Sampling environmental dna from trees and soil to detect cryptic arboreal mammals
Sampling environmental dna from trees and soil to detect cryptic arboreal mammals"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Environmental DNA (eDNA) approaches to monitoring biodiversity in terrestrial environments have largely focused on sampling water bodies, potentially limiting the geographic and
taxonomic scope of eDNA investigations. We assessed the performance of two strictly terrestrial eDNA sampling approaches to detect arboreal mammals, a guild with many threatened and poorly
studied taxa worldwide, within two central New Jersey (USA) woodlands. We evaluated species detected with metabarcoding using two eDNA collection methods (tree bark vs. soil sampling), and
compared the performance of two detection methods (qPCR vs. metabarcoding) within a single species. Our survey, which included 94 sampling events at 21 trees, detected 16 species of mammals,
representing over 60% of the diversity expected in the area. More DNA was found for the 8 arboreal versus 8 non-arboreal species detected (mean: 2466 vs. 289 reads/sample). Soil samples
revealed a generally similar composition, but a lower diversity, of mammal species. Detection rates for big brown bat were 3.4 × higher for qPCR over metabarcoding, illustrating the enhanced
sensitivity of single-species approaches. Our results suggest that sampling eDNA from on and around trees could serve as a useful new monitoring tool for cryptic arboreal mammal communities
globally. SIMILAR CONTENT BEING VIEWED BY OTHERS ASSESSING THE EFFICACY OF EDNA METABARCODING FOR MEASURING MICROBIAL BIODIVERSITY WITHIN FOREST ECOSYSTEMS Article Open access 15 January
2021 COMPARISON OF TRADITIONAL AND DNA METABARCODING SAMPLES FOR MONITORING TROPICAL SOIL ARTHROPODS (FORMICIDAE, COLLEMBOLA AND ISOPTERA) Article Open access 24 June 2022 INTEGRATIVE
ECOLOGICAL AND MOLECULAR ANALYSIS INDICATE HIGH DIVERSITY AND STRICT ELEVATIONAL SEPARATION OF CANOPY BEETLES IN TROPICAL MOUNTAIN FORESTS Article Open access 07 October 2020 INTRODUCTION A
lack of knowledge of species distributions and population trends impedes conservation efforts for terrestrial mammals, a quarter of which are at risk of extinction1,2,3. Cryptic arboreal
mammal species, including tree-roosting bats, are especially lacking in data as a result of labor-intensive survey methods coupled with inadequate resources for detailed investigations4,5,6.
Because robust and long-term monitoring is vital to conservation, developing and improving tools that can more efficiently track population changes and assess species distributions is
critically important2,7. Here we contribute to these efforts by trialing novel and established environmental DNA (eDNA) sampling methods on and around individual trees to detect arboreal
mammals. While a variety of detection methods exist for monitoring cryptic arboreal mammals, most tend to be labor intensive, suffer from poor detection rates4,8,9, and can be biased towards
more easily observed species10. For example, among small bats (Microchiroptera), the demographic datasets available for species that roost in easily accessible locations, such as human
structures, are substantially more robust than those for species roosting in inaccessible locations such as tree canopies and rock crevices6. The combination of capture, visual search, and
telemetry methods necessary to locate bats in tree canopies and rock crevices is labor intensive, requiring hours of diurnal and nocturnal fieldwork to catch and then follow individual
animals to roosts11. Visual transect surveys are another common, fieldwork-intensive survey method for canopy-dwelling mammals that, when targeting nocturnal species, must be conducted at
night via spotlight4,8,9. In the past two decades, advances in technology and an increasingly acute need for monitoring data have spurred a flurry of research into novel methods to enhance
detection of these cryptic arboreal species, including bioacoustics12,13, drone- or canopy-mounted cameras8,14, scent detection dogs15, and eDNA-based methods16,17,18. The ability to survey
for arboreal mammals based on shed DNA may have distinct advantages over conventional methods that require separate surveys for diurnal and nocturnal species (e.g., visual methods), or have
difficulty discriminating among groups of species (e.g., acoustic methods19). Protocols for detecting the presence of aquatic or semi-aquatic vertebrates, including mammals, based upon shed
DNA are now in widespread use20,21. For fully terrestrial species, eDNA detection techniques represent a rapidly expanding area of research22,23,24. Building off of early efforts at
recovering DNA from physical traces such as tracks, hair, and scat25,26, mammal researchers have recently branched out into sampling communities using eDNA that has been deposited or
transported into ponds and rivers16,27,28,29,30 or left on attractants such as natural saltlicks or hollow logs31,32, including for some cryptic arboreal species18. While such approaches are
appealing for their ability to produce community-level inventories at large spatial scales, sampling is limited to wherever such natural features occur and is therefore inadequate for
finer-scale monitoring. For example, such methods cannot be used to survey for cryptic arboreal species at the level of individual forested sites or trees if no water bodies or natural
attractants are present nearby. Such finer scale resolution may be necessary to trigger regulatory protection or management interventions within jurisdictional boundaries (e.g., sites of
proposed development, multi-use public parcels). Fully terrestrial eDNA-based techniques to sample vertebrates (i.e., those not tied to water bodies) have included sampling eDNA found in
soil or air17,23,32, and testing residues left on artificial attractants such as coverboards22,24. Such methods circumvent the geographic limitations of those tied to water by allowing
researchers to decide precisely where in the landscape to take samples. These approaches may be particularly promising in the context of sampling for cryptic arboreal mammals as they allow
targeted sampling near individual trees or other sites with unknown occupancy status. For example, soil and air samples taken from caves and enclosures were demonstrated to readily allow
detection of eDNA from big brown bats (_Eptesicus fuscus_)17. Leempoel et al.23 demonstrated that soil eDNA samples collected at camera trapping stations performed as well as cameras for
larger mammals, and better than cameras for smaller species. Extending these terrestrial eDNA-based techniques to sample for cryptic arboreal species could involve either testing soil
beneath individual trees or using surface sampling techniques to recover eDNA from bark. A newly developed tool for sampling surface eDNA using damp commercially available paint rollers
performed exceptionally well for recovering arthropod DNA from tree bark33 and reptile DNA from cover objects24, and for identifying the vertebrate occupants of tree holes18. However, it has
yet to be extended to sampling the surface of tree trunks to detect arboreal mammals despite its obvious promise. Judging the performance of eDNA survey methods requires quantitative
evaluation and comparison of their effectiveness and efficiency relative to conventional methods8,23,30. We evaluated the mammal communities detected by two eDNA recovery methods—soil
sampling around trees and roller sampling of tree bark—in two New Jersey, USA woodlands. Our primary objectives were to: (1) evaluate if eDNA metabarcoding of bark and soil samples can
effectively characterize arboreal mammal communities, including tree-roosting bats, a group that includes several cryptic and threatened species in our region; (2) quantitatively compare the
metabarcoding detection rates of mammals between bark and soil eDNA samples; and (3) evaluate the extent to which a qPCR molecular detection approach can improve detection rates over
metabarcoding for a single species, the big brown bat. METHODS Our methods consisted of collecting soil and roller eDNA samples at 21 focal trees at two sites; performing eDNA metabarcoding
on these samples with a mammal-focused primer set; using a community occupancy model to compare detection rates among species and between collection methods; and assessing the relative
ability of these methods to characterize the community of likely arboreal and non-arboreal mammals present. We further compared the ability of a targeted qPCR assay to detect eDNA of one
species (big brown bat) in samples relative to metabarcoding. STUDY AREA AND TARGET SPECIES Field sampling occurred within the Rutgers Ecological Preserve (40.52° N, − 74.44° W; ~ 130 ha)
and Morristown National Historical Park (40.77° N, − 74.54° W; ~ 590 ha) in New Jersey, USA. Both sites are dominated by mixed-age second growth Oak-Hickory forest, which is common in the
region34. We built a database of mammal species occurring at the sites from personal records (unpublished data), park websites, the National Park Service’s Inventory and Monitoring Program
database, a camera trapping study35, and iNaturalist36 (see Table 1). This database formed the basis for reference for any species detected. FIELD SAMPLING We cleaned and disinfected the
sampling pole and soil collection spoons prior to each field use, and transported equipment in sterile bags to avoid contamination (Supplementary Table S1). To test for contamination of
field gear, we collected a ‘field negative’ roller sample upon arriving at the field site (Morristown or Rutgers) by going through the same sample handling procedures described below but
without field sampling. Between July and September 2021, we sampled 21 trees: 12 at Morristown and 9 at Rutgers. Trees were selected on their suspected status as diurnal roosts from a
parallel radio-telemetry tracking study involving three species: eastern red bat (_Lasiurus borealis_; n = 17 trees), northern long-eared bat (_Myotis septentrionalis_; n = 2 trees), and big
brown bat (_Eptesicus fuscus_; n = 2 trees) (BM, unpublished data). Trees were deciduous (mainly _Liriodendron, Quercus, Fagus,_ and _Betula_) and 17–81 cm in diameter (mean: 51 cm; see
Supplementary Table S2). At 19 trees, we collected soil and roller eDNA within 24 h after a roosting bat was suspected present, and then again on the following two consecutive days (48 h, 72
h), for a total of three rounds of sampling per tree. Two trees received only the first round of sampling for logistical reasons. Thus, we collected a total of 59 sets of roller and soil
samples at 21 trees. We collected soil samples beneath the canopy of focal trees by establishing four transects, oriented 90 degrees from each other, and extending from the trunk out to ~ 3
m, near the outer edge of the canopy. The orientation of the four-way transects was rotated ~ 30 degrees during each subsequent sampling event to avoid overlapping previous sampling
locations. Along each transect, we collected a ~ 20 g scoop of soil every 1 m (i.e., 4 per transect), and then combined the ~ 320 g of soil for each tree into a 178 × 305 mm sterile plastic
bag. Samples were transported in an ice-filled cooler and stored at − 20 °C until processing. We collected roller samples on each focal tree trunk following Valentin et al. including
measures to avoid sample contamination (Supplementary Table S1). Applying the paint roller firmly to the bark of the tree, we rolled from the base of the trunk to a height of ~ 3 m around
the entire circumference of the tree. Then, we removed the roller from the pole, sealed it in a 304 × 114 mm sterile plastic bag with ~ 400 mL of DI water, and shook the bag while massaging
the roller for ~ 30 s to help move any eDNA into solution. We filtered the water using a self-desiccating filter assembly (Smith-Root, Inc., Vancouver, Washington, USA) equipped with a 10-μm
polycarbonate track-etched (PCTE) filter membrane and a peristaltic pump. We then used sterilized forceps to remove the filter membrane from the assembly and place it into a 1.5 mL tube
filled with 100% non-denatured ethanol. Tubes were stored at − 20 °C until extraction. DNA EXTRACTION AND SEQUENCING OF ENVIRONMENTAL SAMPLES For the 59 roller samples, we evaporated ethanol
from filters using a vacuum centrifuge, extracted DNA using the DNeasy Blood and Tissue kit (Qiagen), and removed impurities using Ampure XP beads (Beckman Coultier, Pasadena, California,
USA). For soil, we extracted DNA from a 5 mL portion of the sample using the DNeasy PowerMax Soil Kit (Qiagen) following manufacturer’s protocols. Unfortunately, we were unable to procure
enough kits to extract all 59 soil samples due to prolonged shipping delays. To overcome this hurdle, we designed a subsampling scheme, based on haphazardly selecting trees from which to
extract DNA from soil samples, until the soil extraction kits in our possession were exhausted. This resulted in a total of 35 soil samples analyzed from 14 trees (see Supplementary Table
S2). We confirmed there was no sampling bias resulting from this subsampling scheme by performing a parallel analysis (see Statistical Analysis and Results). For both soil and roller
samples, one extraction negative control was constructed for each extraction batch, consisting of PCR-grade water in place of the sample, but otherwise treated the same. We prepared
libraries using the Illumina two-step PCR metabarcoding protocol37. For each sample, we ran one initial PCR using the MiMammal-U primer set in replicates of 3 to reduce sample drop-out and
PCR bias. The MiMammal-U primer set targets an ~ 200 bp region of the 12S locus and was created to specifically amplify mammal DNA from environmental samples38. We included at least one PCR
negative control in each PCR run. Reaction preparation for the first PCR followed the Illumina protocol37 and had the following cycling parameters: 98 °C for 3 min, 35 cycles of 95 °C (30
s), 65 °C (30 s), and 72 °C (1 min), followed by final extension at 72 °C for 5 min, and then hold at 4 °C. For the soil samples, we used the same parameters except with an annealing
temperature of 67 °C. The primers also contained partial Illumina adapter sequences to act as binding sites for a second PCR run (described below). We then pooled all the replicates for each
sample. After pooling, we performed a bead clean-up for each sample to remove primer dimers and ran a second PCR to add the remainder of the Illumina adapter sequences and add
sample-specific dual indexes. For the second PCR, reaction preparation and cycle parameters were identical to those in the Illumina protocol37. We performed agarose gel electrophoresis on
each sample, and all libraries that produced a visible band were then cleaned with a second magnetic bead clean-up and quantified using the Qubit dsDNA High sensitivity DNA quantification
assay. Libraries with no visible band on the gel were not analyzed further, with the exception of negatives, of which all were included. We then pooled all samples in equimolar ratios; 5 μl
of each negative were also included in the final sequencing pool. We sent libraries to the Genomics Core Facility at Princeton University for sequencing on an Illumina MiSeq v3 2 × 300nt.
BIOINFORMATICS We processed sequence data and made taxonomic assignments following OBITools39 documentation and Leempoel et al.23 (see Supplementary Table S3). Briefly, we aligned paired
reads, and removed reads with join scores < 40, bases with quality scores < 30, and adapter sequences. We then removed reads with ≥ 21 ambiguous bases, dereplicated reads, removed
sequences with read counts < 10 or with length < 80 bp, and filtered all reads for PCR or sequencing errors. We generated a reference database from all vertebrate sequences available
from ENSEMBL40 and used EcoPCR to match taxa to our sequence reads. For unmatched reads, we assigned taxa manually using BLAST41 and performed a local BLAST search against a supplemental
database of 17 sequences from 9 bat species that we generated from tissue samples (see Supplementary Appendix S1 and Table S4). We then removed all resulting sequences with insert lengths ≥
200 to exclude non-target taxa (e.g., bacteria)23. Additionally, we removed sequences from 10 species that were deemed to be likely contaminants from other projects in our lab (Supplementary
Table S5) and five that are commonly associated with contamination in other metabarcoding studies23,28,30 (_Bos taurus_, _Homo sapiens_, _Mus musculus, Sus scrofa, Gallus gallus_).
Following assignment and filtering of taxa, we controlled for contamination by setting a detection threshold for each OTU based on the number of reads that appeared in the negatives,
removing any counts that appeared in field, extraction, and PCR negatives from the corresponding samples. Finally, we removed any OTU with a read count < 20 as an additional control for
contamination and to reduce noise in the data set. BIG BROWN BAT QPCR METHODS To compare quantitative PCR (qPCR) detection methods with metabarcoding, we re-tested the same 94 extracted
roller and soil samples described above using a highly-sensitive qPCR assay for big brown bat that targets a 91 bp segment of mtDNA in the COI region (limit of detection: 2 copies per
reaction)17. Our qPCR methods matched those of Serrao et al.17, except that we used a StepOne Plus Real-Time PCR System (Applied Biosystems, Inc., Waltham, Massachusetts, USA) and lacked an
internal positive control. qPCR runs included 3 technical replicates each for all samples, a no-template control, and a six-level standard curve of synthetic DNA (10-fold dilution).
Following Serrao et al.17, we confirmed species identification in all positive samples by enzymatically cleaning the resulting qPCR product (ExoSAP-IT™ PCR Product Cleanup Reagent, Applied
Biosystems, Inc.) and performing bidirectional Sanger sequencing using the same primer set as for the qPCR reaction. The resulting sequences were aligned using Geneious Prime (Biomatters,
Inc., Aukland, New Zealand) and identified to species using BLAST as described above. STATISTICAL ANALYSIS We assessed the performance of soil and roller eDNA sampling for metabarcoding
analyses using a Dorazio-Royle (DR) community occupancy model42. These hierarchical models can estimate the effects of covariates on the probability of presence (occupancy) and detection
given presence for multiple species simultaneously. Information is shared among species by treating species-specific covariate effects as originating from common distributions. In our model,
the dependent variable was the detection (1) or non-detection (0) of mammal species at each focal tree during each sampling event. We included sampling method (soil or roller) as a
covariate in the detection sub-model to evaluate differences in detection probability between the two methods_._ In addition, we visualized mammal community data using Venn diagrams,
taxonomic heat trees43, species accumulation curves, and non-metric multidimensional scaling (NMDS) ordination plots44. We compared the performance of qPCR versus metabarcoding methods for
detecting big brown bat eDNA using multimethod occupancy models45. These multilevel models use joint detection information (e.g., from qPCR and metabarcoding) to inform the availability for
detection, _θ_, and detection probability given availability, _P_(_detect_ | _θ_)_._ In this case, availability refers to the probability of big brown bat eDNA presence within a sample. The
dependent variable was the detection (1) or non-detection (0) of big brown bat by each method. We used a covariate for sampling method (soil vs. roller) within the availability sub-model,
and a covariate for molecular approach (qPCR vs. metabarcoding) to estimate the method-specific probability of detecting big brown bat eDNA given availability within the sample. We then
estimate the total ‘per-visit detection rate’ of bats at focal trees by multiplying _θ_ by _P_(_detect_ | _θ_). For both the DR and the multimethod occupancy model, we used the per-visit
detection probability (_p_) to estimate the cumulative probability of detecting a species at least once given _n_ repeated samples using the formula 1− (1 − _p_)_n_ (e.g., Ref.24). All
models were fit in a Bayesian framework with non-informative priors (see Ref.42, pp. 607 and 690) using JAGS and jagsUI in program R46,47. We ran 3 chains of 30,000 iterations each,
including a burn-in period of 10,000, keeping every 10th draw. Model convergence was assessed by examining trace plots and Gelman-Rubin statistics (r-hat < 1.1). We compared estimates of
per-visit detection probability for the various methods by examining and plotting posterior predictive distributions. RESULTS We performed roller and soil eDNA sampling at 21 trees,
obtaining three samples per tree at all except for two trees, which each received a single sample. We analyzed all 59 roller samples from 21 trees, and a subset of 35 soil samples from 14
trees (1–3 samples per tree; mean = 2.5/tree). Forty-seven of 59 roller samples and 35 of 35 soil samples had measurable read counts with a total of 9,852,404 and 4,731,187 reads matched to
roller and soil samples, respectively. After all filtering steps (see Supplementary Table S6), contaminant reads still remained in 5 of our 46 negative control samples, all associated with
two taxa: _Canis lupus familiaris_ (7702 reads of 4 OTUs) and _Glaucomys volans_ (a single read of 1 OTU). After removal of these negative control reads from samples, 2,548,768 total reads
across 46 samples remained for roller samples, and 33,314 total reads across 23 samples remained for soil samples (Supplementary Table S6). A total of 359 OTUs remained after all filtering
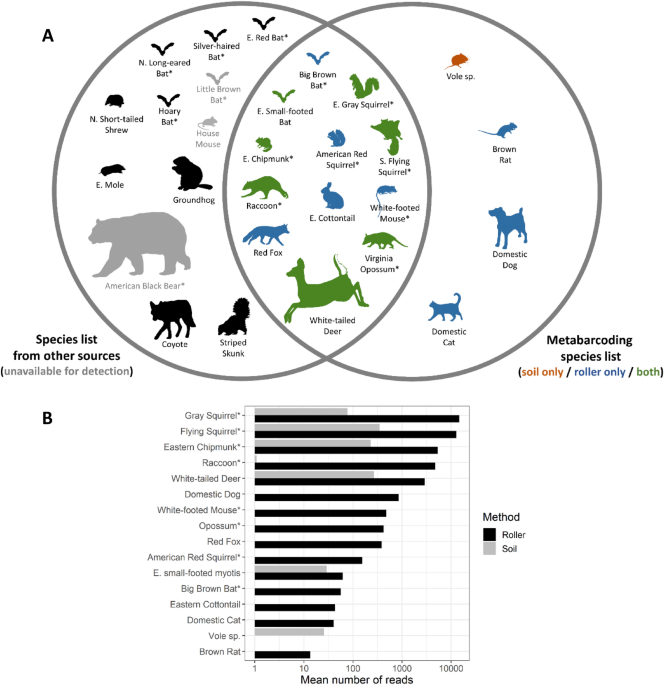
steps, attributable to 16 mammal species: 8 arboreal species (5 detected with soil, 8 with roller) and 8 non-arboreal species (3 detected with soil, 7 with roller; Figs. 1 and 2). We
identified 28 total species (14 arboreal, 14 non-arboreal) as likely to be present at the sites based on our list compiled from external sources in addition to our metabarcoding results
(Table 1, Fig. 1A). Two of those species (house mouse and little brown bat) were not available to be detected using metabarcoding methods as they were excluded due to contamination concerns
(Table 1, Supplementary Table S5), and one species (American black bear) was not available for detection because the MiMammal-U primer set is not effective for bears38. Thus, the 16 species
we detected using metabarcoding represents 64% of the 25 total species expected to be present and available for detection. Of the 9 species that were not detected using metabarcoding, 4 were
considered arboreal, all of which were bats (Table 1, Fig. 1A). We detected 2 of 6 expected bat species: big brown bat, a tree-roosting species, and eastern small-footed bat, a species that
roosts in rock crevices. Four species (brown rat, vole sp., domestic dog, and small-footed bat) were detected by metabarcoding but did not occur in our compiled list of likely species
(Table 1, Fig. 1A). In general, higher read counts were found in roller versus soil samples and for arboreal versus non-arboreal species. For roller samples, the mean number of reads per
sample averaged 4849 ± 2094 (SE) across species for arboreal mammals (range = 56–14,699, n = 8), and 537 ± 353 for non-arboreal mammals (range = 0–2901, n = 8; Fig. 1B). For soil samples,
the number of reads averaged 82 ± 48 for arboreal mammals (range = 0–349, n = 8), and 40 ± 33 (range = 0–270, n = 8) for non-arboreal mammals. The number of reads for bats averaged 59 ± 3
(range = 56–61, n = 2) for roller sampling and 14 ± 14 (range = 0–28, n = 2) for soil sampling. The discrepancy in mean read depth observed between sampling methods is partly explained by
the removal of a large number of microbial reads from soil samples during bioinformatics (> 95% of reads removed for soil compared with ~ 50% of reads for roller; Supplemental Table S6).
Despite having fewer samples and fewer reads per sample, our soil method resulted in a generally similar characterization of mammal community composition to the roller method (Figs. 1 and 2;
see also NMDS ordination plot, Supplementary Fig. S1). Species accumulation curves (Fig. 3) revealed that the number of species detected had not yet leveled off with either method,
suggesting that sampling additional trees likely would have added more species. In addition to mammals, we also detected seven other vertebrates (six birds and one salamander), all of which
were detected using roller methods, but only one of which (American Robin, _Turdus migratorius_) was detected using soil methods (Fig. 2). Arboreal mammal species with the highest detection
probability according to the community occupancy model were also the species with the highest read counts (Figs. 1B and 4): gray squirrel (roller: 0.70; soil: 0.39), southern flying squirrel
(roller: 0.51; soil: 0.21), and eastern chipmunk (roller: 0.50; soil; 0.23). Surprisingly, the non-arboreal white-tailed deer also showed relatively high detection probability estimates
within both roller (0.58) and soil (0.23) samples (Fig. 4). Based on these estimates for southern flying squirrel, the most cryptic non-bat arboreal mammal that we detected, ~ 4 roller
sampling visits to an ‘occupied’ tree would be needed to ensure 95% confidence of detection, compared with ~ 12 visits required with our soil eDNA methods (Fig. 5). The reduced data set
included in our parallel analysis contained two fewer species (lacked American red squirrel and eastern cottontail). Modeling based on this data set yielded very similar results to those of
the full data set (see Supplemental Figs. S2–4). For big brown bat, metabarcoding detections occurred in 3 of 94 samples (all roller samples) at 3 of 21 trees, while qPCR detections occurred
in 10 samples (9 roller samples and 1 soil sample) at 6 trees. Together, metabarcoding and qPCR detected big brown bat DNA in 11 samples at 7 trees, including one of the two trees
identified as likely containing a roosting individual of this species. The multimethod occupancy model revealed that detection probability was 3.4 × higher using qPCR-based detection methods
(49%, 95% CI = [21%, 86%]) than when using metabarcoding methods (15%, [3%, 41%]). Based on these estimates, ~ 7 visits to an occupied tree would be required to establish presence of big
brown bats with 95% confidence using qPCR methods, while ~ 27 visits would be required using metabarcoding methods (Fig. 5). DISCUSSION Fully terrestrial eDNA sampling approaches offer a
potentially powerful addition to biodiversity monitoring efforts23,24. However, protocols for using eDNA-based methods to characterize terrestrial biodiversity, and vertebrate communities in
particular, are still nascent27,28,30. In this study, we show for the first time that an eDNA metabarcoding approach can be used to broadly characterize tree-dwelling mammal communities by
sampling tree trunks and surrounding soil. Our findings add to recent work (e.g., for reptiles22,24) showing that surface eDNA collection methods, which are relatively untested compared with
soil-based eDNA methods, can also be effective at detecting terrestrial vertebrates. Further, we demonstrate that supplementing metabarcoding detection with qPCR-based methods can greatly
improve sensitivity, a potentially important consideration for monitoring schemes focused on rare taxa (e.g., Refs.11,12). Together, our results have significant implications for global
biodiversity conservation as the broader guild of arboreal vertebrates includes highly threatened5,48,49, as well as invasive alien species50, that are often cryptic, inhabit inaccessible
locations, and are therefore challenging to monitor. Our methods captured over 60% of the mammalian diversity expected at the sites, and a similar fraction of the subset of arboreal species,
despite sampling only 21 trees. Species accumulation curves suggest that more species would likely have been added with increased sampling effort. These results broadly agree with those of
Leempoel et al.23 who found that soil eDNA metabarcoding well characterized mammal communities in California chaparral. However, in both our study and that of Leempoel et al.23, some
conspicuous absences were evident. Bats comprised all of the arboreal species that we expected but failed to detect at our sites using metabarcoding (Fig. 1A). Leempoel et al.23 also noted a
lack of bat detections (2 of 14 possible taxa detected), which they suggested could be due to low efficiency of either the 12S primer set or of their soil sampling methods for that order.
While both reasons could also apply to the lack of bats detected in our study (discussed further below), the performance of the 12S primer set very likely contributed to our lack of American
black bear detections as MiMammal-U primers are known to be ineffective at amplifying bear DNA38. These challenges highlight the reality that false negatives and varying detectability among
species are common issues to all survey approaches, including eDNA metabarcoding. Our study represents a rare example among metabarcoding studies in that it uses repeated sampling and
community occupancy models to quantify false negative rates. This quantitative approach, coupled with continued experimentation with different molecular techniques and survey methods (e.g.,
Refs.23,27), will be vital to helping researchers decide how eDNA metabarcoding methods will fit into existing biodiversity monitoring efforts moving forward. Although our results suggest
that sampling for tree-roosting bats using eDNA metabarcoding still requires further research and optimization, our approach likely has application to characterizing communities in a much
broader range of arboreal species globally. Geographic regions with multiple elusive arboreal mammals of management interest—for example, gliders and tree kangaroos in Australasia, or
primates in the global tropics—may be particularly suited for a metabarcoding approach for community-level assessments4,8,9,49. It may be especially useful for rapid biodiversity assessments
(RBAs51) in remote forested environments, where the ability to collect multiple samples relatively rapidly without regard to time of day would be a key advantage27. Existing survey methods
to monitor arboreal mammals tend to be optimized for particular groups of species, often segregated by body size and behavior, with no suitable single method available to characterize all
members of the guild4,8,9,16,49. Diurnal and nocturnal species, for example, often require separate survey methods or timing8. While camera traps capture both diurnal and nocturnal species,
they typically miss smaller species16,23. The need for multiple methods to survey for nocturnal and diurnal, or large and small, species separately raises the cost of sampling and can result
in datasets that are difficult to compare across sites because of inherent sampling biases8. Excluding bats, we found encouraging results for both diurnal and nocturnal arboreal species of
a broad range of body sizes, detecting all seven expected species (Fig. 1A). While more work is needed to assemble robust genetic reference libraries before global arboreal mammal monitoring
with eDNA metabarcoding will be broadly feasible, a clear advantage of the technique remains the power to detect a broad swath of species, with widely varying morphologies and behaviors,
with a single method23,27,28,51. The promise of eDNA metabarcoding approaches for at least some arboreal guilds is well illustrated by our results for southern flying squirrel, _Glaucomys
volans_. Like other flying squirrels (Tribe: Pteromyini), this species is strictly nocturnal, highly arboreal, and tends to get injured in live traps, making it difficult to directly observe
and monitor48,52. Yet _G. volans_ eDNA was readily detectable using metabarcoding in our study, occurring in 19–26% of soil samples and 47–52% of roller samples across both sites. Our
similarly encouraging results for detecting other squirrels (Sciuridae) also bode well for management applications. For example, the methods would enable fine-scale mapping of habitat use in
places such as the United Kingdom where native red squirrels (_Sciurus vulgaris_) are outcompeted by eastern gray squirrels, or the Delmarva peninsula (USA) to support the conservation
efforts for the Delmarva fox squirrel (_Sciurus niger cinereus_)53. Further research is needed to determine the extent to which our results for squirrels generalize to other taxa with
similar active tree-climbing lifestyles (e.g., gliders4, primates49). Our finding that soil samples revealed fewer species, had lower detection probability, and had lower read counts than
roller samples, even for some non-arboreal species like white-tailed deer, likely reflects multiple factors. First, soil and tree bark represent markedly different biological and chemical
environments that likely differ in eDNA quantity by species, eDNA persistence rates54,55, and microorganism abundance. The latter may be especially pertinent to our study as we observed a
relatively large drop in the number of reads after removal of microorganism reads, especially for soil samples. This suggests that performing additional purification steps prior to
sequencing could boost the ability of both methods, and especially soil eDNA, to detect target species by increasing mammalian sequencing depth. Other in-lab factors, such as method of
extraction23 or choice of primers, similarly have the potential to influence the recovery and amplification of target species’ DNA and should be the focus of future research. Next, our focal
trees were not chosen to occur near any special attractants or areas of multi-species use, such as saltlicks or water sources, which has proven successful in other vertebrate eDNA
studies18,25,31,32,56. It is possible that adding a broader range of soil sampling sites, including some targeted towards other guilds (e.g., burrow users32,56), would have yielded a more
complete inventory. Nevertheless, both soil and surface methods have advantages over the much more commonly-used metabarcoding approaches that rely on natural water bodies for assessing
mammal communities16,17,27,28,29,30,38 as they are not limited to where these features occur. Our study is the first to suggest that surface eDNA metabarcoding methods can be a powerful
supplement to established soil-based methods of characterizing mammal communities, especially for arboreal species. As noted, bats were especially lacking from our eDNA metabarcoding
results, with only two of six likely species detected. Notably, our metabarcoding species list lacked two of the bat species that our sampling scheme was designed around (eastern red bat and
northern long-eared bat) and for which we had confirmed recent presence at the sites (Table 1). The lack of northern long-eared bat detections may directly relate to recent precipitous
population declines (~ 99%) caused by white-nose syndrome57. However, the lack of eastern red bat detections was especially surprising as roosting of this species was suspected based on
telemetry in 17 of our 21 target trees. Reasons for this omission may relate to the fact that eastern red bats roost singly on small twigs and in leaf clusters, and therefore may not leave
much DNA on tree trunks. Another possibility is low efficiency of the 12S primer set for bats, although we were unable to find information about this in the literature. It is notable that
Leempoel et al.23 had a similarly poor representation of bats with comparable soil-based methods. However, our metabarcoding results did indicate that we are capable of detecting even
uncommon, or at least unexpected, bat species with our methods. Eastern small-footed bat, which is typically viewed as a rock-roosting species and is considered endangered by the
International Union for Conservation of Nature (IUCN)58, was detected in both soil and surface eDNA samples from Morristown National Historic Park. This species was not otherwise confirmed
as present at the site until a year later, in spring 2022, when it was caught in a mist net (BM, unpublished data). Our results with respect to bat detections, along with those of others23,
underscore the need for further research to adapt eDNA metabarcoding methods to this vulnerable group, which could contribute much needed demographic and distribution information. This is
especially urgent as 18% of bat species are listed as “data deficient” by the IUCN, while 57% lack basic population trend information5,58. Our comparison of qPCR to metabarcoding detection
methods for big brown bat represents a hopeful result for the use of eDNA to monitor rare vertebrates that are of particular conservation interest. It is well-known that qPCR-based eDNA
surveys targeted towards individual species return higher detection probabilities and have greater power at low abundance, than metabarcoding approaches59. Our results agree, showing for the
first time that adding a qPCR step in the analysis of surface and soil eDNA samples can be effective for detecting bats in forested environments. The addition of a qPCR step opens the door
for developing species-specific assays to increase detection power for endangered or elusive bat species, or other cryptic arboreal mammals49. Emerging molecular detection approaches such as
droplet digital PCR have the potential to increase this sensitivity even further59. Like other eDNA-based tools and survey tools in general, careful consideration of sampling effort, the
natural history of target species, and the configuration of different field and molecular methods will be key to optimizing our approach to characterize mammal communities, or to target a
particular species, in different regions. Although eDNA surveys are not inexpensive given the need for both fieldwork and molecular analyses, they can be cheaper than conventional
approaches, especially if such approaches require many hours of fieldwork or expensive equipment60. Thus, the relative cost-effectiveness of surface or soil eDNA surveys will depend heavily
on the mammal communities of interest, the mix of methods that must be employed to effectively sample them, and the purpose of the sampling efforts. However, even if costs are increased,
eDNA surveys can reduce field time to the extent that they can improve detection rates, either by replacing or supplementing conventional sampling methods (e.g., as a supplement to visual
observations). With higher detection rates, fewer visits are required to achieve the same results. This operational efficiency would be especially advantageous when field conditions present
safety risks, are intrusive to sensitive habitats, or are challenging to access. For example, adding surface eDNA sampling to existing visual surveys of eastern wood rats (_Neotoma
floridana_), a cryptic mammal that inhabits steep, rocky slopes in the eastern US, could likely increase detection power, thereby reducing the need for additional risky and costly sampling
visits. More studies involving direct comparisons among methods (e.g., Refs.23,24,30,60), in a variety of ecoregions, are needed to determine the extent to which incorporating our methods
into existing vertebrate monitoring workflows would increase efficiency. Finally, we detected other vertebrates, including seven birds and one salamander, in soil and surface eDNA samples,
despite our use of a mammal-specific primer set. This is similar to results from Leempoel et al.23 in California using the same primer set, in which six bird species were detected. We found
that surface eDNA detected more bird species than soil, perhaps for the same reasons as for mammals (above). Our results provide evidence that surface eDNA surveys, with taxon-specific
primers, could be used to survey bird communities, or used to target particularly rare species in forested ecosystems (e.g., Ref.61). Our detection of a salamander, coupled with recent
promising research into reptile detection using surface eDNA methods22,24 suggests a broader potential for applications with other vertebrates as well. Finally, both surface and soil eDNA
metabarcoding can be expanded beyond forests, providing insight into their effectiveness in other habitats (e.g., caves17 or talus slopes). Our study and others highlight that the potential
of coupling surface and soil eDNA methods for detecting and monitoring mammalian biodiversity, and terrestrial organisms generally, has yet to be fully realized. DATA AVAILABILITY Raw
sequence data for this project are available at NCBI BioProject PRJNA915402: https://www.ncbi.nlm.nih.gov/bioproject/PRJNA915402. Processed data are available on Open Science Foundation:
https://doi.org/10.17605/OSF.IO/A6FYV. CODE AVAILABILITY R code and data to reproduce the results and figures in this paper are available on GitHub (https://github.com/mikeallen-eco/mammal/)
with an archived copy on Zenodo (https://doi.org/10.5281/zenodo.7486742). REFERENCES * Ceballos, G., Ehrlich, P. R., Soberón, J., Salazar, I. & Fay, J. P. Global mammal conservation:
What must we manage?. _Science_ 309, 603–607 (2005). Article ADS CAS Google Scholar * Carwardine, J. _et al._ Cost-effective priorities for global mammal conservation. _Proc. Natl. Acad.
Sci._ 105, 11446–11450 (2008). Article ADS CAS Google Scholar * Visconti, P. _et al._ Future hotspots of terrestrial mammal loss. _Philos. Trans. R. Soc. B Biol. Sci._ 366, 2693–2702
(2011). Article Google Scholar * Cripps, J. K. _et al._ Double-observer distance sampling improves the accuracy of density estimates for a threatened arboreal mammal. _Wildl. Res._ 48,
756–768 (2021). Article Google Scholar * Frick, W. F., Kingston, T. & Flanders, J. A review of the major threats and challenges to global bat conservation. _Ann. N. Y. Acad. Sci._
1469, 5–25 (2020). Article ADS Google Scholar * Weller, T. J., Cryan, P. M. & O’Shea, T. J. Broadening the focus of bat conservation and research in the USA for the 21st century.
_Endanger. Species Res._ 8, 129–145 (2009). Article Google Scholar * Holland, G. J. _et al._ Conservation cornerstones: Capitalising on the endeavours of long-term monitoring projects.
_Biol. Conserv._ 145, 95–101 (2012). Article Google Scholar * Bowler, M. T., Tobler, M. W., Endress, B. A., Gilmore, M. P. & Anderson, M. J. Estimating mammalian species richness and
occupancy in tropical forest canopies with arboreal camera traps. _Remote Sens. Ecol. Conserv._ 3, 146–157 (2017). Article Google Scholar * Pocknee, C. A., Lahoz-Monfort, J. J., Martin, R.
W. & Wintle, B. A. Cost-effectiveness of thermal imaging for monitoring a cryptic arboreal mammal. _Wildl. Res._ 48, 625–634 (2021). Article Google Scholar * Dambly, L. I., Jones, K.
E., Boughey, K. L. & Isaac, N. J. Observer retention, site selection and population dynamics interact to bias abundance trends in bats. _J. Appl. Ecol._ 58, 236–247 (2021). Article
Google Scholar * de Torrez, E. C. B., Ober, H. K. & McCleery, R. A. Use of a multi-tactic approach to locate an endangered Florida bonneted bat roost. _Southeast. Nat._ 15, 235–242
(2016). Article Google Scholar * Murray, S. W. & Kurta, A. Nocturnal activity of the endangered Indiana bat (_Myotis sodalis_). _J. Zool._ 262, 197–206 (2004). Article Google Scholar
* Whisson, D. A., McKinnon, F., Lefoe, M. & Rendall, A. R. Passive acoustic monitoring for detecting the Yellow-bellied Glider, a highly vocal arboreal marsupial. _PLoS ONE_ 16,
e0252092 (2021). Article CAS Google Scholar * Gregory, T., Carrasco Rueda, F., Deichmann, J., Kolowski, J. & Alonso, A. Arboreal camera trapping: Taking a proven method to new
heights. _Methods Ecol. Evol._ 5, 443–451 (2014). Article Google Scholar * Chambers, C. L., Vojta, C. D., Mering, E. D. & Davenport, B. Efficacy of scent-detection dogs for locating
bat roosts in trees and snags. _Wildl. Soc. Bull._ 39, 780–787 (2015). Article Google Scholar * Mena, J. L. _et al._ Environmental DNA metabarcoding as a useful tool for evaluating
terrestrial mammal diversity in tropical forests. _Ecol. Appl._ 31, e02335 (2021). Article Google Scholar * Serrao, N. R., Weckworth, J. K., McKelvey, K. S., Dysthe, J. C. & Schwartz,
M. K. Molecular genetic analysis of air, water, and soil to detect big brown bats in North America. _Biol. Conserv._ 261, 109252 (2021). Article Google Scholar * Newton, J. P., Bateman, P.
W., Heydenrych, M. J., Mousavi-Derazmahalleh, M. & Nevill, P. Home is where the hollow is: Revealing vertebrate tree hollow user biodiversity with eDNA metabarcoding. _Environ. DNA_ 4,
1078–1091 (2022). Article CAS Google Scholar * Luszcz, T. M. _et al._ A blind-test comparison of the reliability of using external morphology and echolocation-call structure to
differentiate between the little brown bat (_Myotis lucifugus_) and Yuma myotis (_Myotis yumanensis_). _Northwest. Nat._ 97, 13–23 (2016). Article Google Scholar * Rees, H. C., Maddison,
B. C., Middleditch, D. J., Patmore, J. R. & Gough, K. C. The detection of aquatic animal species using environmental DNA–a review of eDNA as a survey tool in ecology. _J. Appl. Ecol._
51, 1450–1459 (2014). Article CAS Google Scholar * Padgett-Stewart, T. M. _et al._ An eDNA assay for river otter detection: A tool for surveying a semi-aquatic mammal. _Conserv. Genet.
Resour._ 8, 5–7 (2016). Article Google Scholar * Matthias, L., Allison, M. J., Maslovat, C. Y., Hobbs, J. & Helbing, C. C. Improving ecological surveys for the detection of cryptic,
fossorial snakes using eDNA on and under artificial cover objects. _Ecol. Indic._ 131, 108187 (2021). Article Google Scholar * Leempoel, K., Hebert, T. & Hadly, E. A. A comparison of
eDNA to camera trapping for assessment of terrestrial mammal diversity. _Proc. R. Soc. B_ 287, 20192353 (2020). Article CAS Google Scholar * Kyle, K. E. _et al._ Combining surface and
soil environmental DNA with artificial cover objects to improve terrestrial reptile survey detection. _Conserv. Biol._ 36, e13939 (2022). Article Google Scholar * Kinoshita, G., Yonezawa,
S., Murakami, S. & Isagi, Y. Environmental DNA collected from snow tracks is useful for identification of mammalian species. _Zool. Sci._ 36, 198–207 (2019). Article CAS Google Scholar
* Marucco, F., Boitani, L., Pletscher, D. H. & Schwartz, M. K. Bridging the gaps between non-invasive genetic sampling and population parameter estimation. _Eur. J. Wildl. Res._ 57,
1–13 (2011). Article Google Scholar * Coutant, O. _et al._ Amazonian mammal monitoring using aquatic environmental DNA. _Mol. Ecol. Resour._ 21, 1875–1888 (2021). Article CAS Google
Scholar * Sales, N. G. _et al._ Fishing for mammals: Landscape-level monitoring of terrestrial and semi-aquatic communities using eDNA from riverine systems. _J. Appl. Ecol._ 57, 707–716
(2020). Article CAS Google Scholar * Harper, L. R. _et al._ Environmental DNA (eDNA) metabarcoding of pond water as a tool to survey conservation and management priority mammals. _Biol.
Conserv._ 238, 108225 (2019). Article Google Scholar * Lyet, A. _et al._ eDNA sampled from stream networks correlates with camera trap detection rates of terrestrial mammals. _Sci. Rep._
11, 1–14 (2021). Article Google Scholar * Ishige, T. _et al._ Tropical-forest mammals as detected by environmental DNA at natural saltlicks in Borneo. _Biol. Conserv._ 210, 281–285 (2017).
Article Google Scholar * Ryan, E., Bateman, P., Fernandes, K., van der Heyde, M. & Nevill, P. eDNA metabarcoding of log hollow sediments and soils highlights the importance of
substrate type, frequency of sampling and animal size, for vertebrate species detection. _Environ. DNA_ https://doi.org/10.1002/edn3.306 (2022). Article Google Scholar * Valentin, R. E.
_et al._ Moving eDNA surveys onto land: Strategies for active eDNA aggregation to detect invasive forest insects. _Mol. Ecol. Resour._ 20, 746–755 (2020). Article Google Scholar * Collins,
B. R. & Anderson, K. _Plant Communities of New Jersey: A Study in Landscape Diversity_ (Rutgers University Press, 1994). Google Scholar * Cove, M. V. _et al._ SNAPSHOT USA 2019: A
coordinated national camera trap survey of the United States (2021). * iNaturalist. iNaturalist Research-grade Observations. https://inaturalist.org/. Occurrence dataset
https://doi.org/10.15468/ab3s5x. Accessed via https://gbif.org/. 14 June 2022 (2022). * Illumina, I. 16S Metagenomic sequencing library preparation. _Prep. 16S Ribosomal RNA Gene Amplicons
Illumina MiSeq Syst._ 1, 28 (2013). Google Scholar * Ushio, M. _et al._ Environmental DNA enables detection of terrestrial mammals from forest pond water. _Mol. Ecol. Resour._ 17, e63–e75
(2017). Article CAS Google Scholar * Boyer, F. _et al._ obitools: A unix-inspired software package for DNA metabarcoding. _Mol. Ecol. Resour._ 16, 176–182 (2016). Article CAS Google
Scholar * Cunningham, F. _et al._ Ensembl 2022. _Nucleic Acids Res._ 50, D988–D995 (2022). Article CAS Google Scholar * Johnson, M. _et al._ NCBI BLAST: A better web interface. _Nucleic
Acids Res._ 36, W5-9 (2008). Article CAS Google Scholar * Kéry, M. & Royle, J. A. _Applied Hierarchical Modeling in Ecology: Analysis of Distribution, Abundance and Species Richness
in R and BUGS: Volume 1: Prelude and Static Models_ (Academic Press, 2016). MATH Google Scholar * Foster, Z. S., Sharpton, T. J. & Grünwald, N. J. Metacoder: An R package for
visualization and manipulation of community taxonomic diversity data. _PLoS Comput. Biol._ 13, e1005404 (2017). Article ADS Google Scholar * Oksanen, J. _et al._ ‘vegan’: Community
ecology package. R package version 2.5-7. https://CRAN.R-project.org/package=vegan (2020). * Nichols, J. D. _et al._ Multi-scale occupancy estimation and modelling using multiple detection
methods. _J. Appl. Ecol._ 45, 1321–1329 (2008). Article Google Scholar * Kellner, K. Package ‘jagsUI’: A wrapper around ‘rjags’ to Streamline ‘JAGS’ analyses. R Package Version 1.5.1.
(2019). * R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/ (2021). * Clucas, B. &
Atkins, Z. Using camera traps to survey Humboldt’s flying squirrels in old- and second-growth redwood forests. _Northwest. Nat._ 103(1), 11–19 (2022). Article Google Scholar * Oliver, K.,
Ngoprasert, D. & Savini, T. Assessment of survey protocol for estimates of abundance for elusive nocturnal primates. _Wildl. Res._ 47, 372–380 (2020). Article Google Scholar * Boback,
S. M., Nafus, M. G., Yackel Adams, A. A. & Reed, R. N. Use of visual surveys and radiotelemetry reveals sources of detection bias for a cryptic snake at low densities. _Ecosphere_ 11,
e03000 (2020). Article Google Scholar * Yu, D. W. _et al._ Biodiversity soup: Metabarcoding of arthropods for rapid biodiversity assessment and biomonitoring. _Methods Ecol. Evol._ 3,
613–623 (2012). Article Google Scholar * Suzuki, K. K. & Ando, M. Early and efficient detection of an endangered flying squirrel by arboreal camera trapping. _Mammalia_ 83, 372–378
(2019). Article Google Scholar * Greene, D. U., McCleery, R. A., Wagner, L. M. & Garrison, E. P. A comparison of four survey methods for detecting fox squirrels in the southeastern
United States. _J. Fish Wildl. Manag._ 7, 99–106 (2016). Article Google Scholar * Valentin, R. E., Kyle, K. E., Allen, M. C., Welbourne, D. J. & Lockwood, J. L. The state, transport,
and fate of aboveground terrestrial arthropod eDNA. _Environ. DNA_ 3, 1081–1092 (2021). Article Google Scholar * Barnes, M. A. & Turner, C. R. The ecology of environmental DNA and
implications for conservation genetics. _Conserv. Genet._ 17, 1–17 (2016). Article CAS Google Scholar * Katz, A. D. _et al._ Environmental DNA is effective in detecting the federally
threatened Louisiana Pinesnake (_Pituophis ruthveni_). _Environ. DNA_ 3, 409–425 (2021). Article Google Scholar * Hoyt, J. R., Kilpatrick, A. M. & Langwig, K. E. Ecology and impacts of
white-nose syndrome on bats. _Nat. Rev. Microbiol._ 19, 196–210 (2021). Article CAS Google Scholar * Wieringa, J. G. Comparing predictions of IUCN Red List categories from machine
learning and other methods for bats. _J. Mammal._ https://doi.org/10.1093/jmammal/gyac005 (2022). Article Google Scholar * Wood, S. A. _et al._ A comparison of droplet digital polymerase
chain reaction (PCR), quantitative PCR and metabarcoding for species-specific detection in environmental DNA. _Mol. Ecol. Resour._ 19, 1407–1419 (2019). Article CAS Google Scholar *
Smart, A. S. _et al._ Assessing the cost-efficiency of environmental DNA sampling. _Methods Ecol. Evol._ 7, 1291–1298 (2016). Article Google Scholar * Day, K. _et al._ Development and
validation of an environmental DNA test for the endangered Gouldian finch. _Endanger. Species Res._ 40, 171–182 (2019). Article Google Scholar Download references ACKNOWLEDGEMENTS Primary
funding for this project by ExxonMobil. Funding for bat capture and tracking surveys by the National Park Service Cooperative Research and Training Grant (P20AC00480) and a USDA-NIFA
McIntire-Stennis Capacity Research Project (NJ17385). We especially thank Kathleen Kyle, Kathleen Kerwin, Erin McHale, Morgan Mark, Rebecca Harvey, and a large team of research assistants.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Ecology, Evolution and Natural Resources, Rutgers University, 14 College Farm Road, New Brunswick, NJ, 08901, USA Michael C. Allen, Robert
Kwait, Anthony Vastano, Alex Kisurin, Isabelle Zoccolo, Brooke Maslo & Julie L. Lockwood * ExxonMobil Biomedical Sciences Inc, Annandale, NJ, USA Benjamin D. Jaffe * ExxonMobil Upstream
Research Company, Spring, TX, USA Jordan C. Angle Authors * Michael C. Allen View author publications You can also search for this author inPubMed Google Scholar * Robert Kwait View author
publications You can also search for this author inPubMed Google Scholar * Anthony Vastano View author publications You can also search for this author inPubMed Google Scholar * Alex Kisurin
View author publications You can also search for this author inPubMed Google Scholar * Isabelle Zoccolo View author publications You can also search for this author inPubMed Google Scholar
* Benjamin D. Jaffe View author publications You can also search for this author inPubMed Google Scholar * Jordan C. Angle View author publications You can also search for this author
inPubMed Google Scholar * Brooke Maslo View author publications You can also search for this author inPubMed Google Scholar * Julie L. Lockwood View author publications You can also search
for this author inPubMed Google Scholar CONTRIBUTIONS B.J., B.M., J.A., J.L., A.V., and M.A. planned the study. A.K. and I.Z. conducted field sampling and curated data. R.K., A.V., and I.Z.
performed the lab work. M.A. and R.K. analyzed the data. M.A. drafted the manuscript and all authors contributed substantially to revisions. CORRESPONDING AUTHOR Correspondence to Michael C.
Allen. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1. SUPPLEMENTARY INFORMATION 2. RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third
party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Allen, M.C., Kwait, R.,
Vastano, A. _et al._ Sampling environmental DNA from trees and soil to detect cryptic arboreal mammals. _Sci Rep_ 13, 180 (2023). https://doi.org/10.1038/s41598-023-27512-8 Download citation
* Received: 15 July 2022 * Accepted: 03 January 2023 * Published: 05 January 2023 * DOI: https://doi.org/10.1038/s41598-023-27512-8 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
Trending News
Crescenzi: a scary drop in commercial paperFor a third week, the total amount of commercial paper outstanding plunged, falling a record $94.9 billion in the week e...
ELHERALDO.COEL HERALDO S.A. Prohibida la reproducción y utilización, total o parcial, de los contenidos en cualquier forma o modalid...
Food inflation in sub-saharan africa – analysisInflation is rising around the world. In sub-Saharan Africa, one item is driving the trend more than others: food prices...
Flight secrets: the safest airplane seat revealed – are you booked on?Flight passengers have a variety of options available to them during the booking process for their dream getaway. Aside ...
Narendra modi visits nawaz sharif's home, attends his grand-daughter's wedding* Home * News * Narendra Modi visits Nawaz Sharif’s home, attends his grand-daughter’s wedding INDIA PAKISTAN RELATIONSH...
Latests News
Tzotzil weaver wins mexico’s highest cultural award for her life’s workA weaver from a rural town in Chiapas has a new award in her collection after being named winner of the National Prize i...
Four-day roadtrip through florida for nature loversThe Sunshine State's abundant natural attractions go far beyond beaches, palm trees and the Everglades. Take a four...
Superior metabolic improvement of polycystic ovary syndrome traits after glp1-based multi-agonist therapyABSTRACT Polycystic ovary syndrome (PCOS) is a heterogeneous condition, defined by oligo-/anovulation, hyper-androgenism...
Hancock exposes uk's coronavirus testing nightmare as kits don't workAt yesterday's daily Downing Street briefing, Matt Hancock unveiled a new pledge to reach 100,000 coronavirus tests...
Tips for Booking Hotels, Vacation Rentals and MoreLodging Find expert advice, budget-friendly options, insider tips and the latest trends to make the most of your home aw...