Dna-based biocomputing circuits and their biomedical applications
Dna-based biocomputing circuits and their biomedical applications"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT DNA-based biocomputing circuits are chemical reaction networks with information-processing capability that take advantage of DNA molecular interactions. The high parallelism and
intrinsic biocompatibility of DNA circuits allows liquid-phase computing for various biomedical applications. In this Review, we examine the design rules and implementation strategies of DNA
circuits, outlining the engineering and function of DNA computing units, including switches, logic gates, amplifiers and neurons. We further discuss the integration of these computing units
into DNA circuits by 3D free diffusion, surface-confined diffusion, localized diffusion using DNA nanostructures, and algorithmic assembly. Furthermore, we investigate how the temporal
dynamics of DNA circuits can be regulated and highlight their application in cellular imaging, biosensing and diagnostics, in conditional therapeutics, and for the rewiring of endogenous
gene networks. Finally, we discuss the challenges that remain to be addressed for the clinical translation DNA-based biocomputing, outlining key future research directions. KEY POINTS *
DNA-based computing circuits provide an efficient approach for sensing, processing and responding to biomolecular information. * DNA circuits exhibit a variety of computing functions that
allow the selection of design strategies based on application requirements. * DNA-based computing circuits have found wide applications in bioengineering. * Multiple translational challenges
remain to be addressed for the clinical translation of DNA-based computing. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 digital issues and online access to articles $119.00 per year only $9.92 per issue Learn more
Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS:
* Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS PROSPECTS AND CHALLENGES OF DYNAMIC DNA NANOSTRUCTURES IN
BIOMEDICAL APPLICATIONS Article Open access 23 May 2022 DNA-BASED PROGRAMMABLE GATE ARRAYS FOR GENERAL-PURPOSE DNA COMPUTING Article 13 September 2023 DYNAMIC REGULATION OF DNA
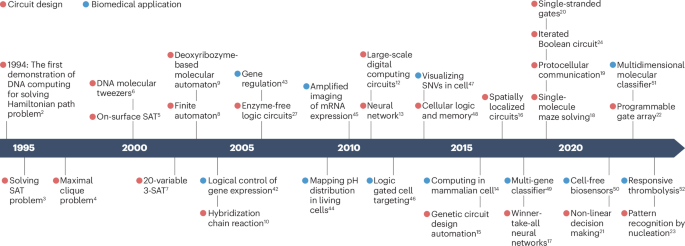
NANOSTRUCTURES BY NONCANONICAL NUCLEIC ACIDS Article Open access 30 April 2021 REFERENCES * Yang, S. et al. DNA as a universal chemical substrate for computing and data storage. _Nat. Rev.
Chem._ 8, 179–194 (2024). Article Google Scholar * Adleman, L. M. Molecular computation of solutions to combinatorial problems. _Science_ 266, 1021–1024 (1994). THIS ARTICLE REPORTS THE
FIRST DEMONSTRATION OF DNA COMPUTING. Article Google Scholar * Lipton, R. J. DNA solution of hard computational problems. _Science_ 268, 542–545 (1995). Article Google Scholar * Ouyang,
Q., Kaplan, P. D., Liu, S. & Libchaber, A. DNA solution of the maximal clique problem. _Science_ 278, 446–449 (1997). Article Google Scholar * Liu, Q. et al. DNA computing on surfaces.
_Nature_ 403, 175–179 (2000). Article Google Scholar * Yurke, B., Turberfield, A. J., Mills, A. P., Simmel, F. C. & Neumann, J. L. A DNA-fuelled molecular machine made of DNA.
_Nature_ 406, 605–608 (2000). Article Google Scholar * Braich, R. S., Chelyapov, N., Johnson, C., Rothemund, P. W. K. & Adleman, L. Solution of a 20-variable 3-SAT problem on a DNA
computer. _Science_ 296, 499–502 (2002). Article Google Scholar * Benenson, Y., Adar, R., Paz-Elizur, T., Livneh, Z. & Shapiro, E. DNA molecule provides a computing machine with both
data and fuel. _Proc. Natl Acad. Sci. USA_ 100, 2191–2196 (2003). Article Google Scholar * Stojanovic, M. N. & Stefanovic, D. A deoxyribozyme-based molecular automaton. _Nat.
Biotechnol._ 21, 1069–1074 (2003). Article Google Scholar * Dirks, R. M. & Pierce, N. A. Triggered amplification by hybridization chain reaction. _Proc. Natl Acad. Sci. USA_ 101,
15275–15278 (2004). Article Google Scholar * Qian, L. & Winfree, E. A simple DNA gate motif for synthesizing large-scale circuits. _J. R. Soc. Interface_ 8, 1281–1297 (2011). Article
Google Scholar * Qian, L. & Winfree, E. Scaling up digital circuit computation with DNA strand displacement cascades. _Science_ 332, 1196–1201 (2011). THIS ARTICLE INTRODUCES A SIMPLE
AND MODULAR DESIGN SCHEME BY ENGINEERING REVERSIBLE STRAND DISPLACEMENT FOR LARGE-SCALE DIGITAL COMPUTING. Article Google Scholar * Qian, L., Winfree, E. & Bruck, J. Neural network
computation with DNA strand displacement cascades. _Nature_ 475, 368–372 (2011). Article Google Scholar * Groves, B. et al. Computing in mammalian cells with nucleic acid strand exchange.
_Nat. Nanotechnol._ 11, 287–294 (2016). Article Google Scholar * Nielsen, A. A. et al. Genetic circuit design automation. _Science_ 352, aac7341 (2016). THIS ARTICLE REPORTS A HARDWARE
DESCRIPTION LANGUAGE FOR THE AUTOMATED DESIGN OF LIVE-CELL GENE CIRCUITS. Article Google Scholar * Chatterjee, G., Dalchau, N., Muscat, R. A., Phillips, A. & Seelig, G. A spatially
localized architecture for fast and modular DNA computing. _Nat. Nanotechnol._ 12, 920–927 (2017). THIS ARTICLE REPORTS THAT THE SPATIAL ARRANGEMENT OF DNA MOLECULES ON DNA ORIGAMI ENABLES
RAPID AND MODULAR DNA COMPUTING. Article Google Scholar * Cherry, K. M. & Qian, L. Scaling up molecular pattern recognition with DNA-based winner-take-all neural networks. _Nature_
559, 370–376 (2018). Article Google Scholar * Chao, J. et al. Solving mazes with single-molecule DNA navigators. _Nat. Mater._ 18, 273–279 (2019). Article Google Scholar * Joesaar, A. et
al. DNA-based communication in populations of synthetic protocells. _Nat. Nanotechnol._ 14, 369–378 (2019). Article Google Scholar * Song, T. et al. Fast and compact DNA logic circuits
based on single-stranded gates using strand-displacing polymerase. _Nat. Nanotechnol._ 14, 1075–1081 (2019). Article Google Scholar * Okumura, S. et al. Nonlinear decision-making with
enzymatic neural networks. _Nature_ 610, 496–501 (2022). Article Google Scholar * Lv, H. et al. DNA-based programmable gate arrays for general-purpose DNA computing. _Nature_ 622, 292–300
(2023). THIS ARTICLE INTRODUCES A GATE ARRAY SCHEME FOR SCALABLE AND PROGRAMMABLE DNA COMPUTING. Article Google Scholar * Evans, C. G., O’Brien, J., Winfree, E. & Murugan, A. Pattern
recognition in the nucleation kinetics of non-equilibrium self-assembly. _Nature_ 625, 500–507 (2024). Article Google Scholar * Woods, D. et al. Diverse and robust molecular algorithms
using reprogrammable DNA self-assembly. _Nature_ 567, 366–372 (2019). Article Google Scholar * Chen, Y. J. et al. Programmable chemical controllers made from DNA. _Nat. Nanotechnol._ 8,
755–762 (2013). Article Google Scholar * Stojanovic, M. N., Mitchell, T. E. & Stefanovic, D. Deoxyribozyme-based logic gates. _J. Am. Chem. Soc._ 124, 3555–3561 (2002). Article Google
Scholar * Seelig, G., Soloveichik, D., Zhang, D. Y. & Winfree, E. Enzyme-free nucleic acid logic circuits. _Science_ 314, 1585–1588 (2006). THIS ARTICLE REPORTS THE CONSTRUCTION OF
LOGIC CIRCUITS USING STRAND DISPLACEMENT. Article Google Scholar * Xiong, X. et al. Molecular convolutional neural networks with DNA regulatory circuits. _Nat. Mach. Intell._ 4, 625–635
(2022). Article Google Scholar * Thubagere, A. J. et al. A cargo-sorting DNA robot. _Science_ 357, eaan6558 (2017). Article Google Scholar * Davidson, E. A. & Ellington, A. D.
Synthetic RNA circuits. _Nat. Chem. Biol._ 3, 23–28 (2007). Article Google Scholar * Chen, Y. Y., Jensen, M. C. & Smolke, C. D. Genetic control of mammalian T-cell proliferation with
synthetic RNA regulatory systems. _Proc. Natl Acad. Sci. USA_ 107, 8531–8536 (2010). Article Google Scholar * Green, A. A., Silver, P. A., Collins, J. J. & Yin, P. Toehold switches:
de-novo-designed regulators of gene expression. _Cell_ 159, 925–939 (2014). Article Google Scholar * Kim, J. et al. De novo-designed translation-repressing riboregulators for multi-input
cellular logic. _Nat. Chem. Biol._ 15, 1173–1182 (2019). Article Google Scholar * Schaffter, S. W. & Strychalski, E. A. Cotranscriptionally encoded RNA strand displacement circuits.
_Sci. Adv._ 8, eabl4354 (2022). Article Google Scholar * Hong, F. et al. Precise and programmable detection of mutations using ultraspecific riboregulators. _Cell_ 180, 1018–1032.e16
(2020). Article Google Scholar * Lajoie, M. J. et al. Designed protein logic to target cells with precise combinations of surface antigens. _Science_ 369, 1637–1643 (2020). Article Google
Scholar * Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. _Nature_ 596, 583–589 (2021). Article Google Scholar * Cabello-Garcia, J., Bae, W., Stan, G. B.
V. & Ouldridge, T. E. Handhold-mediated strand displacement: a nucleic acid based mechanism for generating far-from-equilibrium assemblies through templated reactions. _ACS Nano_ 15,
3272–3283 (2021). Article Google Scholar * Zhang, D. Y. & Winfree, E. Control of DNA strand displacement kinetics using toehold exchange. _J. Am. Chem. Soc._ 131, 17303–17314 (2009).
Article Google Scholar * Srinivas, N. et al. On the biophysics and kinetics of toehold-mediated DNA strand displacement. _Nucleic Acids Res._ 41, 10641–10658 (2013). Article Google
Scholar * Benenson, Y. et al. Programmable and autonomous computing machine made of biomolecules. _Nature_ 414, 430–434 (2001). Article Google Scholar * Benenson, Y., Gil, B., Ben-Dor,
U., Adar, R. & Shapiro, E. An autonomous molecular computer for logical control of gene expression. _Nature_ 429, 423–429 (2004). Article Google Scholar * Rosi, N. L. et al.
Oligonucleotide-modified gold nanoparticles for intracellular gene regulation. _Science_ 312, 1027–1030 (2006). Article Google Scholar * Modi, S. et al. A DNA nanomachine that maps spatial
and temporal pH changes inside living cells. _Nat. Nanotechnol._ 4, 325–330 (2009). Article Google Scholar * Choi, H. M. T. et al. Programmable amplification for multiplexed imaging of
mRNA expression. _Nat. Biotechnol._ 28, 1208–1212 (2010). Article Google Scholar * Douglas, S. M., Bachelet, I. & Church, G. M. A logic-gated nanorobot for targeted transport of
molecular payloads. _Science_ 335, 831–834 (2012). THIS ARTICLE REPORTS DNA CIRCUIT-CONTROLLED TARGETED DRUG RELEASE. Article Google Scholar * Levesque, M. J., Ginart, P., Wei, Y. &
Raj, A. Visualizing SNVs to quantify allele-specific expression in single cells. _Nat. Methods_ 10, 865–867 (2013). Article Google Scholar * Siuti, P., Yazbek, J. & Lu, T. K. Synthetic
circuits integrating logic and memory in living cells. _Nat. Biotechnol._ 31, 448–452 (2013). Article Google Scholar * Lopez, R., Wang, R. & Seelig, G. A molecular multi-gene
classifier for disease diagnostics. _Nat. Chem._ 10, 746–754 (2018). THIS ARTICLE REPORTS A MACHINE LEARNING-ASSISTED MOLECULAR CLASSIFIER FOR GENE PROFILE ANALYSIS. Article Google Scholar
* Jung, J. K., Archuleta, C. M., Alam, K. K. & Lucks, J. B. Programming cell-free biosensors with DNA strand displacement circuits. _Nat. Chem. Biol._ 18, 385–393 (2022). Article
Google Scholar * Yin, F. et al. DNA-framework-based multidimensional molecular classifiers for cancer diagnosis. _Nat. Nanotechnol._ 18, 677–686 (2023). THIS ARTICLE REPORTS A
MULTIDIMENSIONAL MOLECULAR CLASSIFIER THAT CAN PROCESS RNA, PROTEIN AND SMALL-MOLECULE INFORMATION FOR ACCURATE CANCER DIAGNOSIS. Article Google Scholar * Yin, J. et al. An intelligent DNA
nanodevice for precision thrombolysis. _Nat. Mater._ 23, 854–862 (2024). Article Google Scholar * Kang, D. et al. DNA biomolecular-electronic encoder and decoder devices constructed by
multiplex biosensors. _NPG Asia Mater._ 4, e1 (2012). Article Google Scholar * Zhang, C. et al. Cancer diagnosis with DNA molecular computation. _Nat. Nanotechnol._ 15, 709–715 (2020).
Article Google Scholar * Rudchenko, M. et al. Autonomous molecular cascades for evaluation of cell surfaces. _Nat. Nanotechnol._ 8, 580–586 (2013). Article Google Scholar * Gong, X. et
al. A smart multiantenna gene theranostic system based on the programmed assembly of hypoxia-related siRNAs. _Nat. Commun._ 12, 3953 (2021). Article Google Scholar * Wang, F., Liu, X. Q.
& Willner, I. DNA switches: from principles to applications. _Angew. Chem. Int. Ed._ 54, 1098–1129 (2015). Article Google Scholar * Wang, F. et al. Implementing digital computing with
DNA-based switching circuits. _Nat. Commun._ 11, 121 (2020). Article Google Scholar * Machinek, R. R. F., Ouldridge, T. E., Haley, N. E. C., Bath, J. & Turberfield, A. J. Programmable
energy landscapes for kinetic control of DNA strand displacement. _Nat. Commun._ 5, 5324 (2014). Article Google Scholar * Pei, H. et al. Reconfigurable three-dimensional DNA nanostructures
for the construction of intracellular logic sensors. _Angew. Chem. Int. Ed._ 51, 9020–9024 (2012). Article Google Scholar * Zhu, J., Zhang, L., Li, T., Dong, S. & Wang, E. Enzyme-free
unlabeled DNA logic circuits based on toehold-mediated strand displacement and split G-quadruplex enhanced fluorescence. _Adv. Mater._ 25, 2440–2444 (2013). Article Google Scholar *
Elbaz, J. et al. DNA computing circuits using libraries of DNAzyme subunits. _Nat. Nanotechnol._ 5, 417–422 (2010). Article Google Scholar * Orbach, R. et al. A full-adder based on
reconfigurable DNA-hairpin inputs and DNAzyme computing modules. _Chem. Sci._ 5, 3381–3387 (2014). Article Google Scholar * Prokup, A. & Deiters, A. Interfacing synthetic DNA logic
operations with protein outputs. _Angew. Chem. Int. Ed._ 53, 13192–13195 (2014). Article Google Scholar * Yao, G. B. et al. Meta-DNA structures. _Nat. Chem._ 12, 1067–1075 (2020). Article
Google Scholar * Qi, M. Y. et al. Meta-DNA strand displacement for sub-micron-scale autonomous reconfiguration. _J. Am. Chem. Soc._ 145, 16812–16820 (2023). Article Google Scholar *
Amir, Y. et al. Universal computing by DNA origami robots in a living animal. _Nat. Nanotechnol._ 9, 353–357 (2014). Article Google Scholar * Kishi, J. Y., Schaus, T. E., Gopalkrishnan,
N., Xuan, F. & Yin, P. Programmable autonomous synthesis of single-stranded DNA. _Nat. Chem._ 10, 155–164 (2018). Article Google Scholar * Ogasawara, S., Kyoi, Y. & Fujimoto, K.
Nonenzymatic parallel DNA logic circuits. _ChemBioChem_ 8, 1520–1525 (2007). Article Google Scholar * You, M. X., Zhu, G. Z., Chen, T., Donovan, M. J. & Tan, W. H. Programmable and
multiparameter DNA-based logic platform for cancer recognition and targeted therapy. _J. Am. Chem. Soc._ 137, 667–674 (2015). Article Google Scholar * Zhang, J. Y. & Liu, C. C.
CRISPR-powered DNA computing and digital display. _ACS Synth. Biol._ 10, 3148–3157 (2021). Article Google Scholar * Guo, Y. J. et al. pH-controlled detachable DNA circuitry and its
application in resettable self-assembly of spherical nucleic acids. _ACS Nano_ 14, 8317–8327 (2020). Article Google Scholar * Brophy, J. A. & Voigt, C. A. Principles of genetic circuit
design. _Nat. Methods_ 11, 508–520 (2014). Article Google Scholar * Tamsir, A., Tabor, J. J. & Voigt, C. A. Robust multicellular computing using genetically encoded NOR gates and
chemical ‘wires’. _Nature_ 469, 212–215 (2011). Article Google Scholar * Gaber, R. et al. Designable DNA-binding domains enable construction of logic circuits in mammalian cells. _Nat.
Chem. Biol._ 10, 203–208 (2014). Article Google Scholar * Gines, G. et al. Isothermal digital detection of microRNAs using background-free molecular circuit. _Sci. Adv._ 6, eaay5952
(2020). Article Google Scholar * Padirac, A., Fujii, T. & Rondelez, Y. Bottom-up construction of in vitro switchable memories. _Proc. Natl Acad. Sci. USA_ 109, E3212–E3220 (2012).
Article Google Scholar * Han, D. et al. A cascade reaction network mimicking the basic functional steps of adaptive immune response. _Nat. Chem._ 7, 835–841 (2015). Article Google Scholar
* Zhang, D. Y., Turberfield, A. J., Yurke, B. & Winfree, E. Engineering entropy-driven reactions and networks catalyzed by DNA. _Science_ 318, 1121–1125 (2007). Article Google Scholar
* Chen, C. Y. et al. Single-cell whole-genome analyses by Linear Amplification via Transposon Insertion (LIANTI). _Science_ 356, 189–194 (2017). Article Google Scholar * Kim, S. et al.
Nanoparticle-based computing architecture for nanoparticle neural networks. _Sci. Adv._ 6, eabb3348 (2020). Article Google Scholar * Wang, B., Thachuk, C., Ellington, A. D., Winfree, E.
& Soloveichik, D. Effective design principles for leakless strand displacement systems. _Proc. Natl Acad. Sci. USA_ 115, E12182–E12191 (2018). Article Google Scholar * Nikitin, M. P.
Non-complementary strand commutation as a fundamental alternative for information processing by DNA and gene regulation. _Nat. Chem._ 15, 70–82 (2023). Article Google Scholar * Seo, J.,
Kim, S., Park, H. H., Choi, D. Y. & Nam, J. M. Nano-bio-computing lipid nanotablet. _Sci. Adv._ 5, eaau2124 (2019). Article Google Scholar * You, M. X. et al. DNA probes for monitoring
dynamic and transient molecular encounters on live cell membranes. _Nat. Nanotechnol._ 12, 453–459 (2017). Article Google Scholar * Zhang, Y. et al. Activating a DNA nanomachine via
computation across cancer cell membranes for precise therapy of solid tumors. _J. Am. Chem. Soc._ 143, 15233–15242 (2021). Article Google Scholar * Dey, S. et al. DNA origami. _Nat. Rev.
Methods Primers_ 1, 13 (2021). Article Google Scholar * Mendoza, O., Mergny, J. L., Aimé, J. P. & Elezgaray, J. G-quadruplexes light up localized DNA circuits. _Nano Lett._ 16, 624–628
(2016). Article Google Scholar * Masubuchi, T. et al. Construction of integrated gene logic-chip. _Nat. Nanotechnol._ 13, 933–940 (2018). Article Google Scholar * Teichmann, M.,
Kopperger, E. & Simmel, F. C. Robustness of localized DNA strand displacement cascades. _ACS Nano_ 8, 8487–8496 (2014). Article Google Scholar * Liu, L. et al. A localized DNA
finite-state machine with temporal resolution. _Sci. Adv._ 8, eabm9530 (2022). Article Google Scholar * Ji, W. Encoding signal propagation on topology-programmed DNA origami. _Nat. Chem._
16, 1408–1417 (2024). Article Google Scholar * Zhang, Y. A. et al. Prime factorization via localized tile assembly in a DNA origami framework. _Sci. Adv._ 9, eadf8263 (2023). Article
Google Scholar * Tandon, A. et al. Demonstration of arithmetic calculations by DNA tile-based algorithmic self-assembly. _ACS Nano_ 14, 5260–5267 (2020). Article Google Scholar * Mao, C.
D., LaBean, T. H., Reif, J. H. & Seeman, N. C. Logical computation using algorithmic self-assembly of DNA triple-crossover molecules. _Nature_ 407, 493–496 (2000). Article Google
Scholar * Barish, R. D., Schulman, R., Rothemund, P. W. & Winfree, E. An information-bearing seed for nucleating algorithmic self-assembly. _Proc. Natl Acad. Sci. USA_ 106, 6054–6059
(2009). Article Google Scholar * Tian, Y. et al. Ordered three-dimensional nanomaterials using DNA-prescribed and valence-controlled material voxels. _Nat. Mater._ 19, 789–796 (2020).
Article Google Scholar * Meng, W. et al. An autonomous molecular assembler for programmable chemical synthesis. _Nat. Chem._ 8, 542–548 (2016). Article Google Scholar * Zadeh, J. N. et
al. NUPACK: analysis and design of nucleic acid systems. _J. Comput. Chem._ 32, 170–173 (2011). Article Google Scholar * Lakin, M. R., Youssef, S., Polo, F., Emmott, S. & Phillips, A.
Visual DSD: a design and analysis tool for DNA strand displacement systems. _Bioinformatics_ 27, 3211–3213 (2011). Article Google Scholar * Bucci, J., Irmisch, P., Del Grosso, E., Seidel,
R. & Ricci, F. Orthogonal enzyme-driven timers for DNA strand displacement reactions. _J. Am. Chem. Soc._ 144, 19791–19798 (2022). Article Google Scholar * Lapteva, A. P., Sarraf, N.
& Qian, L. L. DNA strand-displacement temporal logic circuits. _J. Am. Chem. Soc._ 144, 12443–12449 (2022). Article Google Scholar * Fern, J. et al. DNA strand-displacement timer
circuits. _ACS Synth. Biol._ 6, 190–193 (2017). Article Google Scholar * Haydell, M. W., Centola, M., Adam, V., Valero, J. & Famulok, M. Temporal and reversible control of a DNAzyme by
orthogonal photoswitching. _J. Am. Chem. Soc._ 140, 16868–16872 (2018). Article Google Scholar * Schaffter, S. W. et al. Standardized excitable elements for scalable engineering of
far-from-equilibrium chemical networks. _Nat. Chem._ 14, 1224–1232 (2022). Article Google Scholar * Haley, N. E. C. et al. Design of hidden thermodynamic driving for non-equilibrium
systems via mismatch elimination during DNA strand displacement. _Nat. Commun._ 11, 2562 (2020). Article Google Scholar * Bucci, J., Irmisch, P., Del Grosso, E., Seidel, R. & Ricci, F.
Timed pulses in DNA strand displacement reactions. _J. Am. Chem. Soc._ 145, 20968–20974 (2023). Article Google Scholar * Schaffter, S. W. & Schulman, R. Building in vitro
transcriptional regulatory networks by successively integrating multiple functional circuit modules. _Nat. Chem._ 11, 829–838 (2019). Article Google Scholar * Srinivas, N., Parkin, J.,
Seelig, G., Winfree, E. & Soloveiehile, D. Enzyme-free nucleic acid dynamical systems. _Science_ 358, eaal2052 (2017). Article Google Scholar * Genot, A. J. et al. High-resolution
mapping of bifurcations in nonlinear biochemical circuits. _Nat. Chem._ 8, 760–767 (2016). Article MathSciNet Google Scholar * Weitz, M. et al. Diversity in the dynamical behaviour of a
compartmentalized programmable biochemical oscillator. _Nat. Chem._ 6, 295–302 (2014). Article Google Scholar * Potvin-Trottier, L., Lord, N. D., Vinnicombe, G. & Paulsson, J.
Synchronous long-term oscillations in a synthetic gene circuit. _Nature_ 538, 514–517 (2016). Article Google Scholar * Kim, J. & Winfree, E. Synthetic in vitro transcriptional
oscillators. _Mol. Syst. Biol._ 7, 465 (2011). Article Google Scholar * Fujii, T. & Rondelez, Y. Predator–prey molecular ecosystems. _ACS Nano_ 7, 27–34 (2013). Article Google Scholar
* Pei, R. J., Matamoros, E., Liu, M. H., Stefanovic, D. & Stojanovic, M. N. Training a molecular automaton to play a game. _Nat. Nanotechnol._ 5, 773–777 (2010). Article Google
Scholar * Wang, L. M., Hall, J. G., Lu, M. C., Liu, Q. H. & Smith, L. M. A DNA computing readout operation based on structure-specific cleavage. _Nat. Biotechnol._ 19, 1053–1059 (2001).
Article Google Scholar * Chirieleison, S. M., Allen, P. B., Simpson, Z. B., Ellington, A. D. & Chen, X. Pattern transformation with DNA circuits. _Nat. Chem._ 5, 1000–1005 (2013).
Article Google Scholar * Piranej, S., Bazrafshan, A. & Salaita, K. Chemical-to-mechanical molecular computation using DNA-based motors with onboard logic. _Nat. Nanotechnol._ 17,
514–523 (2022). Article Google Scholar * Xie, N. L. et al. Scaling up multi-bit DNA full adder circuits with minimal strand displacement reactions. _J. Am. Chem. Soc._ 144, 9479–9488
(2022). Article Google Scholar * Li, J., Green, A. A., Yan, H. & Fan, C. Engineering nucleic acid structures for programmable molecular circuitry and intracellular biocomputation.
_Nat. Chem._ 9, 1056–1067 (2017). Article Google Scholar * Chen, J., Fu, S. N., Zhang, C. Y., Liu, H. Y. & Su, X. DNA logic circuits for cancer theranostics. _Small_ 18, 2108008
(2022). Article Google Scholar * Hao, Y. Y. et al. Programmable live-cell CRISPR imaging with toehold-switch-mediated strand displacement. _Angew. Chem. Int. Ed._ 59, 20612–20618 (2020).
Article Google Scholar * Zou, J. et al. A DNA nanodevice for mapping sodium at single-organelle resolution. _Nat. Biotechnol._ 42, 1075–1083 (2024). Article Google Scholar * Nissim, L.
et al. Synthetic RNA-based immunomodulatory gene circuits for cancer immunotherapy. _Cell_ 171, 1138–1150.e15 (2017). Article Google Scholar * Green, A. A. et al. Complex cellular logic
computation using ribocomputing devices. _Nature_ 548, 117–121 (2017). Article Google Scholar * Zhou, Z. et al. Engineering longevity–design of a synthetic gene oscillator to slow cellular
aging. _Science_ 380, 376–381 (2023). Article Google Scholar * Chakraborty, K., Veetil, A. T., Jaffrey, S. R. & Krishnan, Y. Nucleic acid-based nanodevices in biological imaging.
_Annu. Rev. Biochem._ 85, 349–373 (2016). Article Google Scholar * Zhao, Y. et al. A temporally resolved DNA framework state machine in living cells. _Nat. Mach. Intell._ 5, 980–990
(2023). Article Google Scholar * Del Vecchio, D., Abdallah, H., Qian, Y. & Collins, J. J. A blueprint for a synthetic genetic feedback controller to reprogram cell fate. _Cell Syst._
4, 109–120.e11 (2017). Article Google Scholar * Marken, J. P. & Murray, R. M. Addressable and adaptable intercellular communication via DNA messaging. _Nat. Commun._ 14, 2358 (2023).
Article Google Scholar * Jiao, K. et al. Programming switchable transcription of topologically constrained DNA. _J. Am. Chem. Soc._ 142, 10739–10746 (2020). THIS ARTICLE REPORTS THAT GENE
EXPRESSION CAN BE LOGICALLY CONTROLLED THROUGH STRAND DISPLACEMENT-BASED TOPOLOGICAL ENGINEERING OF DNA. Article Google Scholar * Krawczyk, K. et al. Electrogenetic cellular insulin
release for real-time glycemic control in type 1 diabetic mice. _Science_ 368, 993–1001 (2020). Article Google Scholar * Xiao, M. S. et al. Programming drug delivery kinetics for active
burst release with DNA toehold switches. _J. Am. Chem. Soc._ 141, 20354–20364 (2019). Article Google Scholar * Strimbu, K. & Tavel, J. A. What are biomarkers? _Curr. Opin. HIV AIDS_ 5,
463–466 (2010). Article Google Scholar * Xie, Z., Wroblewska, L., Prochazka, L., Weiss, R. & Benenson, Y. Multi-input RNAi-based logic circuit for identification of specific cancer
cells. _Science_ 333, 1307–1311 (2011). Article Google Scholar * Arter, W. E. et al. Digital sensing and molecular computation by an enzyme-free DNA circuit. _ACS Nano_ 14, 5763–5771
(2020). Article Google Scholar * Ma, D. et al. Multi-arm RNA junctions encoding molecular logic unconstrained by input sequence for versatile cell-free diagnostics. _Nat. Biomed. Eng._ 6,
298–309 (2022). Article Google Scholar * Zhang, C. et al. Logical analysis of multiple single-nucleotide-polymorphisms with programmable DNA molecular computation for clinical diagnostics.
_Angew. Chem. Int. Ed._ 61, e202117658 (2022). Article Google Scholar * Gines, G. et al. Functional analysis of single enzymes combining programmable molecular circuits with droplet-based
microfluidics. _Nat. Nanotechnol._ 19, 800–809 (2024). Article Google Scholar * Bai, M. et al. Intracellular entropy-driven multi-bit DNA computing for tumor progression discrimination.
_Angew. Chem. Int. Ed._ 59, 13267–13272 (2020). Article Google Scholar * Zhang, C. et al. Nicking-assisted reactant recycle to implement entropy-driven DNA circuit. _J. Am. Chem. Soc._
141, 17189–17197 (2019). Article Google Scholar * Gerasimova, Y. V. & Kolpashchikov, D. M. Towards a DNA nanoprocessor: reusable tile-integrated DNA circuits. _Angew. Chem. Int. Ed._
55, 10244–10247 (2016). Article Google Scholar * Bae, W., Stan, G. B. V. & Ouldridge, T. E. Generation of RNA complexes for synthetic molecular strand-displacement circuits in
autonomous systems. _Nano Lett._ 21, 265–271 (2021). Article Google Scholar * Wang, B., Wang, S. S., Chalk, C., Ellington, A. D. & Soloveichik, D. Parallel molecular computation on
digital data stored in DNA. _Proc. Natl Acad. Sci. USA_ 120, e2217330120 (2023). Article MathSciNet Google Scholar * Chen, Z. B. & Elowitz, M. B. Programmable protein circuit design.
_Cell_ 184, 2284–2301 (2021). Article Google Scholar * Xiong, Y. et al. Functional DNA regulated CRISPR-Cas12a sensors for point-of-care diagnostics of non-nucleic-acid targets. _J. Am.
Chem. Soc._ 142, 207–213 (2020). Article Google Scholar * Cao, Y. et al. In situ programmable DNA circuit-promoted electrochemical characterization of stemlike phenotype in breast cancer.
_J. Am. Chem. Soc._ 143, 16078–16086 (2021). Article Google Scholar * Chandrasekaran, A. R. Nuclease resistance of DNA nanostructures. _Nat. Rev. Chem._ 5, 225–239 (2021). Article Google
Scholar * Zhang, Q. et al. Catalytic DNA-assisted mass production of arbitrary single-stranded DNA. _Angew. Chem. Int. Ed._ 62, e202212011 (2023). Article Google Scholar Download
references ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China (T2188102, 22322704, 21991134, 22122406), the National Key R&D Program of China
(2021YFF1200300), the Science Foundation of Shanghai Municipal Science and Technology Commission (23QA1404800), and the New Cornerstone Science Foundation. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Zhangjiang Laboratory, Shanghai, China Sisi Jia (贾思思) * School of Chemistry and Chemical Engineering, Frontiers Science Center for Transformative Molecules, New Cornerstone
Science Laboratory, National Center for Translational Medicine, Shanghai Jiao Tong University, Shanghai, China Sisi Jia (贾思思), Hui Lv (吕慧), Qian Li (李茜), Chunhai Fan (樊春海) & Fei Wang
(王飞) * Institute of Molecular Medicine, Shanghai Key Laboratory for Nucleic Acids Chemistry and Nanomedicine, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai,
China Chunhai Fan (樊春海) Authors * Sisi Jia (贾思思) View author publications You can also search for this author inPubMed Google Scholar * Hui Lv (吕慧) View author publications You can also
search for this author inPubMed Google Scholar * Qian Li (李茜) View author publications You can also search for this author inPubMed Google Scholar * Chunhai Fan (樊春海) View author
publications You can also search for this author inPubMed Google Scholar * Fei Wang (王飞) View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
S.J., F.W. and C.F. wrote the manuscript draft. All authors reviewed and edited the manuscript before submission. CORRESPONDING AUTHORS Correspondence to Chunhai Fan (樊春海) or Fei Wang
(王飞). ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Reviews Bioengineering_ thanks Thomas Ouldridge and the
other anonymous reviewer(s) for their contribution to the peer review of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under
a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such
publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jia, S., Lv, H., Li, Q. _et al._ DNA-based biocomputing circuits and their biomedical
applications. _Nat Rev Bioeng_ (2025). https://doi.org/10.1038/s44222-025-00303-8 Download citation * Accepted: 10 March 2025 * Published: 11 April 2025 * DOI:
https://doi.org/10.1038/s44222-025-00303-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Video. Danish pm gets the giggles during statement on circus elephantsDenmark's Prime Minister Mette Frederiksen roared with laughter when giving a speech during the parliament session ...
Spatio-temporal variation of male sterile frequencies in two natural populations of beta maritimaABSTRACT The spatio-temporal variation of sex phenotype frequencies is investigated within two gynodioecious populations...
Photocurrent-driven transient symmetry breaking in the weyl semimetal taasABSTRACT Symmetry plays a central role in conventional and topological phases of matter, making the ability to optically...
What Works to Prepare Young Children With Disabilities for School?Share article Remove Save to favorites Save to favorites Print Email Facebook LinkedIn Twitter Copy URL A new review of ...
Sprint takes the Treo 755p out behind the wood shedIf our very own Matt “The Stapler” Hickey got you all hot and bothered about buying a Treo 755p thanks to his review yes...
Latests News
Dna-based biocomputing circuits and their biomedical applicationsABSTRACT DNA-based biocomputing circuits are chemical reaction networks with information-processing capability that take...
What to know about a fourth wave of covid-19 casesFor an interactive version of this chart, visit CDC.gov. So far more than 64 million Americans have been fully vaccinate...
AARP Innovation Labs1:07 AARP Videos AARP Innovation Labs AARP has a long history of solving social problems for people 50 plus through crea...
Binding of sap sh2 domain to fynt sh3 domain reveals a novel mechanism of receptor signalling in immune regulationABSTRACT SAP (or SH2D1A), an adaptor-like molecule expressed in immune cells, is composed almost exclusively of a Src ho...
Tennis: depth in women’s game no surprise for top playersTennis: Depth in women’s game no surprise for top players | WTVB | 1590 AM · 95.5 FM | The Voice of Branch County Close ...