Single-atom catalytic growth of crystals using graphene as a case study
Single-atom catalytic growth of crystals using graphene as a case study"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Anchored Single-atom catalysts have emerged as a cutting-edge research field holding tremendous appeal for applications in the fields of chemicals, energy and the environment.
However, single-atom-catalysts for crystal growth is a nascent field. Of the few studies available, all of them are based on state-of-the-art in situ microscopy investigations and
computational studies, and they all look at the growth of monolayer graphene from a single-atom catalyst. Despite the limited number of studies, they do, collectively, represent a new
sub-field of single-atom catalysis, namely single-atom catalytic growth of crystalline solids. In this review, we examine them on substrate-supported and as freestanding graphene
fabrication, as well as rolled-up graphene, _viz_., single-walled carbon nanotubes (SWCNT), grown from a single atom. We also briefly discuss the catalytic etching of graphene and SWCNT’s
and conclude by outlining the future directions we envision this nascent field to take. SIMILAR CONTENT BEING VIEWED BY OTHERS GRAPHENE NANORIBBONS INITIATED FROM MOLECULARLY DERIVED SEEDS
Article Open access 30 May 2022 CYCLODEHYDROGENATION OF MOLECULAR NANOGRAPHENE PRECURSORS CATALYZED BY ATOMIC HYDROGEN Article Open access 15 January 2025 GENERAL SYNTHESIS OF SINGLE-ATOM
CATALYSTS WITH HIGH METAL LOADING USING GRAPHENE QUANTUM DOTS Article 24 June 2021 INTRODUCTION Catalysts are a major contributor to the global economy. Over 90% of chemical processes
require catalysts and there is a continuous demand for the development of more effective, inexpensive, and green catalysts to secure a sustainable future for our society1. As defined by
Berzelius in 1835, a catalyst is a substance capable of accelerating the rate of a chemical reaction without being consumed2. Broadly speaking, there are two groups of catalysts, namely,
homogeneous catalysis in which the catalyst is in the same phase as the reactants during the reaction, and heterogeneous catalysis where the catalyst and reactants are in two distinct
phases. Because of challenges in separating homogenous catalysts from the reaction product as well as the fact that most are somewhat thermally unstable, most industrial catalysts (ca. 80%)
are heterogeneous catalysts1,3. Typically, heterogenous catalytic properties are sensitive to their surface heterogeneity and structure. For instance, metal catalysts are affected by their
terminating atom coordination and so steps sites, kinks, vacancies, staking faults and twin boundaries all affect their performance. Moreover, the size of metal particles is also an
important factor governing performance4 and has helped drive the use of nanoparticles as catalysts. However, because of surface defects it is near impossible to fabricate two identical
nanoparticles. One approach to address this complex issue is to reduce the size of a metal particle down to the atomic level, _viz_., a single atom serving as a catalyst and in this case
there is no longer any surface heterogeneity1. Aside from providing high specific activity, single-atom catalysts (SAC) reduce cost and this is particularly important for expensive noble
metals such as Pt, Pd, Ru, Rh and Ir which are important to the petrochemical industry, medicine production, environmental protection and energy applications5. Single-atom catalysts are
nearly always prepared on a support material (e.g. metal oxide, metal carbide, carbon nitride, graphene and various other two-dimensional (2D) materials)6,7 and can even be produced at scale
now8. SAC’s have compelling properties in that each atom is accessible for the catalytic reaction (100% atomic efficiency), often the metal atom-support interaction enhances charge transfer
between the two and they have unsaturated coordination unlike metal clusters which can lower activation energies7,9. SACs resemble heterogenous catalysts since they are immobilized on
supports that can be relatively easily separated and re-used. While they also resemble homogeneous catalysts since the strong metal atom-support interactions yield electronic properties
similar to those in organo-metallic complexes7. SACs are advantageous in comparison to other heterogeneous catalysts because one can also tune the coordination environment of a single atom.
Typically, this is achieved by altering the type of coordinating atoms on the support6. Indeed, the use of isolated single atoms is the most effective method with which to obtain optimal
active sites in corresponding catalysts so as to maximize the metal atom efficiency and maintain the necessary catalytic performances5,10,11. The field of SACs is a highly dynamic and
fast-evolving field and can be expected to continue to develop strongly. However, despite the growing body of research on SACs, very little research exists on the use of _SINGLE ATOMS FOR
THE CATALYTIC GROWTH OF CRYSTALLINE MATERIALS__._ Remarkably, in our extensive literature survey we only found examples based on the growth of a single crystalline material - graphene.
Perhaps this is not surprising given the large body of research on graphene and moreover, as a single element 2D material it is technically less challenging to investigate. Of the few
studies we found, all are based on state-of-the-art in situ microscopy investigations as well as supporting theoretical studies. Despite the limited number of studies for SAC crystal growth,
they do, collectively, represent a new sub-field of SACs, namely single-atom catalytic growth of crystalline solids. The use of SACs for crystal growth is crucial at the nanoscale, because,
as for bulk solids the material’s crystal structure determines its properties, however, at the nanoscale the location and position of each atom becomes critically important. Thus, to
fabricate such structures usefully for application, both with known and novel and as yet to be discovered materials, a prerequisite is to synthesize these materials with atom precision. Top
down structuring at these levels, while having been so successful (for example in the microelectronics industry) struggles evermore as a manufacturing process at the nanoscale these days as
few atom or single atom precision becomes a precondition. Thus, the ability to develop bottom-up approaches, where material is added with atomic precision is crucial. In this context, it
becomes self-evident that the ability to construct crystals using a single catalyst atom has advantages, because in affect an atom serves as a tool with which to add atoms and the tool is of
the same scale as the bricks (atoms) being used to construct a material. Indeed, the need for precision manufacturing at the atomic scale is ever more pertinent as we discover and develop
new nanomaterials such as clusters (quantum dots), nanowires with variable properties and, of course, the rapidly developing 2D materials. All of these hold great promise for a number of
applications ranging from bio-medicine, energy and electronics12,13,14. The need for atom precise control for materials fabrication is epitomized by the need for atom precise structuring of
2D materials for device fabrication. For example, in a simple case, the edge termination of graphene influences its properties, such as its chemical properties (e.g. hydrogen evolution
reaction)15,16, its magnetic properties17, its mechanical properties18 and its electronic properties19,20,21,22. Similar dependencies are found for other 2D layered materials such as the
transition metal dichalcogenides (TMDs)23,24,25, the few atoms-thick layers of transition metal carbides, nitrides, or carbonitrides, commonly referred to as MXENES26,27,28. Such atom
precise edge termination control will be imperative if we are to develop electronic circuits based on these 2D materials. Thus, to fabricate such materials from a bottom-up approach using a
catalyst it becomes challenging to form the structure using a catalyst particle since the size of the catalyst particle is significantly larger than that of the edge structure and as a
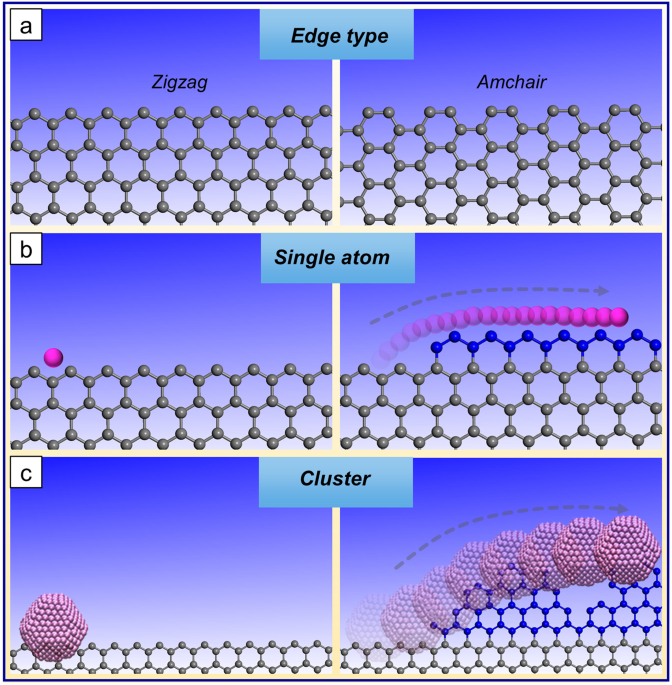
result can’t yield atom precise clean edges29. Using a single atom overcomes this obstacle. This is illustrated in Fig. 1 below using graphene as an example. The two canonical types of
graphene edges are also provided, namely zig-zag (ZZ) and arm-chair (AC) edge termination are firstly presented schematically (panel a). Then a schematic representation of a single atom
growing a single-atom layer on graphene with only a single-atom layer growing is presented (panel b) and in panel c the rough edge termination typically observed from catalyst nanoparticles
and clusters at graphene edges is presented. The use of single-atom catalysts for crystal growth with atomic precision is in its infancy and there remain many challenges. To highlight some
of these challenges let us consider the case of a SAC for the growth of a device from a 2D material, e.g., graphene. To achieve growth one needs to first determine an appropriate catalyst
atom and then nucleate a seed maintaining a SAC at an edge or place the catalyst atom at an edge of an initial seed. For atom precise growth, control of the edge atom will then be required,
as well as systems to provide the appropriate feedstock etc. From these few aspects it is clear there are many hurdles ahead, but overcoming them presents enormous potential for atom scale
crystal growth with many potential applications we have as yet to imagine or discover. At this early stage, we are only just beginning to show how single and even paired atoms can be used to
grow 2D materials (namely graphene) and what factors can be used to control the SACs and even cause them to switch between catalytically growing or catalytically etching a material. The
road ahead appears exciting. In this review, we examine the studies that employ a SAC for the edge growth of graphene on a substrate and as a freestanding structure as well as the case of
rolled-up graphene, _viz_., single-walled carbon nanotubes (SWCNT). We also briefly discuss the catalytic etching of graphene and carbon nanotubes and finally discuss the future directions
we envision this nascent field to take. SINGLE-ATOM SP2 CARBON CRYSTAL GROWTH The potential for a catalytic role from single metal atoms to grow sp2 crystalline carbon was predicted
theoretically for graphene30 and for single-walled carbon nanotubes31. As mentioned in the introduction the potential and relevance of SACs for 2D crystal growth were laid out. In
particular, if one considers the importance of edge terminations on the properties of 2D materials and even device systems relying on single and few atoms, such as single-atom
transistors32,33, molecular electronics34 and thereby quantum electronics and logic at the atomic scale35. If we consider the case of graphene, the fabrication of edge terminations with
atomic control we could construct entire integrated electrical circuits. Equally if we are able to control single atoms to grow and control the chirality of single-walled carbon nanotubes we
could also build entire integrated electronic circuits from carbon nanotubes (see Fig. 2). Such technological developments will be groundbreaking and highlight the importance of developing
the use of SAC’s for crystal growth and in particular sp2 carbon nanomaterials. However, it is only in the last few years that experimental evidence for the growth of graphene from a single
metal atom has started to emerge. This is because both high spatial and temporal resolution are required to provide an understanding of the reaction pathways. Thus far, two microscopy
techniques have demonstrated their potential to provide direct observational information at the atomic scale. They are the transmission electron microscope and scanning tunneling microscopes
for freestanding graphene growth and supported graphene growth studies, respectively. Here, we begin with the case of supported growth. GRAPHENE GROWTH ON A SUBSTRATE Shu et al.36 conducted
a comprehensive study on the stability of several graphene edge configurations on Cu (111) and other metal surfaces during chemical vapor deposition (CVD) growth conditions using density
functional theory (DFT) calculations. The work found that the termination of armchair (AC) edges by individual Cu atoms was energetically preferred as compared to zigzag (ZZ) edges that tend
to keep their pristine structure. Moreover, the study showed that the Cu atom passivation of AC edge sites on the graphene significantly lowers the barrier of incorporating carbon atoms
into the growing graphene edge from 2.5 to 0.8 eV. This results in very fast growth at the AC edge of the graphene. As a consequence, the authors state, that, on the basis of the classical
theory of crystal growth, the ZZ edge should be the dominant edge of growing graphene edges in agreement with broad experimental observations36. In another work, the same group looked at the
role of Ni adatoms at graphene edges (in comparison to Cu) using DFT calculations. The study showed that C species can be inserted (added) through individual Ni atoms at kink sites at the
edges37. Indeed, mobile adatoms which are commonly present on metal surfaces at elevated temperatures are involved in several chemical processes taking place on surfaces and at the
solid-liquid interface38,39. In a detailed high-speed STM characterization study on epitaxial graphene growth on Ni (111) the researchers observed single Ni atoms at kink sites on the
graphene edges at elevated temperatures40. The time resolution was on the order of milliseconds. The edge termination of the epitaxial graphene were identified as zigzag (zz) and Klien (k)
edges. For both edges kink sites (where there are interruptions on the last two C rows) were observed with individual Ni atoms. These kink sites were shown to act as nucleation centers for
graphene growth. Growth was shown to proceed from the kink site by the orderly completion of the two interrupted carbon rows while moving the kink ahead. The observational data pointed to
mobile Ni adatoms diffusing along the edges and that these single Ni atoms at kink sites are directly involved with the growth process. This is in agreement with the above discussed
theoretical study which is in agreement with the above mentioned DFT studies37. Moreover, the experimental team also conducted molecular dynamic (MD) studies. The MD studies at a temperature
of 710 K showed Ni adatoms to move randomly over the bare metal surface until they reached a graphene edge. Once at a graphene edge the Ni atoms diffuse parallel to the edge and showed
considerably longer residence times when encountering a kink site. Experimentally the STM image series showed that, in most cases, the adatoms presence was accompanies by C dimer attachment
nearby strongly suggesting a catalytic role of the single Ni atom. Figure 3 shows a sequence corresponding to the k edge. Two short-lived Ni adatom configurations can be identified at the
kink sites (panels B, C, F, and G). Constant-height simulated images (panels D and H) matched the corresponding STM data. In short, the stable configurations observed by STM, as well as
theory point to individual Ni atoms playing a catalytic role in the insertion of C at kink sites, _viz_. graphene growth while on a metal surface40. While synthetically grown graphene is
mostly achieved on substrates, the above works show that the actual growth process is complicated and that there remains much to study, in particular regards growth on substrates involving a
SAC. The only clear evidence thus far is for a Ni atom, but how other elemental SACs can be used, can SACs be used on differing substrates, what are the roles for differing environmental
aspects (e.g., feedstock, presence of hydrogen, pressure etc), can multiple SACs be employed etc, are but a few of the critical questions that need to be explored when employing SACs for
crystal grow on a substrate. One of the greatest challenges to explore SAC driven crystal growth, at least through direct observation studies, are the limited choices in characterization
tools. STM has proven itself, but is still a challenging approach. We envision that in the future, electron transparent ultrathin substrates could be employed in dynamic in situ
environmental TEM systems. TEM based studies may also pave the way for a broader set of synthesis conditions to be explored than that through STM based studies. FREESTANDING GROWTH AT
GRAPHENE EDGES We now turn our attention to the case of single metal atoms at the edges of freestanding graphene. In all these experimental studies a TEM was used to study the individual
metal atoms at the edge of the freestanding graphene. In some cases additional heating was provided to help drive the catalytic reaction, however, in most cases, electron irradiation is
sufficient to drive the reaction. Details of the electron beam-specimen interactions that help drive the reaction can be found in reference41. The carbon feedstock for growth is obtained
from hydrocarbon contamination within the TEM column42. We begin with the case of single iron atoms at graphene edges43. The iron atoms were provided from FeCl3 which was used as an etchant
during the transfer of the CVD grown graphene44. The Fe atoms attach to the edges more easily because of the larger binding energy of edges as compared to the basal plane of the graphene.
The researchers43 found two ubiquitous configurations of a single Fe atom residing at an AC edge. In the first configuration, an Fe atom incorporated into a five-atom ring forming a pentagon
while in the second an Fe atom incorporated into a five-atom ring to form a distorted pentagon (i.e., substituting a C atom). However, when irradiated by the electron beam the Fe atoms
diffuse along the graphene edge and at times incorporate additional carbon. Figure 4 shows a cycle of the single Fe atom catalytically adding in two carbon atoms. In panels A–D a typical
translocation of a single unit cell for an Fe at is shown. The Fe atoms changes from a pentagon structure and absorbs some carbon atoms. As it absorbs the carbon atoms it moves to the right
(this is observed as a shadow line in panel C as the motion occurred during the image exposure). It then stops at a nearby location, again, forming a pentagon. Stick and ball models show the
different atomic rearrangements of the process in panel E. While panel G shows the corresponding translocations added a micrograph of the process. During the process the single Fe atoms
unit cell shifts to the right on a ZZ edge, the whole cycle represents a single catalytic growth cycle for graphene from a Fe atom incorporating two C atoms43. The external carbon atoms
arise from hydrocarbon contamination with the TEM42. It is also worth noting that the in situ TEM studies on Fe atoms at graphene edges, the researchers also observed the catalytic etching
of graphene, which, is discussed later. A similar study was conducted to investigate the behavior of Cr atoms at graphene edges45. Upon electron beam irradiation the Cr atoms diffuse along
the edge and after their migrations, new carbon atoms were found to have been incorporated between the Cr atoms initial and final position. This led to the formation of new hexagonal
structures leaving a ZZ edge. Figure 5 shows an example of this process. The authors conducted a statistical analysis of the number of new hexagons forming at an open graphene edge and found
one to six formed within a one-frame-per-second micrograph capture rate. Unlike the case for Fe atoms at graphene edges, with Cr atoms no etching of the graphene was observed. The Cr atom
at graphene edge study also included a MD study comparing Fe and Cr atoms at a graphene edge. The MD study showed confirmed both Fe and Cr single atoms can catalytically grow graphene;
however, there were some differences. The Fe atom was shown to be far more dynamic both from a temporal and spatial perspective. These differences were attributed to their energetic and
kinetic stability and these aspects affect the catalytic efficiency of the atoms in that an efficient SAC should have a good balance between stability and migration. Stability is important
to provide a nucleation site, which is needed to attract C atoms for the catalytic process. However, the atoms potential to migrate is also important to keep the catalyst atom active along
the graphene edge and avoid poisoning. The comparative MD study on Cr and Fe atoms at graphene edges indicated that Cr is a more efficient SAC as compared to Fe for graphene edge growth45.
In another study, the role of single Cu atoms at graphene edges was investigated46. They observed Cu atoms frequently in pentagon structures at the graphene edge (similar to the case for Fe
described above). With electron irradiation and heating (150 oC or 300 oC) they observed graphene growth mediated by a single Cu atom. Figure 6 shows examples of the growth. Panels A and B
show before and after examples of the growth. The shaded region in panel B shows extensive growth after 3 min of irradiation. Panels C to G show more details of the actual growth process. In
a previous study by the team, they showed substitutional Cu atoms can promote the rotation of C–C bonds in graphene47. Such C–C rotations can convert pentagon-heptagon pairs into hexagons
which is what they observed in the Cu atoms at graphene edge study (panels C and D) which triggered further rotations as shown in panel E. Panels F and G provide another example of a Cu atom
translocation over a different edge system and the incorporation of new C atoms as it does so, further confirming the catalytic growth of graphene by a single Cu atom. A more recent study
examined the role of Sn atoms at graphene edges48. The study examined single Sn atoms (monomers) as well as Sn atom pairs (dimers) as SACs. Both graphene growth and etching was observed from
both monomer and dimer SACs, and the switching mechanism between growth or etching is discussed a little later. The experimental data and supporting DFT studies showed a good correlation
between the observed frequency of Sn-graphene bonding configurations and their relative energies (i.e., energy difference relative to the most commonly observed configuration of an Sn atom
residing at an armchair position at the graphene edge). In terms of the dynamic diffusion of Sn atom SAC pairs a remarkable tendency was observed, namely, a synchronized diffusion between
the two atoms (for growth and etching) was observed (see Fig. 7). More in-depth experimental and theoretical investigations revealed the synchronized diffusion occurred in a step-wise
shuffle of the two atoms, viz. after the first Sn SAC atom shifts, the second one follows, rather than a hopping process which was also postulated as a possible mechanism. While most
observations showed Sn dimer SAC diffusion to occur synchronously, occasionally the Sn pair would diffuse in opposite directions and the dimer would collapse to two Sn monomers. A detailed
statistical analysis of the dimers diffusion showed the vast majority diffused as a dimer (indicating an attractive interaction which was also supported by DFT studies). Moreover, the study
showed an edge dependency, such that ZZ edges tended to lead to dimer collapse, while AC edges and chiral edges tented to favor dimer synchronized diffusion. Calculations of the relative
binding energies showed two interesting aspects (see Fig. 8). In the first, the relative binding energy for zigzag edges is positive and indicates that ZZ edges favor individual Sn SACs
rather than dimer SACSs. Thus, paired SACs (dimers) are likely to separate (collapse) on diffusing, exactly as was observed experimentally. Secondly, in the case of Sn single and binary
atoms at AC edges, the binding energy for Sn pairs (dimers) was found to be significantly lower than for a single Sn atom. Thus, the study explains why when the binary SAcs are at AC sites
they prefer to synchronize their diffusion such that as soon as one atom moves, the second will follow in the same direction (shuffle) so as to minimize their energy and it explains why,
experimentally, the synchronized diffusion of binary atoms was observed so often and moreover, why this was found predominantly at armchair sites48. Another important SAC is Ni which is a
well-known and commonly used transition metal catalyst material in its bulk form due to its availability and cost. To this end, we have been investigating Ni atoms as SAC candidates for
graphene growth. Initial studies show that Ni SACs can effectively grow graphene in situ in TEM under electron beam irradiation. At times we observe Ni SACs to catalytically etch graphene
(data not shown). An example of a Ni SAC catalytically growing graphene is presented in Fig. 9. Figure 9 (a) shows a Ni atom initially residing in a hexagonal location at a graphene edge
(i.e., the Ni atom substitutes a C atom). After 20.25 sec. the Ni atom shifts to the left and locates in an armchair configuration (pentagon). Its former location now has a C atom,
indication growth. Where this C atom came from is unclear. It could have come from the position the Ni atom translocated to, or from the Klein edge that existed at the far left of the
initial system (panel a), or from the vacuum. Regardless, the translocation confirms the catalytic action of the Ni atom at a graphene edge. To summarize this section a number of metal atoms
(Fe, Cr, Cu, Sn, and Ni) all show the potential as SACs for crystal growth, at least for graphene. Moreover, in all instances a single or few carbon atoms get incorporated during the
catalytic growth cycle of the metal SACs, confirming that in reality SACs offer the potential to grow graphene edges with atom precision. Equally attractive is the potential for single atoms
to grow single-walled carbon nanotubes with atomic precision (and hence chiral control). Reportedly, the Nobel Laurette Richard E. Smalley, proposed a single catalyst atom could grow a
single carbon nanotube by adding C at an open edge (see the bottom left panel in Fig. 4). In the next section, we examine SAC growth of single-walled carbon nanotubes (SWCNT). SWCNT GROWTH
AT EDGES Single-walled carbon nanotubes are, in essence, rolled-up tubules of graphene strips. Depending on the manner in which the graphene strip is rolled (their so called chirality), the
tubes electronic properties can be either semiconducting or conducting. Moreover, SWCNT combining different chirality’s leads to interesting properties such that carbon nanotubes can serve
as (ballistic) interconnects and devices (e.g., rectifiers, transistors etc.)49. Their controlled combinations can yield integrated electronic circuits. However, at this stage, such
integrated circuits remain challenging to produce since it requires fabrication control at the atomic scale. SACs could potentially provide a means to overcome this hurdle. While we did not
find any examples of a SACs growing a single-walled carbon nanotube experimentally, we did find a theoretical study looking at a single Ni atom growing a freestanding SWCNT. The study was
based on density functional total energy calculations. A temperature of 1200 oC was used. Figure 10 shows the intermediate steps involved in the catalytic growth of a SWCNT, as well as the
binding energies. The study looked at the energetics and in the first scenario a Ni atom forms a pentagon structure (panel A1), then a carbon atom gains energy (4.5 eV) by forming a pentagon
defect (panel A2). The high mobility Ni atom nearby reacts with the carbon to form a hexagon (panel A3) The authors argue that an incoming C atom would then push out the substitutional Ni
atom (panel A4). Then, because of the weak attachment energy (inferred from the geometries in panels A4 and A5), the Ni atom can continue its diffusion along the edge and further grow the
SWCNT. Further intermediate steps for carbon insertion are provided in panels A1’ to A5’ for a different catalytic cycle. Both catalytic cycles are exothermic and occur with no activation
barriers which would suggest a high reaction rate. In short, using concerted exchange mechanisms, the single Ni atom at the open SWCNT edge can catalyze growth by the continued formation of
hexagons. As an early step to investigate the propensity if Ni SAC’s to catalytically grow sp2 carbon we explored their SAC behavior in situ in a TEM at graphene edges under electron
irradiation. As discussed at the end of the previous section, indeed, Ni SACs can grow graphene. The presented example (Fig. 9 does not, however, show growth from AC sites as theoretically
predicted31, although we do observe Ni atoms at AC edges (see Fig. 11) and so we may yet observe the predicted growth. Moreover, the synthesis conditions in our studies are rather different
in that the reaction is driven by an electron beam and is conducted at room temperature. We also plan on conducting experimental studies with SACs at the end of open SWCNT. There is much to
be explored regards SACs and SWCNT growth. SINGLE-ATOM SP2 CARBON CRYSTAL ETCHING While the focus of this article is on the growth of graphene from SACs, it is worth briefly looking at SACs
potential to etch graphene catalytically. We begin with the case of Fe SACs which although they can grow graphene under electron beam irradiation, as discussed above, they can also etch
graphene17. In that study, the conditions to determine which catalytic process occurred was not clear since the conditions were more or less the same. Another example is that of Cr, which on
one study was only found to grow graphene and no etching was observed20. While in another study looking at Cr, Ti, Pd, Ni, Al and Au, the authors claim to have only observed etching from
these SACs50. This might be due to differences in the electron beam conditions, given that in the study with Cr SACs where only growth was observed, the imaging mode was in TEM (parallel
imaging) while in the latter study the scanning TEM (STEM) mode was used. Moreover the latter article did not present analytical data confirming the presence of Cr atoms at the graphene
edge. In another TEM study, the use of Pt SACs at graphene edges at the elevated temperatures of 150 oC or 300 oC were investigated and only etching was observed. In the same study, Au atoms
at graphene edges observed in TEM at room temperature showed neither catalytic etching or growth51. Thus, the contradictory results between the different studies highlight a need for
careful studies on the actual conditions implemented. In terms of SAC for graphene etching, Si, a non-metal atom, has also been shown to catalytically etch graphene24. In this study, the
researchers were able to form clean graphene edges or pores from Si SACs under electron beam irradiation (see Fig. 12). A recent study exploring single-atom and binary Sn SACs, an
interesting concept, based on Au nanoparticle catalyst action, was postulated to explain the observation that in some cases SAC’s catalytic behavior followed either growth or etching despite
similar or identical in situ TEM conditions48. To explain this fact they argue, the atoms follow exactly the same catalytic action when growing or etching a graphene edge. The difference
lies in where the SAC obtains a carbon atom from and where it then deposits that captured carbon atom during the dynamic catalytic action. This is illustrated in Fig. 13, panel a. During the
first catalytic step on the left, a C atom from the vacuum is captured by the Sn catalyst atom and then deposited at the graphene edge and this step represents growth. While on the right
example a C atom from the graphene edge is captured by the Sn SAC and then the C is ejected to the vacuum and this cycle represents etching. However, to the Sn SAC there is no difference in
its catalytic action since it simply captures a C atoms and then releases/deposits it somewhere else. However, the more costly cycle energetically will be the capture of a C atom from a
graphene edge, as compared to C from the vacuum, since additional energy tip extract the C from the graphene edge is required. Thus in this argument, by controlling the supply of C atoms one
can control if a SAC grows or etches Graphene. The catalytic process of an Sn SAC in the presence of a C supply in the vacuum (for graphene growth) is shown schematically in panel b, while
in panel c the scenario where there is a lack of C atoms in the vacuum is shown and, thus, C atoms from the graphene edges are consumed (etched). Finally, in panel d the schematic shows the
scenario where only a limited supply of C atoms are present in the vacuum and thus, both growth and etching can be observed. Finally, we turn to the case of a SAC etching a SWCNT, for which
we did not find any experimental evidence, but has been explored theoretically. The study explored the potential of a single Fe atom in an H2 environment52. The computational study suggests
H helps passivate edge C atoms and thus release the Fe atom for the continuous unzipping of the SWCNT. The curvature energy is released during the unzipping process and serves as the main
energy source to support the continuous unzipping. Moreover, it can enable chirality and diameter dependent unzipping behavior. In this way tailored graphene nanoribbons can form. In terms
of SAC atoms at the open end of a SWCNT, we can expect, like graphene, that a SACs catalytic action to etch (or grow) can be controlled by the feedstock supply in the vicinity. In short,
controlling the feedstock supply could be a simple and effective means to switch a SACs behavior between the growth and etching. Other parameters can also be expected to affect a SACs
catalytic activity too. OUTLOOK AND SUMMARY The studies examined in this review provide both unequivocal theoretical and experimental evidence that SAC’s can drive crystal growth and we
anticipate significant developments on SAC based crystal growth over the next decade. Clearly, future studies need to focus on more in-depth investigations to understand the underlying
mechanisms at play and how, armed with that knowledge, controlled crystal growth with atomic accuracy can be achieved. Various aspects need to be developed in this sense. Figure 14
summarizes the different key aspects. One important aspect is understanding the different elemental atoms, such as their divergent catalytic efficiency which depends on the crystal (single
or hetero-crystals) being grown, their variation in terms of the bonding and coordination environment etc. Then there are the synthesis conditions which will involve aspects such as
temperature, background gas/vacuum conditions, electron beam conditions (where relevant), sample purity, substrate choice (where relevant), feedstock type and availability etc. All these
will depend to on the choice and phase of the crystals being grown. For example, forming a OD structure, such as a quantum dot will have differing needs as compared to a 1D nanoribbon or a
2D lateral heterolayer structure (e.g. integrated electronic circuit) or stacked heterolayers and small 3D structures with functional single atoms (e.g., single-atom transistors). Even then
variations for devices at the atom scale will be of significance too. To achieve success there will be a need for numerous systematic and detailed studies, in particular, microscopy-based
studies with supporting characterizations and intense supporting and predictive theoretical investigations will be of great importance. It is not hard to envision how important their use
could be in the fabrication of atom accurate device production in the future. In short, SAC crystal growth could be a game changer to the semiconductor industry, as well as other
technologies. In summary, we have collated a number of experimental and computational works examining the growth of crystalline monolayer graphene from a single-atom serving as the catalyst.
Experimental examples on graphene growth from a SAC are shown for both substrates based growth and freestanding growth. A number of important theoretical studies yield deeper insight into
the underlying mechanisms involved. Collectively, these works represent a new sub-field of SACs, namely SAC growth of crystalline solids. Clearly, a great deal of research into this exciting
new field can be anticipated in the near future and such studies could have significant impact in the controlled growth of atom-level structures and devices fabricated with atomic
precision. DATA AVAILABILITY Data sharing was not applicable to this paper, as no original datasets were generated or analyzed for this review. REFERENCES * Li, J., Stephanopoulos, M. F.
& Xia, Y. Introduction: Heterogeneous Single-Atom Catalysis. _Chem. Rev._ 120, 11699–11702 (2020). Article CAS Google Scholar * Berzelius, J. J. _Årsberättelse om Framstegen i Fysik
och Kemi, Annual Report on Progress in Physics and Chemistry_, Royal Swedish Academy of Sciences (P.A. Norstedt & Söner, Stockholm, Sweden, 1835). * Sun, R. et al. Heterogeneous
catalysts for CO2 hydrogenation to formic acid/formate: from nanoscale to single atom. _Energy Environ. Sci._ 14, 1247–1285 (2021). Article CAS Google Scholar * Chen, M. S. & Goodman,
D. W. The structure of catalytically active gold on titania. _Science_ 306, 252–255 (2004). Article CAS Google Scholar * Cheng, N., Zhang, L., Doyle-Davis, K. & Sun, X. Single-Atom
Catalysts: From Design to Application. _Electro Ener. rev._ 2, 539–573 (2019). Article Google Scholar * Zhang, B., Fan, T., Xie, N., Nie, G. & Zhang, H. Versatile Applications of Metal
Single-Atom @ 2D Material Nanoplatforms. _Adv. Sci._ 6, 1901787 (2019). Article CAS Google Scholar * Weon, S. et al. Environmental Materials beyond and below the Nanoscale: Single-Atom
Catalysts. _ACS EST Eng._ 1, 157–172 (2020). Article Google Scholar * He, X. et al. Mechanochemical Kilogram-Scale Synthesis of Noble Metal Single-Atom Catalysts. _Cell Rep. Phys. Sci._ 1,
100004 (2020). Article Google Scholar * Zhang, L., Ren, Y., Liu, W., Wang, A. & Zhang, T. Single-atom catalyst: a rising star for green synthesis of fine chemicals. _Natl Sci. Rev._
5, 653–672 (2018). Article CAS Google Scholar * Dyck, O. et al. Building Structures Atom by Atom via Electron Beam Manipulation. _Small_ 14, e1801771 (2018). Article Google Scholar *
Yang, X. F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. _Acc. Chem. Res_ 46, 1740–1748 (2013). Article CAS Google Scholar * Zhu, Y., Yuan, D., Zhang, H., Xu,
T. & Sun, L. Atomic-scale insights into the formation of 2D crystals from in situ transmission electron microscopy. _Nano Res._ 14, 1650–1658 (2020). Article Google Scholar * Tapia, M.
A. et al. Phosphorene and other layered pnictogens as a new source of 2D materials for electrochemical sensors. _Trends Anal. Chem._ 139, 116249 (2021). Article CAS Google Scholar *
Yanling, Y., Zhongmin, T., Han, L. & Jianlin, S. Emerging two-dimensional material nanozymes for theranostic nanomedicine. _Biophysics Rep._ 7, 159–172 (2021). Article Google Scholar *
Kumatani, A. et al. Chemical Dopants on Edge of Holey Graphene Accelerate Electrochemical Hydrogen Evolution Reaction. _Adv. Sci._ 6, 1900119 (2019). Article Google Scholar * Jiang, D.
E., Sumpter, B. G. & Dai, S. Unique chemical reactivity of a graphene nanoribbon’s zigzag edge. _J. Chem. Phys._ 126, 134701 (2007). Article Google Scholar * Krompiewski, S. One edge
magnetic configurations in graphene, stanene and phosphorene zigzag nanoribbons. _J. Magn. Magn. Mater._ 534, 168036 (2021). Article CAS Google Scholar * Lopez-Polin, G., Gomez-Navarro,
C. & Gomez-Herrero, J. The effect of rippling on the mechanical properties of graphene. _Nano Mater. Sci_. 10, 1–9 (2021). * Castro Neto, A. H., Guinea, F., Peres, N. M. R., Novoselov,
K. S. & Geim, A. K. The electronic properties of graphene. _Rev. Mod. Phys._ 81, 109–162 (2009). Article CAS Google Scholar * Abdelsalam, H. et al. Electronic and adsorption
properties of extended chevron and cove-edged graphene nanoribbons. _Phys. E Low. Dimens. Syst. Nanostruct._ 126, 114438 (2021). Article CAS Google Scholar * Wakabayashi, K., Takane, Y.,
Yamamoto, M. & Sigrist, M. Edge effect on electronic transport properties of graphene nanoribbons and presence of perfectly conducting channel. _Carbon_ 47, 124–137 (2009). Article CAS
Google Scholar * Saraswat, V., Jacobberger, R. M. & Arnold, M. S. Materials Science Challenges to Graphene Nanoribbon Electronics. _ACS Nano_ 15, 3674–3708 (2021). Article CAS
Google Scholar * Guo, N. et al. Electronic and magnetic properties of group-V TMDs monolayers with defects: a first-principles study. _Comput. Mater. Sci._ 176, 109540 (2020). * Zhang, W.,
Zhang, Y., Qiu, J., Zhao, Z. & Liu, N. Topological structures of transition metal dichalcogenides: A review on fabrication, effects, applications, and potential. _InfoMat_ 3, 133–154
(2020). Article Google Scholar * Wang, Q. H., Kalantar-Zadeh, K., Kis, A., Coleman, J. N. & Strano, M. S. Electronics and optoelectronics of two-dimensional transition metal
dichalcogenides. _Nat. Nanotechnol._ 7, 699–712 (2012). Article CAS Google Scholar * Pang, J. et al. Applications of 2D MXenes in energy conversion and storage systems. _Chem. Soc. Rev._
48, 72–133 (2019). Article CAS Google Scholar * Liu, Z. & Alshareef, H. N. MXenes for Optoelectronic Devices. _Adv. Electron. Mater._ 2100295 (2021). * Fu, B. et al. MXenes:
Synthesis, Optical Properties, and Applications in Ultrafast Photonics. _Small_ 17, e2006054 (2021). Article Google Scholar * Schaffel, F. et al. Atomic Resolution Imaging of the Edges of
Catalytically Etched Suspended Few-Layer Graphene. _ACS Nano_ 5, 1975–1983 (2011). Article CAS Google Scholar * Zhang, X., Xin, J. & Ding, F. The edges of graphene. _Nanoscale_ 5,
2556–2569 (2013). Article CAS Google Scholar * Lee, Y. H., Kim, S. G. & Tománek, D. Catalytic Growth of Single-Wall Carbon Nanotubes: An Ab Initio Study. _Phys. Rev. Lett._ 78,
2393–2396 (1997). Article CAS Google Scholar * Wyrick, J. et al. Atom‐by‐Atom Fabrication of Single and Few Dopant Quantum Devices. _Adv. Funct. Mater_. 29, 1903475 (2019). * Obermair,
C., Xie, F. Q. & Schimmel, T. The Single-Atom Transistor: perspectives for quantum electronics on the atomic-scale. _Europhys. N._ 41, 25–28 (2010). Article CAS Google Scholar * Li,
Z. et al. Towards graphyne molecular electronics. _Nat. Commun._ 6, 6321 (2015). Article CAS Google Scholar * Radsar, T., Khalesi, H. & Ghods, V. Graphene properties and applications
in nanoelectronic. _Opt. Quant. Electron_ 53, 178 (2021). Article CAS Google Scholar * Shu, H., Chen, X., Tao, X. & Ding, F. Edge Structural Stability and Kinetics of Graphene
Chemical Vapor Deposition Growth. _ACS Nano_ 6, 3243–3250 (2012). Article CAS Google Scholar * Wang, L., Zhang, X., Chan, H. L., Yan, F. & Ding, F. Formation and healing of vacancies
in graphene chemical vapor deposition (CVD) growth. _J. Am. Chem. Soc._ 135, 4476–4482 (2013). Article CAS Google Scholar * Zhao, L. et al. In Situ Electron Driven Carbon
Nanopillar-Fullerene Transformation through Cr Atom Mediation. _Nano Lett._ 17, 4725–4732 (2017). Article CAS Google Scholar * Feng, Z. et al. Trapping of Charged Gold Adatoms by Dimethyl
Sulfoxide on a Gold Surface. _ACS Nano_ 9, 8697–8709 (2015). Article CAS Google Scholar * Patera, L. L. et al. Real-time imaging ofadatom-promoted graphene growth on nickel. _Science_
359, 1243–1246 (2018). Article CAS Google Scholar * Rummeli, M. H. et al. New Frontiers in Electron Beam-Driven Chemistry in and around Graphene. _Adv. Mater._ 31, e1800715 (2019).
Article Google Scholar * Rummeli, M. H. et al. In Situ Room Temperature Electron-Beam Driven Graphene Growth from Hydrocarbon Contamination in a Transmission Electron Microscope. _Mater.
(Basel)_ 11, 896 (2018). Article Google Scholar * Zhao, J. et al. Direct in situ observations of single Fe atom catalytic processes and anomalous diffusion at graphene edges. _Proc. Natl
Acad. Sci. USA_ 111, 15641–15646 (2014). Article CAS Google Scholar * Zhao, J. et al. Free-standing single-atom-thick iron membranes suspended in graphene pores. _Science_ 343, 1228–1232
(2014). Article CAS Google Scholar * Ta, H. Q. et al. Single Cr atom catalytic growth of graphene. _Nano Res._ 11, 2405–2411 (2018). Article CAS Google Scholar * Kano, E., Hashimoto,
A. & Takeguchi, M. Opposite effects of Cu and Pt atoms on graphene edges. _Appl. Phys. Express_ 10, 025104 (2017). Article Google Scholar * Kano, E. et al. Interactions between C and
Cu atoms in single-layer graphene: direct observation and modelling. _Nanoscale_ 8, 529–535 (2016). Article CAS Google Scholar * Yang, X. et al. On the Catalytic Activity of Sn Monomers
and Dimers at Graphene Edges and the Synchronized Edge Dependence of Diffusing Atoms in Sn Dimers. _Adv. Funct. Mater_. 31, 2104340 (2021). * Anantram, M. P. & Léonard, F. Physics of
carbon nanotube electronic devices. _Rep. Prog. Phys._ 69, 507–561 (2006). Article CAS Google Scholar * Ramasse, Q. M. et al. Direct Experimental Evidence of Metal-Mediated Etching of
Suspended Graphene. _ACS Nano_ 6, 4063–4071 (2012). Article CAS Google Scholar * Wang, H. et al. Interaction between single gold atom and the graphene edge: a study via
aberration-corrected transmission electron microscopy. _Nanoscale_ 4, 2920–2925 (2012). Article CAS Google Scholar * Ma, L. & Zeng, X. C. Unravelling the Role of Topological Defects
on Catalytic Unzipping of Single-Walled Carbon Nanotubes by Single Transition Metal Atom. _J. Phys. Chem. Lett._ 9, 6801–6807 (2018). Article CAS Google Scholar * Areshkin, D. A. &
White, C. T. Building Blocks for Integrated Graphene Circuits. _Nano Lett._ 7, 3253–3259 (2007). Article CAS Google Scholar * Wang, W. L. et al. Direct observation of a long-lived
single-atom catalyst chiseling atomic structures in graphene. _Nano Lett._ 14, 450–455 (2014). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was financially
supported by the National Science Foundation of China (Grant Nos. 52071225 & 51676154) and the Czech Republic under the ERDF program “Institute of Environmental Technology—Excellent
Research” (No. CZ.02.1.01/0.0/0.0/16_019/0000853). M.H.R. and L.F. thank the Sino–German Research Institute for support (Project No. GZ 1400). FUNDING Open Access funding enabled and
organized by Projekt DEAL. AUTHOR INFORMATION Author notes * These authors contributed equally: Xiaoqin Yang, Yu Liu. AUTHORS AND AFFILIATIONS * Soochow Institute for Energy and Materials
Innovations, College of Energy, Collaborative Innovation Center of Suzhou Nano Science and Technology, Key Laboratory of Advanced Carbon Materials and Wearable Energy Technologies of Jiangsu
Province, Soochow University, Suzhou, 215006, China Xiaoqin Yang, Yu Liu & Mark H. Rümmeli * School of Energy and Power Engineering, Xi’an Jiaotong University, No. 28, Xianning West
Road, Xi’an, Shaanxi, 710049, China Xiaoqin Yang, Jinping Luo & Lijun Liu * Gusu Laboratory of Materials, No. 388, Ruoshui Road, Industrial Park, Suzhou, 215123, China Xiaoqin Yang *
Leibniz Institute for Solid State and Materials Research Dresden, P.O. Box 270116, D-01171, Dresden, Germany Huy Q. Ta, Yue Zhang & Mark H. Rümmeli * School of Mechanical and Materials
Engineering, University College Dublin, Dublin 4, Ireland Ehsan Rezvani * College of Chemistry and Molecular Sciences, Wuhan University, Wuhan, 430072, China Mengqi Zeng & Lei Fu *
Polish Center for Technology Development (PORT), Ul. Stabłowicka 147, Wrocław, 54-066, Poland Alicja Bachmatiuk * Centre of Polymer and Carbon Materials, Polish Academy of Sciences, M.
Curie-Skłodowskiej 34, Zabrze, 41-819, Poland Mark H. Rümmeli * Institute of Environmental Technology, VŠB -Technical University of Ostrava, 17 Listopadu 15, Ostrava, 708 33, Czech Republic
Mark H. Rümmeli Authors * Xiaoqin Yang View author publications You can also search for this author inPubMed Google Scholar * Yu Liu View author publications You can also search for this
author inPubMed Google Scholar * Huy Q. Ta View author publications You can also search for this author inPubMed Google Scholar * Ehsan Rezvani View author publications You can also search
for this author inPubMed Google Scholar * Yue Zhang View author publications You can also search for this author inPubMed Google Scholar * Mengqi Zeng View author publications You can also
search for this author inPubMed Google Scholar * Lei Fu View author publications You can also search for this author inPubMed Google Scholar * Alicja Bachmatiuk View author publications You
can also search for this author inPubMed Google Scholar * Jinping Luo View author publications You can also search for this author inPubMed Google Scholar * Lijun Liu View author
publications You can also search for this author inPubMed Google Scholar * Mark H. Rümmeli View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
M. H. R., X. Y., and Y. L. conceived the manuscript concept. All authors contributed to the manuscript preparation. CORRESPONDING AUTHORS Correspondence to Lijun Liu or Mark H. Rümmeli.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which
permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, X., Liu, Y., Ta, H.Q. _et al._ Single-atom catalytic growth of crystals using graphene as a case study. _npj 2D Mater Appl_ 5, 91
(2021). https://doi.org/10.1038/s41699-021-00267-4 Download citation * Received: 07 May 2021 * Accepted: 29 October 2021 * Published: 03 December 2021 * DOI:
https://doi.org/10.1038/s41699-021-00267-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Image conscious youngsters are ‘desperate’ to ditch fast fashionResearchers who polled 1,500 18-34-year-olds found a total of 59 million unworn items currently languish in Gen Z wardro...
Fourteen leicester city january transfer rumours and what happened nextThe transfer rumour mill hasn’t produced quite so many Leicester City links as is typical for a January window, but perh...
Reading star gives personal guarantee chris coleman will be successfulColeman quit as Wales manager to take charge of the relegation-threatened Black Cats last month. And Wales internationa...
End this gun insanity pleads grieving fatherKatie Cooper, 22, was one of three of the six victims who were named during the day. She died along with her friend Vero...
Undiksha institutional repository system undiksha repositoryCahyani, Ida Ayu Dita Safitri (2025) _TINJAUAN HUKUM HAK ASASI MANUSIA TERHADAP DISKRIMINASI LESBIAN GAY BISEKSUAL DAN T...
Latests News
Single-atom catalytic growth of crystals using graphene as a case studyABSTRACT Anchored Single-atom catalysts have emerged as a cutting-edge research field holding tremendous appeal for appl...
Esther mcvey's comments on lgbt education were far from homophobic | thearticleEsther McVey has suffered a rough few weeks. Her personal photographer expenses were exposed, Lorraine Kelly cast shade ...
Sorry, we can't find that page. | The StandardThe page you are looking for might have been removed, had its name changed, or is temporarily unavailable.The Standard i...
Uttarakhand crisis ebbingThe central leadership worked out a compromise formula under which three loyalists of rebel leader Harish Rawat will be ...
Directed molecular evolution to design advanced red fluorescent proteinsABSTRACT Fluorescent proteins have become indispensable imaging tools for biomedical research. Continuing progress in fl...