Google Scholar * Salmelin, R.
& Hari, R. Spatiotemporal characteristics of sensorimotor neuromagnetic rhythms related to thumb movement. _Neuroscience_ 60, 537–550 (1994). Article CAS Google Scholar * Salenius,
S., Portin, K., Kajola, M., Salmelin, R. & Hari, R. Cortical control of human motoneuron firing during isometric contraction. _J Neurophysiol_ 77, 3401–3405 (1997). Article CAS Google
Scholar * Maris, E. & Oostenveld, R. Nonparametric statistical testing of EEG- and MEG-data. _J Neurosci Methods_. 164, 177–190 (2007). Article Google Scholar * Oostenveld, R., Fries,
P., Maris, E. & Schoffelen, J.-M. FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. _Comput Intell Neurosci_ 2011, 156869
(2010). PubMed PubMed Central Google Scholar * Berens, P. CircStat: A MATLAB toolbox for circular statistics. _J Stat Softw_. 31, 1–21 (2009). Article Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by the German Research Foundation (Grant LA 2400/4-1 to J.L. and SFB 974, B07 to T.J.B.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of
Clinical Neuroscience and Medical Psychology, Medical Faculty, Heinrich-Heine-University, Düsseldorf, 40225, Germany Thomas J. Baumgarten, Alfons Schnitzler & Joachim Lange * Department
of Experimental Psychology, Faculty of Mathematics and Natural Sciences, Heinrich-Heine-University, Düsseldorf, 40225, Germany Sara Königs Authors * Thomas J. Baumgarten View author
publications You can also search for this author inPubMed Google Scholar * Sara Königs View author publications You can also search for this author inPubMed Google Scholar * Alfons
Schnitzler View author publications You can also search for this author inPubMed Google Scholar * Joachim Lange View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS Designed Research: J.L.; Performed research: T.J.B., S.K., J.L.; Analyzed and interpreted data: T.J.B., J.L.; Wrote article: T.J.B., A.S., J.L. CORRESPONDING AUTHOR
Correspondence to Joachim Lange. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES (PDF 162 KB)
RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission
from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Baumgarten, T., Königs, S., Schnitzler, A. _et al._ Subliminal stimuli modulate somatosensory perception rhythmically and provide evidence for discrete perception. _Sci Rep_ 7, 43937
(2017). https://doi.org/10.1038/srep43937 Download citation * Received: 17 August 2016 * Accepted: 31 January 2017 * Published: 09 March 2017 * DOI: https://doi.org/10.1038/srep43937 SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Author correction: the effect of national protest in ecuador on pm pollutionCorrection to: _Scientific Reports_ https://doi.org/10.1038/s41598-021-96868-6, published online 02 September 2021 In th...
Wave functions of the conduction electrons in crystalline aluminiumABSTRACT MANY of the properties of metals are satisfactorily accounted for in terms of relatively simple models. The coh...
Early data suggests coronavirus variant could be more deadlyU.K. Prime Minister Boris Johnson said Friday that there's "some evidence" a surging COVID-19 variant may...
How self-driving cars benefit older driversPerhaps we’ve been thinking about self-driving vehicles the wrong way. Fleets of self-driving personal cars may be year...
Identifying novel mechanisms of biallelic tp53 loss refines poor outcome for patients with multiple myelomaABSTRACT Biallelic _TP53_ inactivation is the most important high-risk factor associated with poor survival in multiple ...
Latests News
Subliminal stimuli modulate somatosensory perception rhythmically and provide evidence for discrete perceptionABSTRACT Despite being experienced as continuous, there is an ongoing debate if perception is an intrinsically discrete ...
Nation in brief : nationwide : weather forecast failures reportedThe National Weather Service has fallen short of its mission to warn the public of savage weather that claims more than ...
Citizens detain 20 national guardsmen, 19 other officials in protestResidents of a small town in the Sierra Sur of Oaxaca detained 20 members of the National Guard, five police officers an...
Once again, remittances set a new record; july's total up 28% at us $4. 54 billionRemittances sent to Mexico hit a new monthly record of US $4.54 billion in July, a 28.6% increase compared to the same m...
After 91 days, security forces clear teachers' rail blockades in michoacánTeachers who blockaded train tracks in Michoacán for the last 91 days were removed by security forces on Sunday. Member...