Viral infection as an nad+ battlefield
Viral infection as an nad+ battlefield"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Coronavirus replication results in expenditure of nicotinamide adenine dinucleotide (NAD+), the central catalyst of cellular metabolism, in the innate response to infection. Repletion of
NAD+ levels has the potential to enhance antiviral responses. Since early 2020, the roughly 30-kb SARS-CoV-2 RNA genome has rewritten the rules for human life. Causing perhaps 250 million
infections and 5 million deaths1, the polyprotein instructions on this positive RNA strand dictate assembly of active viral particles that do a remarkable job of spreading to others through
aerosol droplets, causing life-threatening disease in a wide swath of infected individuals. The ongoing coronavirus pandemic has reorganized social and economic relationships globally. The
potential of pets and wildlife to be currently incubating SARS-CoV-2 variants coupled with persistent vaccine hesitance and problems with vaccine distribution all limit confidence that any
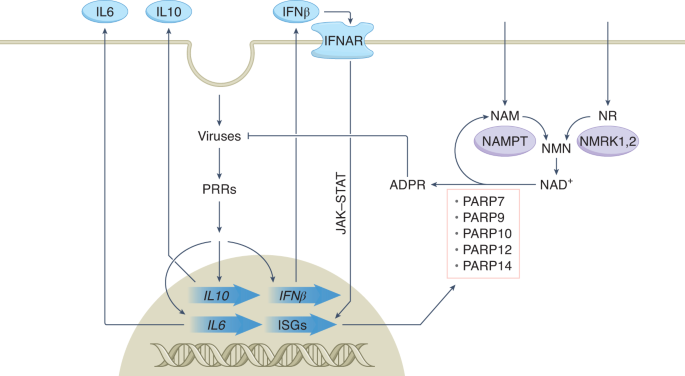
wave of infection is the last. From their earliest replication intermediates, coronaviruses elicit an attack on cellular NAD+. Cellular NAD+ becomes a battlefield in which the innate immune
system and the virus struggle for the upper hand. Based on preclinical work demonstrating transcriptional upregulation of NAD+-consuming enzymes and particular NAD+ biosynthesis pathways2,
early clinical data indicate that NAD+ repletion might constitute an antiviral treatment approach3 that could be generally useful against infectious agents that activate the interferon
system. The caveat—as with all approaches targeted to inflammation—is that inflammatory responses are themselves antiviral, although the severe cytokine storm associated with acute and
chronic viral disease is considered a pathological overreaction. Positive-strand RNA viruses, such as SARS-CoV-2, reveal a double-stranded RNA replication intermediate that binds to a
pattern recognition receptor (PRR), the product of the retinoic acid-inducible gene I (_RIG-I_; also known as _DDX58_)4, and activates an antiviral immune response in naive cells. The RIG-I
system leads to activation of interferon-β (IFNβ) transcription, followed by synthesis of IFN proteins that exit cells and bind to IFN receptors (IFNAR) in infected and neighboring cells.
Interferon signalling proceeds through the JAK–STAT pathway to activate transcription of a set of interferon-stimulated genes (ISGs)5 (Fig. 1). As an innate response, the interferon system
is, by definition, not educated by exposure to specific viral antigens and is highly similar in response to other molecular patterns, including those encoded by bacteria and unrelated
viruses. Multiple ISG products have been shown to have potent antiviral activities. For example, higher circulating levels of 2′–5′ oligoadenylate synthetase 1 (OAS1), a component of
antiviral RNase L activity, are strongly associated with COVID-19 resistance6. Assembly of a set of 71 genes that generate and respond to the NAD+ metabolome7 revealed that SARS-CoV-2
infection strongly induces transcription of several genes with the potential to consume NAD+ while also increasing expression of a subset of NAD+ biosynthetic genes2. Among the
NAD+-consuming enzymes, PARP7, PARP9, PARP10, PARP12 and PARP14 are named for their membership in the poly(ADPribose) polymerase superfamily. However, rather than polymerize ADPribose, these
enzymes transfer single ADPribose units from NAD+ to polypeptide sidechains in order to modify protein functions8. Coronaviruses encode an enzymatic activity that removes ADPribose
modifications9, suggesting that infected cells expend NAD+ in antiviral ADPribosylating activities while the virus attempts to reverse these modifications. Indeed, coronavirus infection
strikingly depletes cellular NAD+ levels, and boosting NAD+ pharmacologically or with NAD+ precursors increases the enzymatic activity of antiviral PARP isozymes and inhibits coronavirus
replication in vitro2. Metabolism is the set of processes that allows us to convert everything we eat into everything we are and everything we do. The underlying chemistry of fuel oxidation,
ATP generation, macromolecule synthesis and the generation and detoxification of reactive oxygen species involves electron flow in which the four NAD+ coenzymes are the literal electrical
conduits. Because cells struggle to maintain levels of NADH, NADP+ or NADPH when NAD+ is being churned by PARP activities, the data suggest that viral infection may generally impair host
metabolic functions. It follows that if the induction of mono(ADPribosylating) enzymes is common to interferon system activation, chronic inflammation may be an indicator of a local or
systemically stressed NAD+ system, and a wide variety of bacterial and viral infections may similarly depress the NAD+ system. Model systems have found numerous examples in which tissue NAD+
levels are depressed, for example in heart failure10, and peripheral11 and central12 neurodegeneration. These disturbances in NAD+ are accompanied by transcriptional induction of
nicotinamide (NAM) riboside (NR) kinase genes13 as part of a homeostatic mechanism to restore NAD+ metabolomes through the AMP kinase alarm system10. In response to coronavirus infection, NR
kinase (NMRK) and NAM phosphoribosyltransferase (NAMPT) genes are upregulated, while NAD+ biosynthetic capacity from other NAD+ precursors is dampened2. These data suggested that NR or NAM
as NAD+ precursors or pharmacological activators of NAMPT might support the mono(ADPribosylating) activities of PARP in infection prevention or control. Consistent with the view that viral
infection leads to a bioenergetic crisis, blood from patients with severe COVID-19 contains lower levels of NAD+ metabolites and higher levels of AMP14. In addition, dysregulation of NAD+
metabolites and AMP in the blood of patients with severe COVID-19 correlates with high circulating levels of the inflammatory cytokines IL-6, IL-10, IL-8, M-CSF and IL-1α14. Notably,
inflammatory cytokine gene expression is downstream of PRR engagement and associated with antiviral defenses. However, persistent high-level expression is a sign of severe disease. Small
clinical studies have shown that oral supplementation with the NAD+ precursor NR lowers circulation of inflammatory cytokines in healthy older adults15 and depresses expression of
inflammatory cytokines in the peripheral blood mononuclear cells of people undergoing heart failure16. Following completion of a positive open-label phase 2 trial, a double-blinded phase 3
trial was completed, in which patients with mild-to-moderate COVID-19 were assigned to standard of care plus placebo (_n_ = 75) or to standard of care plus a combination of four nutritional
supplements, consisting of 3.7 g carnitine, 2.6 g _N_-acetylcysteine, 1 g NR and 12.4 g serine (_n_ = 229), twice a day for two weeks3. Standard of care was either hydroxychloroquine17 or
favipiravir18, the repurposed malaria and flu drugs that do not have activity against SARS-CoV-2 in clinical trials. Those on the supplement cocktail had a time to complete recovery of 5.7
days, compared with 9.2 days for those on placebo. This was significant at _P_ < 2.0 × 10−16 with a hazard ratio of 5.6 (95% confidence interval = 4.1–7.7). Consistent with a known
activity of high-dose NR, those taking the supplement cocktail had reduced circulating levels of IL6, IL10, IFNγ, CXCL10, CCL19, CX3CL1, CXCL11, IL-15RA, IL-17C, MCP-2 and TNFα3. Consistent
with the view that any virus that engages the innate immune system to launch an interferon response attacks the NAD+ system, zika virus infection has been shown to do just this in mouse
brains19. Furthermore, NR protects cortex thickness, brain and body weight, and modestly improves survival of zika-infected mice when provided as an oral supplement19. Mechanistic questions
that remain to be resolved include the definition of the cellular or viral targets of the mono(ADPribosylating) PARP isozymes, and the dissection of how age and pre-existing conditions
modify inflammation, the NAD+ system and antiviral defenses. Trials are being conducted to test whether NAM will protect against lymphopenia (NCT04910230) and the general disease course
(NCT04751604) in COVID-19 and to determine whether NR will protect the kidneys (NCT04818216) and cognitive abilities (NCT04809974) of individuals with COVID-19 and those who have recovered
from it, respectively. We also suggest prevention trials to determine whether fortification of the NAD+ system may represent a relatively inexpensive way to protect exposed healthcare
workers and housemates from viral infection. REFERENCES * Dong, E., Du, H. & Gardner, L. _Lancet Infect. Dis._ 20, 533–534 (2020). Article CAS Google Scholar * Heer, C. D. _J. Biol.
Chem._ 295, 17986–17996 (2020). Article CAS Google Scholar * Altay, O. et al. https://doi.org/10.1101/2020.10.02.20202614_Advanced Science_. * Yamada, T. et al. _Nat. Immunol._ 22,
820–828 (2021). Article CAS Google Scholar * Rehwinkel, J. & Gack, M. U. _Nat. Rev. Immunol._ 20, 537–551 (2020). Article CAS Google Scholar * Zhou, S. et al. _Nat. Med._ 27,
659–667 (2021). Article CAS Google Scholar * Trammell, S. A. & Brenner, C. _Comput. Struct. Biotechnol. J._ 4, e201301012 (2013). Article Google Scholar * Cohen, M. S. _Genes Dev._
34, 254–262 (2020). Article CAS Google Scholar * Alhammad, Y. M. O. & Fehr, A. R. _Viruses_ 12, 384 (2020). Article CAS Google Scholar * Diguet, N. et al. _Circulation_ 137,
2256–2273 (2018). Article CAS Google Scholar * Sasaki, Y., Araki, T. & Milbrandt, J. _J. Neurosci._ 26, 8484–8491 (2006). Article CAS Google Scholar * Vaur, P. et al. _FASEB J._
31, 5440–5452 (2017). Article CAS Google Scholar * Bieganowski, P. & Brenner, C. _Cell_ 117, 495–502 (2004). Article CAS Google Scholar * Xiao, N. et al. _Nat. Commun._ 12, 1618
(2021). Article CAS Google Scholar * Elhassan, Y. S. et al. _Cell Rep_ 28, 1717–1728.e6 (2019). Article CAS Google Scholar * Zhou, B. et al. _J. Clin. Invest._ 130, 6054–6063 (2020).
Article CAS Google Scholar * Horby, P. et al. _N. Engl. J. Med._ 383, 2030–2040 (2020). Article Google Scholar * Hassanipour, S. et al. _Sci. Rep._ 11, 11022 (2021). Article CAS
Google Scholar * Pang, H. et al. _Nat. Metab._ 3, 1109–1124 (2021). Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Beckman Research
Institute, City of Hope National Medical Center, Duarte, CA, USA Charles Brenner Authors * Charles Brenner View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHOR Correspondence to Charles Brenner. ETHICS DECLARATIONS COMPETING INTERESTS C.B. developed intellectual property on nutritional and therapeutic uses of NR, which
were commercialized by ChromaDex. C.B. owns ChromaDex stock and serves as their chief scientific advisor. PEER REVIEW INFORMATION _Nature Metabolism_ thanks Eduardo Chini and the other,
anonymous, reviewers for their contribution to the peer review of this work. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Brenner, C. Viral infection
as an NAD+ battlefield. _Nat Metab_ 4, 2–3 (2022). https://doi.org/10.1038/s42255-021-00507-3 Download citation * Published: 03 January 2022 * Issue Date: January 2022 * DOI:
https://doi.org/10.1038/s42255-021-00507-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
The spatial and temporal reconstruction of a medieval moat ecosystemABSTRACT Moats and other historical water features had great importance for past societies. The functioning of these eco...
David edelstein's top 10 films of 2007_Fresh Air_'s arbiter of things filmic offers his annual year-end movies wrap-up. This time, his Top 10 list has 11...
German construction company avoided paying tax in greeceIn September last year, a court in Athens found that Hochtief, a German construction company running Athens Internationa...
Us star to fire starting gun on 100th le mans 24-hour raceLEBRON JAMES WILL JOIN LIST OF CELEBRITY ‘STARTERS’ WHO SIGNAL THE BEGINNING OF FAMOUS ENDURANCE EVENT US basketball sta...
Bring Her Home | PBSSHARE THIS SHOW * Link Copied to Clipboard HOW TO WATCH BRING HER HOME Bring Her Home is available to stream on pbs.org ...
Latests News
Viral infection as an nad+ battlefieldCoronavirus replication results in expenditure of nicotinamide adenine dinucleotide (NAD+), the central catalyst of cell...
University of london appointmentsABSTRACT THE following appointments have been made in the University of London: Dr. D. H. Hey, since 1941 director of re...
Inside rebel wilson's 'spectacular' birthday weekend in cabo — plus, the villa where she stayedRebel Wilson is living it up in luxury for her 42nd birthday! The Australian actress spent the past few days in Mexico a...
Editorial | Journal of Exposure Science & Environmental EpidemiologyDuring the past few years, an ever-increasing number of authors have requested to submit their work electronically to _t...
Boxer gervonta davis cursed out, sucker punched nyc parking attendant: suitEXPLORE MORE Boxing superstar and lightweight champion Gervonta “Tank” Davis impatiently cursed out and sucker-punched a...