Horses discriminate human body odors between fear and joy contexts in a habituation-discrimination protocol
Horses discriminate human body odors between fear and joy contexts in a habituation-discrimination protocol"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Animals are widely believed to sense human emotions through smell. Chemoreception is the most primitive and ubiquitous sense, and brain regions responsible for processing smells are
among the oldest structures in mammalian evolution. Thus, chemosignals might be involved in interspecies communication. The communication of emotions is essential for social interactions,
but very few studies have clearly shown that animals can sense human emotions through smell. We used a habituation-discrimination protocol to test whether horses can discriminate between
human odors produced while feeling fear _vs._ joy. Horses were presented with sweat odors of humans who reported feeling fear or joy while watching a horror movie or a comedy, respectively.
A first odor was presented twice in successive trials (habituation), and then, the same odor and a novel odor were presented simultaneously (discrimination). The two odors were from the same
human in the fear or joy condition; the experimenter and the observer were blinded to the condition. Horses sniffed the novel odor longer than the repeated odor, indicating they
discriminated between human odors produced in fear and joy contexts. Moreover, differences in habituation speed and asymmetric nostril use according to odor suggest differences in the
emotional processing of the two odors. SIMILAR CONTENT BEING VIEWED BY OTHERS DECOMPOSITION OF AN ODORANT IN OLFACTORY PERCEPTION AND NEURAL REPRESENTATION Article 18 March 2024 NEURAL
MANIFOLDS FOR ODOR-DRIVEN INNATE AND ACQUIRED APPETITIVE PREFERENCES Article Open access 05 August 2023 RELATIONSHIP BETWEEN ASYMMETRIC NOSTRIL USE AND HUMAN EMOTIONAL ODOURS IN CATS Article
Open access 06 July 2023 INTRODUCTION Animals are widely believed to sense human emotions through smell. Chemoreception is the most primitive and ubiquitous sense, and brain regions
responsible for processing smells are among the oldest structures in mammalian evolution1. Thus, chemosignals might be involved in interspecies communication, including emotional
communication. An emotion is defined as “an intense but short-living affective response to an event”2, and the expression and perception of emotions play an essential role in the regulation
of social interactions in mammals3, including human-animal interactions. In the last two decades research on the sociocognitive capacities of domestic mammals associated with human-animal
interactions has increased, providing insight into how animals perceive our emotions4. Domestic mammals have been shown to perceive human emotions through several sensory channels. For
example, horses, dogs, cats and goats react to the emotional facial expressions of humans5,6,7,8,9. Horses, dogs and cats also perceive human emotions in vocalizations10,11,12,13. Moreover,
cross-modal experiments have shown that horses, dogs and cats can integrate visual and vocal stimuli of humans expressing anger and joy, indicating that these species have multimodal mental
representations of these emotions (i.e., they have mental representations of human emotions that combine visual and vocal features12,13,14,15). In addition, these species seem sensitive to
the emotional valence of visual and vocal expressions. Cats and goats showed a preference for expressions of joy rather than anger5,6, and the behavioral and physiological reactions of
horses, dogs and cats to expressions of anger were similar to those observed when these animals experience negative emotions themselves4,16. For instance, horses showed an increase in heart
rates following visual presentation of angry faces compared to happy faces8. In dogs, visual and vocal signals of human fear also seemed to provoke behavioral and physiological
reactions9,10. The processing of human fear by domestic mammals is unclear as research on the perception of human emotions by these species has mostly focused on anger and joy or happiness4,
except for a few studies on other emotions17,18. Moreover, very few studies have investigated the olfactory perception of human emotions by domestic mammals, although olfaction is a
dominant sense for most mammals, including horses19,20. The perception of conspecific odors has been documented among domestic mammals. For example, horses differentiated between samples
bearing the odor of unfamiliar conspecifics in a habituation-discrimination test; these samples were obtained by rubbing a piece of material on the coats of conspecifics21.
Habituation-discrimination tests involve presenting an odor twice in successive trials (habituation phase), and then presenting the same odor and a novel odor simultaneously (discrimination
phase). Horse sniffing duration is expected to decrease during the habituation phase, and to be higher for the novel sample than the repeated sample during the discrimination phase if they
are able to discriminate between the two odors. Preference tests can also be used to assess olfactory perception; for example horses sniffed the odor of defecations produced by conspecifics
who directed more aggression towards them for longer than defecations from other conspecifics in their group22. Additionally, heifers and pigs preferred to eat from a dispenser bearing the
odor of urine from an unstressed conspecific rather than a stressed conspecific23,24. Dogs also showed more stress-related behaviors when sniffing conspecific body odors produced during
isolation, a stress-inducing situation, rather than during play25. These findings highlight the influence of the emotional state of the emitter on the response of the receiver upon sniffing
the olfactory cues. Beyond this sensitivity to conspecific odors, a few studies have reported that domestic mammals are sensitive to human odors, including emotional body odors. Emotional
information, such as fear and happiness, is conveyed by chemosignals produced in the sweat of humans26,27. Apocrine sweat glands in the armpit are thought to release compounds of different
natures and/or quantities in the sweat, such as adrenaline and androstadienone, according to the emotional valence of the emitter28. A few studies have suggested that domestic mammals can
perceive our emotions through olfaction and are influenced by them. For example, cattle sniffed human sweat produced in a non-stressful context for longer than that produced in a stressful
context29, and dogs can distinguish between human odors from baseline and psychological stress conditions30. Dogs also showed more stress-like behaviors25 and interacted less with an
unfamiliar human31,32 after sniffing human sweat collected while watching a fear-inducing video rather than a joy-inducing video. Using the same type of stimuli, in a recent experiment
horses were presented successively with odors of human happiness and fear in the presence of a familiar human17. Horses lifted their head and tended to touch the familiar person more when
sniffing the odor from the fear condition compared to that from the joy condition, suggesting that they perceived fear in the first odor and reacted with a fear-related behavior. These
results are promising and merit further elucidation with fully counterbalanced experiments (i.e., experiments in which the presentation order of stimuli and the collection of samples from
participants is randomized) and incorporation with other behavioral evidence, such as laterality biases. Indeed, the emotional response of domestic mammals to stimuli is revealed not only by
specific behavioral responses, such as the ones described above (preferences and emotional behaviors), but also by detecting brain asymmetries in the processing of emotional expressions, as
the brain hemispheres are differentially involved in emotional processing33. These asymmetries are assessed by observing the preferential use of an ear, eye or nostril, indicating the
preferential involvement of the contralateral hemisphere (for vision and audition) or the ipsilateral hemisphere (for olfaction)34. In general, in domestic mammals, the right hemisphere is
preferentially used for negative or intense stimuli whereas the left hemisphere is favored for positive or familiar stimuli33. For example, horses preferentially used their left ear (right
hemisphere) to listen to a human growl and their right ear (left hemisphere) to listen to laughter11 or voices associated with a positive past experience35; additionally, horses
preferentially looked at a human face expressing anger with their left eye (right hemisphere) rather than their right eye8. Regarding olfactory stimuli, horses have been observed to
preferentially use their right nostril (right hemisphere) to sniff arousing or novel odors36,37. The purpose of the present study was to further explore the olfactory perception of human
emotions by horses. Specifically, we examined (1) whether horses can discriminate between human body odors produced in joy and fear conditions, and (2) whether horses showed any emotional
reaction to these stimuli. We expected that horses would discriminate between the two human emotional odors, and that they would react differently to the odors from the joy and fear
contexts. METHODS ETHICS STATEMENT This study was reported in accordance with ARRIVE guidelines. It was approved by the Val de Loire Ethical Committee (CEEA VdL, Nouzilly, France,
authorization number CE19—2022-1503-2). Animal care and experimental treatments complied with the French and European guidelines for the housing and care of animals used for scientific
purposes (European Union Directive 2010/63/EU) and were performed under authorization and supervision of official veterinary services (agreement number F371752 delivered to the UEPAO animal
facility by the veterinary service of the Département d’Indre et Loire, France). The horses lived in groups, were not food deprived during the experiment and did not undergo any invasive
procedures. Human participation in the experiment was carried out according to the Declaration of Helsinki and was approved by the Institutional Review Board of the University of Tours
(authorization number 2022-029). All participants were fully informed about the general aims and methods of the study, and they provided written informed consent for the collection of
samples as well as their use in the experiment. HORSES The study involved 30 Welsh mares (_Equus caballus_) aged 5.7 ± 2.3 years (mean ± _s.d._) reared and living at the Animal Physiology
Experimental Unit PAO (UEPAO, 37,380 Nouzilly, France, https://doi.org/10.15454/1.55738963217 28955E12), INRAE. These mares lived in groups in indoor stalls bedded with straw and had free
access to an outdoor paddock. Hay and water were available ad libitum. These horses are used only for research purposes and are handled daily by humans. They have the opportunity to
experience human emotions expressed by caregivers and researchers. STIMULI The odor-collection method was adapted from previous studies on human body odor28,29,38. Human axillary sweat odor
was collected from 24 adult participants (6 males and 18 females) who volunteered to take part in the experiment. They were recruited through an e-mail sent to all personnel of our research
facility (660 people). Participants were asked to abstain from consuming products known to influence body odors (i.e., chili pepper, spices, blue cheese, onion, garlic, cabbage, tobacco, and
alcohol), abstain from use of deodorant, perfume or scented lotion, and to wash with a perfume-free soap provided by the experimenters for 2 days before their sweat was collected. The
morning before participants donated their sweat, they were asked to wash their armpits with clear water only. Given the small number of participants, the menstrual cycle of females was not
discriminated. Each participant took part in two individual sessions separated by at least 24 h, during which they watched a 20-min video meant to provoke fear or joy. The clip selected for
the fear condition was an excerpt from the movie _Sinister_39 (judged as the most frightening horror movie in 2020—https://www.broadbandchoices.co.uk/features/science-of-scare). The clips
selected for the joy condition were adapted from those used by de Groot et al.28: “Bare Necessities” from _The Jungle Book_, Kurt Kuene’s short movie _Validation_, and the dance scene from
the film _The Intouchables_. The order of the conditions was chosen randomly for each participant and counterbalanced among participants (half of participants watched the fear-inducing video
first and the other half watched the joy-inducing video first). Immediately before watching the video, participants were required to wash their armpits with wet unscented cotton pads and
dry them with an unscented paper towel. Then, they placed under each armpit two cotton pads (7.5 × 7.5 cm, Euromedis, Neuilly-sous-Clermont, France) that had been previously folded together
and secured them in place with unscented surgical tape. They wore a provided unscented cotton t-shirt that was previously washed without detergent and did not wear any other clothes over it.
After each session, participants placed the cotton pads and t-shirts in airtight sealed bags, which were stored in a freezer at − 20 °C for a maximum of six weeks. Participants rated their
extent of fear and joy while watching the videos on 7-point Likert scales28. Participants also indicated in a questionnaire whether they had thoroughly followed the dietary and hygienic
instructions. The samples from nine participants had to be excluded from the experiment due to lack of compliance with the instructions. The samples from the remaining 15 participants were
used as stimuli presented to horses. PROCEDURE The experiment took place over 2 weeks in January, 2022. SAMPLE PREPARATION One hour before the beginning of a habituation-discrimination test,
the samples were thawed at room temperature in the airtight bags17,28. The stimuli were presented on 150-cm wooden sticks covered on one end by a single-use plastic bag that was changed for
each odor presentation. Over the plastic bag was placed a piece of fabric (30 × 25 cm) from the armpits of a participant’s t-shirt, with an unfolded cotton pad from the same participant and
same condition layered on top. For each horse, four wooden sticks were prepared with pieces of the t-shirt and pads from a single human participant; depending on which odor was used for the
habituation phase, these sticks either consisted of three sticks with the odor from the fear condition and one with the odor from the joy condition, or vice versa. The samples were then
covered with single-use plastic bags. To keep the samples warm despite winter weather, they were placed 15 cm from a heating lamp for 5 min before they were presented to the horse. For each
condition (fear and joy), sweat was collected on four pads for each participant. This design enabled samples from each participant to be prepared for two horses (three pads from the joy
condition and one from the fear condition for one horse and vice versa for the second horse). Thus, each set of four pads from one participant (either three pads from the joy condition and
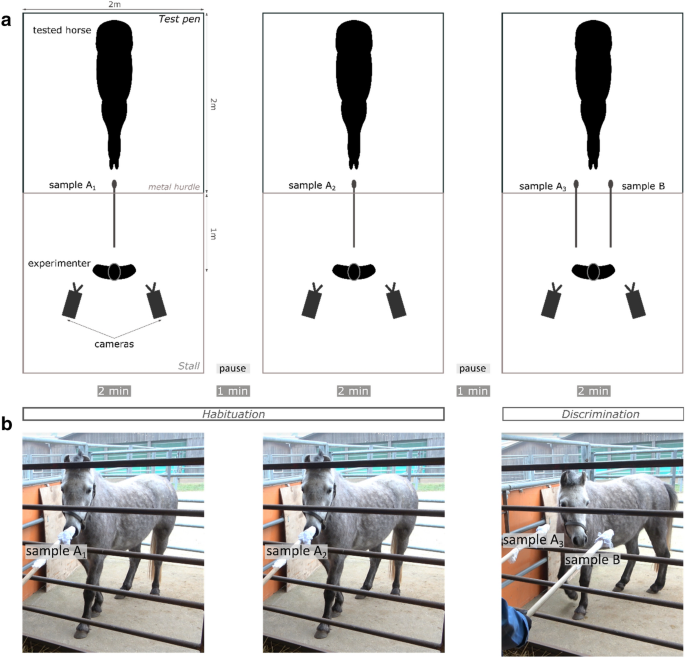
one from the fear condition, or vice versa) was sniffed by one horse. EXPERIMENTAL SETUP The experiment took place in an outdoor pen (2 × 2 m) adjacent to an open stall where the
experimenter stood (Fig. 1a). The wooden sticks bearing the odors were presented through a metal hurdle. Both the sticks and the hurdle were marked to standardize the stimulus presentation
(the distance from the hurdle to the odor and the placements of the sticks on the hurdle were fixed). The experimenter stood 1 m from the hurdle, facing the horse. Two cameras were placed on
to the left and right of the experimenter to film the behavior of the horse during the test. Two assistants who were not visible to the horse gave the samples to the experimenter at the
appropriate time. The experimenter and assistants wore surgical masks hiding their facial expressions and did not wear any perfume. They were as immobile as possible and never looked
directly at the horse. The experimenter looked strictly in front of them, with a 45° angle towards the ground. Importantly, the experimenter presenting the odors to the horse was blind to
the odor condition: they did not take part in the preparation of the sticks and could not discriminate between them once prepared. FAMILIARIZATION The familiarization phase began when the
horse was released in the test pen. This phase lasted at least 30 s, during which the experimenter and the assistants quietly placed themselves. The test could then begin as soon as the
horse was calm (not neighing, trying to escape the pen or circling). All horses met this criterion within two minutes. HABITUATION-DISCRIMINATION TEST The test was a
habituation-discrimination procedure adapted from21 and was comprised of two phases: habituation and discrimination (Fig. 1 and Supplementary Material—Video S1). During the habituation
phase, a sample (A1) with odor A was presented to the horse at the center of the hurdle at a height of 1 m for two minutes; then, a one-minute interval elapsed, before a second sample (A2)
with odor A was presented to the horse for two minutes in the same way. Another one-minute pause was observed between the habituation and discrimination phases. During the discrimination
phase, two samples were presented simultaneously to the horse, 50 cm apart; one of these samples (A3) carried odor A, the repeated odor, and one carried odor B, the novel odor. The two
samples were presented at the same time and speed and were equidistant from the previous sample location (Fig. 1). Half the horses were presented with the odor from the joy condition as
samples A1, A2 and A3 and the odor from the fear condition as sample B, and vice versa for the other half (Table 1). Moreover, during the discrimination phase the location (left or right) of
odors A3 and B and of the odors from the fear and joy conditions were randomly distributed and counterbalanced among horses (Table 1). Throughout the test, if the horse started biting the
cotton pad or catching it with her lips, the experimenter had to move the stick 5 cm to one side to prevent the horse from swallowing the sample, then the stick was returned to the initial
location within 1 s. BEHAVIORAL ANALYSIS The recorded videos of the tests were analyzed using BORIS v. 7.12.240 by a coder who was blind to the side of odor B and to the type of odor (fear
or joy condition) of each stick. The duration that each horse spent sniffing the samples was determined. Valid sniffing of the samples was defined as when the horse had its head turned
towards a sample with a visible dilation of the nostrils and/or when its nose was 15 cm or less from a sample. Moreover, the preferential use of nostrils for sniffing the odors was analyzed;
for each nostril, when it was directed towards the sample (touching or almost touching it) while the other was not directed towards the sample (Fig. 2), the number of nostril dilations and
side of this nostril were noted. When both or neither nostril was directed to the sample, we considered that no nostril was being used preferentially. Flehmen responses (raising of the upper
lip), defecations and neighs were also counted. STATISTICAL ANALYSIS All statistical analyses were performed using R 4.1.241, and figures were generated using the _ggplot2_ package42. The
significance threshold was set at α ≤ 0.05 and tendencies were considered for p values ≤ 0.1. To test whether the human participants experienced different emotions during the two videos, we
compared the rating scores for ‘fearful’ and ‘joyful’ during the fear condition to those during the joy condition, using two-tailed paired permutation tests28 (_symmetry.test_ function from
the package _coin_43 with an exact distribution). Of the 30 horses that participated in the test, 5 did not sniff any of the samples during the habituation phase (neither A1 nor A2) and were
therefore excluded from further analysis. The duration sniffing the odors and the number of nostril dilations were explored with generalized linear mixed models (GLMM) from the package
_glmmTMB_44, using Poisson distributions. The habituation and discrimination phases were analyzed individually. For both variables, an initial model was constructed for each phase, assessing
the effect of the sample presented (A1 or A2, then A3 or B) and the effect of the group (J or F), representing which odor was presented during the habituation phase (i.e., the odor from the
joy condition or fear condition, respectively), and their interaction. In addition, for the number of nostril dilations, the effect of the side of the nostril as well as its interaction
with the other two factors was assessed. Horse identity was added as a random effect to account for individual variation in paired data, as each horse was presented with two samples in each
phase. The variables included in each model were subjected to selection using a model comparison with two-tailed analysis of variance (ANOVA) with the null model and simpler models (without
an interaction, then without each variable of interest). Distributions, within-group variance and homoscedasticity of the residuals were checked using the package _DHARMa_45 for each
selected model, showing that the model assumptions were satisfied. When necessary, a post hoc test based on Tukey’s methods was performed with the package _emmeans_46. The selected models
are presented in Table 2 (see Table S1 for the detailed results of each ANOVA). To further analyze the first reaction of horses according to the type of odor presented, we focused on odor
A1. We calculated a left-nostril bias index measuring the propensity to use the left nostril more than the right. This index was defined as L/(L + R), where L is the number of dilations of
the left nostril, and R the number of dilations of the right nostril. This left-nostril bias could vary from 0 (indicating exclusive use of the right nostril) to 1 (indicating exclusive use
of the left nostril); a score of 0.5 indicated equal use of both nostrils. Five horses did not show preferential nostril use for A1; we therefore were unable to calculate this index for
these horses and excluded them from this analysis, focusing on the remaining twenty horses. We tested whether the horses used their left nostril more than the right nostril for each emotion
(joy: _n_ = 11, fear: _n_ = 9), by comparing this index to 0.5 using one-tailed Wilcoxon tests (_wilcox.test_ function with _mu_ = 0._5_). Flehmen responses, defecations and neighs were
exhibited by too few individuals to be considered in the statistical analysis (Flehmen responses: _n_ = 1, defecations: _n_ = 3, and neighs: _n_ = 2). RESULTS PARTICIPANT EMOTIONS
Participant ratings of their emotions showed that they were significantly more joyful and less fearful after watching the joy-inducing video compared to the fear-inducing video (two-tailed
paired permutation tests, _n_ = 15; joyful: _Z_ = 3.45, _p_ < 0.001; fearful: _Z_ = − 3.50, _p_ < 0.001; Fig. 3). HABITUATION AND DISCRIMINATION OF HORSES TO THE EMOTIONAL ODORS The
GLMMs showed that during the habituation phase, the time duration that horses sniffed the odors was affected by the sample x group interaction. Tukey’s post hoc tests revealed that horses
from group J (i.e., the horses for which odor A was from the joy condition) sniffed A2 for a shorter time than A1, while it was not the case for horses from group F (i.e., the horses for
which odor A was from the fear condition; Fig. 4, group J: _t_ = 5.108, _p_ < 0.0001; group F: _t_ = 1.092, _p_ = 0.28). During the discrimination phase, horses sniffed the novel odor (B)
for significantly longer than the repeated odor (A3), regardless of their group (Fig. 4, _Z_ = 3.388, _p_ = 0.0007). Therefore, horses habituated to the presented odor when it was from the
joy condition but not when it was from the fear condition; and discriminated between the new odor and the repeated odor in all cases. PREFERENTIAL NOSTRIL USE The GLMMs showed that during
the habituation phase, the number of nostril dilations close to the sample was affected by the side of the nostril and by the odor. The number of dilations decreased from A1 to A2 (Fig. 5,
_Z_ = − 2.48, _p_ = 0.013), indicating that the number of nostril dilations was also influenced by habituation. Moreover, during the habituation phase horses preferentially used their left
nostril to sniff the odors (Fig. 5, _Z_ = − 3.03, _p_ = 0.002). During the discrimination phase, the number of nostril dilations was affected by the side of the nostril side x odor
interaction. Tukey’s post hoc tests revealed that horses used their left nostril more than their right nostril to sniff the repeated odor A3, whereas they used their right nostril more than
their left nostril to sniff the novel odor B (Fig. 5, odor A3: _t_ = 2.50, _p_ = 0.014; odor B: _t_ = − 2.21, _p_ = 0.029). The group (i.e., whether the first presented odor was from the joy
or from the fear condition; groups J and F, respectively) did not affect the number of nostril dilations, as it was not included in the selected models. In addition, the left-nostril bias
index was significantly higher than 0.5 for odor A1 when it was the odor from the joy condition but not when it was the odor from the fear condition (joy: _V_ = 52, _p_ = 0.049; fear: _V_ =
23.5, _p_ = 0.80), indicating that for A1 horses showed a significant left-nostril bias when sniffing joy but not when sniffing fear. DISCUSSION The main result of this study was that when
presented with human odors from different emotional contexts in a habituation-discrimination test, horses sniffed the novel odor for longer than the repeated odor. This result is consistent
with that of other habituation-discrimination tests21,47 and shows that horses are able to discriminate human body odors from two distinct emotional contexts when they are presented
simultaneously. Moreover, when the repeated odor and the novel odor were presented simultaneously during the discrimination phase, horses preferentially used their left nostril to sniff the
repeated odor and their right nostril to sniff the novel odor. This finding is consistent with the previous observation that horses preferentially used their right nostril for sniffing novel
objects37, and it supplies additional evidence that horses differentiated between the two odors. These results from our experiment confirm the differential perception of human emotional
odors by horses that had been suggested in the study of Sabiniewicz et al.17, reporting that horses presented with human emotional odors responded by lifting their head more and touching the
familiar person more when sniffing the odor from the fear context compared to that from the joy context. If horses can perceive the emotional odors of humans, this raises the question of
what compounds are the chemical basis for such interspecific communication. In humans, several compounds in sweat, such as adrenaline or androstadienone have been proposed as candidates that
carry emotional information28, and recent findings support the notion of the signal-specificity of axillary odors for distinct emotional states48. The perception of human emotional
information contained in sweat odors implies the existence of receptors for such compounds. These receptors could be present in horses, either as a result of domestication or by inheritance
from a common mammalian ancestor. As several other species of domestic mammals seem to perceive these compounds (namely, dogs, cattle and mice29,31,32), the first hypothesis would entail
multiple appearances of such receptors during the domestication of each of these species. However, olfaction is the most ancient and universal sense, and the cerebral structures that process
odors evolved very early in mammals1. Therefore, the second hypothesis appears more parsimonious. The second hypothesis is also supported by the recent finding that humans could recognize
fear and non-fear odors in horse sweat49, which could occur through existence of common chemical compounds and their receptors in all mammals. During the habituation phase (samples A1 and
A2), we detected a significant decrease in the duration sniffing the stimuli when the odor from the joy condition was presented; however, when the odor from the fear condition was presented,
there was no significant difference in sniffing durations. The smell of fear could be more stimulating for horses than that of joy. Indeed, for humans the odor of fear appears more intense
than that of joy: in a study, participants were more successful at distinguishing fear sweat from neutral sweat than happiness sweat from neutral sweat50. Moreover, studies have shown that
the primitive role of olfactory signaling in humans seems to be the fight-or-flight response26, thus, the odor of fear could be alarming for horses, given their prey nature and reactivity to
flight-triggering stimuli51. As a consequence, a longer duration or repeated presentations of the same stimulus may be necessary for horses to habituate to odors from a fear context
compared to odors from a joy context. This differential processing of two emotional odors by horses suggests different perceptions of human body odors according to the emotional context of
their production. Furthermore, when sniffing the first sample, horses exhibited a left-nostril bias for the odor from the joy condition but not for that from the fear condition. In mammals,
nerve fibers from the left nostril project to the left hemisphere of the brain33; therefore, this result suggests a left hemisphere bias in horses when sniffing the joy-context odor during
the first sample presentation. As a left hemisphere bias was previously observed in horses for listening to human voices associated with a positive past experience35, a human vocalization of
happiness11, and vocalizations of familiar conspecifics52, this pattern suggests that horses perceived the joy-context odor as positive. Together, these results suggest that horses perceive
human body odors from a positive and negative emotional context differently. This sensitivity to the emotional valence of human odors could lead to emotional reactions in horses, akin to
the emotional contagion mechanism reported in humans27,53. Thus, horses’ emotions could also be influenced by those scented on humans as a consequence of either spontaneous responses to the
chemical compounds or a learned association between odors and the situations in which they are encountered1. In this study, we also observed that during the habituation phase, horses used
their left nostril significantly more than their right nostril, suggesting that horses explored these human body odors with a left hemisphere bias. Such bias is usually observed when
exploring positive or familiar stimuli in domestic mammals33; thus, these results indicate that the horses in this experiment perceived human body odors as positive or familiar stimuli,
which can be explained by an overall positive relationship with humans. Moreover, horses used their right nostril significantly more than the left in the discrimination phase. It is possible
that after recognizing in the habituation phase that the two samples were produced in the same emotional state by the same person, horses were somewhat surprised by the different emotional
state they smelled in sample B. Indeed, other studies found the right hemisphere to be preferred for the evaluation of novel stimuli and situations that may request quick reactions54,55,56.
LIMITATIONS OF THE STUDY To avoid multiplying the number of factors included in the analysis, only female horses were involved in this study. In humans, sex differences in the perception of
emotional chemosignals have been established, with women showing better classification of a happiness odor than men57 and showing larger effects of olfactory-induced emotional
contagion27,58. In dogs, sex differences have also been revealed, with females reacting more strongly to a human happiness odor32. However, stallions are more reactive to interspecific odors
than mares and geldings59; therefore, it would be interesting to conduct experiments to assess sex differences in horses regarding the perception of human emotional odors. It would also be
interesting to examine potential variations of horses’ response to human emotional odors according to their temperament, as gusto-olfactory sensitivity and fearfulness are part of the
temperament traits of horses60,61. Further studies could also explore the influence of different stress levels of horses on the perception of human emotional odors, as higher stress levels
were associated with higher olfactory sensitivity in humans62. Hormonal status of the receiver (horses) could play a role as well, considering that odor exploration seems to be influenced by
reproductive status in mares63 and women64. Finally, further studies may consider including a control group (odors A vs A in the discrimination phase) to rule out any effects caused by a
change in the number of samples between the habituation and discrimination phases. CONCLUSION In this study, we showed in habituation-discrimination tests that horses can discriminate
between human odors produced in a joy _vs_. fear context. Moreover, differences in habituation speed and asymmetric nostril use according to odor suggest a differential emotional processing
of the two odors. This study adds olfaction to audition and vision as senses through which horses perceive human emotions and may be influenced by them. These perceptions can affect the
interactions between horses and their owners, riders or caretakers. DATA AVAILABILITY The datasets and R code generated and analyzed during the current study are available in the INRAE data
repository from the following link: https://doi.org/10.57745/SLUKIO. REFERENCES * Semin, G. R., Scandurra, A., Baragli, P., Lanatà, A. & D’Aniello, B. Inter- and intra-species
communication of emotion: Chemosignals as the neglected medium. _Animals_ 9, 887 (2019). Article PubMed PubMed Central Google Scholar * Désiré, L., Boissy, A. & Veissier, I. Emotions
in farm animals: A new approach to animal welfare in applied ethology. _Behav. Processes_ 60, 165–180 (2002). Article PubMed Google Scholar * Briefer, E. F. & Le Comber, S. Vocal
expression of emotions in mammals: mechanisms of production and evidence. _J. Zool._ 288, 1–20 (2012). Article Google Scholar * Jardat, P. & Lansade, L. Cognition and the human–animal
relationship: a review of the sociocognitive skills of domestic mammals toward humans. _Anim. Cogn._ 25, 369–384 (2022). Article PubMed Google Scholar * Galvan, M. & Vonk, J. Man’s
other best friend: domestic cats (F. silvestris catus) and their discrimination of human emotion cues. _Anim. Cogn._ 19, 193–205 (2016). * Nawroth, C., Albuquerque, N., Savalli, C., Single,
M. S. & McElligott, A. G. Goats prefer positive human emotional facial expressions. _R. Soc. Open Sci._ 5, 180491 (2018). Article ADS PubMed PubMed Central Google Scholar * Proops,
L., Grounds, K., Smith, A. V. & McComb, K. Animals remember previous facial expressions that specific humans have exhibited. _Curr. Biol._ 28, 1428-1432.e4 (2018). Article CAS PubMed
Google Scholar * Smith, A. V., Proops, L., Grounds, K., Wathan, J. & McComb, K. Functionally relevant responses to human facial expressions of emotion in the domestic horse (Equus
caballus). _Biol. Lett._ 12, 20150907 (2016). Article PubMed PubMed Central Google Scholar * Siniscalchi, M., D’Ingeo, S., Fornelli, S. & Quaranta, A. Lateralized behavior and
cardiac activity of dogs in response to human emotional vocalizations. _Sci. Rep._ 8, 77 (2018). Article ADS PubMed PubMed Central Google Scholar * Siniscalchi, M., D’Ingeo, S. &
Quaranta, A. Orienting asymmetries and physiological reactivity in dogs’ response to human emotional faces. _Learn. Behav._ 46, 574–585 (2018). Article PubMed Google Scholar * Smith, A.
V. _et al._ Domestic horses (Equus caballus) discriminate between negative and positive human nonverbal vocalisations. _Sci. Rep._ 8, 13052 (2018). Article ADS PubMed PubMed Central
Google Scholar * Trösch, M. _et al._ Horses categorize human emotions cross-modally based on facial expression and non-verbal vocalizations. _Animals_ 9, 862 (2019). Article PubMed PubMed
Central Google Scholar * Quaranta, A., D’ingeo, S., Amoruso, R. & Siniscalchi, M. Emotion recognition in cats. _Animals_ 10, 1107 (2020). * Nakamura, K., Takimoto-Inose, A. &
Hasegawa, T. Cross-modal perception of human emotion in domestic horses (Equus caballus). _Sci. Rep._ 8, 8660 (2018). Article ADS PubMed PubMed Central Google Scholar * Albuquerque, N.
_et al._ Dogs recognize dog and human emotions. _Biol. Lett._ 12, 20150883 (2016). Article PubMed PubMed Central Google Scholar * Briefer, E. F. Vocal contagion of emotions in non-human
animals. _Proc. R. Soc. B Biol. Sci._ 285 (2018). * Sabiniewicz, A., Tarnowska, K., Świątek, R., Sorokowski, P. & Laska, M. Olfactory-based interspecific recognition of human emotions:
Horses (Equus ferus caballus) can recognize fear and happiness body odour from humans (Homo sapiens). _Appl. Anim. Behav. Sci._ 230, 105072 (2020). Article Google Scholar * Baba, C.,
Kawai, M. & Takimoto-Inose, A. Are horses (Equus caballus) sensitive to human emotional cues?. _Animals_ 9, 630 (2019). Article PubMed PubMed Central Google Scholar * Brennan, P. A.
& Kendrick, K. M. Mammalian social odours: attraction and individual recognition. _Philos. Trans. R. Soc. B Biol. Sci._ 361, 2061–2078 (2006). * Saslow, C. A. Understanding the
perceptual world of horses. _Appl. Anim. Behav. Sci._ 78, 209–224 (2002). Article Google Scholar * Péron, F., Ward, R. & Burman, O. Horses (Equus caballus) discriminate body odour cues
from conspecifics. _Anim. Cogn._ 17, 1007–1011 (2014). Article PubMed Google Scholar * Krueger, K. & Flauger, B. Olfactory recognition of individual competitors by means of faeces in
horse (Equus caballus). _Anim. Cogn._ 14, 245–257 (2011). Article PubMed Google Scholar * Boissy, A., Terlouw, C. & Le Neindre, P. Presence of cues from stressed conspecifics
increases reactivity to aversive events in cattle: evidence for the existence of alarm substances in urine. _Physiol. Behav._ 63, 489–495 (1998). Article CAS PubMed Google Scholar *
Vieuille-Thomas, C. & Signoret, J. P. Pheromonal transmission of an aversive experience in domestic pig. _J. Chem. Ecol._ 18, 1551–1557 (1992). Article CAS PubMed Google Scholar *
Siniscalchi, M., D’Ingeo, S. & Quaranta, A. The dog nose ‘KNOWS’ fear: Asymmetric nostril use during sniffing at canine and human emotional stimuli. _Behav. Brain Res._ 304, 34–41
(2016). Article PubMed Google Scholar * Calvi, E. _et al._ The scent of emotions: A systematic review of human intra- and interspecific chemical communication of emotions. _Brain Behav_.
10 (2020). * de Groot, J. H. B., Semin, G. R. & Smeets, M. A. M. On the communicative function of body odors: A theoretical integration and review. _Perspect. Psychol. Sci._ 12, 306–324
(2017). Article PubMed Google Scholar * de Groot, J. H. B. _et al._ A sniff of happiness. _Psychol. Sci._ 26, 684–700 (2015). Article PubMed Google Scholar * Destrez, A. _et al._ Male
mice and cows perceive human emotional chemosignals: A preliminary study. _Anim. Cogn._ 24, 1205–1214 (2021). Article PubMed Google Scholar * Wilson, Id. Dogs can discriminate between
human baseline and psychological stress condition odours. _PLoS ONE_ 17, e0274143 (2022). Article CAS PubMed PubMed Central Google Scholar * D’Aniello, B., Semin, G. R., Alterisio, A.,
Aria, M. & Scandurra, A. Interspecies transmission of emotional information via chemosignals: from humans to dogs (Canis lupus familiaris). _Anim. Cogn._ 21, 67–78 (2018). Article
PubMed Google Scholar * D’Aniello, B. _et al._ Sex differences in the behavioral responses of dogs exposed to human chemosignals of fear and happiness. _Anim. Cogn._ 24, 299–309 (2021).
Article PubMed PubMed Central Google Scholar * Siniscalchi, M., d’Ingeo, S., Quaranta, A., D’Ingeo, S. & Quaranta, A. Lateralized emotional functioning in domestic animals. _Appl.
Anim. Behav. Sci._ 237, 105282 (2021). Article Google Scholar * Rogers, L. & Vallortigara, G. _Lateralized Brain Functions: Methods in Human and Non-Human Species_. vol. 122 (2017). *
D’Ingeo, S. _et al._ Horses associate individual human voices with the valence of past interactions: A behavioural and electrophysiological study. _Sci. Rep._ 9, 11568 (2019). Article ADS
PubMed PubMed Central Google Scholar * Siniscalchi, M., Padalino, B., Aubé, L. & Quaranta, A. Right-nostril use during sniffing at arousing stimuli produces higher cardiac activity in
jumper horses. _Laterality_ 20, (2015). * De Boyer Des Roches, A., Richard-Yris, M.-A. A., Henry, S., Ezzaouïa, M. & Hausberger, M. Laterality and emotions: Visual laterality in the
domestic horse (Equus caballus) differs with objects’ emotional value. _Physiol. Behav._ 94, 487–490 (2008). * Albrecht, J. _et al._ Smelling chemosensory signals of males in anxious versus
nonanxious condition increases state anxiety of female subjects. _Chem. Senses_ 36, 19–27 (2011). Article CAS PubMed Google Scholar * Derrickson, S. _Sinister (VF)—YouTube_. (Wild Bunch
SA, 2001). * Friard, O. & Gamba, M. BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. _Methods Ecol. Evol._ 7, 1325–1330 (2016).
Article Google Scholar * R Core Team. R: A language and environment for statistical computing (2021). * Wickham, H. Ggplot2: Elegant graphics for data analysis. (2016). * Hothorn, T.,
Winell, H., Hornik, K., van de Wiel, M. A. & Zeileis, A. coin: Conditional inference procedures in a permutation test framework (2021). * Brooks, M. E. _et al._ glmmTMB balances speed
and flexibility among packages for zero-inflated generalized linear mixed modeling. _R J._ 9, 378–400 (2017). Article Google Scholar * Hartig, F. DHARMa: Residual diagnostics for
hierarchical (multi-level/mixed) regression models (2021). * Lenth, R. V. emmeans: Estimated Marginal Means, aka Least-Squares Means. (2022). * Hothersall, B., Harris, P., Sörtoft, L. &
Nicol, C. J. Discrimination between conspecific odour samples in the horse (Equus caballus). _Appl. Anim. Behav. Sci._ 126, 37–44 (2010). Article Google Scholar * Smeets, M. A. M. _et al._
Chemical fingerprints of emotional body odor. _Metabolites_ 10, (2020). * Sabiniewicz, A. _et al._ A preliminary investigation of interspecific chemosensory communication of emotions: Can
Humans (Homo sapiens) recognise fear- and non-fear body odour from horses (Equus ferus caballus). _Animal_ 11, 3499 (2021). Article Google Scholar * Zhou, W. & Chen, D. Entangled
chemosensory emotion and identity: Familiarity enhances detection of chemosensorily encoded emotion. _Soc. Neurosci._ 6, 270–276 (2011). Article PubMed Google Scholar * Starling, M.,
McLean, A. & McGreevy, P. The contribution of equitation science to minimising horse-related risks to humans. _Animal_ 6, 15 (2016). Article Google Scholar * Basile, M. _et al._
Socially dependent auditory laterality in domestic horses (Equus caballus). _Anim. Cogn._ 12, 611–619 (2009). Article PubMed Google Scholar * Hatfield, E., Cacioppo, J. T. & Rapson,
R. L. Emotional contagion. _Curr. Dir. Psychol. Sci._ 2, 96–100 (1993). Article Google Scholar * Austin, N. P. & Rogers, L. J. Limb preferences and lateralization of aggression,
reactivity and vigilance in feral horses Equus caballus. _Anim. Behav._ 83, 239–247 (2012). Article Google Scholar * Larose, C., Richard-Yris, M.-A., Hausberger, M. & Rogers, L. J.
Laterality of horses associated with emotionality in novel situations Laterality Asymmetries Body. _Brain Cogn._ 11, 355–367 (2006). Google Scholar * Farmer, K., Krüger, K., Byrne, R. W.
& Marr, I. Sensory laterality in affiliative interactions in domestic horses and ponies (Equus caballus). _Anim. Cogn._ 21, 631–637 (2018). Article PubMed PubMed Central Google
Scholar * Chen, D. & Haviland-Jones, J. Human olfactory communication of emotion. _Percept. Mot. Skills_ 91, 771–781 (2000). Article CAS PubMed Google Scholar * de Groot, J. H. B.,
Semin, G. R. & Smeets, M. A. M. Chemical communication of fear: A case of male-female asymmetry. _J. Exp. Psychol. Gen._ 143, 1515–1525 (2014). Article PubMed Google Scholar *
Marinier, S. L., Alexander, A. J. & Waring, G. H. Flehmen behaviour in the domestic horse: Discrimination of conspecific odours. _Appl. Anim. Behav. Sci._ 19, 227–237 (1988). Article
Google Scholar * Lansade, L., Pichard, G. & Leconte, M. Sensory sensitivities: Components of a horse’s temperament dimension. _Appl. Anim. Behav. Sci._ 114, 534–553 (2008). Article
Google Scholar * Lansade, L., Bouissou, M. F. & Erhard, H. W. Fearfulness in horses: A temperament trait stable across time and situations. _Appl. Anim. Behav. Sci._ 115, 182–200
(2008). Article Google Scholar * Hoenen, M., Wolf, O. T. & Pause, B. M. _The impact of stress on odor perception._ https://doi.org/10.1177/030100661668870746,366-376 (2017). Article
PubMed Google Scholar * Rørvang, M. V., Nicova, K. & Yngvesson, J. Horse odor exploration behavior is influenced by pregnancy and age. _Front. Behav. Neurosci._ 16, 295 (2022). Article
Google Scholar * Doty, R. L. & Cameron, E. L. Sex differences and reproductive hormone influences on human odor perception. _Physiol. Behav._ 97, 213–228 (2009). Article CAS PubMed
PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank the staff from the UEPAO (Unité Expérimentale de Physiologie Animale de l’Orfrasière) for
technical help. FUNDING This study was funded by Institut Français du Cheval et de l’Equitation (IFCE), Grant Number 32 000809-Cognition Equine. This funding source had no role in the study
design, data collection and analysis, or preparation and submission of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * CNRS, IFCE, INRAE, Université de Tours, PRC, F-37380,
Nouzilly, France Plotine Jardat, Zoé Menard--Peroy, Céline Parias, Matthieu Keller, Ludovic Calandreau & Léa Lansade * Developmental Ethology and Cognitive Psychology Laboratory, Centre
des Sciences du Goût et de l’Alimentation, Institut Agro Dijon, CNRS, Université de Bourgogne-Franche-Comté, Inrae, Dijon, France Alexandra Destrez * Development of Olfactory Communication
and Cognition Laboratory, Centre des Sciences du Goût et de l’Alimentation, Institut Agro Dijon, CNRS, Université de Bourgogne-Franche-Comté, Inrae, Dijon, France Fabrice Damon * UEPAO,
INRAE, F-37380, Nouzilly, France Philippe Barrière Authors * Plotine Jardat View author publications You can also search for this author inPubMed Google Scholar * Alexandra Destrez View
author publications You can also search for this author inPubMed Google Scholar * Fabrice Damon View author publications You can also search for this author inPubMed Google Scholar * Zoé
Menard--Peroy View author publications You can also search for this author inPubMed Google Scholar * Céline Parias View author publications You can also search for this author inPubMed
Google Scholar * Philippe Barrière View author publications You can also search for this author inPubMed Google Scholar * Matthieu Keller View author publications You can also search for
this author inPubMed Google Scholar * Ludovic Calandreau View author publications You can also search for this author inPubMed Google Scholar * Léa Lansade View author publications You can
also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors devised the protocol. P.J., Z.M., C.P., P.B. and L.L. implemented the protocol. Z.M., P.J. and L.L. coded the
videos and analyzed data. P.J., Z.M., A.D., F.D., M.K., L.C. and L.L. revised the analysis and report. CORRESPONDING AUTHORS Correspondence to Plotine Jardat or Léa Lansade. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1. Supplementary Video 1. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jardat, P., Destrez, A., Damon, F. _et al._ Horses discriminate
human body odors between fear and joy contexts in a habituation-discrimination protocol. _Sci Rep_ 13, 3285 (2023). https://doi.org/10.1038/s41598-023-30119-8 Download citation * Received:
07 December 2022 * Accepted: 15 February 2023 * Published: 25 February 2023 * DOI: https://doi.org/10.1038/s41598-023-30119-8 SHARE THIS ARTICLE Anyone you share the following link with will
be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
Trending News
Us doubles h-1b visa fee, modi had raised concern with obamaSnapshot * US Congress imposed a special fee of $4,000 on certain categories of H-1B visas and $4,500 on L-1 visas * The...
Pfizer bids again for astra in pharma mega-deal?The plan was to re-domicile the American company to the UK where corporation tax is lower, in a process known as ‘invers...
Trump promotes gop tax plan in indiana[The stream has ended.] President Donald Trump is set to promote the newly released Republican tax plan in a speech in I...
White sox charities | grant recipients | chicago white soxThe Chicago White Sox announced cumulative charitable donations of nearly $1.5 million in the past year. Donations inclu...
Uk lawmaker alleges sexual harassment by pm boris johnson's fatherNokes, who is the chair of the British parliament's Women and Equalities Select Committee, added that the man she w...
Latests News
Horses discriminate human body odors between fear and joy contexts in a habituation-discrimination protocolABSTRACT Animals are widely believed to sense human emotions through smell. Chemoreception is the most primitive and ubi...
Bernard minet: come-back againPar Marianne Lesdos Le 11 mars 2014 Bernard Minet, le chanteur et comédien du groupe _Les Musclés_ tente une énième sort...
Specificity of the agglutinin in extracts of wheat germABSTRACT EXTRACTS of wheat germ prepared to contain lipase have been shown to have the additional property of agglutinat...
Wouldn't get away with that on bbc: susanna reid goes casual for goodThe 44-year-old was dressed head-to-toe in high street attire as she presented Good Morning Britain today with Ben Sheph...
What is france’s ‘franchise médicale’ fee and why might it go up soon?THE CHARGE COULD DOUBLE IN PRICE, THE FRENCH GOVERNMENT HAS SUGGESTED France’s _franchise médicale_, the €0.50 paid on e...