Nucleophilic reactivity of a copper(ii)-hydroperoxo complex
Nucleophilic reactivity of a copper(ii)-hydroperoxo complex"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Copper(II)-hydroperoxo species are often detected as key intermediates in metalloenzymes and biomimetic compounds containing copper. However, the only reactivity has previously been
observed for the copper(II)-hydroperoxo complexes is electrophilic, occurring through O-O bond cleavage. Here we report that a mononuclear end-on copper(II)-hydroperoxo complex, which has
been successfully characterized by various physicochemical methods including UV-vis, rRaman, CSI-MS and EPR, is a reactive oxidant that utilizes a nucleophilic mechanism. In addition, DFT
calculations fully support the electronic structure of this complex as a copper(II)-hydroperoxo complex with trigonal bipyramidal coordination geometry. A positive Hammett _ρ_ value (2.0(3))
is observed in the reaction of copper(II)-hydroperoxo complex with _para_-substituted acyl chlorides, which clearly indicates nucleophilic character for the copper(II)-hydroperoxo complex.
The copper(II)-hydroperoxo complex is an especially reactive oxidant in aldehyde deformylation with 2-PPA and CCA relative to the other metal-bound reactive oxygen species reported so far.
The observation of nucleophilic reactivity for a copper(II)-hydroperoxo species expands the known chemistry of metal-reactive oxygen species. SIMILAR CONTENT BEING VIEWED BY OTHERS TRAPPING
OF A PHENOXYL RADICAL AT A NON-HAEM HIGH-SPIN IRON(II) CENTRE Article 12 January 2024 COPPER-DEPENDENT HALOGENASE CATALYSES UNACTIVATED C−H BOND FUNCTIONALIZATION Article 29 January 2025
IRON-CATALYZED ALIPHATIC C–H FUNCTIONALIZATION TO CONSTRUCT CARBON–CARBON BONDS Article Open access 20 May 2025 INTRODUCTION Copper-bound reactive oxygen adducts, such as Cu-superoxo,
-peroxo, -hydroperoxo, and -oxyl radical species, have been proposed to be reactive intermediates in biological systems1,2. One such intermediate, specifically a Cu(II)-hydroperoxo complex,
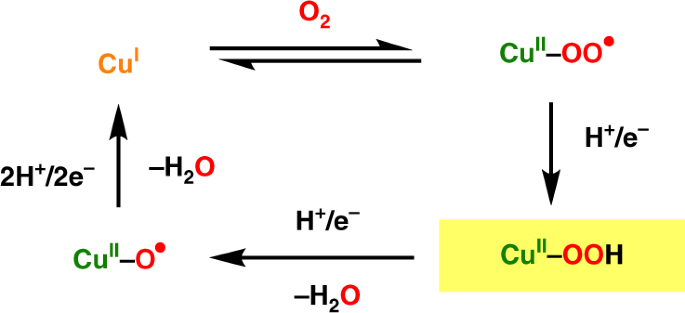
has received much attention since it has been hypothesized to play an important role in the catalytic cycle of certain copper-containing enzymes (Fig. 1). In
peptidylglycine-_α_-hydroxylating monooxygenase (PHM) and dopamine-_β_-monooxygenase (D_β_M), it has been proposed that Cu(II)-hydroperoxo species were converted to Cu(II)-oxyl radicals and
water after electron and proton transfers3,4,5. The reactive species in lytic polysaccharide monooxygenases (LPMOs) has also been proposed to be a Cu(II)-hydroperoxo intermediate that
directly reacts with polysaccharides6,7,8,9. In synthetic chemistry, a number of Cu(II)-OOH species have been prepared and characterized by spectroscopic and crystallographic methods.
Masuda’s group reported the first crystal structure of the Cu(II)-OOH complex bearing a trigonal bipyramidal ligand where the hydroperoxo bound Cu(II) core was stabilized through
intramolecular hydrogen bonding interactions10. Most recently, stabilization of [Cu(biot-et-dpea)(OOH)]+ has been achieved in artificial proteins by using antigen-antibody interaction11. In
general, the reaction of Cu(II)-OOH complexes with substrates occurs through O–O bond cleavage to give putative Cu(II)-oxyl radical or Cu(III)-oxo species. Detailed studies on the ligand
hydroxylation by the putative intermediates have been reported by Karlin and co-workers12. Recently, it was also reported that the cleavage of Cu–O bond in Cu(II)-OOH species results in the
_N_-dealkylation through the Fenton chemistry13. Notably, to our knowledge no reports of direct reactions of Cu(II)-OOH species with external substrates, i.e., prior to O–O bond cleavage,
have yet appeared in the literature, although numerous examples of electrophilic reactivity in Cu(II)-OOH complexes have been shown to occur via O–O bond cleaved intermediates14,15,16. By
contrast the nucleophilic reaction of Cu(II)-alkylperoxo complexes was reported very recently17. Herein, we report the nucleophilic reactivity of the Cu(II)-hydroperoxo complex,
[Cu(_i_Pr3-tren)(OOH)]+ (1, _i_Pr3-tren = tris[2-(isopropylamino)ethyl]amine), which we find is capable of oxidation of organic carbonyl compounds directly. 1 was successfully characterized
by various spectroscopic methods such as UV-vis, resonance Raman (rRaman), and electron paramagnetic resonance (EPR) spectroscopy together with cold spray ionization mass spectrometry
(CSI-MS). The nucleophilic reactivity of 1 has also been investigated by kinetic studies including a Hammett analysis. RESULTS SYNTHESIS AND CHARACTERIZATION OF COPPER COMPLEXES As a
starting material, the copper(II) complex, [Cu(_i_Pr3-tren)(CH3CN)]2+, was synthesized by combining _i_Pr3-tren and Cu(ClO4)2·H2O in acetonitrile and characterized using UV-vis, electrospray
ionization mass spectrometry (ESI-MS) and X-ray crystallography (see the Supplementary Methods, Supplementary Tables 1 and 2, and Supplementary Figs. 1 and 2). The copper(II)-hydroperoxo
complex, [Cu(_i_Pr3-tren)(OOH)]+ (1), was generated by the addition of 5 equiv of H2O2 and 2 equiv of TEA to [Cu(_i_Pr3-tren)(CH3CN)]2+ in CH3OH at –50 °C (Fig. 2). Spectrophotometric
titration used to monitor the formation of 1 revealed that 5 equiv of H2O2 is required for full formation (Supplementary Fig. 3). The intermediate 1 is observed to be metastable (e.g., ca.
5% decay for 1 h at –50 °C). The color of the solution changed from blue to green, with the final solution showing an intense band at 360 nm (_ε_ = 1300 M–1 cm–1) and two weak bands at 656
(_ε_ = 230 M–1 cm–1) and 830 nm (_ε_ = 270 M–1 cm–1) in the UV-vis spectrum, which are characteristic features of Cu(II)-hydroperoxo complexes (Fig. 3a)18,19,20,21,22,23,24. The former band
has been assigned to a LMCT band of the Cu(II)-OOH species, and the latter bands to the d-d transitions of the Cu(II) ion bearing a trigonal bipyramidal structure. The CSI-MS spectrum of 1
supported the formation of Cu(II)-hydroperoxo species, [Cu(_i_Pr3-tren)(OOH)]+ (1-16O2H) at a mass-to-charge ratio (_m/z_) of 368.2 (calcd _m/z_ 368.2; Fig. 3b, red line), together with some
unidentified species presumably resulting from the thermal instability of 1 (Supplementary Fig. 4). When the reactions were carried out with isotopically labeled H218O2 in CH3OH and 2H2O2
in CH3O2H, mass peaks corresponding to [Cu(_i_Pr3-tren)(18O18OH)]+ (1-18O2H) (Fig. 3b, blue line) and [Cu(_i_Pr3-tren)(OO2H)]+ (1-O22H) (Fig. 3b, green line) were observed at _m/z_ 372.2
(calcd _m/z_ 372.2) and 369.2 (calcd _m/z_ 369.2), respectively. The four-mass unit shift upon substitution of 16O with 18O demonstrates that 1 contains two oxygen atoms. The one-mass unit
shift in a deuterium isotope experiment verifies the existence of a hydroperoxide ligand in 1. The rRaman spectrum of 1 was collected using 405 nm excitation in CH3OH at –30 °C (Fig. 3a,
inset). 1-16O2H exhibits two isotope sensitive bands at 834 and 501 cm−1, which shifted to 790 and 482 cm−1, respectively, in 1-18O2H. The results support the assignments of these features
as O–O and Cu–O stretching vibration on the basis of 16-18Δ value of 44 and 19 cm−1 (16-18Δ (calcd) = 48 and 23 cm−1 for diatomic harmonic oscillator), respectively18,19,20,21,22,23,24. Upon
2H-substitution in 1, the characteristic Cu–O and O–O stretching vibrational features exhibited a 2-cm−1 downshift each (Supplementary Fig. 5), which is comparable to those of a number of
metal-hydroperoxo species (Supplementary Table 3)24,25,26,27,28,29,30. The EPR spectrum of a frozen solution of 1 at 113 K shows a reverse axial signal with g┴ = 2.26 (_A_┴ = 108 G) and g║ =
2.06 (_A_║ = 70 G), which is typical for trigonal bipyramidal five-coordinate Cu(II) complexes (Fig. 3c; see also Supplementary Fig. 6)2,18,31,32,33,34,35,36,37. Spin quantification finds
that the EPR signal corresponds to 91(6)% of the total copper content in the sample (see Supplementary Methods). All the spectroscopic results clearly show that 1 is assigned to be a
Cu(II)-hydroperoxo complex in trigonal bipyramidal geometry, which is further supported by DFT calculations (vide infra). COMPUTATIONAL STUDY To investigate the geometric structure of 1, DFT
calculations were conducted at the spin-unrestricted B3LYP level of theory (see Supplementary Methods). The optimized structure of 1 reveals the trigonal bipyramidal geometry whose axial
site is occupied by the hydroperoxo group in the manner of end-on replacing an acetonitrile of [Cu(_i_Pr3-tren)(CH3CN)]2+ (Fig. 2, and see also Supplementary Table 4). This result is in
agreement with the result of EPR spectroscopy described above. The calculated O–O bond distance is determined to be 1.46 Å, which is very close to that determined crystallographically for
Masuda’s Cu(II)-hydroperoxo complex (1.460 Å)10 and also that observed for homoprotocatechuate 2,3-dioxygenase (1.5 Å)38. TD-DFT calculations were employed to investigate the electronic
structure of 1. The calculated spectrum exhibits an intense peak at 368 nm and multiple weak peaks in the range from 650 to 850 nm which are seen in experimental UV-Vis spectrum
(Supplementary Fig. 7). The peak at 368 nm was comprised of electron transitions from overlapped orbitals between the ligand and Cu d-orbitals to σ* orbital of the dz2 orbital in Cu and pz
orbital in O2H. The minor peaks in the range from 650 to 850 nm are assigned as electron transitions between π* orbitals of Cu-OOH moieties. The singly occupied molecular orbital (SOMO) for
1 was observed to be σ* orbital whose spin density distribution was calculated indicating that about half a radical belongs to Cu center and the rest is contained in the O2H group and ligand
in doublet state (Supplementary Fig. 8; Supplementary Table 5). Natural population analysis (NPA) depicted atomic charges for 1, which demonstrates that the proximal oxygen atom possesses
more negative charge than the distal oxygen atom (Supplementary Fig. 9)39. Thus, overall calculations are indicative of a trigonal bipyramidal Cu(II)-hydroperoxo intermediate for 1.
REACTIVITY STUDY OF 1 The reactivity of 1 was examined in oxidation reaction with organic substrates. The electrophilic reactivity of 1 was tested in the oxidation of triphenylphosphine
(i.e., oxygen atom transfer) and cyclohexadiene (i.e., hydrogen atom abstraction). Upon addition of the substrates to 1 in CH3OH at –50 °C, 1 remained intact without showing any spectral
changes (Supplementary Fig. 10). Only trace amounts of products were detected in the product analysis of the reaction solutions. These results indicate that 1 is not capable of oxidizing
substrates in electrophilic oxidation under the reaction conditions. NUCLEOPHILIC REACTION WITH ACYL CHLORIDE The nucleophilic character of 1 was established in oxidation of acyl chlorides,
which are electrophile substrates. On the addition of 100 equiv of benzoyl chloride (PhCOCl) to 1 in CH3OH at –50 °C, the characteristic absorption bands of 1 disappeared through a
first-order decay profile (Fig. 4a). After the reaction was completed, product analysis revealed the formation of benzoic acid in a quantitative yield (Supplementary Table 6). Reaction of 1
with a series of _para_-substituted benzoyl chlorides (_para_-X-PhCOCl, X = OCH3, CH3, F, H, Cl) gave a linear correlation of the Hammett plot of the first-order rate constant against _σ_p+
resulting a _ρ_ value of 2.0(3) (Fig. 4b). The positive _ρ_ value indicates nucleophilic character for 1. ALDEHYDE DEFORMYLATION REACTION It is well known that metal-peroxo species are
frequently capable of nucleophilic reactivity. Since it has been reported that the iron-hydroperoxo species is much more reactive than the corresponding iron-peroxo species in aldehyde
deformylation40, the reactivity of 1 was further examined in the oxidation of 2-phenylpropionaldehyde (2-PPA) and cyclohexylcarboxaldehyde (CCA). Kinetic studies of 1 with CCA in CH3OH at
–70 °C exhibit a pseudo-first-order reaction profile (Supplementary Fig. 11). A plot of the first-order rate constants against the concentration of CCA shows a linear correlation, giving a
second-order rate constant (_k_2) of 6.5(3) M–1 s–1 (Fig. 5a). Similarly, the _k_2 value of 1.5(1)×10–1 M–1 s–1 was determined in the reaction of 1 and 2-PPA at –50 °C (Supplementary Fig.
12b). Cyclohexene (84(9)%) and acetophenone (93(7)%) were obtained in the oxidation of CCA and 2-PPA, respectively, as final products. Also 1 changed back to the Cu(II) precursor after
completion of aldehyde deformylation reaction (Supplementary Fig. 13). The _k_2 values were dependent on the reaction temperature where the activation parameters for oxidation of aldehydes
by 1 were determined to be _ΔH_‡ = 23(1) kJ mol–1 and _ΔS_‡ = −112(2) J mol–1 K–1 in the range of 193–223 K for CCA (Fig. 5b), and _ΔH_‡ = 31(1) kJ mol–1 and _ΔS_‡ = −118(7) J mol–1 K–1 in
the range of 213–243 K for 2-PPA (Supplementary Fig. 12c). A bimolecular mechanism for the nucleophilic oxidative reaction by 1 is suggested by the observed negative entropy and the
second-order kinetics. Compared with the reactivity of a previously reported iron(III)-hydroperoxo complex40 and copper(II)-alkylperoxo adducts17, the reactivity of 1 (_k_2 = 3.6 × 10–1 M–1
s–1 at –40 °C) is higher than that of the iron(III)-hydroperoxo species (_k_2 = 1.3 × 10–1 M–1 s–1 at –40 °C) and those of copper(II)-alkylperoxo complexes (_k_2 = 1.2–1.8 × 10–1 M–1 s–1 at
–40 °C) in aldehyde deformylation of 2-PPA (Supplementary Table 7). It should be noted that the reactivity of 1 (_k_2 = 3.4 M–1 s–1 at –80 °C) with CCA is also higher than that of a
Cu(II)-superoxo complex, [Cu(_β_DK)(O2)]– (_k_2 = 1.4 M–1 s–1 at –80 °C), which was a highly reactive oxidant in aldehyde deformylation reported so far (Supplementary Table 8)41. Very
recently, it has been reported that an anionic copper(II)-superoxo complex acts as a base than a nucleophile in the aldehyde deformylation of wet 2-PPA42. Furthermore, nucleophilic
reactivity has been observed for an Fe(III) porphyrin peroxo complex in the epoxidation of electron-deficient olefins43,44. Thus, we also explored epoxidation of olefins such as cyclohexene
and 2-cyclohexene-1-one, which is an electron-deficient olefin, by 1 in CH3OH at –50 °C (Supplementary Figs. 14 and 15). Product analyses of the reaction solutions with olefins did not show
oxidized products. These results suggest that 1 lacked reactivity in the epoxidation of electron-deficient olefins. DISCUSSION Based on the results of kinetic and product analyses, there are
two possible initial steps for the nucleophilic reaction, a proximal oxygen attack and a distal oxygen attack by 1 (Fig. 6). The formation of a peroxyhemiacetal intermediate initially
proceeds via an attack of the proximal oxygen to the carbonyl center of aldehydes, while the formation of a [Cu(_i_Pr3-tren)]2+ bound peroxyhemiacetal-like species occurs through a proton
transfer and a distal oxygen attack to the aldehydes. The calculated atomic charge distribution of the proximal oxygen (–0.60) from NPA is more negative than that of the distal oxygen
(–0.49) (Supplementary Fig. 8), supporting the proximal oxygen attack mechanism. However, the negative charge difference is small. Thus, further detailed DFT studies such as reaction
coordinate calculations are under investigation. In this study, we have demonstrated the oxidation of organic carbonyl compounds by 1, giving the first, to our knowledge, example of
nucleophilic reactivity of a copper(II)-hydroperoxo complex. 1 was characterized by various methods such as UV-vis, CSI-MS, rRaman and EPR. These results clearly indicate that 1 is an end-on
Cu(II)-hydroperoxo complex with trigonal bipyramidal coordination geometry, which is also supported by isotope labeling experiments and DFT calculations. The nucleophilic character of 1 was
confirmed by a positive slop in the Hammett plot for the reaction of 1 and _para_-substituted acyl chlorides. Kinetic studies represent that 1 is a very reactive nucleophilic oxidant in
aldehyde deformylation. We believe the observed oxidative nucleophilic reactivity of 1 demonstrates a new class of reactivity for Cu(II)-hydroperoxo intermediates. METHODS PREPARATION OF
[CU(_I_PR3-TREN)(CH3CN)](CLO4)2 [Cu(_i_Pr3-tren)(CH3CN)](ClO4)2 was prepared by reacting Cu(ClO4)2·6H2O (0.185 g, 0.50 mmol) and tris[2-(isopropylamino)ethyl]amine (_i_Pr3-tren) (176.42
_μ_L, 0.50 mmol) in CH3CN (3.0 mL). The mixture was stirred for 12 h, giving a blue solution. Et2O (40 mL) was added to the resulting solution to yield a blue powder, which was collected by
filtration, washed with Et2O, and dried in a vacuo. Yield: 97% (0.2799 g). X-ray crystallographically suitable crystals were obtained by slow diffusion of Et2O into a solution of the complex
in CH3CN (Supplementary Table 1 and 2; Supplementary Fig. 1). ESI-MS in CH3CN (Supplementary Fig. 2): _m/z_ 167.6 for [Cu(_i_Pr3-tren)]2+, _m/z_ 188.2 for [Cu(_i_Pr3-tren)(CH3CN)]2+, _m/z_
208.7 for [Cu(_i_Pr3-tren)(CH3CN)2]2+, and _m/z_ 434.3 for [Cu(_i_Pr3-tren)](ClO4)+. Anal. Calcd for C17H39CuN5O8: C, 35.45; H, 6.82; N, 12.16. Found: C, 35.54; H, 7.05; N, 12.16. The
effective magnetic moment of _μ_eff = 1.8 B.M. was determined by 1H NMR Evans method in CD3CN at 25 °C. GENERATION OF [CU(_I_PR3-TREN)(OOH)]+ (1) Treatment of [Cu(_i_Pr3-tren)(CH3CN)](ClO4)2
(1 mM) with 5 equiv of H2O2 in the presence of 2 equiv of triethylamine (TEA) in CH3OH at −50 °C afforded a green solution. [Cu(_i_Pr3-tren)(18O18OH)]+ and [Cu(_i_Pr3-tren)(OO2H)]+ were
prepared by adding 5 equiv of H218O2 (36 μL, 95% 18O-enriched, 2.2% H218O2 in water) and 5 equiv of 2H2O2 (30% in 2H2O), respectively, to a solution containing
[Cu(_i_Pr3-tren)(CH3CN)](ClO4)2 (1 mM) and 2 equiv of TEA in CH3OH at −50 °C. UV-vis in CH3OH: _λ_max (_ε_) = 360 nm (1300 M−1 cm−1), 656 nm (230 M−1 cm−1) and 830 nm (270 M−1 cm−1). X-RAY
CRYSTALLOGRAPHY See Supplementary Methods, Supplementary Fig. 1, and Supplementary Tables 1 and 2. COMPUTATIONAL DETAILS See Supplementary Methods, Supplementary Figs. 6, 7, and 8, and
Supplementary Tables 4 and 5. REACTIVITY STUDIES See Supplementary Methods, Supplementary Figs. 9–14, and Supplementary Tables 6–8. DATA AVAILABILITY The X-ray crystallographic coordinates
for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number CCDC-1879280. These data can be obtained free of
charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/ data_request/cif. All other data are available from the corresponding author upon reasonable request.
REFERENCES * Elwell, C. E. et al. Copper−oxygen complexes revisited: structures, spectroscopy, and reactivity. _Chem. Rev._ 117, 2059–2107 (2017). Article CAS Google Scholar * Solomon, E.
I. et al. Copper active sites in biology. _Chem. Rev._ 114, 3659–3853 (2014). Article CAS Google Scholar * Pettingill, T. M., Strange, R. W. & Blackburn, N. J. Carbonmonoxy dopamine
_β_ -hydroxylase. _J. Biol. Chem._ 266, 16996–17003 (1991). CAS PubMed Google Scholar * Prigge, S. T., Kolhekar, A. S., Eipper, B. A., Mains, R. E. & Amzel, L. M. Amidation of
bioactive peptides: the structure of peptidylglycine _α_-hydroxylating monooxygenase. _Science_ 278, 1300–1305 (1997). Article CAS Google Scholar * Klinman, J. P. The copper-enzyme family
of dopamine _β_-monooxygenase and peptidylglycine _α_-hydroxylating monooxygenase: resolving the chemical pathway for substrate hydroxylation. _J. Biol. Chem._ 281, 3013–3016 (2006).
Article CAS Google Scholar * Luisa, C., Gideon, J. D., William, B. T. & Paul, H. W. Bracing copper for the catalytic oxidation of C–H bonds. _Nat. Catal._ 1, 571–577 (2018). Article
Google Scholar * Hemsworth, G. R., Henrissat, B., Davies, G. J. & Walton, P. H. Discovery and characterization of a new family of lytic polysaccharide monooxygenases. _Nat. Chem. Biol._
10, 122–126 (2014). Article CAS Google Scholar * Kim, S., Stahlberg, J., Sandgren, M., Paton, R. S. & Beckham, G. T. Quantum mechanical calculations suggest that lytic polysaccharide
monooxygenases use a copper-oxyl, oxygen-rebound mechanism. _Proc. Natl Acad. Sci. USA_ 111, 149–154 (2014). Article CAS Google Scholar * Hedegård, E. D. & Ryde, U. Molecular
mechanism of lytic polysaccharide monooxygenases. _Chem. Sci._ 9, 3866–3880 (2018). Article Google Scholar * Wada, A. et al. Structural and spectroscopic characterization of a mononuclear
hydroperoxo - copper(II) complex with tripodal pyridylamine ligands. _Angew. Chem. Int. Ed._ 37, 798–799 (1998). Article CAS Google Scholar * Mann, S. I., Heinisch, T., Ward, T. R. &
Borovik, A. S. Peroxide activation Regulated by hydrogen bonds within artificial Cu proteins. _J. Am. Chem. Soc._ 139, 17289–17292 (2017). Article CAS Google Scholar * Maiti, D., Lucas,
H. R., Narducci Sarjeant, A. A. & Karlin, K. D. Aryl hydroxylation from a mononuclear copper-hydroperoxo species. _J. Am. Chem. Soc._ 129, 6998–6999 (2007). Article CAS Google Scholar
* Kim, S. et al. Amine oxidative N-dealkylation via cupric hydroperoxide Cu–OOH homolytic cleavage followed by site-specifc fenton chemistry. _J. Am. Chem. Soc._ 137, 2867–2874 (2015).
Article CAS Google Scholar * Maiti, D., Sarjeant, A. A. N. & Karlin, K. D. Copper-hydroperoxo-mediated N-debenzylation chemistry mimicking aspects of copper monooxygenases. _Inorg.
Chem._ 47, 8736–8747 (2008). Article CAS Google Scholar * Li, L., Sarjeant, A. A. N. & Karlin, K. D. Reactivity study of a hydroperoxodicopper(II) complex: hydroxylation,
dehydrogenation, and ligand cross-link reactions. _Inorg. Chem._ 45, 7160–7172 (2006). Article CAS Google Scholar * Li, L. et al. Exogenous nitrile substrate hydroxylation by a new
dicopper-hydroperoxide complex. _J. Am. Chem. Soc._ 127, 15360–15361 (2005). Article CAS Google Scholar * Kim, B., Jeong, D. & Cho, J. Nucleophilic reactivity of
copper(II)–alkylperoxo complexes. _Chem. Commun._ 53, 9328–9331 (2017). Article CAS Google Scholar * Choi, Y. J. et al. Spectroscopic and computational characterization of CuII–OOR (R = H
or cumyl) complexes bearing a Me6-tren ligand. _Dalton Trans._ 40, 2234–2241 (2011). Article CAS Google Scholar * Yamaguchi, S. et al. Synthesis, characterization, and thermal stability
of new mononuclear hydrogenperoxocopper(II) complexes with N3O-type tripodal ligands bearing hydrogen-bonding interaction sites. _Bull. Chem. Soc. Jpn_ 78, 116–124 (2005). Article CAS
Google Scholar * Yamaguchi, S. et al. Thermal stability of mononuclear hydroperoxocopper(II) species. Effects of hydrogen bonding and hydrophobic field. _Chem. Lett._ 33, 1556–1557 (2004).
Article CAS Google Scholar * Fujii, T. et al. Mononuclear copper(II)–hydroperoxo complex derived from reaction of copper(I) complex with dioxygen as a model of D_β_M and PHM. _Chem.
Commun._ 0, 4428–4430 (2006). Article CAS Google Scholar * Yamaguchi, S. et al. Copper hydroperoxo species activated by hydrogen-bonding interaction with its distal oxygen. _Inorg. Chem._
42, 6968–6970 (2003). Article CAS Google Scholar * Kim, S., Saracini, C., Siegler, M. A., Drichko, N. & Karlin, K. D. Coordination chemistry and reactivity of a cupric hydroperoxide
species featuring a proximal H‑bonding substituent. _Inorg. Chem._ 51, 12603–12605 (2012). Article CAS Google Scholar * Chen, P., Fujisawa, K. & Solomon, E. I. Spectroscopic and
theoretical studies of mononuclear copper(II) alkyl- and hydroperoxo complexes: electronic structure contributions to reactivity. _J. Am. Chem. Soc._ 122, 10177–10193 (2000). Article CAS
Google Scholar * Denisov, I. G., Mak, P. J., Makris, T. M., Sligar, S. G. & Kincaid, J. R. Resonance raman characterization of the peroxo and hydroperoxo intermediates in cytochrome
P450. _J. Phys. Chem. A_ 112, 13172–13179 (2008). Article CAS Google Scholar * Mak, P. J., Thammawichai, W., Wiedenhoeft, D. & Kincaid, J. R. Resonance Raman spectroscopy reveals
pH-dependent active site structural changes of lactoperoxidase compound 0 and its ferryl heme O–O bond cleavage products. _J. Am. Chem. Soc._ 137, 349–361 (2015). Article CAS Google
Scholar * Ibrahim, M., Denisov, I. G., Makris, T. M., Kincaid, J. R. & Sligar, S. G. Resonance raman spectroscopic studies of hydroperoxo-myoglobin at cryogenic temperatures. _J. Am.
Chem. Soc._ 125, 13714–13718 (2003). Article CAS Google Scholar * Mak, P. J. et al. Resonance Raman detection of the hydroperoxo intermediate in the cytochrome P450 enzymatic cycle. _J.
Am. Chem. Soc._ 129, 6382–6383 (2007). Article CAS Google Scholar * He, C. et al. Diiron complexes of 1,8-naphthyridine-based dinucleating ligands as models for hemerythrin. _J. Am. Chem.
Soc._ 122, 12683–12690 (2000). Article CAS Google Scholar * Mak, P. J. & Kincaid, J. R. Resonance Raman spectroscopic studies of hydroperoxo derivatives of cobalt-substituted
myoglobin. _J. Inorg. Biochem._ 102, 1952–1957 (2008). Article CAS Google Scholar * Lucchese, B. et al. Mono-, bi-, and trinuclear CuII-Cl containing products based on the
tris(2-pyridylmethyl)amine chelate derived from copper(I) complex dechlorination reactions of chloroform. _Inorg. Chem._ 43, 5987–5998 (2004). Article CAS Google Scholar * Barbucci, R.,
Bencini, A. & Gatteschi, D. Electron spin resonance spectra and spin-hamiltonian parameters for trigonal-bipyramidal nickel(I) and copper(II) complexes. _Inorg. Chem._ 16, 2117–2120
(1977). Article CAS Google Scholar * Kooter, I. M. et al. EPR characterization of the mononuclear Cu-containing _aspergillus japonicus_ quercetin 2,3-dioxygenase reveals dramatic changes
upon anaerobic binding of substrates. _Eur. J. Biochem._ 269, 2971–2979 (2002). Article CAS Google Scholar * Addison, A. W., Hendriks, H. M. J., Reedijk, J. & Thompson, L. K. Copper
complexes of the “tripod” ligand tris(2-benzimidazolylmethyl)amine: five- and six-coordinate copper(II) derivatives and some copper(I) derivatives. _Inorg. Chem._ 20, 103–110 (1981). Article
CAS Google Scholar * Thompson, L. K., Ramaswamy, B. S. & Dawe, R. D. Nickel(II) and copper(II) complexes of the ‘tripod’ ligand tris(2-benzimidazylmethy1)amine. _Can. J. Chem._ 56,
1311–1318 (1978). Article CAS Google Scholar * Senyukova, G. A., Mikheikin, I. D. & Zamaraev, K. I. EPR spectra of the complex [Cu(tren)OH]+ which has a trigonal bipyramidal
structure. _J. Struct. Chem._ 11, 18–21 (1970). Article Google Scholar * Jiang, F., Karlin, K. D. & Peisach, J. An electron spin echo envelope modulation (ESEEM) study of
electron-nuclear hyperfine and nuclear quadrupole interactions of dz 2 ground state copper(II) complexes with substituted imidazoles. _Inorg. Chem._ 32, 2576–2582 (1993). Article CAS
Google Scholar * Kovaleva, E. G. & Lipscomb, J. D. Crystal structures of Fe2+ dioxygenase superoxo, alkylperoxo, and bound product intermediates. _Science_ 316, 453–457 (2007). Article
CAS Google Scholar * Panda, C. et al. Nucleophilic versus electrophilic reactivity of bioinspired superoxido nickel(II) complexes. _Angew. Chem. Int. Ed._ 57, 14883–14887 (2018). Article
CAS Google Scholar * Cho, J. et al. Structure and reactivity of a mononuclear non-haem iron(III)-peroxo complex. _Nature_ 478, 502–505 (2011). Article CAS Google Scholar * Pirovano,
P. et al. Nucleophilic reactivity of a copper(II)–superoxide complex. _Angew. Chem. Int. Ed._ 53, 5946–5950 (2014). Article CAS Google Scholar * Bailey, W. D. et al. Revisiting the
synthesis and nucleophilic reactivity of an anionic copper-superoxide complex. _Inorg. Chem._ 58, 4706–4711 (2019). Article CAS Google Scholar * Sisemore, M. F., Burstyn, J. N. &
Valentine, J. S. Epoxidation of electron-deficient olefins by a nucleophilic iron(III) peroxo porphyrinato complex, peroxo(tetramesitylporphyrinato)ferrate(1-). _Angew. Chem. Int. Ed. Engl._
35, 206–208 (1996). Article CAS Google Scholar * Selke, M. & Valentine, J. S. Switching on the nucleophilic reactivity of a ferric porphyrin peroxo complex. _J. Am. Chem. Soc._ 120,
2652–2653 (1998). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The research was supported by NRF (2017R1A2B4005441 and 2018R1A5A1025511 to J.C.) and the Ministry of
Science, ICT and Future Planning (CGRC 2016M3D3A01913243 to J.C.) of Korea, and the Ministry of Education, Culture, Sports, Science and Technology of Japan through the “Strategic Young
Researcher Overseas Visits Program for Accelerating Brain Circulation to T.O.”. We thank Profs. Sachiko Yanagisawa and Minoru Kubo for assistance in acquiring rRaman spectra. AUTHOR
INFORMATION Author notes * Takehiro Ohta Present address: Faculty of Engineering, Department of Applied Chemistry, Sanyo-Onoda City University, 756-0884, Sanyo-Onoda, Yamaguchi, Japan
AUTHORS AND AFFILIATIONS * Department of Emerging Materials Science, DGIST, 42988, Daegu, Korea Bohee Kim, Donghyun Jeong & Jaeheung Cho * Picobiology Institute, Graduate School of Life
Science, University of Hyogo, RSC-LP Center, 679-5148, Hyogo, Japan Takehiro Ohta Authors * Bohee Kim View author publications You can also search for this author inPubMed Google Scholar *
Donghyun Jeong View author publications You can also search for this author inPubMed Google Scholar * Takehiro Ohta View author publications You can also search for this author inPubMed
Google Scholar * Jaeheung Cho View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS B.K. and J.C. conceived and designed the experiments; B.K.,
D.J., and T.O. performed the experiments; B.K., D.J., and J.C. analyzed the data; B.K., D.J., and J.C. co-wrote the paper. CORRESPONDING AUTHOR Correspondence to Jaeheung Cho. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 RIGHTS AND PERMISSIONS
OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kim, B., Jeong, D., Ohta,
T. _et al._ Nucleophilic reactivity of a copper(II)-hydroperoxo complex. _Commun Chem_ 2, 81 (2019). https://doi.org/10.1038/s42004-019-0187-3 Download citation * Received: 22 April 2019 *
Accepted: 24 June 2019 * Published: 18 July 2019 * DOI: https://doi.org/10.1038/s42004-019-0187-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
High stakes get build-to-rent star claire solon going at greystarA business trip to London about 10 years ago gave Claire Solon a glimpse of a future that she believes will fail. At the...
Shelter home abuse case: cbi starts excavation of burial ground; suspect grave of victim girlsThe CBI has started excavation of a grave in Bihar's Muzzafarpur, where, it is suspected, remains of some of the vi...
What we’ve learnt from building africa’s biggest genome libraryThe human genome was first sequenced in 2003 by multiple research centres across the world. The breakthrough was hailed ...
At an average of 1,200 passengers per day, maya train numbers far from eventual targetWill the Maya Train railroad eventually become a popular mode of travel between beach resorts, colonial cities and archa...
Over 500 chiapas residents flee cartel violence into guatemalaHundreds of Mexican families fleeing cartel violence in Mexico’s southern state of Chiapas have sought refuge across the...
Latests News
Nucleophilic reactivity of a copper(ii)-hydroperoxo complexABSTRACT Copper(II)-hydroperoxo species are often detected as key intermediates in metalloenzymes and biomimetic compoun...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
'drug smuggling' teen's pregnancy fears and 'consequence' as she makes complaintBELLA MAY CULLEY, A REPORTEDLY PREGNANT TEENAGER WHO FACES CHARGES OF DRUG SMUGGLING BEHIND THE DECAYING WALLS OF A NOTO...
Dementia risk: is this common medicine is linked to disease?Dementia is the leading cause of death in England and Wales, and experts are conducting in-depth research to consider wh...
City look frightening – roseDanny Rose described Manchester City's recent form as "frightening" but said Tottenham are relishing the ...