Potential for re-emergence of wheat stem rust in the united kingdom
Potential for re-emergence of wheat stem rust in the united kingdom"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Wheat stem rust, a devastating disease of wheat and barley caused by the fungal pathogen _Puccinia graminis_ f. sp. _tritici_, was largely eradicated in Western Europe during the
mid-to-late twentieth century. However, isolated outbreaks have occurred in recent years. Here we investigate whether a lack of resistance in modern European varieties, increased presence of
its alternate host barberry and changes in climatic conditions could be facilitating its resurgence. We report the first wheat stem rust occurrence in the United Kingdom in nearly 60 years,
with only 20% of UK wheat varieties resistant to this strain. Climate changes over the past 25 years also suggest increasingly conducive conditions for infection. Furthermore, we document
the first occurrence in decades of _P. graminis_ on barberry in the UK . Our data illustrate that wheat stem rust does occur in the UK and, when climatic conditions are conducive, could
severely harm wheat and barley production. SIMILAR CONTENT BEING VIEWED BY OTHERS A LANDSCAPE-SCALE FIELD SURVEY DEMONSTRATES THE ROLE OF WHEAT VOLUNTEERS AS A LOCAL AND DIVERSIFIED SOURCE
OF LEAF RUST INOCULUM Article Open access 21 November 2023 GENOMICS ACCELERATED ISOLATION OF A NEW STEM RUST AVIRULENCE GENE–WHEAT RESISTANCE GENE PAIR Article 22 July 2021 A COSMOPOLITAN
FUNGAL PATHOGEN OF DICOTS ADOPTS AN ENDOPHYTIC LIFESTYLE ON CEREAL CROPS AND PROTECTS THEM FROM MAJOR FUNGAL DISEASES Article Open access 19 August 2020 INTRODUCTION Wheat stem rust, caused
by the fungal pathogen _Puccinia graminis_ f. sp. _tritici_, has recently re-emerged in Europe. In 2013, Germany experienced its first major outbreak in decades after an unusually cold
spring was followed by high early summer temperatures1. In addition, both bread and durum wheat were ravaged by stem rust in Sicily in 2016, marking the largest European outbreak for many
years2. Stem rust is a long-standing threat to wheat and barley production. A cornerstone of the Green Revolution in the mid-to-late twentieth century was breeding for resistance against
stem rust3. However, new supervirulent wheat stem rust isolates such as the notorious Ug99 race group have emerged in Africa and their impending spread poses a significant threat to global
food security4. In addition, as climate conditions shift, the earlier-maturing wheat varieties that were once bred to avoid inoculum build-up5 could be at risk, as evidenced by recent
reports of stem rust outbreaks in Europe. Beyond breeding for resistance, large-scale removal of the alternate host barberry (_Berberis_ spp.)6 reduced the potential for enhancing the
pathogen’s genetic diversity and the spawning of new races, e.g., radically reducing the number of _P. graminis_ f. sp. _tritici_ races in the United States from 17 to 8 per year after
eradication3. Over the past decade, however, barberry planting has been reinitiated and is increasing rapidly in many major wheat-growing regions3. The presence of common barberry has the
potential not only to enhance the pathogen’s genetic diversity but also to provide a seasonal bridge for stem rust in temperate zones7. Dormant stem rust spores may overwinter and germinate
in the spring to infect the alternate host barberry, providing inoculum to re-infect primary grass and cereal hosts. Barberry eradication in the United Kingdom during the late nineteenth and
early twentieth century was a massive success, breaking the disease cycle and driving wheat stem rust to almost complete extinction8, with the last recorded epidemic in the United Kingdom
in 19559. Accordingly, overwintering has been perceived as unlikely in Europe for decades due to the absence of both _P. graminis_ f. sp. _tritici_ and the alternate host in most areas.
However, in 2017 Sweden reported the first occurrence of a sexual population of wheat stem rust that was derived from barberry signifying a worrying turn for wheat stem rust in Europe10.
Here we report the first record of wheat stem rust in the United Kingdom in nearly 60 years, and that only 20% of UK wheat varieties are resistant to this strain. We also identified for the
first time in many decades a stem rust fungus on its alternate host common barberry in the United Kingdom, where it was identified within meters of a barley field. Our results indicate that,
with alterations in climatic conditions over the past 25 years, suggesting increasingly conducive conditions for fungal pathogen growth and infection, wheat stem rust is becoming an
increasing threat to European wheat and barley production. RESULTS UK-01 BELONGS TO THE EPIDEMIC RACE ‘DIGALU’ In 2013, we found a single wheat plant in southern England infected with stem
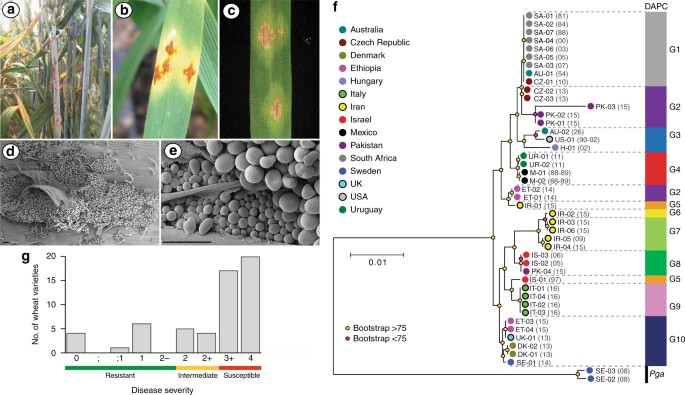
rust. This UK isolate, which we named UK-01, induced characteristic _P. graminis_ f. sp. _tritici_ uredinia on wheat, which were erumpent, diamond-shaped, and full of spiny oval
urediniospores on the stem and leaves (Fig. 1a–e). To compare UK-01 with global stem rust populations, we carried out comparative population genetic analysis using 42 _P. graminis_ f. sp.
_tritici_ isolates from fourteen countries and two _P. graminis_ f. sp. _avenae_ isolates as outliers (Supplementary Table 1). First, we undertook either full-genome or transcriptome
sequencing on all isolates, including UK-01. High-quality reads were aligned to the _P. graminis_ f. sp. _tritici_ reference genome11 and phylogenetic analysis undertaken using 7,348,046
sites and a maximum-likelihood approach (Fig. 1f and Supplementary Data 1). To evaluate genetic subdivisions within this population, we used 306,960 synonymous single-nucleotide polymorphism
(SNP) sites and discriminant analysis of principal components to define genetic groups (Supplementary Fig. 1), which assigned the isolates to 10 groups of homogeneous individuals (Fig. 1f
and Supplementary Fig. 2). Notably, UK-01 was most closely related to _P. graminis_ f. sp. _tritici_ isolates from Ethiopia collected in 2014 and 2015, and Danish and Swedish isolates
detected in single locations in 2013 and 2014, respectively, with all aforementioned isolates clustering in a single genetic group with little diversity (Fig. 1f and Fig. 2; median
nucleotide diversity 1.46 × 103). The collection of the Ethiopian isolates in 2014–15 succeeded a severe stem rust epidemic facilitated by the widespread planting of a single bread wheat
variety. ‘Digalu’ was planted on ~ 30% of the wheat acreage and then succumbed to stem rust infection in late 2013, leading to rapid, wide-scale production losses12. Originally detected in
Turkey, the ‘Digalu’-infecting race, TKTTF, has spread across the Middle East12 and recently into Europe, where it was the dominant race in the 2013 German outbreak1. The close genetic
proximity between the UK isolate and the Ethiopian, Danish, and Swedish TKTTF-like variants13 suggests that UK-01 belongs to the TKTTF (or a closely related) race. This relationship was
further supported through virulence profiling, where UK-01 was inoculated onto a series of differential wheat varieties known as the North American Wheat Stem Rust Differential set and
disease severity recorded in seedling tests 14–16 days post inoculation. This analysis showed that UK-01 behaved identically to the TKTTF race (Table 1). We speculate that the TKTTF race
likely spread across Europe from south to north via wind-borne urediniospore dispersal along the west European track14 from a common source in 2013. UK-01 MAY INFECT OVER 80% OF CURRENT UK
WHEAT VARIETIES To explore the potential threat stem rust poses to UK wheat production, we assessed the susceptibility of current UK wheat varieties to UK-01. We inoculated UK-01 onto
seedlings of 43 wheat varieties from the UK Recommended List15 and 14 older varieties that are still grown on a small scale. Of these 57 varieties, 37 showed a high degree of susceptibility
in seedling tests, 9 displayed an intermediate reaction, and 11 were resistant to some degree to infection (Fig. 1g and Supplementary Table 2). Thus, only 20% of wheat varieties currently
grown in the United Kingdom are estimated to be resistant to the stem rust isolate UK-01. IDENTIFICATION OF _P. GRAMINIS_ INOCULUM ON BARBERRY In the United Kingdom, replanting of the
alternate host of stem rust, common barberry (_Berberis vulgaris_), is keenly advancing, particularly due to a habitat conservation programme for the endangered barberry carpet moth
_Pareulype berberata_16 (Supplementary Fig. 3). To examine the potential hazard represented by barberry as a source of inoculum, we examined bushes in three locations in the east of England
in June 2017. At one location, we identified a hedgerow that was intermixed with _B. vulgaris_ within a meter of a barley field (Supplementary Fig. 4). We found numerous yellow, tube-like
aecial structures on the abaxial side of leaves (Fig. 3a–d), which are typical of cluster cup rust of barberry caused by _P. graminis_17. Genotypic characterization of the internal
transcribed spacer (ITS) region from four aecia confirmed the identification of _P. graminis_ (Genbank MF684370-3). Subsequent phylogenetic analysis grouped two aecial sequences in a clade
with _P. graminis_ f. sp. _tritici_ from wheat_, P. graminis_ f. sp. _secalis_ from wild rye (_Secale strictum_ subsp. _africanum_), and _P. graminis_ from couch grass (_Elymus_ spp.), which
are too similar to distinguish using classical gene sequence analysis18, but are all capable of infecting barley and, to differing degrees, wheat19,20. The other two sequences were more
closely related to _P. graminis_ from wild grasses (Fig. 3e). To evaluate the ability of selected aecia to cause disease on wheat and barley, we carried out controlled infection assays with
the resulting aeciospores on one barley and two wheat varieties. None of the selected aecia induced symptoms on the two selected wheat varieties. However, spores from 5 of 9 aecia tested
were able to infect the selected barley variety (Supplementary Fig. 4c), thereby suggesting a potential threat to the adjacent barley crop. If confirmed as _P. graminis_ f. sp. _tritici_,
this would be of particular concern as large-scale screening of barley germplasm over the years has only identified seven resistance loci21, most of which have been overcome22. Further
evidence is needed to establish the risk to barley of any UK-derived stem rust isolates. However, this does constitute the first evidence for many decades that the stem rust fungus is
overwintering in the UK and able to infect its alternate host common barberry in the spring. The planting of thousands of common barberry plants across the United Kingdom continues to
accelerate (Supplementary Fig. 3) and each medium-sized barberry bush is capable of producing over 20,000 seeds that can remain dormant for up to 10 years3,23. Thus, the bushes will be
increasingly available to harbor rust pathogens that utilize barberry as a sexual host. Indeed, following the repeal of the barberry exclusion law in Sweden, the oat stem rust fungus for
which common barberry is an alternate host has substantially increased in genetic diversity24. Furthermore, Sweden recently reported the first occurrence of a sexual population of wheat stem
rust derived from barberry for the first time in decades10. In the United Kingdom, the gravest concern regards the well-established wheat yellow rust pathogen, _Puccinia striiformis_ f. sp.
_tritici_, which is closely related to _P. graminis_ f. sp. _tritici_. Although not currently known to undergo sexual reproduction in Europe, the unusually high quantities of teliospores
produced by recent emergent _P. striiformis_ races25 could potentially expedite infection as common barberry becomes increasingly prevalent. INCREASING CLIMATIC RISK OF STEM RUST
RE-EMERGENCE IN THE UNITED KINGDOM To determine whether alterations in climatic conditions could further enhance the risk of wheat stem rust in the United Kingdom, we developed a
probabilistic model for spore germination rates, appressorium formation and penetration rates over the past quarter century, and drove the model using microclimate estimates from the JRA-55
climate re-analysis26 (Supplementary Fig. 5). These growth stages of the fungus require liquid moisture on the leaf surface. The warm temperatures and high light levels required for stem
rust penetration27 suggest that the disease is most likely to occur in the summer; therefore, we focused on weather data for June–August from 1990 to 2016. The estimated canopy liquid
surface water was above zero 30–40% of the time during the summer months, a value that was slightly greater in the far south and northwest of the wheat-growing region (Fig. 4a). The warmest
temperatures during the wet periods were found in the central parts of the wheat growing region (Fig. 4b). The fraction of time the canopy was wet increased significantly from 1990 to 2016,
suggesting increasingly conducive conditions for fungal pathogen growth and infection (Fig. 4c). The modelled spore germination and appressorium formation rates were strongly determined by
leaf wetness, as the optimal temperature range for these processes is wide (see Methods). The predicted rates of penetration, which is dependent on higher temperatures and light levels, as
well as on leaf moisture, were an order of magnitude lower than predicted appressorium formation rates (Fig. 4d)27. Overall, the model for germination and appressorium formation during wet
periods from 1990 to 2016 indicated a trend of increasing risk to 2006, levelling off in the past few years with the exception of the very wet year in 2012 (Fig. 4d). Next, we considered
climate change projections for 2050 that predict very slight drying (Fig. 4e) and slight warming (Fig. 4f) of the central part of the wheat-growing area in the United Kingdom28. This
analysis suggested that although the risk of spore germination and appressorium formation may increase, the wet conditions required for leaf penetration are unlikely to become more common in
the mid-term. However, the high levels of sexual recombination possible via barberry infection could enhance the likelihood of emergence of _P. graminis_ f. sp. _tritici_ variants that are
adapted to prevailing conditions. Worryingly, the Ug99 race has already been reported in preliminary analysis to have a higher level of aggressiveness at cooler temperatures compared to
other wheat stem rust races29. This ability to adapt could facilitate proliferation into new geographic regions in a similar manner to the high temperature-tolerant races of _P. striiformis_
f. sp. _tritici_30. CONCLUSIONS The Nobel laureate Norman Borlaug foresaw that “the greatest ally of the pathogen is our short memory”3. We recommend the re-initiation of resistance
breeding and a review of the mass plantation of common barberry to preclude re-planting near arable land and thereby limit the ability of the pathogen to rapidly overcome any introduced
resistance and/or climatic constraints to safeguard European cereals from a large-scale re-emergence of wheat stem rust. METHODS RNA-SEQ OF _P. GRAMINIS_-INFECTED LEAF SAMPLES A total of 12
_P. graminis_-infected wheat leaf samples were collected and stored to maintain nucleic acid integrity in RNAlater solution (Thermo Fisher Scientific, Paisley, UK; Supplementary Table 1).
Samples were subsequently subjected to RNA extraction using a Qiagen RNeasy Mini Kit (Qiagen, UK) and the quality and quantity of extracted RNA assessed using an Agilent 2100 Bioanalyzer
(Agilent Technologies, UK). Complementary DNA libraries were prepared using the Illumina TruSeq RNA Sample Preparation Kit (Illumina, USA) and sequenced on the Illumina HiSeq 2500 machine at
the Earlham Institute, UK. Adapter and barcode trimming and quality filtering were performed on the 97–101 bp paired-end reads using the FASTX-Toolkit (version 0.0.13.2). Reads were then
aligned to the _P. graminis_ assembly11 and SNP calling undertaken using SAMtools (version 0.1.19)31, considering only sites with a minimum depth of coverage of 20 ×. Allelic frequencies
were determined for each SNP site and those ranging from 0.2 to 0.8 were classified as heterokaryotic sites and those with other frequencies classified as homokaryotic sites. SNP sites that
induced synonymous and non-synonymous substitutions were identified using SnpEff, version 3.632. GENOME SEQUENCING OF _P. GRAMINIS_ ISOLATES Genomic DNA was extracted from dried
urediniospores of 31 _P. graminis_ isolates (Supplementary Table 1) using the cetyl trimethyl ammonium bromide (CTAB) method33. The gDNA libraries were prepared using the Illumina TruSeq DNA
Sample preparation Kit (Illumina) and library quality confirmed before sequencing using the Agilent 2100 Bioanalyzer (Agilent Technologies). Libraries were sequenced on the Illumina HiSeq
2500 machine at the Earlham Institute or Novogene, China. We also included in our analysis publicly available genome sequence data from two _P. graminis_ isolates collected in Australia34.
Following data filtering, the 76–150 bp pair-end reads for each sample were independently aligned to the _P. graminis_ assembly11 and SNP calling performed as described above but with a
minimum threshold of 10 × depth of coverage. PHYLOGENETIC ANALYSIS OF _P. GRAMINIS_ ISOLATES Phylogenetic analyses of _P. graminis_ isolates were performed using gene sequences to avoid
over-representation of isolates subjected to full genome sequencing (compared with those used for transcriptome sequencing) and a maximum likelihood approach. First, nucleotide sites that
differed from the reference genome were identified and recorded if they had a minimum of 10 × depth of coverage for gDNA samples and 20 × depth of coverage for RNA-seq samples. Next, sites
that were identical to the reference were recorded if they satisfied a minimum of 2 × coverage. Using these data, synthetic gene sets were generated that incorporated these sites for each
isolate using the method described previously35. The third codon position of 16,482 genes was used to generate maximum likelihood trees using RaxML 8.0.20 with 100 replicates using the rapid
bootstrap algorithm36. Phylogenetic trees were visualized in MEGA 7.037. POPULATION GENETIC ANALYSIS The existence of population subdivisions was investigated using nonparametric
multivariate clustering. This method allows the clustering of isolates without a priori knowledge (e.g., geographical locations or date of collection) that prevents different genetic
lineages being grouped together when identified in the same region and thereby interfering with the detection of admixture events38. To reduce any potential bias of selection, only sites
that introduced a synonymous change in at least one isolate were listed and the nucleotide at this position extracted for all isolates. Multivariate analyses were performed using
discriminant analyses of principal components (DAPCs) implemented in the Adegenet package in the R environment39, which is a non-parametric approach used without any predetermined genetic
model. The number of population clusters (Kmax) were identified using the Bayesian information criterion (BIC), as suggested39. Next, the synthetic gene sets per isolate that were generated
for the phylogenetic analysis were combined in one file for all isolates within a genetic group following the grouping identified using DAPC. Nucleotide diversity was then determined using
these gene sequences and DnaSP, version 5.10.140 for each genetic group. The diversity within each genetic group was calculated using the statistic _Pi_ divided by the number of analysed
sites (only sites with < 5% of missing data were included: max_missing_freq = 0.05). To determine the proportion of total genetic variance attributable to inter-population polymorphisms,
the synthetic gene sets of all isolates were combined in one file and the Weir and Cockeram _F_ST (egglib statistics: WCst) calculated pairwise for all population pairs with max_missing_freq
= 0.05. For both calculations, the number of analyzed sites and mutations was determined using the Egglib statistics lseff and S, respectively. VIRULENCE PROFILING OF _P. GRAMINIS_ ISOLATES
First, _P. graminis_ isolate UK-01 was screened for its virulence phenotype across the North American Wheat Stem Rust Differential set, which includes the host resistance genes _Sr5_,
_Sr21_, _Sr9e_, _Sr7b_ (set 1),_ Sr11_,_ Sr6_,_ Sr8a_,_ Sr9g_ (set 2), _Sr36_,_ Sr9b_,_ Sr30_,_ Sr17_ (set 3), _Sr9a_,_ Sr9d_,_ Sr10_,_ SrTmp_ (set 4), and _Sr24_, _Sr31_, _Sr38_, and
_SrMcN_ (set 5). Five plants for each of the 20 lines were tested under controlled environmental conditions, with two independent biological replicates. Spores were distributed onto test
plants in a mixture with talcum powder and plants were incubated for 48 h in polythene bags containing a small amount of water at 18 °C (8 h night) and 24 °C (16 h day), before being removed
and grown for a further 14 days. Infection types were assessed on the first seedling leaf using the United States Department of Agriculture scoring system41, where 0, ;, ;1, 1, and 2– were
considered as representing an incompatible interaction, 2 and 2+ were considered intermediate and 3+ and 4 represented a compatible interaction between the host genotype and pathogen. For
the purpose of detailed virulence phenotyping, intermediate reactions were considered as intermediate incompatible. Next, _P. graminis_ isolate UK-01 was screened for its virulence phenotype
across wheat cultivars from the UK AHDB Recommended List15 and other wheat varieties that have historically been widely grown across the UK. Infection assays and scoring were performed as
described above. _P. GRAMINIS_ AECIOSPORE INFECTION ASSAYS A total of 35 aecia were collected from _B. vulgaris_ at a single location in Brandon, UK. Nine aecia were selected for infection
assays and stored in damp conditions to induce release of aeciospores for up to 3 h before being applied with gentle rubbing to the leaves of the wheat varieties Vuka and Solstice and the
barley variety Cassata at the seedling stage. After infection, seedlings were kept in the dark at 10 °C and high relative humidity for 24 h. Plants were then moved to a controlled
environment room under long-day conditions (16 h light/8 h dark) and 19/14 °C cycle. Symptoms were recorded 14 d post infection. _P. GRAMINIS_ ITS SEQUENCE ANALYSIS DNA was extracted from
four aecia collected on _B. vulgaris_ using the CTAB method33 and the ITS region amplified using the primers 5ITS-SR: (5′-ATTAAAAGAATTAGAGTGCACTTT-3′) and 3ITS-SR
(5′-AGATGGCAAGTGTTTTACTACT-3′). PCR products were cloned into the pGEMT-Easy vector system (Promega, USA) according to the manufacturer’s instructions. Inserts of six recombinant plasmids
per amplicon were bi-directionally sequenced (GATC, Germany) and a sequence alignment of the ITS region from these aecia and 27 ITS sequences from _P. graminis_ f. sp.20,42 was generated
using MUSCLE43. Phylogenetic analysis was performed in MEGA 7.037 using a neighbor-joining approach with bootstrap values determined from 1,000 replicates. SCANNING ELECTRON MICROSCOPY
Samples were mounted on aluminium stubs using Tissue TekR (BDH Laboratory Supplies, Poole, England). The stubs were then immediately plunged into liquid nitrogen slush at approximately − 210
°C to cryopreserved the material. The samples were transferred onto the cryostage of an ALTO 2500 cryotransfer system (Gatan, Oxford, England) attached to a Zeiss Supra 55 VP FEG scanning
electron microscope (Zeiss SMT, Germany) or the same type of cryo-system on an FEI Nova NanoSEM 450 (FEI, Eindhoven, The Netherlands). Sublimation of surface frost was performed at − 95 °C
for ~ 3 min before the samples were sputter coated with platinum for 2 min at 10 mA, at colder than − 110 °C. After sputter-coating, the samples were moved onto the cryo-stage in the main
chamber of the microscope, held at − 125 °C. The samples were imaged at 3 kV and digital TIFF files were stored. PROBABILISTIC MODEL OF INFECTION RISK We modelled leaf infection risk in
response to microclimate using a probabilistic model44 parameterized for stem rust19. We modelled specifically germination of urediniospores on the wheat surface and subsequent penetration
through stomata, as these stages are strongly constrained by free water availability14,19,27, in common with other rust fungi. Therefore, results provide estimates only of infection risk,
not of full development of potential epidemics. The reported cardinal temperatures for spore germination, germling growth and appressorium formation are _T_min = 2 °C, _T_opt = 15–24 °C, and
_T_max = 30 °C19. The reported cardinal temperatures for penetration are _T_min = 15 °C, _T_opt = 29 °C, and _T_max = 35 °C. Hence, the temperature range for penetration is considerably
higher than that for germination and appressorium formation. In addition, high light availability is required for the penetration stage, reported as illumination of >10,000 lux, or
approximately that received in the shade under a clear sky at noon. This reflects the pathogen likely germinating following dewfall overnight and then infecting in the morning as
temperatures rise, stomata open, and dew slowly dries19. A beta function was used to estimate relative rates of germination and penetration based on cardinal temperatures44,45, modified for
germination to account for the wide optimal temperature range46. We modelled the transition of spores to appressoria, and appressoria to penetrations, as survival processes following a
Weibull distribution44. In the absence of appropriate empirical data, we estimated the Weibull parameters from qualitative descriptions of the time taken for germination and penetration to
occur19. At optimal temperatures, the Weibull processes gave near-completion of appressorium formation after 8 h and penetration in a further 3 h. The hazard functions were multiplied by the
temperature-dependent rates to reduce germination and penetration rates at sub-optimal temperatures, with zero activity outside of the cardinal temperatures (Supplementary Fig. 5). Both
processes occur only when leaves are wet and germinated spores die if leaves dry out. In the UK, wheat is planted mainly in the east of England and winter wheat accounts for nearly all the
wheat grown. Winter wheat is planted between September and November, tillering occurs over winter, stem elongation in spring, flowering in June, grain filling in July, and collecting in
August–September. The warm temperatures required for penetration strongly suggest that the disease is most likely to strike in the summer. We obtained historical weather estimates for the
summer months (June, July, and August) in the major wheat-growing regions of the United Kingdom (a rectangular grid covering 1.97°W to 1.97°E, 50.0°N to 55.0°N) from the beginning of 1990 to
the end of 2016, at 3 h intervals and 0.5625° spatial resolution, from the Japanese 55-year Reanalysis (JRA55)26. Data were downloaded from the Research Data Archive at the National Center
for Atmospheric Research, Computational and Information Systems Laboratory47. Weather variables required for modelling were canopy temperature (oC), canopy surface water content (kg m−2),
solar irradiance (W m−2) and cloud cover fraction. The 3 h observations were linearly interpolated to give hourly estimates for modeling. We assumed a constant number of spores available for
germination in each hour and that germination and penetration could take place only if canopy surface water content was greater than zero. The total relative number of appressoria formed in
an hour was the sum of appressoria formed by all germinating cohorts. These appressoria were then able to penetrate if moisture and light conditions allowed. JRA55 irradiance estimates were
converted to estimates of illuminance (lux) using a rule-of-thumb factor of 126.6, which suggested that sufficient sunlight for penetration was available between the hours of 0800 and 2100
h. The relative number of penetrations in an hour was the sum of all penetrating cohorts and was taken as an indicator of relative infection risk. Although 3 h projections are available for
air temperature from the CMIP-5 (Coupled Model Intercomparison Project) family of Global Circulation Models (GCM)48, other products such as relative humidity (which can be used as an
indicator of leaf wetness) are available only at coarser temporal resolutions from current repositories. Therefore, driving our germination and infection model with future projections was
not possible without bespoke GCM runs. Instead, we inspected random realizations of CMIP-5 projections at daily temporal resolution provided by the Marksim weather generator49. We obtained
99 ensemble averages for 17 CMIP-5 GCMs for the years 2020 and 2050 under the RCP4.5 representative concentration pathway scenario for a point near Cambridge, UK, which lies near the center
of the wheat-growing region of the UK, and compared temperature and precipitation estimates or the summer months for these time points. CODE AVAILABILITY All custom computer code is
available at https://github.com/vbuens/Field_Pathogenomics. DATA AVAILABILITY The raw sequence data and ITS sequence data that support the findings of this study have been deposited in the
European Nucleotide Archive (ENA; PRJEB22223) and Genbank (MF684370-3), respectively. REFERENCES * Olivera Firpo, P. D. et al. Characterization of _Puccinia graminis_ f. sp. _tritici_
isolates derived from an unusual wheat stem rust outbreak in Germany in 2013. _Plant Pathol._ https://doi.org/10.1111/ppa.12674 (2017). * Bhattacharya, S. Deadly new wheat disease threatens
Europe’s crops. _Nature_ 542, 145–146 (2017). Article CAS PubMed Google Scholar * Peterson, P. D. Stem rust of wheat: from ancient enemy to modern foe. _Am. Phytopathol._ _Soc._ (APS
Press, St. Paul, 2001). * Singh, R. P. et al. The emergence of Ug99 races of the stem rust fungus is a threat to world wheat production. _Annu. Rev. Phytopathol._ 49, 465–481 (2011). Article
CAS PubMed Google Scholar * Roelfs, A. P. in _The Cereal Rusts_. Vol. II (eds A. P. Roelfs & W. R. Bushnell) Ch. Wheat and Rye Stem Rust (Academic Press, Inc., Orlando, Florida,
1985). * Zhao, J., Wang, M., Chen, X. & Kang, Z. Role of alternate hosts in epidemiology and pathogen variation of cereal rusts. _Annu. Rev. Phytopathol._ 54, 207–228 (2016). Article
CAS PubMed Google Scholar * Smith, K. et al. US preparations for potential introduction of Ug99 strains of wheat stem rust. _Outlooks Pest Manag._ 20, 148–152,
https://doi.org/10.1564/20aug02 (2009). Article Google Scholar * Stakman, E. C. _Barberry Eradication Prevents Black Rust in Western Europe_. (United States Department of Agriculture,
Washington D.C., 1923). * Hessayon, D. G. _The Cereal Disease Expert_. 1st edn, 32 (TBS The Book Service Ltd, UK, 1982). * Berlin, A. Stem rust attacks in Sweden heralds the return of a
previously vanquished foe. https://www.slu.se/en/ew-news/2017/11/stem-rust-attacks-in-sweden-heralds-the-return-of-a-previously-vanquished-foe/ (2017). * Duplessis, S. et al. Obligate
biotrophy features unraveled by the genomic analysis of rust fungi. _Proc. Natl Acad. Sci. USA_ 108, 9166–9171 (2011). Article CAS PubMed PubMed Central Google Scholar * Olivera, P. et
al. Phenotypic and genotypic characterization of race TKTTF of _Puccinia graminis_ f. sp. _tritici_ that caused a wheat stem rust epidemic in southern Ethiopia in 2013-14. _Phytopathology_
105, 917–928 (2015). Article PubMed Google Scholar * Hovmoller, M. S. _Global Rust Reference Centre_. http://wheatrust.org/ (2017). * Hogg, W. H., Hounam, C. E., Mallik, A. K. &
Zadoks, J. C. _Meterological Factors Affecting the Epidemiology of Wheat Rusts_ (World Meteorological Organization, Geneva, 1969). * Agriculture and Horticulture Development Board
Recommended Lists. https://cereals.ahdb.org.uk/varieties/ahdb-recommended-lists.aspx (2017). * Waring, P. Successes in conserving the Barberry Carpet moth _Pareulype berberata_ (D. & S.)
(Geometridae) in England. _J. Insect Conserv._ 8, 167–171 (2004). Article Google Scholar * Berlin, A. _Population_ _Biology of_ Puccinia graminis (Department of Forest Mycology and Plant
Pathology, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2012). * Berlin, A., Kyaschenko, J., Justesen, A. F. & Yuen, J. Rust fungi forming aecia on Berberis spp. in
Sweden. _Plant Dis._ 97, 1281–1287 (2013). Article Google Scholar * Singh, R. P., Huerta-Espino, J. H. & Roelfs, A. P. in _Bread Wheat: Improvement and Production_ (Food &
Agriculture Organization of the UN, Rome, 2002). * Pretorius, Z. A., Bender, C. M. & Visser, B. The rusts of wild rye in South Africa. _S Afr. J. Bot._ 96, 94–98 (2015). Article Google
Scholar * Uauy, C., Wulff, B. B. H. & Dubcovsky, J. Combining traditional mutagenesis with new high-throughput sequencing and genome editing to reveal hidden variation in polyploid
wheat. _Annu. Rev. Genet._ 51, 435–454 (2017). Article CAS PubMed Google Scholar * Steffenson, B. J. et al. Vulnerability of barley to African pathotypes of _Puccinia graminis_ f. sp.
_tritici_ and sources of resistance. _Phytopathology_ 107, 950–962 (2017). Article CAS PubMed Google Scholar * Roelfs, A. P. Effects of barberry eradication on stem rust in the United
States. _Plant Dis_. 66, 177–181 (1982). * Berlin, A. et al. Disease development and genotypic diversity of _Puccinia graminis_ f. sp. _avenae_ in Swedish oat fields. _Plant Pathol._ 62,
32–40 (2013). Article CAS Google Scholar * Rodriguez-Algaba, J., Walter, S., Sorensen, C. K., Hovmoller, M. S. & Justesen, A. F. Sexual structures and recombination of the wheat rust
fungus _Puccinia striiformis_ on _Berberis vulgaris_. _Fungal Genet. Biol._ 70, 77–85 (2014). Article CAS PubMed Google Scholar * Kobayashi, S. et al. The JRA-55 reanalysis: general
specifications and basic characteristics. _J. Meteorol. Soc. Jpn_ 93, 5–48 (2015). Article Google Scholar * Burrage, S. W. Environmental factors influencing the infection of wheat by
_Puccinia graminis_. _Ann. Appl. Biol._ 66, 429–440 (1970). Article Google Scholar * Sillmann, J., Kharin, V. V., Zhang, X., Zwiers, F. W. & Bronaugh, D. Climate extremes indices in
the CMIP5 multimodel ensemble: Part 1. Model evaluation in the present climate. _J. Geophys. Res. Atmos._ 118, 1716–1733 (2013). Article Google Scholar * Rouse, M. & Jin, Y. in
_Abstract 12th International Cereal Rusts Powdery Mildew Conference_ (ed. Akkaya M. S.) (Antalya, Turkey, 2009). * Hovmoller, M. S., Walter, S. & Justesen, A. F. Escalating threat of
wheat rusts. _Science_ 329, 369 (2010). Article CAS PubMed Google Scholar * Li, H. et al. The Sequence Alignment/Map format and SAMtools. _Bioinformatics_ 25, 2078–2079,
https://doi.org/10.1093/bioinformatics/btp352 (2009). Article PubMed PubMed Central Google Scholar * Cingolani, P. et al. A program for annotating and predicting the effects of single
nucleotide polymorphisms, SnpEff: SNPs in the genome of _Drosophila melanogaster_ strainw1118; iso-2; iso-3. _Fly_ 6, 80–92 (2012). Article CAS PubMed PubMed Central Google Scholar *
Chen, X. M., Line, R. F. & Leung, H. Relationship between virulence variation and DNA polymorphism in _Puccinia striiformis_. _Phytopathology_ 83, 1489–1497 (1993). Article CAS Google
Scholar * Hubbard, A. et al. Field pathogenomics reveals the emergence of a diverse wheat yellow rust population. _Genome Biol._ 16, 23 (2015). Article PubMed PubMed Central Google
Scholar * Upadhyaya, N. M. et al. Comparative genomics of Australian isolates of the wheat stem rust pathogen _Puccinia graminis_ f. sp. _tritici_ reveals extensive polymorphism in
candidate effector genes. _Front Plant Sci._ 5, 759 (2014). PubMed Google Scholar * Stamatakis, A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and
mixed models. _Bioinformatics_ 22, 2688–2690 (2006). Article CAS PubMed Google Scholar * Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version
7.0 for bigger datasets. _Mol. Biol. Evol._ 33, 1870–1874 (2016). Article CAS PubMed Google Scholar * Dutech, C., Fabreguettes, O., Capdevielle, X. & Robin, C. Multiple introductions
of divergent genetic lineages in an invasive fungal pathogen, _Cryphonectria parasitica_, in France. _Heredity_ 105, 220–228 (2010). Article CAS PubMed Google Scholar * Jombart, T.
adegenet: a R package for the multivariate analysis of genetic markers. _Bioinformatics_ 24, 1403–1405 (2008). Article CAS PubMed Google Scholar * Librado, P. & Rozas, J. DnaSPv5: a
software for comprehensive analysis of DNA polymorphism data. _Bioinformatics_ 25, 1451–1452 (2009). Article CAS PubMed Google Scholar * Stakman, E. C., Stewart, D. M. & Loegering,
W. Q. _Identification of Physiologic Races of_ Puccinia graminis _var._ tritici (U.S. Department of Agriculture, Agricultural Research Service, Minnesota, USA, 1962). * Barnes, C. W. &
Szabo, L. J. Detection and identification of four common rust pathogens of cereals and grasses using real-time polymerase chain reaction. _Phytopathology_ 97, 717–727 (2007). Article CAS
PubMed Google Scholar * Edgar, R. C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. _Nucleic Acids Res_ 32, 1792–1797 (2004). Article CAS PubMed PubMed
Central Google Scholar * Bebber, D. P., Castillo, A. D. & Gurr, S. J. Modelling coffee leaf rust risk in Colombia with climate reanalysis data. _Philos. Trans. R Soc. Lond. B Biol.
Sci._ 371, https://doi.org/10.1098/rstb.2015.0458 (2016). * Yan, W. K. & Hunt, L. A. An equation for modelling the temperature response of plants using only the cardinal temperatures.
_Ann. Bot. Lond._ 84, 607–614 (1999). Article Google Scholar * Launay, M. et al. Climatic indicators for crop infection risk: application to climate change impacts on five major foliar
fungal diseases in Northern France. _Agr. Ecosyst. Environ._ 197, 147–158 (2014). Article Google Scholar * Agency, J. M. (ed Computational and Information Systems Laboratory Research Data
Archive at the National Center for Atmospheric Research) https://rda.ucar.edu (2013). * Rogelj, J., Meinshausen, M. & Knutti, R. Global warming under old and new scenarios using IPCC
climate sensitivity range estimates. _Nat. Clim. Change_ 2, 248–253 (2012). Article Google Scholar * Jones, P. G. & Thornton, P. K. Generating downscaled weather data from a suite of
climate models for agricultural modelling applications. _Agr. Syst._ 114, 1–5 (2013). Article Google Scholar Download references ACKNOWLEDGEMENTS We thank Les Szabo (United States
Department of Agriculture, USA), Frank Ordon (Julius Kuehn Institute, Germany), Amangeldy Tarkalievich Sarybaev (Kazakh Institute for Land Management and Plant Breeding, Almaty, Kazakhstan),
Liliya Serazetdinova (Knowledge Transfer Network, UK), and Olga Baranova (All-Russia Institute of Plant Protection, St. Petersburg, Pushkin, Russia) for assistance in sourcing samples for
this study, Tom G. Fetch (Agriculture and Agri-Food Canada, Canada) for providing differential wheat lines for screening stem rust, Sreya Ghosh (JIC, UK) for assistance in preparing samples
for imaging, James Brown (JIC, UK) for commenting on the manuscript, and the staff of the Biotechnology Biological Sciences Research Council (BBSRC) National Capability in Genomics at the
Earlham Institute (EI, UK), including Leah Catchpole and Daniel Swan, for assistance with sequencing, and the NBI Computing infrastructure for Science (CiS) group. This project was funded by
an institute development grant from the EI (Norwich, UK), an Industrial Partnership Award (BB/M025519/1) from the BBSRC, a European Research Council Starting Grant awarded to D.G.O.S.
(number 715638), H2020 project EMPHASIS (number 634179), by the BBSRC Institute Strategic Programmes BB/J004553/1 and BB/P012574/1, the John Innes Foundation, and an African Women in
Agricultural Research and Development (AWARD) fellowship to R.N.K. AUTHOR INFORMATION Author notes * Clare M. Lewis, Antoine Persoons and Daniel P. Bebber contributed equally to this work.
AUTHORS AND AFFILIATIONS * John Innes Centre, Norwich Research Park, Norwich, NR4 7UH, UK Clare M. Lewis, Antoine Persoons, Rose N. Kigathi, Jens Maintz, Kim Findlay, Vanessa Bueno-Sancho,
Pilar Corredor-Moreno, Sophie A. Harrington, Ngonidzashe Kangara, Brande B. H. Wulff & Diane G. O. Saunders * University of Exeter, Exeter, EX4 4QD, UK Daniel P. Bebber * Pwani
University, 195-80108, Kilifi, Kenya Rose N. Kigathi * Department of Forest Mycology and Plant Pathology, Swedish University of Agricultural Sciences, Uppsala, 750 07, Sweden Anna Berlin *
Instituto Nacional de Investigación Agropecuaria (INIA) La Estanzuela, Mailbox 39173, Colonia, Uruguay Richard García & Silvia E. Germán * Crop Research Institute, Ruzyně, 161 06 Praha
6, Czech Republic Alena Hanzalová * International Maize and Wheat Improvement Center (CIMMYT), 5689, Addis Ababa, Ethiopia David P. Hodson * Aarhus University, Flakkebjerg, 4200, Denmark
Mogens S. Hovmøller, Annemarie F. Justesen & Mehran Patpour * Campo Experimental Valle de México INIFAP, Texcoco, C. P. 56237, Mexico Julio Huerta-Espino * CIMMYT-Pakistan, Islamabad,
44000, Pakistan Muhammed Imtiaz * Crop Disease Research Program, National Agriculture Research Center, Islamabad, 44000, Pakistan Javed Iqbal Mirza * Wageningen University, Wageningen, 6700,
The Netherlands Rients E. Niks * Faculty of Agriculture, Department of Plant Breeding and Biotechnology, University of Tabriz, Tabriz, 5166616471, Iran Ali Omrani * University of the Free
State, Bloemfontein, 9301, South Africa Zacharias A. Pretorius & Botma Visser * Seed and Plant Improvement Institute, Agricultural Research, Education and Extension Organization (AREEO),
4119, Karaj, Iran Ramin Roohparvar * Tel Aviv University, Tel Aviv, 69978, Israel Hanan Sela * CIMMYT, Apdo. Postal 6-641, D. F. México, 06600, Mexico Ravi P. Singh * University of
Minnesota, St. Paul, 55455, MN, USA Brian Steffenson * Limagrain UK Ltd, Woolpit, IP30 9UP, UK Paul M. Fenwick * National Institute of Agricultural Botany, Cambridge, CB3 0LE, UK Jane Thomas
Authors * Clare M. Lewis View author publications You can also search for this author inPubMed Google Scholar * Antoine Persoons View author publications You can also search for this author
inPubMed Google Scholar * Daniel P. Bebber View author publications You can also search for this author inPubMed Google Scholar * Rose N. Kigathi View author publications You can also
search for this author inPubMed Google Scholar * Jens Maintz View author publications You can also search for this author inPubMed Google Scholar * Kim Findlay View author publications You
can also search for this author inPubMed Google Scholar * Vanessa Bueno-Sancho View author publications You can also search for this author inPubMed Google Scholar * Pilar Corredor-Moreno
View author publications You can also search for this author inPubMed Google Scholar * Sophie A. Harrington View author publications You can also search for this author inPubMed Google
Scholar * Ngonidzashe Kangara View author publications You can also search for this author inPubMed Google Scholar * Anna Berlin View author publications You can also search for this author
inPubMed Google Scholar * Richard García View author publications You can also search for this author inPubMed Google Scholar * Silvia E. Germán View author publications You can also search
for this author inPubMed Google Scholar * Alena Hanzalová View author publications You can also search for this author inPubMed Google Scholar * David P. Hodson View author publications You
can also search for this author inPubMed Google Scholar * Mogens S. Hovmøller View author publications You can also search for this author inPubMed Google Scholar * Julio Huerta-Espino View
author publications You can also search for this author inPubMed Google Scholar * Muhammed Imtiaz View author publications You can also search for this author inPubMed Google Scholar * Javed
Iqbal Mirza View author publications You can also search for this author inPubMed Google Scholar * Annemarie F. Justesen View author publications You can also search for this author
inPubMed Google Scholar * Rients E. Niks View author publications You can also search for this author inPubMed Google Scholar * Ali Omrani View author publications You can also search for
this author inPubMed Google Scholar * Mehran Patpour View author publications You can also search for this author inPubMed Google Scholar * Zacharias A. Pretorius View author publications
You can also search for this author inPubMed Google Scholar * Ramin Roohparvar View author publications You can also search for this author inPubMed Google Scholar * Hanan Sela View author
publications You can also search for this author inPubMed Google Scholar * Ravi P. Singh View author publications You can also search for this author inPubMed Google Scholar * Brian
Steffenson View author publications You can also search for this author inPubMed Google Scholar * Botma Visser View author publications You can also search for this author inPubMed Google
Scholar * Paul M. Fenwick View author publications You can also search for this author inPubMed Google Scholar * Jane Thomas View author publications You can also search for this author
inPubMed Google Scholar * Brande B. H. Wulff View author publications You can also search for this author inPubMed Google Scholar * Diane G. O. Saunders View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS D.G.O.S. wrote the manuscript with contributions from A.P., D.B. and B.B.H.W. C.M.L., A.P., D.P.B., R.N.K., J.M., K.F., V.B-S.,
P.C-M., S.A.H., N.K., P.M.F., J.T. and D.G.O.S. performed the experiments and analysed the data. A.B., R.G., S.E.G., A.H., D.P.H., M.S.H., J.H-E., M.I., J.I.M., A.F.J., R.E.N., A.O., M.P.,
Z.A.P., R.R., H.S., R.P.S., B.S., B.V. and P.M.F. collected, purified, and propagated stem rust isolates for sequencing. B.B.H.W. and D.G.O.S. conceived and designed the experiments. All
authors read and approved the final manuscript. CORRESPONDING AUTHORS Correspondence to Brande B. H. Wulff or Diane G. O. Saunders. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing financial interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lewis, C.M., Persoons, A., Bebber,
D.P. _et al._ Potential for re-emergence of wheat stem rust in the United Kingdom. _Commun Biol_ 1, 13 (2018). https://doi.org/10.1038/s42003-018-0013-y Download citation * Received: 06
September 2017 * Accepted: 11 January 2018 * Published: 08 February 2018 * DOI: https://doi.org/10.1038/s42003-018-0013-y SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
Trending News
Muslim Women Group Says Their Petition To End Triple Talaq Has Collected 50,000 SignaturesMumbai-based NGO, Bharatiya Muslim Mahila Andolan (BMMA) has sought the support of the National Commission for Women (NC...
Southern california wildfires: new blaze erupts at camp pendletonReporting from San Diego — As firefighters began to get the upper hand on several wildfires in San Diego County, a new b...
What is the green credits scheme, which pm modi mentioned at cop28Prime Minister Narendra Modi Friday launched an initiative focusing on generating Green Credits through plantation on de...
The age of the outsider | thearticleEach month about 5,000 people in the UK are interviewed for the Labour Force Survey. The Office for National Statistic...
Kelsey Beveridge | Pew Research CenterABOUT PEW RESEARCH CENTER Pew Research Center is a nonpartisan, nonadvocacy fact tank that informs the public about the ...
Latests News
Potential for re-emergence of wheat stem rust in the united kingdomABSTRACT Wheat stem rust, a devastating disease of wheat and barley caused by the fungal pathogen _Puccinia graminis_ f....
Ranking the top 10 safeties in the 2024 nfl draftThe Post’s Ryan Dunleavy gives his top 10 safeties in this year’s NFL draft, based on evaluations and conversations with...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
Kim huybrechts' wife called out 'lazy' darts star obsessed with football managerKim Huybrechts is one of the most gifted darts players to come out of mainland Europe. However, there is a prevailing se...
Evergrande dodges second default by paying an overdue couponChina Evergrande Group has staved off a potential default for the second time in a week, after paying an overdue offshor...