Genetic affinities of the jewish populations of india
Genetic affinities of the jewish populations of india"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Due to the lack of written records or inscription, the origin and affiliation of Indian Jewish populations with other world populations remain contentious. Previous genetic studies
have found evidence for a minor shared ancestry of Indian Jewish with Middle Eastern (Jewish) populations. However, these studies (relied on limited individuals), haven’t explored the
detailed temporal and spatial admixture process of Indian Jewish populations with the local Indian populations. Here, using large sample size with combination of high resolution biparental
(autosomal) and uniparental markers (Y chromosome and mitochondrial DNA), we reconstructed genetic history of Indian Jewish by investigating the patterns of genetic diversity. Consistent
with the previous observations, we detected minor Middle Eastern specific ancestry component among Indian Jewish communities, but virtually negligible in their local neighbouring Indian
populations. The temporal test of admixture suggested that the first admixture of migrant Jewish populations from Middle East to South India (Cochin) occurred during fifth century. Overall,
we concluded that the Jewish migration and admixture in India left a record in their genomes, which can link them to the ‘Jewish Diaspora’. SIMILAR CONTENT BEING VIEWED BY OTHERS UNIQUE
DEMOGRAPHIC HISTORY AND POPULATION SUBSTRUCTURE AMONG THE COORGS OF SOUTHERN INDIA Article Open access 05 May 2025 MIDDLE EASTERN GENETIC LEGACY IN THE PATERNAL AND MATERNAL GENE POOLS OF
CHUETAS Article Open access 08 December 2020 GENE POOL PRESERVATION ACROSS TIME AND SPACE IN MONGOLIAN-SPEAKING OIRATS Article Open access 11 April 2024 INTRODUCTION The Jewish communities
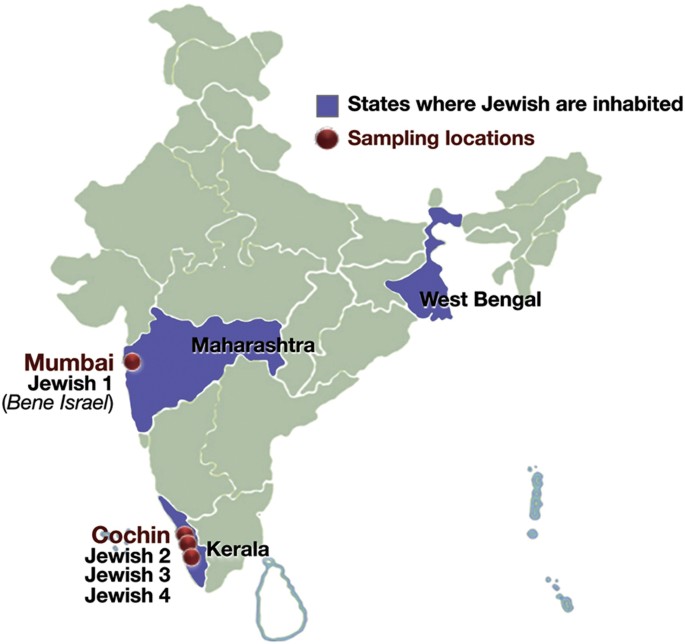
are distributed in most parts of the world1, however, of all the Jewish Diaspora community, Indian Jewish are one of the least known people2,3,4. There are three main distinct groups of
Jewish living in India (Fig. 1)- the Jewish of Cochin in Kerala, South India; the Bene Israel in Mumbai, West India and Baghdadi Jewish in Kolkata, East India4. Apart from these three
distinct groups, there is fourth group known as Paradesi Jewish, who are supposed to be migrated from Portugal and Spain during 15-16th Century and now integrated in to Cochin Jewish
community3. It was observed that each of these communities are socially linked to their neighbours than one another3. There are several legendry stories about their migrations to India
(Supplementary text), but because lack of written records and inscriptions, the origin and migrations of Indian Jewish remain moot2,3,4,5. The Cochin Jewish are considered as the oldest and
the first Jewish group appeared in India, who migrated during fifth or sixth century. Whereas the Bene Israel, the largest among all, were supposed to have migrated roughly one thousand
years ago to the Maharashtra coast2,3,5,6. The recent newcomers were Baghdadi Jewish who migrated to East India during English regime (19th Century)7. Yet, Indian Jewish are one of the early
offshoot of Jewish Diaspora, their complex history, the presence of multiple subgroups on various regions of India and persistence of ancestral social system, decorate them with several
unique characteristics2,3,4,6,7. Distinct to other Jewish communities8,9,10,11,12,13,14,15,16,17, Indian Jewish has not been the subject of extensive genetic study11,18,19,20. Nevertheless,
being a distant outlier, the Indian Jewish have added a unique dimension to the Jewish Diaspora11,18,19. Previous genetic studies have utilised low resolution and limited samples from Cochin
Jewish and Bene Israel, primarily as a reference population11,16,20. The first study on Cochin Jewish by utilising classical markers suggested their allele sharing both with Yemenite Jewish
and South Indian populations18. Analysis of haploid and genomewide data on Cochin and Bene Israel communities, reported their clustering with the neighbouring autochthonous
populations11,19. More specifically the mtDNA studies so far have identified largely South Asian autochthonous lineages among Jewish communities of India11,19, whereas the Y chromosomal
investigation have reported both Indian and Middle Eastern specific lineages11. Recently, the Y chromosome analysis of Bene Israel linked them to Levant20, whilst the R1a-M582 clade frequent
in Askenazi Jewish were absent among Indian Jewish6. All the above findings strongly indicate either high level of admixture of migrant Jewish with the local populations, or (and) religious
integration of the local indigenous populations. Moreover, the extent of gene flow from Middle East, associated with the spread of Judaism in the India is still largely unknown. Therefore,
to study the genetic signature and gain a better temporal and spatial understanding of their admixture with the native Indians, we present a detailed genetic characterization of the major
Indian Jewish communities (Cochin Jewish and Bene Israel), by the combination of high resolution haploid and diploid genetic markers analysis. RESULTS AUTOSOMAL SNP ANALYSIS For the ease of
understanding, we first classified different Indian Jewish groups present in our combined dataset (Supplementary Table 1). For autosomal analysis, we merged our data with the data coming
from nine different studies11,12,21,22,23,24,25,26,27. The combined data was representing the Indian Jewish populations from two distinct geographical regions of India (Fig. 1). We renamed
Bene Israel (Mumbai Jewish)11, coming from the West of India, to Jewish 1 and three South Indian Jewish groups11,12,23 as Jewish 2, Jewish 3 and Jewish 4 (Supplementary Table 1 and
Supplementary text). In the present study we have analysed samples from Cochin Jewish and Mumbai Jewish (Bene Israel) groups and referred them collectively as Indian Jewish (Fig. 1). To
measure the genetic differentiation of Indian Jewish in terms of inter and intra-regional as well as at population level, we first calculated _F_st (Fig. 2a and Supplementary Table 2). The
populationwise comparison analysis showed that the Indian Jewish share close affinity with their local South Asian neighbours, except for Indian Jewish 2 and Indian Jewish 4 (sampled from
the same Indian Jewish territory) (Fig. 2a and Supplementary Table 2). We further used principle component analysis (PCA) to capture the genetic variation of Indian Jewish along the two axes
covering the Eurasian landscape (Fig. 2b). Consistent with the _F_st analysis, the Indian Jewish were dispersed over the South Asian Indo-European-Dravidian cline. In agreement with the
previous study11, the model based clustering method ADMIXTURE identified, three ancestral components among Indian Jewish populations (Fig. 3 and Supplementary Fig. 1). Supporting the _F_st
and PCA results, the Indian ancestry was overwhelmingly dominant among Indian Jewish, however substantial traces of Middle Eastern ancestry (dark blue component) was also evident. The
relative proportion of Middle Eastern component among Indian Jewish was observed between the range of 3–20% (Table 1). Conversely, the Middle Eastern ancestry was largely negligible among
their neighbouring Indo-European and Dravidian populations. The spatial worldwide distribution of Middle Eastern (dark blue) component also showed elevation among Indian Jewish populations,
which cannot be explained by the isolation by distance scenario, where one can expect the Middle Eastern ancestry gradient in India along the West-East and North-South axes (Supplementary
Fig. 2). The Indian Jewish populations differ with each other in the context of harbouring the Near Eastern ancestry, as well as their placement in the PCA plot and _F_st variation (Fig. 2
and Supplementary Table 2). Therefore, regardless of the potential existence of a Near Eastern genetic link of Indian Jewish, substantial proportions of their genomes ancestry is shared with
Indian populations. To validate the Middle Eastern admixture in India Jewish populations, we have applied three (_f3_) and four (_f4_) population tests23,25,28. The outgroup _f3_ statistics
test showed significantly higher shared genetic drift of three Indian Jewish groups (Jewish 1, Jewish 2, Jewish 4), with the Middle Eastern, than their neighbouring local populations
(Supplementary Fig. 3 and 4 and Table 2). Jewish 3 showed lowest affinity to Middle East in comparison with the rest Indian Jewish groups, nevertheless it was significantly higher than most
of their neighbouring Dravidian populations (Table 2). The ANI (Ancestral North Indian) admixture proportion calculated from the _f4_ ancestry test was consistently higher among Indian
Jewish populations than their Indian neighbours (Supplementary Table 3). We also found higher number and longer length of segments of ROH among Indian Jewish 1 and Indian Jewish 4 groups,
whilst the Indian Jewish 2 had lowest ROH segments among all the Indian Jewish populations (Supplementary Fig. 5). To identify the population structure of Indian Jewish based on haplotypes
and recombination across the genome, we have further used ChromoPainter and performed fineSTRUCTURE analysis29. Consistent with the above analyses, all the Indian Jewish groups receive more
number/length of chunks with local South Asian populations than their parental Middle Eastern populations (Fig. 4 and Table 3). Nevertheless, the neighbouring Dravidian local populations
have significantly received lower number/length of Middle Eastern chunks in comparison with the Indian Jewish populations (two tailed p value <0.0001) (Table 3), which supports their
(Indian Jewish) affinity with the Middle Eastern populations. Among all the four Jewish groups the attraction with the Middle Eastern ancestry was in Jewish 1 > Jewish 2 > Jewish 4
> Jewish 3 order (Fig. 4 and Table 3). Notably, we didn’t find any significant difference of chunk number/length sharing of Indian Jewish with Middle Eastern Jewish _vs_. non-Jewish
populations (Table 3). We applied LD based Alder method30, to estimate the time of admixture between Indian Jewish and their neighbouring local Indian populations. We have used Yemeni Jewish
and Druze populations as Middle Eastern, while GIH, Paniya and Kurumba as local Indian surrogate populations (Supplementary Table 4). The Alder analysis of Indian Jewish 1 (by considering a
generation time of 30 years), has yielded ~1100 years as the time of admixture with GIH population (Table 4). For three groups of Kerala Jewish (Jewish 2, Jewish 3 and Jewish 4), the time
of admixture was oldest for Indian Jewish 4 (1590 years) whereas, Indian Jewish 3 showed a time of admixture of 1100 years. Surprisingly, the admixture time for Indian Jewish 2 was
relatively recent (480 years). MTDNA AND Y CHROMOSOMAL ANALYSIS To gain more insight about the sex specific Middle Eastern ancestry among Indian Jewish, we examined maternally inherited
mitochondrial DNA (mtDNA) and paternally inherited Y chromosome biallelic markers in large sample sizes (Tables 5, 6 and Supplementary Tables 5,6). Consistent with the autosomal analysis,
the mtDNA and Y chromosomal haplogroups were frequently South Asia specific (Tables 5 and 6). Apart from South Asian specific lineages (M2-6, M18, M30, M33, M35-37, M39-40, M64, N5, R5-6,
R8, R30 and U2), the Indian Jewish also share 4.6% East Eurasian and 21.1% West Eurasian maternal lineages (Table 5). Among the West Eurasian lineages, subclades of haplogroup H, HV1, J, K,
N1a and U5 were absent in their local neighbouring populations, which were otherwise predominant among Middle Eastern Jewish populations. (Table 5 and Supplementary Table 5). Interestingly,
subclade K1a1b1a is also detected in Indian Jewish 3, which is one of the major founder lineage of the Jewish diaspora8,17, but was not observed among local Indian populations. The PC
(Principle Component) analysis for mtDNA placed Indian Jewish 1 within the Indian cluster, whilst Jewish 3 and Jewish 4 were distracted away from the Indian core cluster because of higher
proportion of genetic lineages of Middle East origin (Fig. 5a). Similar to maternal haplogroup distribution, the paternal ancestry of Indian Jewish were also composed with some exclusive
Middle East specific haplogroups (E,G, J(xJ2) and I) (Table 6). However, at the present level of resolution, it is not possible to link other common lineages (e.g. haplogroups J2 and R1a),
which might have Middle Eastern roots (Table 6). The PC analysis was not well differentiated as in case of mtDNA, because of overwhelming presence of South Asian autochthonous lineages (Fig.
5b). Indian Jewish 3 and Jewish 4 clustered loosely to the South Asian knot, whereas Jewish 1 was located between Middle Eastern Jewish and South Asian populations (Fig. 5b). DISCUSSION
According to the oral traditions, the first Jewish migrant to India arrived in South India (Kerala state), followed by Bene Israel in West India (Maharashtra), whereas Paradesi Jewish and
Baghdadi Jewish in more recent times2,3,4,6,7. Genetic studies on classical markers as well as on uniparental and biparental markers have hitherto been found at some extant the Middle
Eastern Genetic affinity of Indian Jewish populations8,11,18,19. However, the detailed analysis on the information about their origin, admixture and migration is largely lacking. Therefore,
in this study by adding large number of Indian Jewish samples and groups, we reconstructed their history and showed that they have inherited their ancestry from Middle Eastern and Indian
populations. The population differentiation (_F_st) analysis suggested admixture of Indian Jewish with local Indian populations with some degree of relative isolation (Fig. 2a). The affinity
with the local South Asian populations advocated their excessive admixture with the local Indian populations. All the Indian Jewish are far apart from each other except for the Jewish 2 and
Jewish 4, who are closest to each other, likely because of sharing same geographical territory (Fig. 1). Our result on ADMIXTURE analysis agrees on the presence of South Asian and Middle
Eastern ancestral components among Indian Jewish populations (Fig. 3). Together with ADMIXTURE results, the PC analysis suggested overwhelming South Asia ancestry among Indian Jewish,
responsible for their placement over the South Asian cline (Fig. 2a). Although we could not segregate Middle Eastern ancestry from the ANI (Ancestral North India), nonetheless it is one of
the major denominator to elevate ANI ancestry among Indian Jewish populations, with respect to the local Indian populations (Supplementary Table 3). The Middle Eastern ancestry component
over the geographical landscape of India is only well visible among Indian Jewish populations, whereas among local Indian populations it is largely absent (Tables 1, 2 and Supplementary Fig.
2). This argues against any major geneflow from the migrant Jewish populations towards their local neighbouring populations. However, the marked difference of effective population sizes of
migrant Jewish and local Indian populations could easily dilute this signal in few generations. The haplotype oriented analysis (Fig. 4) was in congruent with the allele frequency based
analysis by showing minor (but significant) Middle Eastern signals in to the Indian Jewish populations. The time of admixture analysis has revealed that the first migration of Jewish in
India (Cochin Jewish) has happened more than 1500 years ago, followed by the Bene Israel (Mumbai Jewish) roughly 1000 years ago (Table 4 and Supplementary Table 4). Therefore, the molecular
time of admixture is largely consistent with the historical interpretations of Jewish migration in India3. Surprisingly, one of the South Indian Jewish group (Jewish 2) showed a recent time
of admixture (480 years), which we can’t rule out as a migration of Paradesi Jewish group from Spain and Portugal. However, this group was more distant and didn’t share any excess of
haplotypes with Sephardic Jewish than Middle Eastern populations (Figs. 2a, 4 and Supplementary Table 2). For the sex-specific markers (mtDNA and Y chromosome), the distribution of various
haplogroups are variable in each of the Indian Jewish group, not only when comparing with other Indian and world populations but also within the Indian Jewish (Tables 5, 6 and Supplementary
Tables 5, 6). In a recent study it was suggested that one of the major maternal founder of European Jewish (haplogroup K1a1b1a) lineage has likely assimilated in Europe17. Surprisingly, its
minor presence in Cochin Jewish (Jewish 3) group, who don’t show any recent European influx is intriguing (Table 5 and Supplementary Tables 5). The introgression of haplogroup K1a1b1a motif
to Jewish 3 may have been either transmitted through Paradesi Jewish or via Middle East during the initial settlement. The combined results of uniparental and biparental markers didn’t find
any Middle Eastern specific signal in to the local Indian populations, suggesting that the direction of geneflow or population assimilation was largely unidirectional i.e. from local Indians
to Indian Jewish. These results also invoke that the admixture of migrant Jewish with local Indian populations was not a continuous process. It was mainly driven by the religious conversion
of the local populations. In case of prolonged geneflow, the ROH segments of Indian Jewish should have been very similar to the neighbouring Indian populations, which is not the case here
(Supplementary Fig. 5). Moreover, the reduced diversity among Indian Jewish in all the genetics system testifies this scenario. Overall, our pooled analysis of genetic variation among
various groups of Indian Jewish populations, involving high-resolution sex-linked and autosomal markers, provides traces of Middle Eastern ancestry together with more likely unidirectional
geneflow/admixture from their contemporary Indian populations. However, the Indian Jewish carry overwhelmingly South Asian ancestry and the proportion of Middle Eastern genetic ancestry was
minor, regardless of the genetic system explored. Moreover, sharing of specific mtDNA and Y chromosomal haplogroups between all the studied Indian Jewish and their abscence among other local
Indian populations can be seen as a remnant of shared ancestry with Middle Eastern populations. The molecular data supports the model of migration of the Indian Jewish from Middle East,
followed by the religious conversion and admixture with the local South Asian populations. The extensive admixture and assimilation can be seen clearly in our autosomal analysis with a rapid
loss of Middle Eastern signals over the timeline. Nevertheless the rooted ancestry to their ancestral place can be testified because of a higher proportion of genetic lineages of Middle
East origin. MATERIAL AND METHODS SAMPLING About 5–10 ml blood samples with informed written consent were collected from 305 Cochin Jewish and 302 individuals from their seven neighbouring
local populations (Kurchian, Ulladan, Malayan, Adiyan, Paniya, Kuruman and Kattunaikan), belonging to southern state Kerala of India (Fig. 1). With detailed interview procedure we have
avoided individuals related minimum to three generations. This project was carried out in accordance with the approved guidelines and also permitted by the Institutional Ethical Committee of
the Centre for Cellular and Molecular Biology-CSIR, Hyderabad, India. All experimental protocols were also approved by the Committee of the Centre for Cellular and Molecular Biology-CSIR,
Hyderabad, India. SAMPLE GROUPING Since four different Jewish groups (coming from two geographical locations) have been analysed in this study, we have numbered them and used those numbers
throughout the manuscript. We named Jewish 1 to Bene Israel (Mumbai Jewish) coming from West part of India. Jewish 1 data is extracted from Behar _et al_.11. Rest of the three Jewish group
from South India are named as Jewish 2, Jewish 3 and Jewish 4. Jewish 2 is from Atzmon _et al_.12. Jewish 3 is our collection from Cochin (Kerala) and Jewish 4 is Cochin Jewish published in
Behar _et al_.11. The details of SNPs and populations have been mentioned in Supplementary Table 1. GENOTYPING We sequenced the Hypervariable segment I (HVS-I) of mtDNA by Sanger Sequencing
method, utilising 23F and 23R markers described by Reider _et al_.31 Variations were scored the against the r-CRS32 and Reconstructed Sapiens Reference Sequence (RSRS)33. We further
genotyped coding region diagnostic mutations and assigned their haplogroup based on combined information (Supplementary Tables 5 and 6). They were further confirmed by genotyping the coding
regions mutations published till date in PhyloTree build 1634. For Y chromosome analysis, we genotyped more than 40 biallelic markers published elsewhere35 to assign the haplogroups among
male individuals (Table 6). For all the markers, we have used direct Sanger sequencing method and assembled it with the reference to mark the variation. For autosomal genotyping, we used 15
Indian Jewish samples on Affymetrix (SNP 6.0) arrays by using standard protocols. We removed duplicate samples and filtered out individuals having less than 99% genotyping36. To include all
the Indian Jewish populations we have merged the data published in nine different studies11,12,21,22,23,24,25,26,27. The merged data has yielded 98189 SNPs after quality control which we
have used in our statistical analysis (Supplementary Table 1). MTDNA AND Y-CHROMOSOME ANALYSIS For mtDNA and Y chromosome analysis, we ran the Principle Component Analysis (PCA), using
POPSTR (kindly provided by H. Harpending), to infer the relationship of populations based on haplogroup frequencies. AUTOSOMAL ANALYSIS We have used different numbers of populations and
datasets for various analyses (Supplementary Table 1). A check for closely related individuals was carried out within each study population by calculating average IBS (identity by state)
scores for all pairs of individuals36. First, we sought to investigate the extent of population structure and admixture for Indian Jewish, embedded in their autosomal genomes. We used PLINK
1.0736, to filter the combined dataset to include only SNPs on the 22 autosomal chromosomes with minor allele frequency >1% and genotyping success >99%. Because background linkage
disequilibrium (LD) can affect both PCA37 and “Structure-like” (ADMIXTURE) analysis38, we thinned the dataset by removing one SNP of any pair in strong LD r2 > 0.4 in a window of 200 SNPs
(sliding the window by 25 SNPs at a time). The pruned dataset has yielded 75594 SNPs (Supplementary Table 1). We first calculated mean pairwise _F_st differences between different
population groups using the method of Cockerham and Weir39. Next we carried out PC analysis on pruned data using smartpca program (with default settings) of the EIGENSOFT package37to capture
genetic variability described by the first 5 PCs. The fraction of the total variation described by a PC is the ratio of its eigenvalue to the sum of all eigenvalues. In the final setting we
ran ‘Structure-like’ unsupervised ADMIXTURE, with a random seed number generator, on the LD pruned dataset twenty-five times at K = 2 to K = 12. Because the top values of the resulting
log-likelihood scores were stable (virtually identical) within the runs of each K from K = 2 to K = 7 we can with some confidence argue, that convergence at global maximum was reached. The
loglikelihood values showed seven ancestral populations as the best K value. Thus we omitted runs at K = 10 to K = 12 from further analysis. We have plotted the worldwide geographic
distribution of Middle Eastern specific ancestry by utilising geographic coordinates of the studied populations by using Surfer 8 of Golden Software (Golden Software Inc., Golden, Colorado),
following the Kriging procedure. From the result of PC analysis we have removed two outlier samples of Indian Jewish 3 in further population based analysis. The outgroup _f_328 statistics
(Table 2 and Supplementary Fig. 3) was calculated by taking Paniya population (which was attaining outlier position among Indian Dravidians), as an outgroup _f_3 = (Paniya; Druze/Yemen
Jewish, X), where X was Indian Jewish and their neighbouring local population. Subsequently, we have plotted the _f_3 results for shared drift with Middle East Jew outlier (Yemen Jewish)
_vs_. Indian outlier (South Munda) (Supplementary Fig. 4). The ANI (Ancestral North Indian) ancestry was calculated using _f4_ ancestry estimation test28 test (Supplementary Table 3). The
Runs of Homozygosity (ROH) was calculated using Plink (Supplementary Fig. 5). For ROH calculations, we applied 1000kb windows size, a minimum of 100 SNPs per window allowing one heterozygous
and five missing calls per window. For haplotype based analysis (fineSTRUCTURE)29, samples were phased with Beagle 3.3.240. A coancestry matrix was constructed using ChromoPainter29 with
the default settings. The mean chunk lengths of Indian Jewish with other populations was estimated. To estimate the admixture time we used the ALDER software30, between Indian Jewish and
local Indian populations. From Middle Eastern side we have used Yemen Jewish as well as Druze populations as parental for Indian Jewish, whereas from Indian side, we have used different
regional populations. For Jewish 1 (Bene Israel) we had only GIH (Gujarati) as surrogate, whilst for Jewish 2, Jewish 3 and Jewish 4 (all from South India), we have used Kurumba and Paniya
populations (Table 4 and Supplementary Table 4). ADDITIONAL INFORMATION HOW TO CITE THIS ARTICLE: Chaubey, G. _et al_. Genetic affinities of the Jewish populations of India. _Sci. Rep_. 6,
19166; doi: 10.1038/srep19166 (2016). CHANGE HISTORY * _ 23 MAY 2016 A correction has been published and is appended to both the HTML and PDF versions of this paper. The error has not been
fixed in the paper. _ REFERENCES * Ehrlich, M. A. _Encyclopedia of the Jewish Diaspora_ (ABC-CLIO, 2009). * Slapak, O. _The Jews of India_ (UPNE, 1995). * Katz, N. Who Are the Jews of India?
(Univ of California Press, 2000). * Israel, R. R. The Jews of India, (Canada Publisher: Mosaic Books, 2004). * Fernandes, E. The Last Jews Of Kerala (Granta Books, 2011). * Roland, J. G.
The Jewish Communities of India (Transaction Publishers, 1998). * V.ail, S. India’s Jewish heritage (Marg Publications, 2002). * Behar, D. M. et al. The matrilineal ancestry of Ashkenazi
Jewry: portrait of a recent founder event. Am J Hum Genet. 78, 487–497 (2006). Article CAS Google Scholar * Sutton, W. K. et al. Toward resolution of the debate regarding purported
crypto-Jews in a Spanish-American population: evidence from the Y chromosome. Ann Hum Biol. 33, 100–111 (2006). Article Google Scholar * Feder, J., Ovadia, O., Glaser, B. & Mishmar, D.
Ashkenazi Jewish mtDNA haplogroup distribution varies among distinct subpopulations: lessons of population substructure in a closed group. Eur J Hum Genet. 15, 498–500 (2007). Article CAS
Google Scholar * Behar, D. M. et al. The genome-wide structure of the Jewish people. Nature. 466, 238–242 (2010). Article CAS ADS Google Scholar * Atzmon, G. et al. Abraham’s children
in the genome era: major Jewish diaspora populations comprise distinct genetic clusters with shared Middle Eastern Ancestry. Am J Hum Genet. 86, 850–859 (2010). Article CAS Google Scholar
* Moorjani, P. et al. The history of african gene flow into southern europeans, levantines and jews. PLoS Genet. 7, e1001373 (2011). Article CAS Google Scholar * Campbell, C. L. et al.
North African Jewish and non-Jewish populations form distinctive, orthogonal clusters. Proc Natl Acad Sci USA. 109, 13865–13870 (2012). Article CAS ADS Google Scholar * Carmi, S. et al.
Sequencing an Ashkenazi reference panel supports population-targeted personal genomics and illuminates Jewish and European origins. Nature communications. 5, 4835 (2014). Article CAS ADS
Google Scholar * Metspalu, M. et al. Most of the extant mtDNA boundaries in south and southwest Asia were likely shaped during the initial settlement of Eurasia by anatomically modern
humans. BMC Genet. 5, 26 (2004). Article Google Scholar * Costa, M. D. et al. A substantial prehistoric European ancestry amongst Ashkenazi maternal lineages. Nature communications. 4,
2543 (2013). Article ADS Google Scholar * Cohen, T. et al. Genetic studies on Cochin Jews in Israel: 1. Population data, blood groups, isoenzymes and HLA determinants. Am J Med Genet. 6,
61–73 (1980). Article CAS Google Scholar * Behar, D. M. et al. Counting the founders: the matrilineal genetic ancestry of the Jewish Diaspora. PLoS ONE. 3, e2062 (2008). Article ADS
Google Scholar * Rootsi, S. et al. Phylogenetic applications of whole Y-chromosome sequences and the Near Eastern origin of Ashkenazi Levites. Nature communications. 4, 2928 (2013). Article
ADS Google Scholar * Li, J. Z. et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science. 319, 1100–1104 (2008). Article CAS ADS Google Scholar *
Yunusbayev, B. et al. The Caucasus as an Asymmetric Semipermeable Barrier to Ancient Human Migrations. Mol Biol Evol. 29, 359–365 (2012). Article CAS Google Scholar * Moorjani, P. et al.
Genetic evidence for recent population mixture in India. Am J Hum Genet. 93, 422–438 (2013). Article CAS Google Scholar * International HapMap 3 Consortium et al. Integrating common and
rare genetic variation in diverse human populations. Nature. 467, 52–58 (2010). * Reich, D., Thangaraj, K., Patterson, N., Price, A. L. & Singh, L. Reconstructing Indian population
history. Nature. 461, 489–494 (2009). Article CAS ADS Google Scholar * Chaubey, G. et al. Population Genetic Structure in Indian Austroasiatic speakers: The Role of Landscape Barriers
and Sex-specific Admixture. Mol Biol Evol. 28, 1013–1024 (2011). Article CAS Google Scholar * Metspalu, M. et al. Shared and unique components of human population structure and
genome-wide signals of positive selection in South Asia. Am J Hum Genet. 89, 731–744 (2011). Article CAS Google Scholar * Patterson, N. et al. Ancient admixture in human history.
Genetics. 192, 1065–1093 (2012). Article Google Scholar * Lawson, D. J., Hellenthal, G., Myers, S. & Falush, D. Inference of population structure using dense haplotype data. PLoS
Genet. 8, e1002453 (2012). Article CAS Google Scholar * Loh, P. R. et al. Inferring admixture histories of human populations using linkage disequilibrium. Genetics. 193, 1233–1254 (2013).
Article Google Scholar * Rieder, M. J., Taylor, S. L., Tobe, V. O. & Nickerson, D. A. Automating the identification of DNA variations using quality-based fluorescence re-sequencing:
analysis of the human mitochondrial genome. Nucleic Acids Res. 26, 967–73 (1998). Article CAS Google Scholar * Andrews, R. M. et al. Reanalysis and revision of the Cambridge reference
sequence for human mitochondrial DNA. Nat Genet. 23, 147 (1999). Article CAS Google Scholar * Behar, D. M. et al. A “Copernican” reassessment of the human mitochondrial DNA tree from its
root. The American Journal of Human Genetics. 90, 675–684 (2012). Article CAS Google Scholar * van Oven, M. & Kayser, M. Updated comprehensive phylogenetic tree of global human
mitochondrial DNA variation. Hum Mutat. 30, E386–E394 (2009). Article Google Scholar * Karafet, T. M. et al. New binary polymorphisms reshape and increase resolution of the human Y
chromosomal haplogroup tree. Genome Res. 18, 830–838 (2008). Article CAS Google Scholar * Purcell, S. et al. PLINK: a tool set for whole-genome association and population-based linkage
analyses. Am J Hum Genet. 81, 559–575 (2007). Article CAS Google Scholar * Patterson, N., Price, A. L. & Reich, D. Population structure and eigenanalysis. PLoS Genet. 2, e190 (2006).
Article Google Scholar * Alexander, D. H., Novembre, J. & Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009). Article CAS
Google Scholar * Cockerham, C. C. & Weir, B. S. Covariances of relatives stemming from a population undergoing mixed self and random mating. Biometrics. 40, 157–164 (1984). Article CAS
Google Scholar * Browning, S. R. & Browning, B. L. Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype
clustering. Am J Hum Genet. 81, 1084–1097 (2007). Article CAS Google Scholar * Thangaraj, K. et al. The influence of natural barriers in shaping the genetic structure of Maharashtra
populations. PloS one. 5, e15283 (2010). Article CAS ADS Google Scholar * Kivisild, T. et al. The genetic heritage of the earliest settlers persists both in Indian tribal and caste
populations. Am J Hum Genet. 72, 313–332 (2003). Article CAS Google Scholar * Sengupta, S. et al. Polarity and temporality of high-resolution y-chromosome distributions in India identify
both indigenous and exogenous expansions and reveal minor genetic influence of Central Asian pastoralists. Am J Hum Genet. 78, 202–221 (2006). Article CAS Google Scholar * Trivedi, R. et
al. Genetic Imprints of Pleistocene Origin of Indian Populations: A Comprehensive Phylogeographic Sketch of Indian Y-Chromosomes. Int J Hum Genet. 8, 97–118 (2008). Article Google Scholar
Download references ACKNOWLEDGEMENTS We are grateful to Prof. Richard Villems for his comments. We thank to Prof. Gill Atzmon for data sharing. We are grateful to all the volunteer donors
for their participation in this study. KT was supported by the Council of Scientific and Industrial Research, Government of India (GENESIS: BSC0121) and (BSC 0208). GC acknowledges the
financial support from European Union European Regional Development Fund through the Centre of Excellence in Genomics to Estonian Biocentre and University of Tartu by Tartu University grant
(PBGMR06901), by Estonian Personal grants PUT-766 and Estonian Institutional Research grants IUT24-1. LS was supported by Bhatnagar (CSIR) and JC Bose (DST) Fellowships, Government of India.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. AUTHOR INFORMATION Author notes * Chaubey Gyaneshwer, Singh
Manvendra, Rai Niraj and Kariappa Mini contributed equally to this work. AUTHORS AND AFFILIATIONS * CSIR-Centre for Cellular and Molecular Biology, Hyderabad, 500 007, India Gyaneshwer
Chaubey, Manvendra Singh, Niraj Rai, Mini Kariappa, Kamayani Singh, Ashish Singh, Deepankar Pratap Singh, Rakesh Tamang, Deepa Selvi Rani, Alla G. Reddy, Vijay Kumar Singh, Lalji Singh &
Kumarasamy Thangaraj * Department of Evolutionary Biology, Estonian Biocentre, Riia23b, Tartu, 51010, Estonia Gyaneshwer Chaubey * Department of Anatomy, Amala Institute of Medical
Sciences, Thrissur, 680555, India Mini Kariappa * Genome Foundation, Hyderabad, 500007, India Lalji Singh Authors * Gyaneshwer Chaubey View author publications You can also search for this
author inPubMed Google Scholar * Manvendra Singh View author publications You can also search for this author inPubMed Google Scholar * Niraj Rai View author publications You can also search
for this author inPubMed Google Scholar * Mini Kariappa View author publications You can also search for this author inPubMed Google Scholar * Kamayani Singh View author publications You
can also search for this author inPubMed Google Scholar * Ashish Singh View author publications You can also search for this author inPubMed Google Scholar * Deepankar Pratap Singh View
author publications You can also search for this author inPubMed Google Scholar * Rakesh Tamang View author publications You can also search for this author inPubMed Google Scholar * Deepa
Selvi Rani View author publications You can also search for this author inPubMed Google Scholar * Alla G. Reddy View author publications You can also search for this author inPubMed Google
Scholar * Vijay Kumar Singh View author publications You can also search for this author inPubMed Google Scholar * Lalji Singh View author publications You can also search for this author
inPubMed Google Scholar * Kumarasamy Thangaraj View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS M.K., L.S., K.T. and G.C. has conceived and
designed the study. M.S., N.R., M.K., K.S., A.S., D.P.S., R.T., D.S.R., A.G.R. and V.K.S. did mtDNA and Y chromosome genotyping and analysis. L.S. and K.T. has contributed the reagents.
M.S., N.R. and D.S.R. did autosomal data genotyping. G.C. and M.S. performed statistical analysis. G.C., M.S., N.R., M.K., V.K.S. and K.T. performed data interpretation and manuscript
writing. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS This
work is licensed under a Creative Commons Attribution 4.0 International License. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to
reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chaubey, G., Singh,
M., Rai, N. _et al._ Genetic affinities of the Jewish populations of India. _Sci Rep_ 6, 19166 (2016). https://doi.org/10.1038/srep19166 Download citation * Received: 08 July 2015 *
Accepted: 25 November 2015 * Published: 13 January 2016 * DOI: https://doi.org/10.1038/srep19166 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Video Author Angeline Boulley talks about her new book, 'Firekeeper’s Daughter' - ABC NewsABC NewsVideoLiveShowsShopStream onTrump at West Point LatestLatestNew Orleans jailbreak Memorial Day weekend Boeing dea...
Real estate news headlines - 9NewsBuyer coughs up $3.6 million for six car spots in Sydney CBDOne lucky buyer has just spent a jaw-dropping amount of mone...
Life quickly finds a way: the surprisingly swift end to evolution’s big bangThe Cambrian explosion more than 500 million years ago is often considered biology’s “big bang”. Virtually all the major...
The despicable history of imperial food and drink still casts a shadow todayTHE DESPICABLE HISTORY OF IMPERIAL FOOD & DRINK STILL CASTS A SHADOW TODAY STEPHEN COLEGRAVE DELVES INTO THE DARK CO...
Prince harry was phone-hacking victim, london court rulesBritain's Prince Harry, Duke of Sussex, leaves the High Court in London, Britain March 27, 2023. Henry Nicholls | R...
Latests News
Genetic affinities of the jewish populations of indiaABSTRACT Due to the lack of written records or inscription, the origin and affiliation of Indian Jewish populations with...
Guide to the classics news, research and analysis - the conversationMay 13, 2025 Christopher D Parkinson, _The University of Melbourne_ One of the world’s oldest cookbooks, De Re Coquinari...
Societies and Academies | NatureABSTRACT LONDON. Chemical Society, December 13, 1900.—Prof. Thorpe, President, in the chair.—Prof. H. A. Miers delivered...
Vellore election result 2024 live updates: dmk's d. M. Kathir anand has won this lok sabha seatVELLORE LOK SABHA ELECTION RESULT 2024 LIVE UPDATES: With the counting of votes for the 2024 Lok Sabha elections underwa...
J&k decision making under questionThe decision making of J&K Ranji trophy team management has come under severe criticism following another blunder in...