Exhaled breath metabolites reveal postmenopausal gut-bone cross-talk and non-invasive markers for osteoporosis
Exhaled breath metabolites reveal postmenopausal gut-bone cross-talk and non-invasive markers for osteoporosis"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Menopause driven decline in estrogen exposes women to risk of osteoporosis. Detection of early onset and silent progression are keys to prevent fractures and associated
burdens. METHODS In a discovery cohort of 120 postmenopausal women, we combined repeated quantitative pulse-echo ultrasonography of bone, assessment of grip strength and serum bone markers
with mass-spectrometric analysis of exhaled metabolites to find breath volatile markers and quantitative cutoff levels for osteoporosis. Obtained markers and cutoffs were validated in an
independent cohort of 49 age-matched women with six months apart seasonal follow-ups. RESULTS Here, within the discovery cohort, concentrations of exhaled end-tidal dimethyl sulfide (DMS),
allyl-methyl sulfide, butanethiol and butyric acid are increased (p ≤ 0.005) pronouncedly in subjects with bone mineral density (BMD) at high-risk of osteoporosis and fracture, when compared
to subjects with normal BMD. Increased age and decreased grip strength are concomitant. All changes are reproduced during independent validation and seasonal follow-ups. Exhaled metabolite
expressions remain age independent. Serum markers show random expressions without reproducibility. DMS exhalations differs between patients with recent, old and without fractures. Metabolite
exhalations and BMDs are down-regulated during winter. ROC analysis in discovery cohort yields high classification accuracy of DMS with a cutoff for osteoporosis, which predicts subjects at
high-risk within the independent validation cohort with >91% sensitivity and specificity. CONCLUSIONS Non-invasive analysis of exhaled DMS allowed more reliable classification of
osteoporosis risk than conventional serum markers. We identified associations of exhaled organosulfur and short-chain fatty acids to bone metabolism in postmenopausal osteoporosis via a
gut-bone axis. PLAIN LANGUAGE SUMMARY It is estimated globally that one-third of women aged >50 years old experience fractures (breaks in their bones) from osteoporosis (bone weakening
and brittleness). It is difficult to diagnose this condition which makes it hard to put in place measures to help prevent fractures. Here, we investigate links between volatile organic
chemicals detectable in exhaled breath, blood bone markers and the risk of osteoporosis (tested by measuring bone strength). We discover that chemicals coming from the gut are strongly
associated to postmenopausal bone health. Our non-invasive analysis is faster and more reliable than standard blood markers currently used in diagnosing osteoporosis and identifies a
connection between the gut and bones not previously shown. These findings offer easier assessment of osteoporosis risk and paths towards new therapeutic targets. SIMILAR CONTENT BEING VIEWED
BY OTHERS ASSOCIATION BETWEEN URINARY VOLATILE ORGANIC COMPOUND METABOLITES AND SARCOPENIA IN THE US GENERAL POPULATION: A CROSS-SECTIONAL NHANES STUDY FROM 2011 TO 2018 Article Open access
28 March 2025 SELECTED ION FLOW TUBE MASS SPECTROMETRY FOR TARGETED ANALYSIS OF VOLATILE ORGANIC COMPOUNDS IN HUMAN BREATH Article 04 June 2021 NON-VOLATILE ORGANIC COMPOUNDS IN EXHALED
BREATH PARTICLES CORRESPOND TO ACTIVE TUBERCULOSIS Article Open access 13 May 2022 INTRODUCTION Bone is a highly dynamic tissue undergoing lifelong remodeling and serving endocrine,
biochemical, and mechanical functions simultaneously1. Both, bone turnover and mineral homeostasis are predominantly regulated by the interplay between bone cells and the endocrine system
involving estrogen, parathormone, thyrocalcitonin, fibroblast growth factor 23 (FGF-23) and vitamin-D32. Menopause driven decline in estrogen exposes women to metabolic bone disorder leading
towards rapid bone loss3. Compromised bone health due to increased bone resorption, impaired bone formation and alterations of the extracellular matrix gradually turn into post-menopausal
osteoporosis Type-1—an advanced stage of compromised bone stability and a high susceptibility for bone fragility and fracture4. In general, onset and progression of postmenopausal
osteoporosis is clinically silent and mainly remain unnoticed or minimally painful until the first low-energy fracture takes place5. Roughly, one-third of world women population aged >50
years experiences osteoporotic fractures, out of which those of the distal radius (wrist) are often the first sign of osteoporosis and bone fragility6. After the first fracture, women are at
a two-fold increased risk to experience a second low-energy fracture within the next year. In women aged >45 years, osteoporosis accounts for longer periods of hospitalization than many
other diseases, including diabetes, cardiovascular disease or breast cancer7. This employs enormous clinical, economic and public health burdens globally – estimated €37 billion in Europe in
2010. Fracture managements represent >60% of such burden. Numbers of osteoporosis cases are expected to rise by >20% until 2025 in the EU8. Thus, timely diagnosis is indispensable to
administer early therapy and prevent fractures. So far as clinical diagnosis is concerned, the dual-energy X-ray absorptiometry (DXA) scan for the determination of bone mineral density (BMD)
is the WHO-suggested gold standard9. Nevertheless, it exposes patients to X-ray radiation and is neither recommended to screen healthy or even asymptomatic individuals nor covered as a
regular investigation by general health insurances. Eventually, FRAX questionnaires, bone echography, bone markers in blood and hand grip strength have been introduced to risk
assessment8,10,11,12. While bone echography (e.g., BINDEX® system based) exhibits 90% accuracy (with respect to the gold-standard DXA scan) to detect risk of osteoporosis and fracture in
clinical studies, it requires skilled physicians for accurate measurements and critical interpretations to correctly categorize subjects13,14. Similarly, assessment of bone markers (e.g.,
CTX, PINP, BAP, TRAP5b, sclerostin, FGF-23 and Klotho etc.) are based on invasive blood sampling. Beyond this, there is no clear consensus on the selection of optimal markers and the
interpretation of results are frequently confounded by the fasting state as well as intra-subject and/or intra- and inter-assay variations15,16,17. Though hand grip strength measurement is
considered for musculoskeletal integrity assessment in osteoporosis18, various confounding physio-metabolic effects from subject’s overall biological aging dilute a clear clinical
relationship only to bone health19,20. Therefore, novel, inexpensive, non-invasive and repeatable diagnostic tools (with high-accuracy and reproducibility) are necessary for reliable risk
assessments at the point-of-care and for personalized monitoring. Various substance classes of endogenous volatile organic compounds (VOCs) are produced/regulated via diverse in vivo
metabolic processes at the cellular and organ levels as well as by systemic microbiota21. These VOCs are readily transported to the lung and exhaled through our breath. Real-time
mass-spectrometry based breathomics offers rapid and repeated non-invasive assessment and continuous monitoring of various physiological22,23,24, metabolic25,26,27, pathological28,29,30 and
therapeutic31,32,33 conditions/effects via exhaled VOCs. Years of development in real-time alveolar sampling and breath-resolved analysis allowed us to see minute systemic effects beyond the
normal and confounding intra- and inter-individual physio-metabolic noise34,35. Nevertheless, well-designed prospective studies and independent validations are the prerequisites to define
reliable breath markers and translate those into routine clinical practice for pathophysiological and therapeutic monitoring. Here, in a real-life screening scenario, we report on the
combination of bone echography, grip strength and invasive bone marker measurements with proton transfer reaction – time-of-flight – mass-spectrometric (PTR-ToF-MS) analysis of exhaled
breath VOCs within a large discovery cohort and an age-matched independent validation cohort (with seasonal follow-ups) of postmenopausal women. Within the discovery cohort, we defined
breath markers and quantitative cutoff values for osteoporosis risk assessment and applied those onto the validation cohort. Observed unprecedented associations between postmenopausal bone
health and exhaled metabolic markers revealed gut-bone cross-talk and are reproduced within the independent validation cohort during repeated follow-ups. Our findings offered a new
non-invasive option with high test accuracy and translational potential for point-of-care and personalized monitoring of postmenopausal osteoporosis via breath biomarkers and opened up
investigative avenues towards new therapeutic targets. METHODS STUDY DESIGN This is a real-life observational screening study with independent prospective validation, conducted at Rostock
University Medical Center, Rostock, Germany. The design contains a large discovery cohort of postmenopausal women (aged ≥49 years) and an age-matched independent validation cohort of women
with repeated seasonal follow-ups. Within the discovery cohort, we identified breath markers and corresponding quantitative cutoff values for osteoporosis risk assessment and applied those
onto the validation cohort including seasonal follow-ups. SAMPLE SIZE ESTIMATION IN BOTH COHORTS Sample size in each cohort was calculated via analysis of variance (ANOVA) coupled with
Bonferroni correction for multiple comparisons (between three groups of bone densities). Given the high mass-resolving power of the PTR-ToF-MS used in this study, a minimal detectable
difference in mean VOC intensities of 20 ncps (normalized counts pers second) allowed us to detect <5% difference in quantified trace (up to mid pptV range) concentrations of exhaled
endogenous metabolites in both cohorts. As per the real-life screening scenario and interindividual variations, we estimated a relatively high standard deviation of ± 30 ncps for the
discovery cohort. Thus, in order to attain a test-power of 0.99 at 95% confidence interval (i.e., represented by a Bonferroni corrected alpha value of 0.016) the sample size of the discovery
cohort resulted in 118 subjects. Within the independent validation cohort, we applied the actual standard deviation of ±17 ncps obtained from the discovery cohort outcomes. Consequently,
within the validation cohort, the alpha value of the discovery cohort was also readjusted further via Bonferroni correction and resulted as 0.005. Therefore, to achieve a test-power of 0.99
at 95% confidence interval the sample size in the independent validation cohort resulted in 45 subjects. RECRUITMENT OF POSTMENOPAUSAL SUBJECTS Ethical approvals (EA-No.: _A 2017-0183_ and
_A 2019-0040_ for the discovery- and validation study, respectively) for clinical measurements were obtained from the institutional ethics committee (IEC) of Rostock University Medical
Center (Germany) and all experiments were carried out in accordance with the amended Declaration of Helsinki guidelines. All postmenopausal subjects were recruited from our clinical
screening campaign of osteoporosis. Prior to participation, the study design and conduct were clearly explained to each participant by the principal investigator and a written and signed
informed consent was obtained from each subject. These participants appeared to the clinic either by seeing the online advertisements of ongoing bone health screening campaign or due to a
sudden radial fracture. Local travel costs of the participants were covered by institutional funds. All recruitments and repeated (twice in a month) measurements within the discovery cohort
took place from March until September 2018. The majority of these measurements were executed during the summer months (June – August 2018). Initial recruitments in the independent validation
cohort took place from October 2019 until March 2020. The majority of these initial measurements were executed during the winter months (December 2019 – February 2020). Thereafter, in order
to abide by the mandatory safety regulations of the COVID-19 pandemic, we had to completely pause the follow-up measurements for 7 months. After resuming measurements during October –
November 2020, these subjects were followed-up 6 months apart (during summer months of 2021) to observe any seasonal effect. EXCLUSION CRITERIA AND MEASURED PARAMETERS In order to explicitly
focus on bone health and to reduce extrinsic and intrinsic confounding factors, subjects with any severe acute or chronic comorbidity (excluding diabetes, hypothyroidism and hypertension),
chronic/regular smoking and alcohol drinking habits, use of any special diet, supplement, medication (except oral medications for diabetes, hypothyroidism and hypertension) or therapy
(except those with mandatory SARS-CoV-2 vaccinations and fracture treatments within the validation cohort) were excluded from final analysis. Subjects with abnormal respiratory rate (e.g.,
respiratory hyperventilation) were excluded. Similarly, in order to concentrate on early onset of the disease, subjects with immediate bone fracture other than in the radius were also
excluded from data analysis. Thus, we considered 120 subjects (out of 127 recruitments) from the discovery cohort and another 49 subjects (amongst 52 recruitments) from the independent
validation cohort for actual data analysis and interpretations. These numbers also satisfied the above-estimated sample sizes. After recording demography, lifestyle and clinical history
related information, assessment of bone density index (via pulse-echo ultrasonography of bone) and grip strength were executed by experienced clinicians at the Dept. of Traumatology, Hand
and Reconstructive Surgery. Within the next hour, real-time mass-spectrometric (PTR-ToF-MS) analysis of exhaled breath metabolites in these subjects were carried out at the Dept. of
Anesthesiology, Intensive Care Medicine and Pain Therapy by an experienced medical scientist. Peripheral venous blood samples were collected for timely analysis of serum bone markers via
commercially available biochemical assays at the Dept. of Pediatrics. In case of follow-ups, approximately the same measurement time of the day was used for the same participant. ASSESSMENT
OF BONE DENSITY The Bindex® device (Bone Index Finland, Kuopio, Finland) was used for determination of bone density essentially as described previously 36. Briefly, measurements were taken
at the distal radius and at two positions from the ipsilateral tibia. The ultrasound probe was placed at one third of radial length, i.e., the distance between the olecranon and the ulna
styloid as well as one third of the tibial length below and above the top of the medial condyle and the medial malleolus, respectively. Ultrasound gel was applied and the probe was gently
moved at the region of interest (ROI) and orthogonal to the surface of the underlying bone. Per ROI three valid signals were recorded. The proprietary software provided with the instrument
(Bindex® software version 2.5) translated this information together with individual data on age, weight and height into a bone density [g/cm2] and a probability of osteoporosis12. As per
age, gender and ethnicity-based recommendations of the Bindex® software, we considered BMD scores of >0.876 g/cm2, 0.803 – 0.876 g/cm2 and <0.803 g/cm2 as normal, at risk (i.e.,
osteopenia) and at high-risk of osteoporosis, respectively. GRIP FORCE MONITORING The Jamar® dynamometer (Type G200 from Biometrics Ltd., Newport, UK) was used for quantitative determination
of grip force essentially as described37. Briefly, participants were placed on a chair with the shoulder in a neutral position, the elbow 90° flexed and loosely gripping the handle of the
dynamometer38. Participants were asked to grab the dynamometer with maximum strength for 5 seconds, rest for 10 seconds and three repetitions per hand37. The mean of three measurements was
calculated and converted into Newton (i.e., equals to 0.224 pound). LABORATORY INVESTIGATIONS Blood was sampled in a plastic serum separator tube (Sarstedt, Nümbrecht, Germany), immediately
transferred to the laboratory, allowed to clot at room temperature for approximately 20 min and centrifuged (2000 g, 15 min). Subsequently, samples were aliquoted and stored at -80 °C until
further analysis. In particular, the activities of the bone specific phosphatase (BAP) and tartrate-resistant acid phosphatase (TRAP5b) as well as the concentrations of sclerostin, soluble
α-Klotho and intact fibroblast growth factor-23 (FGF23) were determined. To this purpose, assays for BAP and TRAP5b were purchased from IDS (Immunodiagnostic Systems Limited, Boldon
Colliery, UK), the ones used for quantification of sclerostin, soluble α-Klotho and iFGF23 were obtained from TECO Medical Group (Sissach, Swiss), IBL (Immuno-Biological Laboratories,
Minneapolis, USA) and Kainos Laboratories (Tokyo, Japan), respectively. All assays were used essentially as described and all samples were tested in duplicate39,40. BREATH SAMPLING, VOC DATA
ANALYSIS AND QUANTIFICATION Spontaneously breathing postmenopausal women maintained sitting posture41 and performed oral breathing42 via a customized mouthpiece43 by following our
state-of-the-are sampling procedure44. Continuous side-stream sampling (flow: 20 mL/min) from the mouthpiece was performed via the heated (75 °C) transfer-line of a PTR-ToF-MS-8000 (Ionicon
Analytik GmbH, Innsbruck, Austria) under optimized conditions45,46. We used a PTR time-resolution of 200 ms, drift-tube temperature of 75 °C, pressure of 2.3 mbar and voltage of 610 V in
order to reach the E/N ratio of 139 Td23,28,47. The mass scale was recalibrated automatically based on three masses viz., 21.0226 (H3O+-isotope), 29.998 (NO+) and 59.049 (protonated C3H6O)
after each minute of data acquisition. A PTR-MS viewer software (version 3.228) was used for raw data processing. VOCs were measured continuously in counts per second (cps), which were
normalized onto the corresponding counts (i.e., to obtain VOC data in ncps) of the primary ion (H3O+). Breath-by-breath assignment of inspired (room air) and expired (alveolar/end-tidal)
phases of breath were performed via custom-made ‘breath tracker’ algorithm48,49. Here, an endogenous VOC (e.g., acetone) with orders of magnitudes higher concentration in exhalation than in
room air was used as the tracker mass. We quantified the VOCs either via reaction rate constant (_k_-rates) between the volatile and H3O+ ion (at the E/N ratio of 140 Td) or via
multi-component VOC standard mixture under breath adapted humidity and CO2 conditions by using a liquid calibration unit (LCU, Ionicon Analytik GmbH, Innsbruck, Austria)50. NORMALIZATION OF
MEASURED PARAMETERS TO GENERATE OVERVIEW HEATMAPS In order to have an initial overview of relative differences in subjects’ BMD scores, biological age, hand strength, serum bone markers and
exhaled alveolar concentrations of volatile metabolites, each parameter is normalized onto the corresponding maximum value (from an entire cohort) and is executed independently within each
cohort. Group wise means of normalized values are used to generate color coded heatmaps. STATISTICAL ANALYSIS For statistical comparisons of bone health, postmenopausal subjects in either
cohort were categorized separately upon BMD scores and biological age. BMD wise categorization of subjects was based on normal ( > 0.876 g/cm2), at risk (0.803 – 0.876 g/cm2) and at
high-risk ( < 0.803 g/cm2) ranges of bone density scores. Age wise distributions of subjects were based on 49 – 59 years, 60 – 69 years and 70 – 90 years. In order to observe any effect
of fractures, subjects from both cohorts with old fractures (i.e., occurring >1 year before the recruitment), recent radius fracture (i.e., occurring within 12 months prior to enrolment)
and without a history of fractures were categorized upon three levels of BMD sores. In order to check the type of distribution, either by a Kolmogorov-Smirnov test (where N ≥ 50) or a
Shapiro-Wilk (where N < 50) test for normality (that failed at _p_ < 0.050) was applied. In case of parametric data, we used mean values and statistically significant differences
between study groups were tested independently within each cohort by means of one-way ANOVA test followed by a pairwise multiple comparisons test via post-hoc Dunn’s method (at _p_ <
0.05) due to unequal group sizes. In case of non-parametric data, we used median values and statistically significant differences between study groups were tested independently within each
cohort by means of Kruskal-Wallis H test (i.e., a one-way ANOVA on Ranks test for non-parametric data) followed by the pairwise multiple comparisons test via post-hoc Dunn’s method (at _p_
< 0.05) due to unequal group sizes. Receiver operating characteristic (ROC) curves were generated from the discovery cohorts, based on subjects’ biological age, grip strength, bone
markers and exhaled alveolar concentrations of dimethyl sulfide, allyl-methyl sulfide, butanethiol, and butyric acid. Area under the curve (AUC) and cutoff values were calculated with
corresponding standard error and statistical significance (_p_ < 0.05). Discovery cohort derived cutoff values of parameter(s) with high classification accuracy (where AUC value was
>0.85, standard error was <5% and asymptotic significance level was _p_ < 0.005) were applied to predict the subjects with “BMD at high-risk” of osteoporosis within the validation
cohort including follow-ups. Resulting ROC curves with corresponding test sensitivity and specificity are presented. REPORTING SUMMARY Further information on research design is available in
the Nature Portfolio Reporting Summary linked to this article. RESULTS RECRUITED POSTMENOPAUSAL SUBJECTS, DEMOGRAPHY AND LIFESTYLE HABITS Number (n = ) of postmenopausal females recruited in
discovery and independent validation cohorts from our bone-health screening campaign along with their bone-health status (based on BMD scores) are presented in Table 1. Subject’s ethnicity,
biological age, body mass index (BMI), habit of active smoking, consumption of alcohol (i.e., daily/ occasional), daily exercise, intake of special diet/ supplement and any other notable
activities are also presented in Table 1. Due to the parametric distribution, age and BMI values are presented group wise as mean ± SD. Reproducibility in the group wise distributions of
subjects’ age, BMI and BMD within both cohorts depicted the strength of our real-life screening design. CLINICAL AND THERAPEUTIC ATTRIBUTES Number (n = ) of subjects with old ( > 1 year
from the participation date) and recent ( ≤ 1 year from the participation date) radius fractures and number (n = ) of subjects undertaking bone-health related treatments (vitamin D3, oral
calcium, bisphosphonate, anti RNLK and hormone therapy) are listed in Table 1. BONE, BLOOD AND BREATH MARKERS Quantitative values of BMD and blood markers for bone metabolism (BAP, TRAP5b,
sclerostin, FGF23 and Klotho) are presented (parametric data) in terms of group wise mean ± SD in Table 1. Predominantly endogenous volatile metabolites in breath are also presented
(non-parametric data) in terms of group wise median (minimum – maximum) in Table 1. Relevant exhaled endogenous VOCs are comprised of organosulfur (dimethyl sulfide/DMS, allyl-methyl
sulfide/AMS and butanethiol), short-chain fatty acid/SCFA (acetic acid, propionic acid and butyric acid), aldehyde (acetaldehyde, butyraldehyde, pentanal, hexanal), alcohol (ethanol), ketone
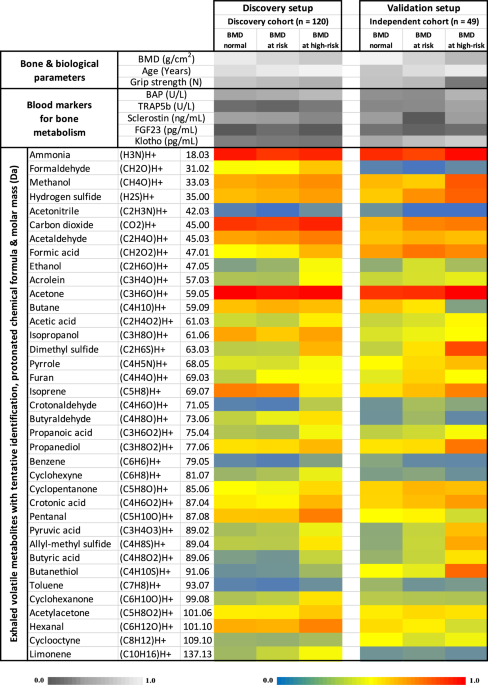
(acetone) and hemiterpene (isoprene) substance classes. INITIAL OVERVIEW OF MEASURED PARAMETERS IN BOTH COHORTS BMD (normal, at risk and at high-risk of osteoporosis) wise categorization of
measured parameters in both cohorts is presented as heatmap (Fig. 1). Relative differences in subjects’ BMD scores, biological age, hand strength, blood markers for bone metabolism and
exhaled alveolar concentrations of volatile metabolites (with putative identification, protonated chemical formula and molar mass) are presented here. The heatmap represents non-quantitative
expressions of each parameter normalized (independently in each cohort) onto the corresponding maximum value of an entire cohort. Here, both cohorts represent group wise means of normalized
values from the initial recruitments (i.e., without seasonal follow-ups). Parameters are coded in different colors to depict relative differences. Amongst the identified VOCs, only
potentially endogenous substances are considered for quantitative statistical analysis (Fig. 2 and Supplementary Fig. 1). Exogenous VOCs appearing due to environmental exposures, subjects’
lifestyle, smoking habits, diet, cosmetics, disinfectant etc. are excluded from analysis. REPRODUCIBILITY OF QUANTITATIVE DIFFERENCES BASED ON BMD OR AGE Subjects’ BMD and age wise
categorizations of quantitative parameters differed significantly (_p_ < 0.05) and reproducibly in both cohorts including seasonal follow-up (Fig. 2). All other parameters are presented
in the Supplementary Fig. 1. While categorized upon three groups of BMDs (left-side panels), statistically significant differences with respect to “BMD at high-risk” group are reproduced
only in subjects’ biological age, grip strength (Fig. 2a) and exhaled alveolar concentrations of DMS, AMS, butanethiol and butyric acid (Fig. 2b). While categorized upon three groups of
biological age (right-side panels), apart from BMD scores and grip strength, all other significant differences (seen in BMD wise categorization) with respect to the age group “70 – 90 years”
and their reproducibility disappeared (Fig. 2). Thus, exhaled organosulfur and SCFA compounds that are predominantly originated from the gut, depicted associations to subjects’ bone health
rather than to their biological age. In all kind of categorizations, serum bone markers behaved at random and did not show any reproducibility (Supplementary Fig. 1). POTENTIAL SEASONAL
INFLUENCE ON BMDS AND VOCS While plotting all individual BMD scores (n = 358) from the discovery- (including repeated measures) and independent validation cohort (including seasonal
follow-ups), as per the 12 calendar months of actual recruitments, those distributions closely mirrored the yearly profiles of regional sunlight and temperature exposures (Supplementary Fig.
2). Within the independent validation cohort, exhalations of endogenous VOCs were down-regulated during the winter months (Fig. 2 and Supplementary Fig. 1). These seasonal patterns
represent the effects of surrounding climate on subjects’ bone metabolism (e.g., effect of sunlight on vitamin D3 metabolism) and overall effects on various underlying processes at cellular,
organ and systemic microbial levels. COMPARISONS BETWEEN SUBJECTS WITH AND WITHOUT RADIUS FRACTURE Quantitative values of subjects’ biological age, hand strength and exhaled alveolar
concentrations of DMS, AMS, butanethiol and butyric acid are presented based on the radius fracture status (no fractures, old fractures and recent fractures) and three groups BMD scores
(Fig. 3). Within the “Recent fractures” cohort, subjects with “BMD at high-risk” range are of significant clinical concern/ attention. Clinically relevant and statistically significant (_p_
< 0.05) differences with respect to the “high-risk” group are only observed in exhaled DMS concentrations. This depicted further associations of DMS to postmenopausal bone health.
OSTEOPOROSIS RISK CLASSIFICATION ACCURACY BENCHMARKING WITH INDEPENDENT VALIDATIONS Amongst all parameters, exhaled alveolar DMS concentrations yielded high AUC value of 0.86 (AUC bounds:
0.790 – 0.931 at 95% CI, standard error of 0.037 and asymptotic _p_ < 0.0001 under non-parametric assumptions) with a quantitative cutoff value of 15.88 ppbV for high-risk of osteoporosis
in the discovery cohort (Fig. 4a). Application of the discovery cohort derived quantitative cutoff onto the independent validation cohort resulted in >91% test accuracy (sensitivity of
91.7% and specificity of 91.3%) with respect to quantitative pulse-echo ultrasonography of bone (via Bindex® device) in detecting subjects at high-risk of osteoporosis even during their
seasonal follow-ups (Fig. 4b). Further ROC analysis confirmed a high risk-classification accuracy (AUC = 0.97, bounds: 0.94 – 0.99) of exhaled DMS for osteoporosis in independent validation
cohort including seasonal follow-ups (Fig. 4c). Thus, continuous monitoring of exhaled DMS would enable assessment of postmenopausal bone health and risk of early onsets and/or asymptomatic
progressions of osteoporosis. Any adverse event is neither reported by the subjects nor observed by the investigators during and at the end of study participation. DISCUSSION In this
real-life observational screening study, we discovered and independently validated non-invasive breath VOC biomarkers for classifying postmenopausal osteoporosis risk. Combining bone
ultrasonography, grip strength, bone marker measurements and PTR-ToF-MS analysis of exhaled breath VOCs within a large discovery cohort of postmenopausal women we identified breath markers
and corresponding quantitative cutoffs for osteoporosis risk assessment. Applying these markers and cutoffs onto an age-matched independent cohort with seasonal follow-ups, we reproduced and
validated the high accuracy of exhaled organosulfur compounds in addressing osteoporosis risk. Reproducibility of demographic parameters as per BMD wise categorization denominates the
strength of our real-life bone health screening campaign in both cohorts. As expected, subjects’ hand grip strength decreased with their compromised BMD and with increased age18,51 depicting
the effect of aging on musculoskeletal health. Observed under-expressions of BMD scores and breath markers during the winter season indicate potential influences of sunlight and temperature
on bone metabolism and thereby, justify the seasonal follow-up of the independent validation cohort. Surprisingly, in our study the anticipated seasonal effects52 were absent on the serum
bone markers. In routine clinical practice, a diagnostic marker must express differentially beyond the normal physio-metabolic limits as well as, intra- and inter-subject variations under
the presence of a disease/condition. Although serum bone markers correlate with bone biopsies and radiotracer kinetics etc. in assessing bone turnover and thereby, may contribute to
diagnosis and management of various conditions including osteoporosis15, they have limited value to predict fractures in individuals. Further studies are needed that use the recommended bone
markers and standardized ways of evaluating those and estimating the risk. Here, we observed random expressions of these markers with relatively large variations and no tracible
reproducibility in both BMD and age wise categorizations. This is in line with already reported confounding effects from intra-subject variations16,17 that hamper optimal bone marker
selection in routine practice. In contrast to invasively sampled bone markers, non-invasively sampled breath markers depicted close associations to postmenopausal bone health. Menopause
driven estrogen decline causes rapid bone turnover in postmenopausal women whereas, a concomitant age-related bone loss remains slower and indifferent in both genders4,9. Reproducibility of
exhaled organosulfur and SCFA expressions only in BMD wise categorizations indicates causal relationships of these VOCs to metabolic effects from postmenopausal bone health and not to the
cumulative effects from subjects’ age. Based on various observations from in vitro and ex vivo studies, DMS, AMS, butanethiol and butyric acid are known to predominantly originate from the
gut53,54,55. Although the systemic transport and alveolar release of these VOCs in human exhalation are not yet investigated mechanistically in vivo, earlier we identified and quantified the
presence of organosulfur such as, DMS in the systemic circulation of mechanically ventilated humans and pigs56. Recently, we observed significant suppression in DMS exhalation throughout
the menstrual cycle in healthy adult females undertaking daily oral contraceptive pills26, depicting the bacteriolytic activity (i.e., known to cause dysbiosis in the gut) of synthetic
progesterone. An absence of such effect on DMS exhalation in absence of the contraceptive pills ascertains the systemic transport and alveolar release of gut derived VOCs. There are
evidences that a systemic microbiota-gut-bone axis regulates skeletal homeostasis by affecting host metabolism, gut permeability and nutrient absorption, cell mediated immunity, and
endocrine functions57,58,59. SCFAs (e.g., butyric acid) are produced via anaerobic microbial lysis of complex carbohydrates (e.g., undigested fibers/starch) and then diffuse from the gut
lumen into the blood stream. Besides providing energy to the intestinal epithelial cells, promoting inflammatory response, enhancing gut-barrier integrity and Ca2+ and Mg2+ ions absorptions,
butyric acid plays an important role in bone remodeling by directly promoting osteoblast activation and suppressing pre-osteoclast differentiation58. While the links of SCFAs to bone health
are repeatedly reported, such connections between bone metabolism and gut-related organosulfur compounds (e.g., DMS) are yet unknown and remained unexplored. DMS is believed to be produced
mainly during anaerobic microbial methylation of L-methionine in the lower gut53,60,61. Earlier, we reported that AMS and butanethiol (/methyl-propyl sulfide) are largely produced from the
oral and nasal cavity microbiota, respectively42. Thus, unlike exhaled AMS and butanethiol profiles, DMS exhalations remained unaffected while switching between oral and nasal breathing.
Hence, in line with our previous pre-clinical and clinical observations26,42,56, the pronounced, significant and reproducible behavior of exhaled DMS with respect to systemic effects in our
present study, indicates its steady and predominant endogenous origin from the intestinal methionine metabolism. While we executed oral breath sampling as per our state-of-the art protocol
primarily to minimize physiological effects (e.g., ventilatory variations)44, this also reduced certain confounding VOC fractions from the nasal cavity microflora42. Studies have also shown
that dietary supplementation of methionine improves bone health and its long-term restriction deteriorate the same62. In a pre-clinical model, methionine facilitated mRNA and protein
expressions of important genes responsible for estrogen synthesis within primary ovarian granulosa cells63. Methionine is a sulfur-containing essential amino acid that converts to
homocysteine after donating the methyl group in cellular methylations64. In vitro assays suggested that estrogen may regulate homocysteine levels through conjugation processes65,66. Although
the systemic interactions between methionine/homocysteine and estrogen (its receptors) are complex and unclear67, based on clinical investigations comparing pregnant, premenopausal and
postmenopausal women with estrogen replacement therapy (ERT) with age-matched men and postmenopausal women without ERT, the 3rd National Health and Nutrition Examination Survey has
recognized the inverse relationship between serum estrogen and homocysteine68. In line with that, randomized (placebo-)controlled trials also demonstrated that exogenous estrogen
intervention in postmenopausal women inversely influences homocysteine–methionine metabolism69,70. However, vivid clinical investigations are lacking to address definite relationships
between methionine metabolism, estrogen and bone health. Previously, we demonstrated that in healthy premenopausal adults (without oral contraceptive pills), DMS exhalation tends to rise
from the ovulation phase, soon after the estrogen level starts to decline physiologically. As already described above, DMS exhalation decreases significantly and progressively in presence of
daily oral contraceptives pills – containing synthetic estrogen and/or progesterone26. While evaluating the physio-metabolic effects of healthy female aging, we observed that exhaled
abundances of DMS tend to rise with age in postmenopausal cohorts23. Such an ascending trend of DMS profiles with age is also noticed in the present study under age wise categorization and
is observed significantly within the discovery cohort. These facts indicate that an increase (physiologically or administered artificially) in systemic estrogen level reduces in vivo DMS
production in women. As menopause driven progressive decline in physiological estrogen level gradually leads to progressive bone resorption (corresponding to bone loss rates of 3 – 5% within
5 – 10 years), significantly increased exhaled DMS concentrations in subjects at risk (/with osteopenia) and at high-risk of osteoporosis suggest a unique gut-bone metabolic axis – i.e., a
potential and yet unexplored interplay between methionine and estrogen. Here, we hypothesize that DMS may act as a gut-microbial signaling molecule to actively regulate bone homeostasis
under compromised BMD and, irrespective of an osteoporotic fracture event. Our hypothesis is further supported by the fact that DMS expressions did not differ significantly between “BMD at
high-risk” groups based on their radius fracture status. We must remember that except for isoprene, the endogenous origin of any other breath VOC is rather putative/postulated. Our precedent
multi-omic investigations has finally unraveled the true origin of isoprene in human breath27 and thereby, dismissed the long-believed erroneous source47 that has mislead clinical
interpretations over the last four decades. Thus, our above hypothesis on DMS must be confirmed via further down-stream and system-wide investigations. In order to focus explicitly on the
physio-metabolic effects of osteoporosis, we excluded subjects with any severe acute/chronic comorbidities from the final analysis. Nevertheless, the age-related common comorbidities e.g.,
diabetes, hypertension and hypothyroidism could not be ruled out entirely. Noteworthy that our subjects with those comorbidities received corresponding medication/therapy and, therefore,
were not experiencing any disease symptoms. They consented having a normal and healthy life. In this context, breath acetone i.e., the putative byproduct of glycolysis and/or lipolysis71 did
not differ between groups in either cohort. Breath ethanol i.e., partly produced by microbial breakdown of carbohydrates in the small intestine23, also remained indifferent. Similarly,
breath aldehydes are regarded as markers for oxidative stress. Though, exhaled butanal and pentanal depicted a significant rise in the “high-risk” groups within the discovery cohort,
reproducibility tests failed within the independent cohort. Being originated from the skeletal muscular lipolysis27, exhaled isoprene remained unaffected by bone health status. Therefore, we
could not consider any effect from well-treated comorbidities onto breath markers. Any disease/condition specific endogenous VOC biomarker is not yet found (or translated to routine
clinical practice) in the human breath. Therefore, changes and/or differences in exhaled VOC concentrations are rather more important than anticipating the presence/absence of unique
biomarkers. Exhaled expressions of VOCs are also affected by many extrinsic and/or intrinsic factors. For instance, the endogenous origins of our observed breath markers are not strictly
specific to human gut and/or its microbiome and can partly be influenced by genetic predisposition, systemic-microbial diversity/activity, local contributions from upper-airways (including
the nasal and oral cavities) microflora, presence of an acute/chronic comorbidity, medication/therapy, diet, lifestyle habits and geographical/environmental factors etc. Consequently, as a
potential limitation, the outcomes and observed classification limits and quantitative cutoffs for gut-related VOCs may vary in another ethnicity/population. Therefore, for generalizability,
the identified marker(s) need to be reproduced in large independent screening studies on other ethnic populations. Further to that, our observations are restricted to postmenopausal bone
health and cannot be generalized for all kinds of osteoporosis—especially, in male population. Due to ethical obligations, we could not include DXA scan within our screening study design and
had to rely on a DXA instrument calibrated BINDEX pulse-echo ultrasonography (i.e., 90% accurate compared to the DXA method) for BMD classifications. The state-of-the-art knowledge on the
production/metabolism of VOCs in the human gut and specific microbial colonies are largely based on in vitro and ex vivo observations. Longitudinal in vivo multi-omic investigations are
indispensable to enhance our fundamental, mechanistic and system-wide (from host and microbial genome towards exhaled volatile metabolome) understanding of individual/demographic group
specific relationship and biochemical milieus between volatile markers and systemic microbiome under compromised bone health and/or other conditions/comorbidities of interest. Observed
associations between postmenopausal bone health and exhaled metabolic markers revealed certain unexplored avenues of gut-bone axis. As postmenopausal osteoporosis progresses silently or with
no/minimal symptoms, women do not recognize and/or often ignore the underlying risk. Thus, they do not seek a bone health checkup—until the first low-energy fracture occurs. Such a fracture
in turn confirms the pronounced manifestation of osteoporosis. After the first fracture, the risk to experience a second fracture increases two fold, especially within the next year7,72.
According to the EU guidelines for prevention and management of osteoporosis, physiological (or sufficient) level of 25(OH)D and calcium have to be achieved and the patients are encouraged
towards physical activity to stimulate bone formation. Beyond this, pharmacotherapy for inhibiting osteoclast maturation and/or activation is achieved via clinical administrations of
bisphosphonates, human monoclonal antibody (directed against RANKL) and selective estrogen receptor modulators73. Though, these therapies may support the healing of an acute fracture and are
directed to lower the risk for subsequent fractures, the improvement of BMD and formation of new bone mass at the site of a fracture is time consuming. Consequently, those measures cannot
completely prevent the occurrence of a fracture in all cases74. Thus, due to the delayed therapeutic intervention (even before a low-energy fracture), bone health gradually deteriorates
towards severe and irreversible osteoporosis. Here, our findings offered an independently validated non-invasive option with high test accuracy (as benchmarking) for predicting osteoporosis
risk and depicted translational potential for both point-of-care (PoC) and personalized monitoring of postmenopausal bone health. Despite the seasonal effects on subjects’ BMD distributions
and VOC exhalations, our test accuracy based on breath DMS was not hampered and it allowed more robust, reliable and rapid stratification of postmenopausal osteoporosis risk than the
conventional and invasive methods e.g., serum bone markers measurement. Thus, breath biomarkers based immediate and repeatable assessments of disease severity will enable timely preventive
measures to improve quality of life as well as will allow quick selection of subjects at the PoC for further costly diagnostic methods (e.g., DXA scan), if necessary. Subsequent development
and optimization of target VOC specific real-time breath sensors will facilitate daily self-monitoring (even at home) of early onset and/or asymptomatic progression of osteoporosis by
postmenopausal women in order to seek precise medical attention – well ahead the disease silently worsens towards a low-energy fracture. Thus, our findings have the potential to reduce the
raising public health burden and global economic expenditure, spent relentlessly to manage postmenopausal osteoporosis and associated fractures. In a perspective, our results also indicate
unexplored investigative avenues towards new therapeutic targets e.g., via gut-microbiome specific pre-/probiotics. DATA AVAILABILITY All disclosable data including source data are available
in the main text or the Supplementary Data files 1-4. Raw and processed experimental data (i.e., not under ethical restrictions) is available from the corresponding author upon reasonable
request. However, source data for individual bone mineral density (BMD) quantified values are linked to patient’s original record with potentially identifying and sensitive information that
could compromise the privacy of research participants. Therefore, BMD values cannot be made publicly available due to the General Data Protection Regulation (GDPR) of the European Union and
ethical constrain. REFERENCES * Harada, S. & Rodan, G. A. Control of osteoblast function and regulation of bone mass. _Nature_ 423, 349–355 (2003). Article CAS PubMed Google Scholar
* Fukumoto, S. & Martin, T. J. Bone as an endocrine organ. _Trends Endocrinol. Metab._ 20, 230–236 (2009). Article CAS PubMed Google Scholar * Teitelbaum, S. L. Bone resorption by
osteoclasts. _Science_ 289, 1504–1508 (2000). Article CAS PubMed Google Scholar * Eastell, R. et al. Postmenopausal osteoporosis. _Nat. Rev. Dis. Primers_ 2, nrdp201669 (2016). Article
Google Scholar * Alami, S., Hervouet, L., Poiraudeau, S., Briot, K. & Roux, C. Barriers to effective postmenopausal osteoporosis treatment: a qualitative study of patients’ and
practitioners’ views. _PLoS ONE_ 11, https://doi.org/10.1371/journal.pone.0158365 (2016). * Sözen, T., Özışık, L. & Başaran, N. Ç. An overview and management of osteoporosis. _Eur. J.
Rheumatol._ 4, 46–56 (2017). Article PubMed Google Scholar * Kanis, J. A. et al. Identification and management of patients at increased risk of osteoporotic fracture: outcomes of an ESCEO
expert consensus meeting. _Osteoporos Int._ 28, 2023–2034 (2017). Article CAS PubMed PubMed Central Google Scholar * Kanis, J. A. & Scientific Advisory Board of the European
Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF), et al.
European guidance for the diagnosis and management of osteoporosis in postmenopausal women. _Osteoporos Int_. 24, 23–57 (2013). * Walker, M. D. & Shane, E. Postmenopausal osteoporosis.
_New England J. Med._ 389, 1979–1991 (2023). Article Google Scholar * Christenson, R. H. Biochemical markers of bone metabolism: an overview. _Clin. Biochem._ 30, 573–593 (1997). Article
CAS PubMed Google Scholar * Dixon, W. G., and on behalf of the European Prospective Osteoporosis Study Group, et al. Low grip strength is associated with bone mineral density and
vertebral fracture in women. _Rheumatology_ 44, 642–646 (2005). Article CAS PubMed Google Scholar * Karjalainen, J. P., Riekkinen, O., Töyräs, J., Jurvelin, J. S. & Kröger, H. New
method for point-of-care osteoporosis screening and diagnostics. _Osteoporos Int._ 27, 971–977 (2016). Article CAS PubMed Google Scholar * Schousboe, J. T., Riekkinen, O. &
Karjalainen, J. Prediction of hip osteoporosis by DXA using a novel pulse-echo ultrasound device. _Osteoporos Int._ 28, 85–93 (2017). Article CAS PubMed Google Scholar * Lewiecki, E. M.
Pulse-echo ultrasound identifies caucasian and hispanic women at risk for osteoporosis. _J. Clin. Densitometry_ 24, 175–182 (2021). Article Google Scholar * Schini, M., Vilaca, T.,
Gossiel, F., Salam, S. & Eastell, R. Bone turnover markers: basic biology to clinical applications. _Endocr. Rev._ 44, 417–473 (2023). Article PubMed Google Scholar * Filella, X.
& Guañabens, N. Clinical use of bone markers: a challenge to variability. Advances in Laboratory Medicine/Avances en Medicina de Laboratorio. https://doi.org/10.1515/almed-2023-0092
(2023). * Koch, L. Assay variability of biochemical markers of bone turnover. _Nat. Rev. Endocrinol._ 5, 587–587 (2009). Google Scholar * Pratt, J. et al. Grip strength performance from
9431 participants of the GenoFit study: normative data and associated factors. _GeroScience_ 43, 2533–2546 (2021). Article CAS PubMed PubMed Central Google Scholar * Richardson, J. K.
& Ellmers, T. J. The relationship between clinical measures of cognitive function and grip strength in healthy older adults. _BMC Geriatr._ 22, 907 (2022). Article PubMed PubMed
Central Google Scholar * Carson, R. G. Get a grip: individual variations in grip strength are a marker of brain health. _Neurobiol. Aging_ 71, 189–222 (2018). Article PubMed Google
Scholar * Miekisch, W., Sukul, P. & Schubert, J. K. Chapter 2 Origin and Emission of Volatile Biomarkers in Breath: Basics and Dynamic Aspects. In _Volatile Biomarkers for Human Health:
From Nature to Artificial Senses_, 22–38 (The Royal Society of Chemistry, 2023). * Sukul, P. et al. Effects of COVID-19 protective face-masks and wearing durations onto
respiratory-haemodynamic physiology and exhaled breath constituents. _Eur. Resp. J._ 60, 2200009 (2022). Article Google Scholar * Sukul, P. et al. Physiological and metabolic effects of
healthy female aging on exhaled breath biomarkers. _iScience_ 25, 103739 (2022). Article CAS PubMed PubMed Central Google Scholar * Mochalski, P., King, J., Mayhew, C. A. &
Unterkofler, K. A review on isoprene in human breath. _J. Breath Res._ https://doi.org/10.1088/1752-7163/acc964 (2023). * Pugliese, G. et al. Real-time metabolic monitoring under exhaustive
exercise and evaluation of ventilatory threshold by breathomics: Independent validation of evidence and advances. Frontiers in Physiology 13 (2022). * Sukul, P., Schubert, J. K., Trefz, P.
& Miekisch, W. Natural menstrual rhythm and oral contraception diversely affect exhaled breath compositions. _Scientific Reports_ 8, 10838 (2018). Article PubMed PubMed Central Google
Scholar * Sukul, P., Richter, A., Junghanss, C., Schubert, J. K. & Miekisch, W. Origin of breath isoprene in humans is revealed via multi-omic investigations. _Commun. Biol._ 6, 1–12
(2023). Article Google Scholar * Remy, R. et al. Profiling of exhaled volatile organics in the screening scenario of a COVID-19 test center. iScience, 105195 (2022). * Ibrahim, W. et al.
Visualization of exhaled breath metabolites reveals distinct diagnostic signatures for acute cardiorespiratory breathlessness. _Sci. Transl. Med._ 14, eabl5849 (2022). Article CAS PubMed
PubMed Central Google Scholar * Trefz, P. et al. Non-invasive assessment of metabolic adaptation in paediatric patients suffering from type 1 Diabetes Mellitus. _J. Clin. Med_. 8,
https://doi.org/10.3390/jcm8111797 (2019). * Löser, B. et al. Changes of exhaled volatile organic compounds in postoperative patients undergoing analgesic treatment: a prospective
observational study. _Metabolites_ 10, 321 (2020). Article PubMed PubMed Central Google Scholar * Trefz, P. et al. Drug detection in breath: non-invasive assessment of illicit or
pharmaceutical drugs. _J. Breath Res._ 11, 024001 (2017). Article PubMed Google Scholar * Brock, B. et al. Monitoring of breath VOCs and electrical impedance tomography under pulmonary
recruitment in mechanically ventilated patients. _J. Breath Res._ 11, 016005 (2017). Article PubMed Google Scholar * Sukul, P. & Trefz, P. Physio-metabolic monitoring via breath
employing real-time mass spectrometry: importance, challenges, potentials, and pitfalls. In _Bioanalytical Reviews_, 1–18 (Springer, 2022). * Modak, A. S. Why have only a handful of breath
tests made the transition from R&D to clinical practice? _J. Breath Res._ 18, 012001 (2023). Article Google Scholar * Behrens, M. et al. The Bindex® ultrasound device: reliability of
cortical bone thickness measures and their relationship to regional bone mineral density. _Physiol. Meas._ 37, 1528 (2016). Article PubMed Google Scholar * Mühldorfer-Fodor, M. et al.
Grip force monitoring on the hand: Manugraphy system versus Jamar dynamometer. _Arch. Orthop. Trauma Surg._ 134, 1179–1188 (2014). Article PubMed Google Scholar * Mathiowetz, V., Weber,
K., Volland, G. & Kashman, N. Reliability and validity of grip and pinch strength evaluations. _J. Hand Surg._ 9, 222–226 (1984). Article CAS Google Scholar * Doyon, A. et al. Markers
of bone metabolism are affected by renal function and growth hormone therapy in children with chronic kidney disease. _PLoS ONE_ 10, e0113482 (2015). Article PubMed PubMed Central Google
Scholar * Fischer, D.-C. et al. Hemodiafiltration is associated with reduced inflammation and increased bone formation compared with conventional hemodialysis in children: the HDF, Hearts
and Heights (3H) Study. _Kidney Int. Rep._ 6, 2358–2370 (2021). Article PubMed PubMed Central Google Scholar * Sukul, P., Trefz, P., Kamysek, S., Schubert, J. K. & Miekisch, W.
Instant effects of changing body positions on compositions of exhaled breath. _J. Breath Res._ 9, 047105 (2015). Article PubMed Google Scholar * Sukul, P., Oertel, P., Kamysek, S. &
Trefz, P. Oral or nasal breathing? Real-time effects of switching sampling route onto exhaled VOC concentrations. _J. Breath Res._ 11, 027101 (2017). Article PubMed Google Scholar *
Sukul, P., Schubert, J. K., Kamysek, S., Trefz, P. & Miekisch, W. Applied upper-airway resistance instantly affects breath components: a unique insight into pulmonary medicine. _J.
Breath Res._ 11, 047108 (2017). Article PubMed Google Scholar * Sukul, P. et al. Exhaled breath compositions under varying respiratory rhythms reflects ventilatory variations: translating
breathomics towards respiratory medicine. _Sci. Rep._ 10, 14109 (2020). Article CAS PubMed PubMed Central Google Scholar * Sukul, P., Trefz, P., Schubert, J. K. & Miekisch, W.
Advanced setup for safe breath sampling and patient monitoring under highly infectious conditions in the clinical environment. _Sci. Rep._ 12, 17926 (2022). Article CAS PubMed PubMed
Central Google Scholar * Trefz, P. et al. Continuous real time breath gas monitoring in the clinical environment by proton-transfer-reaction-time-of-flight-mass spectrometry. _Anal. Chem._
85, 10321–10329 (2013). Article CAS PubMed Google Scholar * Sukul, P., Richter, A., Schubert, J. K. & Miekisch, W. Deficiency and absence of endogenous isoprene in adults,
disqualified its putative origin. _Heliyon_ 7, e05922 (2021). Article CAS PubMed PubMed Central Google Scholar * Schwoebel, H. et al. Phase-resolved real-time breath analysis during
exercise by means of smart processing of PTR-MS data. _Anal. Bioanal Chem._ 401, 2079–2091 (2011). Article CAS PubMed Google Scholar * Sukul, P., Trefz, P., Schubert, J. K. &
Miekisch, W. Immediate effects of breath holding maneuvers onto composition of exhaled breath. _J. Breath Res._ 8, 037102 (2014). Article CAS PubMed Google Scholar * Trefz, P., Schubert,
J. K. & Miekisch, W. Effects of humidity, CO2 and O2 on real-time quantitation of breath biomarkers by means of PTR-ToF-MS. _J. Breath Res._ 12, 026016 (2018). Article PubMed Google
Scholar * Sirola, J., Tuppurainen, M., Honkanen, R., Jurvelin, J. S. & Kröger, H. Associations between grip strength change and axial postmenopausal bone loss—a 10-year population-based
follow-up study. _Osteoporos Int._ 16, 1841–1848 (2005). Article PubMed Google Scholar * Rapuri, P. B., Kinyamu, H. K., Gallagher, J. C. & Haynatzka, V. Seasonal changes in
calciotropic hormones, bone markers, and bone mineral density in elderly women. _J. Clin. Endocrinol. Metab._ 87, 2024–2032 (2002). Article CAS PubMed Google Scholar * Tangerman, A.
Measurement and biological significance of the volatile sulfur compounds hydrogen sulfide, methanethiol and dimethyl sulfide in various biological matrices. _J. Chromatogr. B_ 877, 3366–3377
(2009). Article CAS Google Scholar * Silva, Y. P., Bernardi, A. & Frozza, R. L. The role of short-chain fatty acids from gut microbiota in gut-brain communication. _Front.
Endocrinol_. 11, https://doi.org/10.3389/fendo.2020.00025 (2020). * Wang, L. et al. Acetic acid and butyric acid released in large intestine play different roles in the alleviation of
constipation. _J. Funct. Foods_ 69, 103953 (2020). Article CAS Google Scholar * Miekisch, W., Schubert, J. K., Vagts, D. A. & Geiger, K. Analysis of volatile disease markers in blood.
_Clin. Chem._ 47, 1053–1060 (2001). Article CAS PubMed Google Scholar * Zaiss, M. M., Jones, R. M., Schett, G. & Pacifici, R. The gut-bone axis: how bacterial metabolites bridge the
distance. _J. Clin. Invest._ 129, 3018–3028 (2019). Article PubMed PubMed Central Google Scholar * Tu, Y., Yang, R., Xu, X. & Zhou, X. The microbiota-gut-bone axis and bone health.
_J. Leukocyte Biol._ 110, 525–537 (2021). Article CAS PubMed Google Scholar * He, Y. & Chen, Y. The potential mechanism of the microbiota-gut-bone axis in osteoporosis: a review.
_Osteoporos Int._ 33, 2495–2506 (2022). Article PubMed Google Scholar * Kalantar-Zadeh, K., Berean, K. J., Burgell, R. E., Muir, J. G. & Gibson, P. R. Intestinal gases: influence on
gut disorders and the role of dietary manipulations. _Nat. Rev. Gastroenterol. Hepatol._ 16, 733–747 (2019). Article CAS PubMed Google Scholar * Mutuyemungu, E., Singh, M., Liu, S. &
Rose, D. J. Intestinal gas production by the gut microbiota: a review. _J. Funct. Foods_ 100, 105367 (2023). Article Google Scholar * Navik, U. et al. Methionine as a double-edged sword
in health and disease: current perspective and future challenges. _Ageing Res. Rev._ 72, 101500 (2021). Article CAS PubMed Google Scholar * Yang, G. et al. Dietary methionine
supplementation during the estrous cycle improves follicular development and estrogen synthesis in rats. _Food Funct._ 15, 704–715 (2024). Article CAS PubMed Google Scholar * Troen, A.
M., Lutgens, E., Smith, D. E., Rosenberg, I. H. & Selhub, J. The atherogenic effect of excess methionine intake. _Proc. Natl. Acad. Sci. USA_ 100, 15089–15094 (2003). Article CAS
PubMed PubMed Central Google Scholar * Shah, S., Bell, R. J. & Davis, S. R. Homocysteine, estrogen and cognitive decline. _Climacteric_ 9, 77–87 (2006). Article CAS PubMed Google
Scholar * Gaikwad, N. W. Mass spectrometry evidence for formation of estrogen–homocysteine conjugates: estrogens can regulate homocysteine levels. _Free Rad. Biol. Med._ 65, 1447–1454
(2013). Article CAS PubMed Google Scholar * Dimitrova, K. R., DeGroot, K., Myers, A. K. & Kim, Y. D. Estrogen and homocysteine. _Cardiovasc. Res._ 53, 577–588 (2002). Article CAS
PubMed Google Scholar * Morris, M. S., Jacques, P. F., Selhub, J. & Rosenberg, I. H. Total homocysteine and estrogen status indicators in the third national health and nutrition
examination survey. _Am. J. Epidemiol._ 152, 140–148 (2000). Article CAS PubMed Google Scholar * Smolders, R. G. V. et al. Hormone replacement influences homocysteine levels in the
methionine-loading test: a randomized placebo controlled trial in postmenopausal women. _Eur. J. Obstet. Gynecol. Reprod. Biol._ 117, 55–59 (2004). Article CAS PubMed Google Scholar *
Cagnacci, A., Generali, M., Pirillo, D., Baldassari, F. & Volpe, A. Effects of low- or high-dose hormone therapy on fasting and post-methionine homocysteine levels in postmenopausal
women. _Climacteric_ 9, 388–395 (2006). Article CAS PubMed Google Scholar * Kalapos, M. P. On the mammalian acetone metabolism: from chemistry to clinical implications. _Biochim.
Biophys. Acta_ 1621, 122–139 (2003). Article CAS PubMed Google Scholar * Cappola, A. R. & Shoback, D. M. Osteoporosis therapy in postmenopausal women with high risk of fracture.
_JAMA_ 316, 715–716 (2016). Article PubMed Google Scholar * Kanis, J. A., Cooper, C., Rizzoli, R. & Reginster, J.-Y. European guidance for the diagnosis and management of osteoporosis
in postmenopausal women. _Osteoporos Int._ 30, 3–44 (2019). Article CAS PubMed Google Scholar * McNamara, L. M. Perspective on post-menopausal osteoporosis: establishing an
interdisciplinary understanding of the sequence of events from the molecular level to whole bone fractures. _J. R. Soc. Interface_ 7, 353–372 (2010). Article CAS PubMed Google Scholar
Download references ACKNOWLEDGEMENTS We thank all participants for their voluntary participation in this study. This study was supported by European Union fund for regional development
(EFRE), EU Horizon-2020 grant H2020-PCH-HEARTEN project 643694, European Union’s Horizon 2020 Marie Skłodowska-Curie research and innovation programme 674911-IMPACT and University Medicine
Rostock’s FORUN programme (2018) 889003. Funders have no role in study design, conduct and dissemination of the outcome. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Rostock Medical Breath
Research Analytics and Technologies (ROMBAT), Department of Anesthesiology, Intensive Care Medicine and Pain Therapy, Rostock University Medical Center, Rostock, Germany Pritam Sukul, Daniel
A. Reuter, Jochen K. Schubert & Wolfram Miekisch * Department of Pediatrics, Rostock University Medical Center, Rostock, Germany Dagmar-Christiane Fischer, Celine Broderius, Simon
Grzegorzewski & Anja Rahn * Department of Traumatology, Hand and Reconstructive Surgery, Rostock University Medical Center, Rostock, Germany Celine Broderius, Simon Grzegorzewski &
Thomas Mittlmeier * Institute of Medical Microbiology, Virology and Hygiene, Rostock University Medical Center, Rostock, Germany Bernd Kreikemeyer Authors * Pritam Sukul View author
publications You can also search for this author inPubMed Google Scholar * Dagmar-Christiane Fischer View author publications You can also search for this author inPubMed Google Scholar *
Celine Broderius View author publications You can also search for this author inPubMed Google Scholar * Simon Grzegorzewski View author publications You can also search for this author
inPubMed Google Scholar * Anja Rahn View author publications You can also search for this author inPubMed Google Scholar * Thomas Mittlmeier View author publications You can also search for
this author inPubMed Google Scholar * Bernd Kreikemeyer View author publications You can also search for this author inPubMed Google Scholar * Daniel A. Reuter View author publications You
can also search for this author inPubMed Google Scholar * Jochen K. Schubert View author publications You can also search for this author inPubMed Google Scholar * Wolfram Miekisch View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS P.S. conceived the idea and with D.C.F. conceptualized the study. P.S., D.C.F., T.M., J.K.S.,
W.M. designed the study. P.S., D.C.F., T.M., D.A.R., B.K., J.K.S., W.M. developed the analytical methods. C.B., S.G., P.S. recruited the volunteers and conducted the investigations. P.S.,
C.B., S.G., A.R. analyzed the data and prepared the results. P.S., D.C.F., D.A.R., B.K., W.M. visualized the results. P.S., D.C.F., T.M., B.K., D.A.R., J.K.S., W.M. interpreted outcomes.
P.S., D.C.F., T.M., D.A.R., J.K.S., W.M. acquired all resources. P.S., D.C.F., T.M., J.K.S., W.M. administered the project. P.S., D.C.F., T.M., J.K.S., W.M. supervised the project. P.S.
wrote the original draft. D.C.F., C.B., S.G., A.R., T.M., B.K., D.A.R., J.K.S., W.M., P.S. reviewed and edited the original draft. All authors approved the final version of the manuscript.
CORRESPONDING AUTHOR Correspondence to Pritam Sukul. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Communications
Medicine_ thanks Michael Schoenemann, Patrik Spanel and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION PEER
REVIEW FILE SUPPLEMENTARY MATERIALS DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4 REPORTING SUMMARY RIGHTS
AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use,
sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or
other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sukul, P., Fischer,
DC., Broderius, C. _et al._ Exhaled breath metabolites reveal postmenopausal gut-bone cross-talk and non-invasive markers for osteoporosis. _Commun Med_ 4, 279 (2024).
https://doi.org/10.1038/s43856-024-00723-4 Download citation * Received: 30 April 2024 * Accepted: 19 December 2024 * Published: 28 December 2024 * DOI:
https://doi.org/10.1038/s43856-024-00723-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Suspected qaeda militant killed in yemen drone strikeA suspected Al-Qaeda member died today in a drone strike on a region of Yemen hit by multiple US air raids over the past...
News - Breaking News, Latest News, India News, World News, Bollywood, Sports, Business and Political News | Times of IndiaWe use cookies and other tracking technologies to provide services while browsing the Website to show personalise conten...
Stephen king says mypillow will “pretty soon” be twitter’s “only advertiser left” & elon musk reactsStephen King is back at calling out Twitter‘s new direction as Elon Musk took the reins of the company. This time, the b...
Bears suffer devastating double doink on potential game-winning field goal attemptYou can't make this stuff up. The Chicago Bears were looking to lock up a playoff win against the Philadelphia Eagl...
Test Your Knowledge With AARP’s August 15 News QuizRob Dobi Facebook Twitter LinkedIn Question 1 of 8 True or false? An AARP survey found that about a third of older LGBTQ...
Latests News
Exhaled breath metabolites reveal postmenopausal gut-bone cross-talk and non-invasive markers for osteoporosisABSTRACT BACKGROUND Menopause driven decline in estrogen exposes women to risk of osteoporosis. Detection of early onset...
Oregon's cash-strapped counties reject public safety leviesTwo Oregon counties have reportedly rejected property tax increases that would have funded law enforcement and public sa...
Fisherman's wife seeks govt's help to get his body from pakJivuben Chauhan, a resident of Paldi village in Una tehsil of Gir Somnath district, wrote a letter in this connection to...
Amitabh bachchan and madhuri dixit nene find a place in the 2018 pakistan elections and twitterati has a field day!It's a weird day when you wake up to see Bollywood superstars endorsing Pakistani politicians... The Pakistan Elect...
Texas cities see recovery's strongest growthHouston's skyline. Aaron M. Sprecher | Bloomberg | Getty Images Metropolitan areas in Texas saw the fastest economi...