A genome-wide screen for acrophobia susceptibility loci in a finnish isolate
A genome-wide screen for acrophobia susceptibility loci in a finnish isolate"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Acrophobia, an abnormal fear of heights, is a specific phobia characterized as apprehension cued by the occurrence or anticipation of elevated spaces. It is considered a complex
trait with onset influenced by both genetic and environmental factors. Identification of genetic risk variants would provide novel insight into the genetic basis of the fear of heights
phenotype and contribute to the molecular-level understanding of its aetiology. Genetic isolates may facilitate identification of susceptibility alleles due to reduced genetic heterogeneity.
We took advantage of an internal genetic isolate in Finland in which a distinct acrophobia phenotype appears to be segregating in pedigrees originally ascertained for schizophrenia. We
conducted parametric, nonparametric, joint linkage and linkage disequilibrium analyses using a microsatellite marker panel, genotyped in families to search for chromosomal regions correlated
with acrophobia. Our results implicated a few regions with suggestive evidence for linkage on chromosomes 4q28 (LOD = 2.17), 8q24 (LOD = 2.09) and 13q21-q22 (LOD = 2.22). We observed no
risk haplotypes shared between different families. These results suggest that genetic predisposition to acrophobia in this genetic isolate is unlikely to be mediated by a small number of
shared high-risk alleles, but rather has a complex genetic architecture. SIMILAR CONTENT BEING VIEWED BY OTHERS COMPLEX TRAIT SUSCEPTIBILITIES AND POPULATION DIVERSITY IN A SAMPLE OF 4,145
RUSSIANS Article Open access 23 July 2024 POLYGENIC CONTRIBUTION TO THE RELATIONSHIP OF LONELINESS AND SOCIAL ISOLATION WITH SCHIZOPHRENIA Article Open access 10 January 2022 INSIGHTS FROM
THE LARGEST DIVERSE ANCESTRY SEX-SPECIFIC DISEASE MAP FOR GENETICALLY PREDICTED HEIGHT Article Open access 27 February 2025 INTRODUCTION Acrophobia is a pervasive mental disorder, also known
as an irrational fear of heights, affecting approximately five percent of the world’s population1. It is a disproportional reaction to a common, rational fear, and can be characterized as
apprehension, triggered by elevated spaces or anticipation of them. Acrophobia is classified as a specific phobia under the Diagnostic and Statistical Manual of Mental Disorders (DSM-V), and
its aetiology is influenced by both genetic and environmental factors2,3. While demonstrating high comorbidity rates with various psychiatric disorders and diseases, such as different
anxiety disorders or major depression4,5, acquisition of acrophobia is believed to differ from other phobias. It may be mediated through a non-associative pathway6, rather than conditioning
or learning from negative or traumatic experiences7. The symptoms of individuals suffering from acrophobia involve changes in behavioural, cognitive and physiological functioning, such as
confusion and dizziness, when exposed to heights7. Physiologically associated with anomalies in balance control and avoidance behaviour, acrophobia is a consequence of an underlying
biological anomaly involving impaired visual detection of body sway8. In healthy subjects, posture control is obtained by integrated processing of vestibular, visual and proprioceptive
inputs9. However, in people suffering from acrophobia, dysfunction in one of the feedback mechanisms may lead to increased dependence on other stimuli. In particular, the presence of
vestibular dysfunction causes increased dependence on visual or proprioceptive information to keep balance and constant anticipation of matching the natural oscillation of body sway with
visual flow stimulation7. This matching is the root cause of the feeling of lack of stability, especially when exposed to high places10. Although the biological mechanisms of acrophobia have
been thoroughly investigated8, little is known about its molecular basis. As in the case of other complex psychiatric diseases, efforts to localize genetic variants predisposing to
acrophobia are hindered by the complexity of the clinical phenotype and the heterogeneity of the studied samples11. Therefore, isolated populations offer several advantages in studying the
genetic architecture of such complex human diseases, both when rare12,13 or common14 variants are thought to be involved in the genetic aetiology of the condition. Firstly, the enrichment of
the risk alleles in an isolated population might help enable their identification. Furthermore, the shared environmental and cultural homogeneity is highly beneficial as potent exposures to
environmental risks during the lifetime, possibly influencing the aetiology of the disorder, are not routinely evaluated and might therefore be difficult to detect11. To reduce genetic
heterogeneity we have used an isolated homogenous population from north-eastern Finland with small number of founders. This population, described elsewhere15,16, has been intensively
characterized using multiple high-quality historical and health-care registries and was previously studied due to increased lifetime risk of schizophrenia, compared with the rest of
Finland17. While the collection of the schizophrenia sample was ongoing, we observed that a large number of family members had distinct and severe acrophobia that segregated independently
from schizophrenia. In this study, we aimed to map genetic loci predisposing to this specific phenotype. We hypothesized that predisposition to acrophobia may be mediated by a small number
of high risk susceptibility variants segregating in this isolate, and that we could possibly identify them through a genome-wide linkage scan using microsatellite markers, which had been
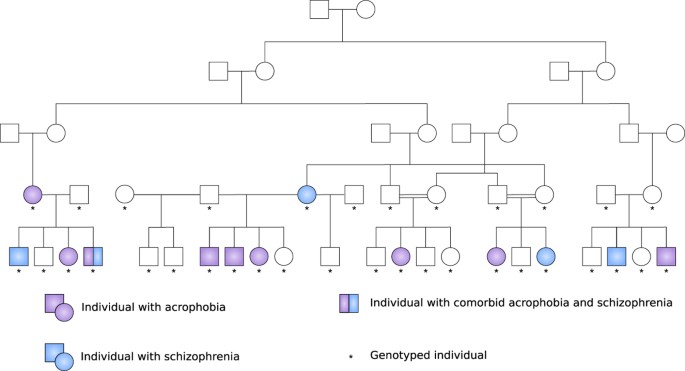
genotyped as a part of earlier linkage-based gene mapping studies17,18. RESULTS Our sample was composed mostly of large multigenerational pedigrees with multiple individuals affected with
acrophobia and at least one parent born in the isolate (Fig. 1). It included 642 people, 42 of them affected only with acrophobia (6.5%) and 75 with acrophobia comorbid with schizophrenia
(11.7%; Table 1). As part of earlier studies17,18, we had genotype information available from 367 individuals, assessed by a genome-wide microsatellite marker panel. As parametric and
nonparametric (model-free) methods have different strengths and weaknesses in detecting linkage19, we applied both techniques across the genome. Two-point parametric analysis has been proven
to be more powerful than nonparametric analysis, provided that the trait and marker locus are specified correctly. However, in case the parametric model is incorrect, the power of the
parametric linkage might be exceeded by nonparametric methods. As the inheritance pattern in our study is unknown, we decided to conduct analyses with both methods. In the parametric
analysis, we used dominant and recessive models due to unknown inheritance pattern, followed by nonparametric analysis, and joint linkage and linkage disequilibrium analysis (Fig. 2). While
two-point analysis examines linkage of a disease to a single marker locus at a time, multipoint analysis evaluates linkage to multiple markers simultaneously. This results in a greater power
to detect linkage, but also an inflation of type I error (false positive) if marker-related information is incorrect20. We performed multipoint, but not two-point nonparametric analysis,
because of the limitations in the size of the pedigrees, which can be analysed, without being divided, by currently available software21. Due to the significant number of individuals with
acrophobia comorbid with schizophrenia, we analysed all individuals with acrophobia, but also conducted separate analyses with individuals with pure acrophobia and pure schizophrenia to
investigate whether any signal was mainly coming from genetic factors influencing susceptibility to schizophrenia independent of acrophobia. PARAMETRIC TWO-POINT RECESSIVE AND DOMINANT
LINKAGE ANALYSIS We first carried out parametric two-point linkage analysis to identify genetic regions linked with acrophobia. The strongest evidence for linkage in the acrophobia sample
(including cases with comorbid schizophrenia) was obtained with marker D5S2115 (LOD = 2.16) using a dominant model. In the pure acrophobia sample, the same marker yielded a LOD score of 0.58
(dominant model), suggesting that this finding may be mainly driven by the schizophrenia phenotype. This was further confirmed by analysing all individuals with schizophrenia without
comorbid acrophobia (LOD = 1.57, dominant model). For the pure acrophobia sample, we obtained the highest LOD score with marker D8S373 (LOD = 2.09) adopting a recessive inheritance model.
Since in the acrophobia with comorbid schizophrenia sample the same marker yielded a LOD score of 0.51 (recessive model) and in the pure schizophrenia sample a LOD score of 0.00 (recessive
model), this signal may be produced mainly by the acrophobia phenotype. Table 2 shows the results of the two-point analyses for markers giving the strongest evidence of linkage (LOD score of
>1.6). PARAMETRIC MULTIPOINT LINKAGE ANALYSIS We carried out parametric multipoint analysis for chromosomes with markers and models which yielded LOD score of >2.0 in at least one
parametric two-point analysis (chromosome 5: dominant model in acrophobia with comorbid schizophrenia; and chromosome 8: recessive model in pure acrophobia). In the acrophobia sample
(including cases with comorbid schizophrenia), marker D5S2115 yielded a maximum LOD score of 0.054 (α = 0.15, dominant model). In the pure acrophobia sample the highest LOD score on
chromosome 8 was with marker D8S373 (0.533, α = 1.00, recessive model). NONPARAMETRIC MULTIPOINT LINKAGE ANALYSIS We next carried out multipoint nonparametric genome-wide linkage analysis
with empirical NPL_ALL model (Fig. 3), measuring if a few founder-alleles are overly represented in affected individuals. Among the acrophobia sample (including cases with comorbid
schizophrenia), the maximum LOD score of 2.91 was detected with marker D13S173, while for the pure acrophobia sample the same marker reached LOD score of 2.11. In the pure acrophobia sample
the most significant evidence of linkage (LOD score = 2.22) was observed with marker D13S162. Markers D13S173 and D13S162 are located 37.8 cM apart and are therefore not strongly linked.
Figure 4 shows the results from the multipoint nonparametric linkage analysis with empirical NPL_PAIR model measuring the sum of conditional kinship coefficients for all affected pairs. For
acrophobia sample (including cases with comorbid schizophrenia), NPL_PAIR approach yielded the maximum LOD score with marker D1S2817 (LOD = 2.52), while in the pure acrophobia sample the LOD
score for the same marker was 0.47. The strongest finding for NPL_PAIR analysis in the pure acrophobia sample was a LOD score of 2.17 with marker D4S2394. JOINT LINKAGE AND LINKAGE
DISEQUILIBRIUM ANALYSIS We hypothesized that reduced genetic heterogeneity in the genetic isolate would lead to the majority of the cases carrying the same predisposing variant, detectable
as linkage disequilibrium (LD). Therefore, we performed PSEUDOMARKER analysis of LD conditional on linkage (Fig. 5). The strongest evidence for association to acrophobia (including cases
with comorbid schizophrenia) was detected for markers D4S2431 (P = 0.0003, recessive model) and D14S267 (P = 0.0019, dominant model). In the pure acrophobia sample the same markers yielded P
values of 0.1704 and 0.0244, respectively. For the pure acrophobia sample the strongest association was obtained to markers D17S2196 (P = 0.0054, dominant model) and D1S235 (P = 0.0054,
recessive model). These results are consistent with the lack of evidence of linkage in the sample. POWER SIMULATION To test the statistical power of the analysed sample, we performed
simulation with SLINK22 software. We obtained average maximum LOD scores of 5.77 and 5.03 with the dominant and recessive model, respectively. Furthermore, 31% of replicates under the
dominant and 12% of replicates under the recessive model reached the conventional LOD score threshold of 3.0. Therefore, this sample has adequate statistical power to obtain significant
evidence for linkage to major loci predisposing to acrophobia. DISCUSSION We aimed to find genetic loci predisposing to acrophobia in a genetically isolated population with the hypothesis
that due to reduced genetic heterogeneity, a few risk alleles predisposing to the phenotype might be identified. While several loci attained LOD score of >2.0, we observed no genome-wide
significant evidence for linkage at any of the studied markers. We detected the strongest evidence of linkage to acrophobia on chromosomal region 13q21-q22 with a peak on marker D13S162 (LOD
= 2.22; pure acrophobia sample). To our knowledge, this region has not been previously associated with phobias or other anxiety disorders. SNP rs2323266, located 14.01 Mb from marker
D13S162 and close to the protocadherin 20 (_PCDH20_) gene in this region, has been previously connected to positive symptom dimension in a genome-wide association study (GWAS) of
schizophrenia (P = 3 × 10−6)23. However, markers D13S162 and rs2323266 are not located within the same LD block. Thus, in our sample, the finding in this chromosomal region seemed to be
specific for acrophobia. We obtained the second strongest evidence of linkage for acrophobia on chromosomal region 4q28 for marker D4S2394 (LOD = 2.17; pure acrophobia sample). Again, in our
study, this signal appeared to be mainly coming from acrophobia and not schizophrenia phenotype (LOD = 0.52; pure schizophrenia sample). This region has not been previously associated with
anxiety disorders or schizophrenia. Chromosomal region 8q24.2-q24.3 with marker D8S373 (LOD = 2.09) was the third region most strongly linked to acrophobia. It has previously been associated
with bipolar disorder24,25,26 and schizophrenia27. However, in our samples of acrophobia with comorbid schizophrenia (LOD = 0.51) and pure schizophrenia (LOD = 0.00) this region did not
provide evidence for linkage. This region encompasses 49 genes, including several candidate genes for psychiatric disorders: potassium voltage-gated channel subfamily KQT member 3
(_KCNQ3_)24,28 coding for a voltage-gated potassium channel; adenylate cyclase 8 (_ADCY8_)24 involved in fear learning and memory, and long-term memory consolidation29; Ly6/Neurotoxin 1
(_LYNX1_)30 (located on 8q24.3 near D8S373) involved in the development of visual perception including binocular vision and motor learning during the critical period31,32,33,34; and
activity-regulated cytoskeleton-associated protein (_ARC_)29 connected to long-term potentiation and the consolidation of long-term memory. Our strongest evidence for LD conditional on
linkage (P = 0.0054, dominant model) was observed with markers located in chromosomal region 17p11. The region harbours hundreds of genes, however, to our knowledge, none of them have been
previously directly connected to phobias or other anxiety disorders. Several of the chromosomal regions we identified seemed mainly specific for schizophrenia. The most significant of those
regions were 5q31 and 1q32, discussed above for markers D5S2115 and D1S2817. Both of them have been previously associated with schizophrenia in numerous studies35,36,37,38. Furthermore,
several markers on the long arm of chromosome 13, localized between 13q31 and 13q33, showed evidence of linkage (LOD > 2.0) to both pure acrophobia and acrophobia with comorbid
schizophrenia, and also weak linkage to pure schizophrenia (maximum LOD = 1.26). Therefore, this region may harbour variants influencing susceptibility to both phenotypes. As specific phobia
subtypes have high comorbidity with other anxiety disorders4 and psychiatric disorders, such as schizophrenia39, the same genetic variants affecting brain function may be involved in both
diseases partially explaining the high comorbidity rates and overlapping biological symptoms. Intriguingly, several shared symptoms related to balance control and avoidance behaviour,
central in acrophobia, have been described in schizophrenia and depressive disorders. Most importantly, irregularities in eye movement and head coordination propensities associated with
difficulties in classification of relevant visual stimuli have been observed in schizophrenia patients40. Furthermore, the primary vestibular disfunction leading to chronic dizziness occurs
both in specific phobias and depressive disorders41. Lastly, acrophobia is a significant predictor of later depressive episodes, generalized anxiety and panic attacks42. Together, these
observations may be related to partially shared genetic predisposition to acrophobia and other comorbid psychiatric diseases, including schizophrenia. We recognise the hypothesis-generating
character of our study. It included a large number of genotyped markers (570) and analysed models (6). Although this serves to strengthen the study, it inevitably lead to multiple
statistical testing. However, as the tests carried out are not completely independent, the adjustment for multiple testing is not straightforward43. Consequently, avoidance of the type I
error might unintentionally inflate the type II error (false negative). Therefore, we decided to provide the LOD scores and their corresponding uncorrected nominal P values, when applicable,
and rely on the future studies of acrophobia in independent samples to assess whether the chromosomal regions identified in this study harbor true predisposing variants. In recent years,
single nucleotide polymorphisms (SNPs) have replaced microsatellite markers due to their lower genotyping costs. This technical advance has enabled genome-wide association studies (GWASs) in
which thousands of individuals are genotyped. Consequently, the usage of microsatellite markers, a class of short tandem repeats (STRs), has severely decreased. However, due to their highly
polymorphic nature microsatellites are still considered more informative than the diallelic SNPs44 in studying the genetic architecture of complex human diseases. Unlike in the
population-based association studies, the enrichment of the risk alleles in isolated populations and higher degree of LD may enable the identification of genetic risk factors in a smaller
sample and with smaller number of markers, as shown previously for neuropsychiatric diseases, such as schizophrenia and autism11,17,45. In conclusion, our findings suggest that the genetic
basis of acrophobia is highly complex, even in this genetic isolate, as we were not able to identify high-risk variants shared by several families. However, we identified several chromosomal
regions with suggestive evidence for linkage which could be investigated further in other acrophobia samples and meta-analyses of such datasets having an increased statistical power.
METHODS STUDY SAMPLE The sample was composed mostly of large multigenerational pedigrees with multiple affected individuals and at least one parent born in the isolate (Fig. 1). It comprised
57 families including 642 people, 75 of them affected with acrophobia and schizophrenia (11.7%) and 42 with pure acrophobia (6.5%). All pedigrees are part of a Finnish severe mental
disorders family collection of the National Institute for Health and Welfare17,18,46,47. We traced ancestors from Finnish Population Registries and performed a genealogical study in
accordance with published criteria47. Data from psychiatric case notes and treatment facilities concerning affected individuals and blood samples were collected between 1991 and 200248.
Structured Clinical Interviews for DSM-IV (SCID-I)49 followed by a diagnostic assessment performed in accordance with the Diagnostic and Statistical Manual of Mental Disorders, 4th edition
(DSM-IV) criteria50 and Operational Criteria Checklist (OPCRIT)44 were conducted at a later time, between 1998 and 200248. Following the interviews, assessment was undertaken independently
by two psychiatrists (T.Par. and R.A.). In case of disagreement, third psychiatrist (J.L.), reanalysed the case in order to gain consensus17. The sample used in the study is detailed in
Table 1. The ethical review board of the National Institute for Health and Welfare (THL), formerly the National Public Health Institute of Finland (KTL), approved all experimental protocols
in this study and the methods used were carried out in accordance with the approved guidelines. Informed consent was obtained from all participants before enrolment in the study. GENOTYPING
We analysed 575 autosomal microsatellite markers across the genome that had been genotyped as a part of earlier linkage-based gene mapping studies17,48. We had genotype information available
from all affected and 292 unaffected individuals. STATISTICAL ANALYSES We performed statistical analyses separately for acrophobia with comorbid schizophrenia and pure acrophobia sample to
discriminate between the possible shared and separate genetic signals associated with the phenotypes. We further followed with statistical analysis of the pure schizophrenia sample for the
most interesting results. We first checked all genotypes for Mendelian inconsistencies with PedCheck software51 and removed erroneous genotypes, which accounted for less than 0.08% of all
genotypes. We performed all analyses as affected-only, meaning that all phenotypes of healthy individuals and those with unknown affection status were treated as _unknown_, due to the fact
that unaffected family members were not systematically assessed52. PARAMETRIC TWO-POINT RECESSIVE AND DOMINANT LINKAGE ANALYSIS We performed two-point parametric linkage analysis with
statistical software package FASTLINK 4.1 P under a recessive and dominant mode of inheritance with, respectively, penetrance of 0.001% and 90%, disease allele frequency of 0.00001 and 0.01,
and phenocopy rates of 0 and 0.01. FASTLINK 4.1 P program was implemented in a helper program AUTOGSCAN53. PARAMETRIC AND NONPARAMETRIC MULTIPOINT LINKAGE ANALYSIS We carried out multipoint
parametric and nonparametric linkage analysis with SimWalk2 version 2.9654,55,56. For the nonparametric analysis, two statistics were examined: empirical NPL_ALL model, measuring if few
founder-alleles are overly represented in affected individuals, and NPL_PAIR, measuring the sum of conditional kinship coefficients for all affected pairs. In all analyses, pedigrees with
just one, non-related affected, were not analysed by the program, limiting the total number of pedigrees evaluated to five for pure acrophobia and 15 for acrophobia with comorbid
schizophrenia sample. Files for SimWalk2 software were prepared with free data conversion program Mega257. STAGE III: JOINT LINKAGE AND LINKAGE DISEQUILIBRIUM (LD) ANALYSIS We performed the
linkage disequilibrium conditional on linkage analysis with PSEUDOMARKER software package under default dominant and recessive models58,59. PSEUDOMARKER program uses a likelihood-based
method, which combines pedigrees of heterogeneous relationship structures, singletons and others, into one unified analysis. POWER SIMULATION The power of the analysed pedigrees to detect
linkage was estimated with SLINK simulation program and the replicates were analysed with ISIM analysis program implemented in SLINK package23. The analysis was performed under PSEUDOMARKER
recessive and dominant models with the assumption of complete linkage (θ = 0.0) between marker and trait locus, and with penetrance and disease allele frequency identical to those used in
the linkage analysis. We assumed the disease prevalence of five percent1 for acrophobia. The analysis was performed using 100 replications. ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE:
Misiewicz, Z. _et al_. A genome-wide screen for acrophobia susceptibility loci in a Finnish isolate. _Sci. Rep._ 6, 39345; doi: 10.1038/srep39345 (2016). PUBLISHER'S NOTE: Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. REFERENCES * Coelho, C. M. & Wallis, G. Deconstructing acrophobia:
physiological and psychological precursors to developing a fear of heights. Depress. Anxiety 27, 864–870 (2010). Article PubMed Google Scholar * Loken, E. K., Hettema, J. M., Aggen, S. H.
& Kendler, K. S. The structure of genetic and environmental risk factors for fears and phobias. Psychol. Med. 44, 2375–2384 (2014). Article CAS PubMed PubMed Central Google Scholar
* Van Houtem, C. M. et al. A review and meta-analysis of the heritability of specific phobia subtypes and corresponding fears. J. Anxiety Disord. 27, 379–388 (2013). Article CAS PubMed
Google Scholar * Curtis, G. C., Magee, W. J., Eaton, W. W., Wittchen, H. U. & Kessler, R. C. Specific fears and phobias. Epidemiology and classification. Br. J. Psychiatry 173, 212–217
(1998). Article CAS PubMed Google Scholar * Choy, Y., Fyer, A. J. & Lipsitz, J. D. Treatment of specific phobia in adults. Clin. Psychol. Rev. 27, 266–286 (2007). Article PubMed
Google Scholar * Boffino, C. C. et al. Fear of heights: cognitive performance and postural control. Eur. Arch. Psychiatry Clin. Neurosci. 259, 114–119 (2009). Article PubMed Google
Scholar * Hüweler, R., Kandil, F. I., Alpers, G. W. & Gerlach, A. L. The impact of visual flow stimulation on anxiety, dizziness, and body sway in individuals with and without fear of
heights. Behav. Res. Ther. 47, 345–352 (2009). Article PubMed Google Scholar * Brandt, T., Kugler, G., Schniepp, R., Wuehr, M. & Huppert, D. Acrophobia impairs visual exploration and
balance during standing and walking. Ann. N. Y. Acad. Sci. 1343, 37–48 (2015). Article ADS PubMed Google Scholar * Maurer, C., Mergner, T. & Peterka, R. J. Multisensory control of
human upright stance. Exp. Brain Res. 171, 231–250 (2006). Article CAS PubMed Google Scholar * Nakahara, H., Takemori, S. & Tsuruoka, H. Influence of height on the spatial
orientation and equilibrium of the body. Otolaryngol. Head. Neck. Surg. 123, 501–504 (2000). Article CAS PubMed Google Scholar * Peltonen, L., Palotie, A. & Lange, K. Use of
population isolates for mapping complex traits. Nat. Rev. Genet. 1, 182–190 (2000). Article CAS PubMed Google Scholar * Sullivan, P. F., Daly, M. J. & O’Donovan, M. Genetic
architectures of psychiatric disorders: the emerging picture and its implications. Nat. Rev. Genet. 13, 537–551 (2012). Article CAS PubMed PubMed Central Google Scholar * Ott, J., Wang,
J. & Leal, S. M. Genetic linkage analysis in the age of whole-genome sequencing. Nat. Rev. Genet. 16, 275–284 (2015). Article CAS PubMed PubMed Central Google Scholar * Stoll, G.
et al. Deletion of TOP3beta, a component of FMRP-containing mRNPs, contributes to neurodevelopmental disorders. Nat. Neurosci. 16, 1228–1237 (2013). Article CAS PubMed PubMed Central
Google Scholar * Hovatta, I. et al. Schizophrenia in the genetic isolate of Finland. Am. J. Med. Genet. 74, 353–360 (1997). Article CAS PubMed Google Scholar * Varilo, T. et al. Linkage
disequilibrium in isolated populations: Finland and a young sub-population of Kuusamo. Eur. J. Hum. Genet. 8, 604–612 (2000). Article CAS PubMed Google Scholar * Hovatta, I. et al. A
genomewide screen for schizophrenia genes in an isolated Finnish subpopulation, suggesting multiple susceptibility loci. Am. J. Hum. Genet. 65, 1114–1124 (1999). Article CAS PubMed PubMed
Central Google Scholar * Wedenoja, J. et al. Replication of linkage on chromosome 7q22 and association of the regional Reelin gene with working memory in schizophrenia families. Mol.
Psychiatry 13, 673–684 (2008). Article CAS PubMed Google Scholar * Ott, J. Methods of analysis and resources available for genetic trait mapping. J. Hered. 90, 68–70 (1999). Article CAS
PubMed Google Scholar * Kruglyak, L., Daly, M. J., Reeve-Daly, M. P. & Lander, E. S. Parametric and nonparametric linkage analysis: a unified multipoint approach. Am. J. Hum. Genet.
58, 1347–1363 (1996). PubMed PubMed Central CAS Google Scholar * Dudbridge, F. A survey of current software for linkage analysis. Hum. Genomics 1, 63–65 (2003). Article CAS PubMed
PubMed Central Google Scholar * Weeks, D. E., Ott, J. & Lathrop, G. M. SLINK: a general simulation program for linkage analysis. Am. J. Hum. Genet. 47, A204 (1990). Google Scholar *
Fanous, A. H. et al. Genome-wide association study of clinical dimensions of schizophrenia: polygenic effect on disorganized symptoms. Am. J. Psychiatry 169, 1309–1317 (2012). Article
PubMed PubMed Central Google Scholar * Avramopoulos, D. et al. Linkage of bipolar affective disorder on chromosome 8q24: follow-up and parametric analysis. Mol. Psychiatry 9, 191–196
(2004). Article CAS PubMed Google Scholar * Gonzalez, S. et al. A genome-wide linkage scan of bipolar disorder in Latino families identifies susceptibility loci at 8q24 and 14q32. Am. J.
Med. Genet. B. Neuropsychiatr. Genet. 165B, 479–491 (2014). Article PubMed CAS Google Scholar * Kaminsky, Z. et al. DNA methylation and expression of KCNQ3 in bipolar disorder. Bipolar
Disord. 17, 150–159 (2015). Article CAS PubMed Google Scholar * Holmans, P. A. et al. Genomewide linkage scan of schizophrenia in a large multicenter pedigree sample using single
nucleotide polymorphisms. Mol. Psychiatry 14, 786–795 (2009). Article CAS PubMed PubMed Central Google Scholar * Wang, H. S. et al. KCNQ2 and KCNQ3 potassium channel subunits: molecular
correlates of the M-channel. Science 282, 1890–1893 (1998). Article ADS CAS PubMed Google Scholar * Wolf, E. J. et al. A genome-wide association study of clinical symptoms of
dissociation in a trauma-exposed sample. Depress. Anxiety 31, 352–360 (2014). Article CAS PubMed PubMed Central Google Scholar * Hu, J. et al. A novel maternally inherited 8q24.3 and a
rare paternally inherited 14q23.3 CNVs in a family with neurodevelopmental disorders. Am. J. Med. Genet. A. 167, 1921–1926 (2015). Article CAS Google Scholar * Miwa, J. M. & Walz, A.
Enhancement in motor learning through genetic manipulation of the Lynx1 gene. PLoS One 7, e43302 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Morishita, H., Miwa, J.
M., Heintz, N. & Hensch, T. K. Lynx1, a cholinergic brake, limits plasticity in adult visual cortex. Science 330, 1238–1240 (2010). Article ADS CAS PubMed PubMed Central Google
Scholar * Hensch, T. K. Critical period regulation. Annu. Rev. Neurosci. 27, 549–579 (2004). Article CAS PubMed Google Scholar * Parker, A. J. Binocular depth perception and the
cerebral cortex. Nat. Rev. Neurosci. 8, 379–391 (2007). Article CAS PubMed Google Scholar * Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from
108 schizophrenia-associated genetic loci. Nature 511, 421–427 (2014). * McClay, J. L. et al. Genome-wide pharmacogenomic study of neurocognition as an indicator of antipsychotic treatment
response in schizophrenia. Neuropsychopharmacol. 36, 616–626 (2011). Article CAS Google Scholar * Betcheva, E. T. et al. Whole-genome-wide association study in the Bulgarian population
reveals HHAT as schizophrenia susceptibility gene. Psychiatr. Genet. 23, 11–19 (2013). Article CAS PubMed Google Scholar * International Schizophrenia Consortium et al. Common polygenic
variation contributes to risk of schizophrenia and bipolar disorder. Nature 460, 748–752 (2009). Article PubMed Central CAS Google Scholar * Huppert, J. D. & Smith, T. E. Anxiety and
schizophrenia: the interaction of subtypes of anxiety and psychotic symptoms. CNS Spectr. 10, 721–731 (2005). Article PubMed Google Scholar * Proudlock, F. A. & Gottlob, I.
Physiology and pathology of eye-head coordination. Prog. Retin. Eye Res. 26, 486–515 (2007). Article PubMed Google Scholar * Peluso, E. T., Quintana, M. I. & Gananca, F. F. Anxiety
and depressive disorders in elderly with chronic dizziness of vestibular origin. Braz J. Otorhinolaryngol. 82, 209–214 (2016). Article PubMed Google Scholar * Kapfhammer, H. P., Huppert,
D., Grill, E., Fitz, W. & Brandt, T. Visual height intolerance and acrophobia: clinical characteristics and comorbidity patterns. Eur. Arch. Psychiatry Clin. Neurosci. 265, 375–385
(2015). Article PubMed Google Scholar * Freimer, N. & Sabatti, C. The use of pedigree, sib-pair and association studies of common diseases for genetic mapping and epidemiology. Nat.
Genet. 36, 1045–1051 (2004). Article CAS PubMed Google Scholar * Schaid, D. J. et al. Comparison of Microsatellites Versus Single-Nucleotide Polymorphisms in a Genome Linkage Screen for
Prostate Cancer Susceptibility Loci. Am. J. Hum. Genet. 75, 948–965 (2004). Article CAS PubMed PubMed Central Google Scholar * Lauritsen, M. B. et al. A genome-wide search for alleles
and haplotypes associated with autism and related pervasive developmental disorders on the Faroe Islands. Mol. Psychiatry 11, 37–46 (2006). Article CAS PubMed Google Scholar * Paunio, T.
et al. Linkage analysis of schizophrenia controlling for population substructure. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 150B, 827–835 (2009). Article PubMed PubMed Central Google
Scholar * Varilo, T. et al. The age of human mutation: genealogical and linkage disequilibrium analysis of the CLN5 mutation in the Finnish population. Am. J. Hum. Genet. 58, 506–512
(1996). PubMed PubMed Central CAS Google Scholar * Arajärvi, R. et al. Psychosis among “healthy” siblings of schizophrenia patients. BMC Psychiatry 6, 6 (2006). Article PubMed PubMed
Central Google Scholar * First, M. B., Spitzer, R. L., Gibbon, M. & Williams, J. B. W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition
(SCID-I/P) (Biometrics Research, New York State Psychiatric Institute, 2002). * McGuffin, P., Farmer, A. & Harvey, I. A polydiagnostic application of operational criteria in studies of
psychotic illness. Development and reliability of the OPCRIT system. Arch. Gen. Psychiatry 48, 764–770 (1991). Article CAS PubMed Google Scholar * O’Connell, J. R. & Weeks, D. E.
PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am. J. Hum. Genet. 63, 259–266 (1998). Article PubMed PubMed Central Google Scholar * Wedenoja,
J. et al. Replication of association between working memory and Reelin, a potential modifier gene in schizophrenia. Biol. Psychiatry 67, 983–991 (2010). Article CAS PubMed Google Scholar
* Hiekkalinna, T., Terwilliger, J. D., Sammalisto, S., Peltonen, L. & Perola, M. AUTOGSCAN: powerful tools for automated genome-wide linkage and linkage disequilibrium analysis. Twin
Res. Hum. Genet. 8, 16–21 (2005). Article PubMed Google Scholar * Sobel, E. & Lange, K. Descent graphs in pedigree analysis: applications to haplotyping, location scores, and
marker-sharing statistics. Am. J. Hum. Genet. 58, 1323–1337 (1996). PubMed PubMed Central CAS Google Scholar * Sobel, E., Sengul, H. & Weeks, D. E. Multipoint estimation of
identity-by-descent probabilities at arbitrary positions among marker loci on general pedigrees. Hum. Hered. 52, 121–131 (2001). Article CAS PubMed Google Scholar * Lange, E. M. &
Lange, K. Powerful allele sharing statistics for nonparametric linkage analysis. Hum. Hered. 57, 49–58 (2004). Article PubMed Google Scholar * Mukhopadhyay, N., Almasy, L., Schroeder, M.,
Mulvihill, W. P. & Weeks, D. E. Mega2: data-handling for facilitating genetic linkage and association analyses. Bioinformatics 21, 2556–2557 (2005). Article CAS PubMed Google Scholar
* Hiekkalinna, T. et al. PSEUDOMARKER: a powerful program for joint linkage and/or linkage disequilibrium analysis on mixtures of singletons and related individuals. Hum. Hered. 71,
256–266 (2011). Article PubMed PubMed Central Google Scholar * Gertz, E. M. et al. PSEUDOMARKER 2.0: efficient computation of likelihoods using NOMAD. BMC Bioinformatics 15, 47 (2014).
Article PubMed PubMed Central CAS Google Scholar * Wigginton, J. E. & Abecasis, G. R. PEDSTATS: descriptive statistics, graphics and quality assessment for gene mapping data.
Bioinformatics 21, 3445–3447 (2005). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr. Ritva Arajärvi, M.D., Ph.D., for help with data collection, Marc
Llort Asens for assistance with formatting the figures, and Lea Urpa for proofreading the manuscript. This work has been supported by Doctoral Program Brain & Mind, University of
Helsinki (Z.M.), Academy of Finland (I.H., J.T.), and Sigrid Jusélius Foundation (I.H.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Biosciences, University of Helsinki,
Helsinki, Finland Zuzanna Misiewicz & Iiris Hovatta * Department of Health, National Institute for Health and Welfare, Helsinki, Finland Tero Hiekkalinna, Tiina Paunio, Joseph D.
Terwilliger, Timo Partonen & Iiris Hovatta * Institute for Molecular Medicine Finland, University of Helsinki, Helsinki, Finland Tero Hiekkalinna * Department of Psychiatry, University
of Helsinki and Helsinki University Hospital, Helsinki, Finland Tiina Paunio * Development of Work and Work Organizations, Finnish Institute of Occupational Health, Helsinki, Finland Tiina
Paunio * Department of Medical Genetics, University of Helsinki, Helsinki, Finland Teppo Varilo * Department of Psychiatry, Department of Genetics and Development, and Gertrude H. Sergievsky
Center, Columbia University, New York, NY, USA Joseph D. Terwilliger * Division of Medical Genetics, New York State Psychiatric Institute, New York, NY, USA Joseph D. Terwilliger Authors *
Zuzanna Misiewicz View author publications You can also search for this author inPubMed Google Scholar * Tero Hiekkalinna View author publications You can also search for this author
inPubMed Google Scholar * Tiina Paunio View author publications You can also search for this author inPubMed Google Scholar * Teppo Varilo View author publications You can also search for
this author inPubMed Google Scholar * Joseph D. Terwilliger View author publications You can also search for this author inPubMed Google Scholar * Timo Partonen View author publications You
can also search for this author inPubMed Google Scholar * Iiris Hovatta View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS T.Par. and I.H.
designed the study. T.Pau., T.V., T.Par. and I.H. collected the data. Z.M., T.H. and T.Pau. analysed the data. T.H. and J.D.T. assisted with statistical analyses. Z.M. and I.H. wrote the
manuscript. All authors read and commented on the manuscript, and approved the final version. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests.
RIGHTS AND PERMISSIONS This work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission
from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Misiewicz, Z., Hiekkalinna, T., Paunio, T. _et al._ A genome-wide screen for acrophobia susceptibility loci in a Finnish isolate. _Sci Rep_ 6, 39345 (2016).
https://doi.org/10.1038/srep39345 Download citation * Received: 17 February 2016 * Accepted: 16 November 2016 * Published: 20 December 2016 * DOI: https://doi.org/10.1038/srep39345 SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Pardon Our InterruptionPardon Our Interruption As you were browsing something about your browser made us think you were a bot. There are a few ...
Royal operators for the teeth: the hemetsABSTRACT The influence of the Huguenots upon the practice of dentistry in England has received so little attention that ...
Obama 'modestly optimistic' on cliff deal; 'he won,' says grahamPresident Obama went on the air to levy pressure on Congress Sunday as Senate leaders worked to negotiate a deal to aver...
A machine learning model for ranking candidate hla class i neoantigens based on known neoepitopes from multiple human tumor typesABSTRACT Tumor neoepitopes presented by major histocompatibility complex (MHC) class I are recognized by tumor-infiltrat...
Controlling Speech | NatureResearch in Verbal Behaviour and Some Neurophysiological Implications Edited by Kurt Salzinger and Susanne Salzinger. Pp...
Latests News
A genome-wide screen for acrophobia susceptibility loci in a finnish isolateABSTRACT Acrophobia, an abnormal fear of heights, is a specific phobia characterized as apprehension cued by the occurre...
Nutritional management in hospital setting during sars-cov-2 pandemic: a real-life experienceTO THE EDITOR: Coronaviruses are a family of pathogens that primarily affect the human respiratory tract. In December 20...
Diet quality and breast cancer incidence in the multiethnic cohortABSTRACT This study investigated the relation of diet quality indexes (DQI) with breast cancer incidence among women fro...
Why israel is attacking gaza’s al-shifa hospitalThe Gaza Strip’s largest and most advanced hospital, al-Shifa, is no longer functioning due to loss of power and water s...
Comparison of single-slice ct and dxa-derived measures of central adiposity in south african womenABSTRACT BACKGROUND Visceral adipose tissue (VAT) accumulation is a known risk factor for cardiometabolic diseases. Effi...