Sirolimus-eluting cobalt–chrome alloy stent suppresses stent-induced tissue hyperplasia in a porcine eustachian tube model
Sirolimus-eluting cobalt–chrome alloy stent suppresses stent-induced tissue hyperplasia in a porcine eustachian tube model"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Various preclinical studies with developed Eustachian tube (ET) stents are in progress but have not yet been clinically applied. ET stent is limited by stent-induced tissue
hyperplasia in preclinical studies. The effectiveness of sirolimus-eluting cobalt–chrome alloy stent (SES) in suppressing stent-induced tissue hyperplasia after stent placement in the
porcine ET model was investigated. Six pigs were divided into two groups (i.e., the control and the SES groups) with three pigs for each group. The control group received an uncoated
cobalt–chrome alloy stent (_n_ = 6), and the SES group received a sirolimus-eluting cobalt–chrome alloy stent (_n_ = 6). All groups were sacrificed 4 weeks after stent placement. Stent
placement was successful in all ETs without procedure-related complications. None of the stents was able to keep its round shape as original, and mucus accumulation was observed inside and
around the stent in both groups. On histologic analysis, the tissue hyperplasia area and the thickness of submucosal fibrosis were significantly lower in the SES group than in the control
group. SES seems to be effective in suppressing stent-induced tissue hyperplasia in porcine ET. However, further investigation was required to verify the optimal stent materials and
antiproliferative drugs. SIMILAR CONTENT BEING VIEWED BY OTHERS TAPERED SELF-EXPANDABLE METALLIC STENT OPTIMIZED FOR EUSTACHIAN TUBE MORPHOLOGY IN A PORCINE ET MODEL Article Open access 24
November 2022 SIROLIMUS-COATED EUSTACHIAN TUBE BALLOON DILATATION FOR TREATING EUSTACHIAN TUBE DYSFUNCTION IN A RAT MODEL Article Open access 16 April 2024 COMPARISON OF THREE KINDS OF
SELF-EXPANDABLE METALLIC STENTS INDUCED GRANULATION TISSUE HYPERPLASIA IN THE RABBIT TRACHEA Article Open access 30 November 2021 INTRODUCTION Eustachian tube (ET) has essential functions in
the middle ear (e.g., ventilation, protection against pathogenic microorganisms, and secretion transport into the nasopharynx)1. Protection from nasopharyngeal sound and reflux is also
included2. ET is usually closed but it opens when swallowing, yawning, or chewing. However, ET dysfunction may occur when the tube does not open or close appropriately3,4. Dilatory
(obstructive) ET dysfunction inhibits ET functions, and acute or chronic otitis media may develop if these functions cannot be preserved, which is one of the most common disorders in
otolaryngology practice5. Current ET dysfunction treatments (e.g., nasal surgery, ventilation tube insertion, and pharmacologic agents) have been applied in patients. However, these
treatments were limited in their effectiveness and may result in ET obstruction, infection, and permanent perforation of the tympanic membrane3,6,7. Balloon Eustachian tuboplasty has been
introduced as an alternative dilatory ET dysfunction treatment8. Some patients fail to respond to dilation although several studies since 2010 have reported that balloon Eustachian
tuboplasty is superior to conventional ET dysfunction treatment8,9,10,11. Thus, stent placement can serve as an effective therapeutic option12,13. Despite many ongoing preclinical studies
that evaluate the technical feasibility and tissue response after stent placement in ET, stent-induced tissue hyperplasia caused by mechanical injury still represents a significant
postprocedural complication14,15,16,17,18,19. Drug-eluting stents loaded with antiproliferative agents are believed to improve this situation. Drug-eluting stents have been used to suppress
in-stent restenosis caused by tissue and neointimal hyperplasia after stent placement20,21,22,23. In general, stent struts or stent-covering membranes are coated with pharmacologic agents
(e.g., everolimus, paclitaxel, and sirolimus)20,23,24. Sirolimus, which is a representative drug for antiproliferation, can inhibit several phases of the restenosis cascade (e.g.,
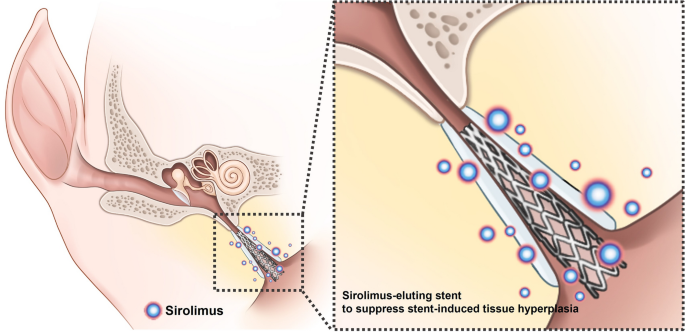
inflammation, neointimal hyperplasia formation, and collagen synthesis)25. Therefore, this study hypothesized that sirolimus-coated stent may prevent stent-induced tissue hyperplasia in the
porcine ET (Fig. 1). This study aims to investigate the effectiveness of sirolimus-eluting cobalt–chrome alloy stent (SES) in suppressing stent-induced tissue hyperplasia after stent
placement in the porcine ET model. METHODS PREPARATION OF SIROLIMUS-ELUTING STENT The cobalt–chrome (Co–Cr) alloy stent was manufactured by laser cutting a Co–Cr alloy tube (Genoss Co.,
Ltd., Suwon, Korea). The stent platform featured open-cell two links with a uniform architecture that permits a high level of flexibility with optimal radial force, shortening, and
conformability. The stent was 3 mm in diameter and 18 mm in length with a strut thickness of 78 µm (Fig. 2a). The Co–Cr alloy stent size was determined based on our previous study19.
Sirolimus was coated on the stent surface using the ultrasonic spray technique. SES is designed to release nearly 70% of its initial drug payload (1.15 µg/mm2) within the first 30 days
following placement. A 3-µm ultrathin coating is applied only to the abluminal side of the stent to attain the desired drug release profile and minimize the polymer amount; this
biodegradable coating comprises a proprietary blend of poly (lactic-co-glycolic acid) and poly (l-lactic acid)26,27. The Co–Cr alloy stent was crimped onto a 3-mm (diameter) and 28-mm
(length) balloon catheter (Genoss Co., Ltd.; Fig. 2b). The stents are commercially available for coronary artery diseases in Korea. METALLIC GUIDING SHEATH A newly developed metallic guiding
sheath for use in the porcine ET model was made of stainless steel (Fig. 2c). This sheath had an inner and outer diameter of 2 and 2.5 mm, respectively, and a total length of 250 mm. The
distal 30 mm of the sheath was curved into a J shape at a 15° angle to the axis to enable easy access from the nose to the nasopharyngeal orifice of the ET in the porcine model. ANIMAL STUDY
DESIGN This study was approved by the Institutional Animal Care and Use Committee of Asan institute for Life Sciences (Seoul, Korea) and conformed to the US National Institutes of Health
guidelines for humane handling of laboratory animals (IACUC-2020-12-189). The study was conducted in compliance with the ARRIVE guidelines. This study used 12 ETs in six pigs weighing
33.8–36.4 kg at 3 months. The six pigs were divided into two groups (i.e., the control and the SES groups) with three pigs in each group. The control group received an uncoated Co–Cr alloy
stent, while the SES group received a sirolimus-eluting Co–Cr alloy stent. All pigs were supplied with water and food ad libitum and were maintained at a temperature of 24 °C ± 2 °C with a
12-h day–night cycle. Subsequently, all pigs were sacrificed 4 weeks after stent placement. STENT PLACEMENT INTO THE PORCINE ET AND ENDOSCOPIC EXAMINATION All pigs were anesthetized
immediately before stent placement using a mixture of 50 mg/kg zolazepam, 50 mg/kg tiletamine (Zoletil 50; Virbac, Carros, France), and 10 mg/kg xylazine (Rompun; Bayer HealthCare,
Leverkusen, Germany). An endotracheal tube was then placed, and anesthesia was administered by inhalation of 0.5–2% isoflurane (Ifran®; Hana Pharm. Co., Seoul, Korea) with 1:1 oxygen (510
mL/kg per min). The pig was in the prone position and baseline endoscopic examination (VISERA 4K UHD Rhinolaryngoscope; Olympus, Tokyo, Japan) was performed to check the nasopharyngeal
orifice of the ET. The metallic guiding sheath was then advanced through the nostril to the nasopharyngeal orifice of the ET under endoscopic guidance (Fig. 3a,b). A balloon catheter, which
was a crimped stent, was inserted through the sheath into the ET until its tip met resistance in the bony cartilaginous isthmus of the ET (Fig. 3c). The balloon catheter was fully inflated
with saline to 9 atm as determined by a pressure gauge monitor (Fig. 3d). The balloon catheter was removed after stent placement (Fig. 3e), and the nasopharyngeal orifice was carefully
evaluated for any procedure-related complications by endoscopy (Fig. 3f). All pigs underwent an endoscopic examination before and immediately after stent placement and 4 weeks after stent
placement to evaluate the stent position with patency and the secretion presence around the stent. HISTOLOGIC EXAMINATION All pigs were sacrificed with 75 mg/kg of potassium chloride via
marginal ear vein injection. The head of the pigs was midsagittally sectioned using an electric saw, and the stented ET tissue samples were then carefully extracted for histologic
examination (Supplementary Fig. 1a,b). The ET tissue samples were fixed in 10% neutral-buffered formalin for 24 h. The ET tissue samples were sequentially dehydrated with alcohols of
different concentrations. Samples were embedded in a resin block by infiltrations with glycol methacrylate (Technovit 7200® VLC; Heraus Kulzer GMBH, Wertheim, Germany). Embedded ET tissue
samples were axially sectioned at the proximal and distal portions (Supplementary Fig. 1c). The resin blocks were then mounted on acrylic slides. Using a griding system (Apparatebau GMBH,
Hamburg, Germany), microgrinding and polishing of the resin block slides were performed with silicon carbide papers of different thicknesses until reaching 20 µm thick. All slides were
stained with hematoxylin and eosin stains for histologic evaluation. Histologic evaluations were performed to assess the tissue hyperplasia percentage, the submucosal fibrosis thickness, and
the inflammatory cell infiltration degree. The percentage of tissue hyperplasia of ET cross-sectional area stenosis was calculated by solving the equation: $$100 \times \left(1 -
\frac{\text{Stenotic} \; \text{area} \; \text{of} \; \text{stent }({\text{mm}}^{2})}{\text{Original} \; \text{area} \; \text{of} \; \text{stent }({\text{mm}}^{2})}\right)$$ The thickness of
submucosal fibrosis was vertically measured from the stent strut to the submucosal layer. The degree of inflammatory cell infiltration was subjectively determined according to the
distribution and density of inflammatory cells, i.e., grade 1 (mild), visible occasional infiltration of single leukocytes; grade 2 (mild-to-moderate), patchy infiltration of leukocytes;
grade 3 (moderate), coalescing leukocytes such that individual loci could not be distinguished; grade 4 (moderate-to-severe) diffuse infiltration of leukocytes throughout the submucosal
layer; and grade 5 (severe), diffuse infiltration with multiple necrotic foci28. The thickness of submucosal fibrosis and degree of inflammatory cell infiltration were obtained by averaging
eight points around the circumference. Histologic analysis of the ET was performed using a microscope (BX51; Olympus, Tokyo, Japan). Measurements were acquired using the CaseViewer software
(CaseViewer; 3D HISTECH Ltd., Budapest, Hungary). The analysis of histological findings was based on the consensus of three observers blinded to the study. STATISTICAL ANALYSIS The
Mann–Whitney _U_ test was used to analyze the differences between the groups as appropriate. A _p_ < 0.05 was considered statistically significant. A Bonferroni-corrected Mann–Whitney
_U_-test was performed for _p_ values < 0.05 to detect group differences (_p_ < 0.008 as statistically significant). Statistical analyses were performed using SPSS software (version
27.0; SPSS, IBM, Chicago, IL, USA). RESULTS TECHNICAL OUTCOMES OF STENT PLACEMENT INTO THE PORCINE ET Stent placement was technically successful in all pigs. Metallic guiding sheaths were
successfully located in the nasopharyngeal orifice of the ET under endoscopic guidance although mucosal injury with touch bleeding was observed in four (33.3%) of the 12 specimens during the
metallic sheath insertion. The touch bleeding was spontaneously resolved at 4 weeks of follow-up. All pigs survived until the end of the study without stent-related complications.
ENDOSCOPIC FINDINGS The endoscopic findings are shown in Fig. 4. The stents were kept in place in all pigs at 4 weeks of follow-up study. Mucus accumulation was observed inside and around
the stented ET in all (100%) ETs in the control group and three (50%) of six ETs in the SES groups, and the incidence rates did not differ between the two groups (_p_ = 0.182). All placed
stents were unable to keep the round shape. HISTOLOGICAL FINDINGS The histological findings are shown in Fig. 5 and Supplementary Fig. 2. The tissue hyperplasia and the submucosal fibrosis
proliferated between the stent strut in the ET lumen in both groups. The mean percentage of tissue hyperplasia area was significantly larger in the control group than in the SES group
(79.48% ± 6.82% vs. 48.36% ± 10.06%, _p_ < 0.001). Moreover, the mean thickness of submucosal fibrosis was also significantly higher in the control group than in the SES group (1.41 ±
0.25 vs. 0.56 ± 0.20 mm, _p_ < 0.001). However, no significant difference in the degree of inflammatory cell infiltration was noted between the two groups (control group [3.50 ± 0.55] vs.
SES group [3.00 ± 0.89], _p_ = 0.270). DISCUSSION Drug-eluting stents have contributed to a high rate of stent patency, inhibiting stent-in-restenosis20,21,22,23,24. Stent-induced stenosis
is caused by the formation of granulation tissue with fibrotic tissue changes in various nonvascular organs, including the esophagus, trachea, gastrodeodenum, and bile duct29,30,31,32,33.
Pharmacologic agents such as dexamethasone, paclitaxel, gemcitabine, EW-7197, and sirolimus are coated onto the surface of stent wire meshes or covering membrane to prevent or treat tissue
hyperplasia after stent placement29,30,34,35,36. More recent stent innovations via multi-functionalized stents using fusion technologies are actively investigated to treat nonvascular
obstructive disorders37,38,39. In a previous study, stent-induced tissue hyperplasia was observed in the porcine ET model. Although stent development in the ET has not been sufficiently
investigated, tissue responses after stent placement similar to those of other nonvascular luminal organs have been observed19. In the present study, SES was used to suppress stent-induced
tissue hyperplasia in the porcine ET model. Sirolimus is toxic to islets and β-cell lines and can reduce cell viability and increase cell apoptosis40,41. Such effects may help suppress
tissue hyperplasia formation by stimulating cell death. In our study, using the drug-eluting stent for the first time in the ET demonstrated to be effective in inhibiting the stent-induced
tissue hyperplasia of the ET. Balloon-expandable Co–Cr alloy stents used in the present study were readily available because it was commonly used in coronary arterial diseases42. Moreover,
Co–Cr alloy has mechanical properties (e.g., high radial strength and nonelastic force)43. According to the endoscopic examination of the current study, Co–Cr alloy stents used for the
porcine ET were unable to keep the round shape in all pigs due to insufficient elasticity without the ability to self-expand. The shape of the inserted stents may also conceivably be altered
by movements surrounding the ET in a living animal (e.g., chewing and swallowing). The mechanical properties of Co–Cr alloy stents have become a disadvantage during stent placement in
porcine ET. In addition, stent placement to the isthmus portion may lead to a permanently open ET. A permanently open ET, or a patulous ET, allows speech and nasopharyngeal sounds, a reflux
from the gastrointestinal tract44, and pathogenic microorganisms1 to ascend into the middle ear, causing mucosal irritation and infections. Thus, the permanent nasopharyngeal opening should
be prevented. Therefore, given the structure of the cartilaginous ET, it would be better if the stent is made of shape-memory alloy with superelastic properties (e.g., nitinol-based alloy).
In general, severe secretion was detected inside and around the stent in the nasopharyngeal orifice. Accumulation of secretions in the stent protruding from the nasopharyngeal orifice is
expected due to the blockage of the normal mucociliary movement of the mucus. Prevention of ascending infections to the middle ear is one of the main ET tasks45, and the placement method in
which the stent protrudes out of the ET should be avoided because the direct stent contact with the bacterial flora in the nasopharynx may lead to increased ascending infection. Balloon
Eustachian tuboplasty via the nasopharyngeal orifice represents a novel, minimally invasive, therapeutic option for ET dysfunction that aims to open and expand the cartilaginous portion of
the ET8,9,10,46. However, the underlying treatment mechanism has not yet been revealed47, and its long-term outcomes can be suboptimal8,9,11,46. In this case, temporary metallic stent
placement can serve as an effective therapeutic option for patients who failed to respond to balloon Eustachian tuboplasty, and the feasibility of stenting in the ET has been verified by
many preclinical studies. To assess in vivo tolerability and degradation profile, poly-l-lactide stents were implanted in chinchillas and rabbits through the tympanic membrane17,18.
Moreover, a sheep model was established to evaluate the in vivo profile of a balloon-expandable metallic stent15. Our previous study developed a porcine ET model to investigate technical
feasibility and assess stent-induced complications19, which laid a solid foundation for this study to examine the efficacy of SES using the previously established technique. In the present
study, SES was successfully located in the cartilaginous portion to effectively suppress tissue hyperplasia. No stent-related complications occurred; however, mucosal injury with touch
bleeding caused by the metallic guiding sheath was observed and resolved spontaneously within 4 weeks. Considering the possible complications from the metallic guiding sheath, improvement in
the SES delivery system is urgent and crucial. This study has some limitations. Although the histological findings reached significant differences between the groups, the number of animals
in this study was too small to perform a robust statistical analysis. Although three observers conducted blinded analyses to evaluate interobserver variability, the degree of submucosal
inflammatory cell infiltration was subjectively determined according to the distribution and density of inflammatory cells, as counting the inflammatory cells was difficult. Because our
study was conducted using a limited number of large animals, a single dose of the drug was used, and in vivo pharmacokinetic study was not performed. Further studies should be performed to
verify the optimal drug dosage and safety of sirolimus for the ET. Finally, the 4-week follow-up period was also a study limitation; thus, a study on the long-term efficacy of SES is needed.
The results of the present study demonstrated that SESs effectively suppressed tissue hyperplasia formation caused by mechanical injury after placing a balloon-expandable Co–Cr alloy stent
in a porcine ET model. Stent-induced tissue hyperplasia-relative variables, including the tissue hyperplasia area and thickness of submucosal fibrosis, were significantly lower at 4 weeks
after stent placement in the SES group than in the control group. SES appears to be effective in suppressing stent-induced tissue hyperplasia in the porcine ET. Although further
investigation was required to verify the optimal stent materials and drug candidate with dosage, SES has localized therapeutic potential in preventing tissue hyperplasia in the ET after
stent placement. REFERENCES * Di Martino, E. F. Eustachian tube function tests: An update. _HNO_ 61, 467–476. https://doi.org/10.1007/s00106-013-2692-5 (2013). Article PubMed Google
Scholar * Adil, E. & Poe, D. What is the full range of medical and surgical treatments available for patients with Eustachian tube dysfunction?. _Curr. Opin. Otolaryngol. Head Neck
Surg._ 22, 8–15. https://doi.org/10.1097/moo.0000000000000020 (2014). Article PubMed Google Scholar * Llewellyn, A. _et al._ Interventions for adult Eustachian tube dysfunction: A
systematic review. _Health Technol. Assess._ 18(1–180), v–vi. https://doi.org/10.3310/hta18460 (2014). Article Google Scholar * Schilder, A. G. _et al._ Eustachian tube dysfunction:
Consensus statement on definition, types, clinical presentation and diagnosis. _Clin. Otolaryngol._ 40, 407–411. https://doi.org/10.1111/coa.12475 (2015). Article CAS PubMed PubMed
Central Google Scholar * Bluestone, C. D. Pathogenesis of otitis media: Role of eustachian tube. _Pediatr. Infect. Dis. J._ 15, 281–291. https://doi.org/10.1097/00006454-199604000-00002
(1996). Article CAS PubMed Google Scholar * McCoul, E. D., Singh, A., Anand, V. K. & Tabaee, A. Balloon dilation of the Eustachian tube in a cadaver model: Technical considerations,
learning curve, and potential barriers. _Laryngoscope_ 122, 718–723. https://doi.org/10.1002/lary.23181 (2012). Article PubMed Google Scholar * Norman, G. _et al._ Systematic review of
the limited evidence base for treatments of Eustachian tube dysfunction: A health technology assessment. _Clin. Otolaryngol._ 39, 6–21. https://doi.org/10.1111/coa.12220 (2014). Article CAS
PubMed Google Scholar * Ockermann, T., Reineke, U., Upile, T., Ebmeyer, J. & Sudhoff, H. H. Balloon dilation Eustachian tuboplasty: A feasibility study. _Otol. Neurotol._ 31,
1100–1103. https://doi.org/10.1097/MAO.0b013e3181e8cc6d (2010). Article PubMed Google Scholar * Randrup, T. S. & Ovesen, T. Balloon Eustachian tuboplasty: A systematic review.
_Otolaryngol. Head Neck Surg._ 152, 383–392. https://doi.org/10.1177/0194599814567105 (2015). Article PubMed Google Scholar * Song, H. Y. _et al._ Fluoroscopic balloon dilation using a
flexible guide wire to treat obstructive Eustachian tube dysfunction. _J. Vasc. Interv. Radiol._ 30, 1562–1566. https://doi.org/10.1016/j.jvir.2019.04.041 (2019). Article PubMed Google
Scholar * Silvola, J., Kivekäs, I. & Poe, D. S. Balloon dilation of the cartilaginous portion of the Eustachian tube. _Otolaryngol. Head Neck. Surg._ 151, 125–130.
https://doi.org/10.1177/0194599814529538 (2014). Article PubMed Google Scholar * Song, H. Y. _et al._ Retrievable covered nitinol stents: Experiences in 108 patients with malignant
esophageal strictures. _J. Vasc. Interv. Radiol._ 13, 285–293. https://doi.org/10.1016/s1051-0443(07)61722-9 (2002). Article PubMed Google Scholar * Song, H. Y. _et al._ Self-expandable
metallic stents in high-risk patients with benign prostatic hyperplasia: Long-term follow-up. _Radiology_ 195, 655–660. https://doi.org/10.1148/radiology.195.3.7538681 (1995). Article CAS
PubMed Google Scholar * Schnabl, J. _et al._ Sheep as a large animal model for middle and inner ear implantable hearing devices: A feasibility study in cadavers. _Otol. Neurotol._ 33,
481–489. https://doi.org/10.1097/MAO.0b013e318248ee3a (2012). Article PubMed Google Scholar * Pohl, F. _et al._ Stenting the Eustachian tube to treat chronic otitis media—A feasibility
study in sheep. _Head Face Med._ 14, 8. https://doi.org/10.1186/s13005-018-0165-5 (2018). Article CAS PubMed PubMed Central Google Scholar * Park, J. H. _et al._ Transnasal placement of
a balloon-expandable metallic stent: Human cadaver study of the Eustachian tube. _J. Vasc. Interv. Radiol._ 29, 1187–1193. https://doi.org/10.1016/j.jvir.2018.03.029 (2018). Article PubMed
Google Scholar * Litner, J. A. _et al._ Tolerability and safety of a poly-l-lactide Eustachian tube stent using a chinchilla animal model. _J. Int. Adv. Otol._ 5, 290–293 (2009). Google
Scholar * Presti, P., Linstrom, C. J., Silverman, C. A. & Litner, J. The poly-l-lactide Eustachian tube stent: Tolerability, safety and resorption in a rabbit model. _J. Int. Adv.
Otol._ 7, 1–3 (2011). Google Scholar * Kim, Y. _et al._ Technical feasibility and histological analysis of balloon-expandable metallic stent placement in a porcine Eustachian tube. _Appl.
Sci._ 11, 1359 (2021). Article CAS Google Scholar * Shin, J. H. _et al._ Tissue hyperplasia: Influence of a paclitaxel-eluting covered stent–preliminary study in a canine urethral model.
_Radiology_ 234, 438–444. https://doi.org/10.1148/radiol.2342040006 (2005). Article PubMed Google Scholar * Shin, J. H. _et al._ Influence of a dexamethasone-eluting covered stent on
tissue reaction: An experimental study in a canine bronchial model. _Eur. Radiol._ 15, 1241–1249. https://doi.org/10.1007/s00330-004-2564-1 (2005). Article PubMed Google Scholar * Kim, E.
Y. _et al._ IN-1233-eluting covered metallic stent to prevent hyperplasia: Experimental study in a rabbit esophageal model. _Radiology_ 267, 396–404. https://doi.org/10.1148/radiol.12120361
(2013). Article PubMed Google Scholar * Bünger, C. M. _et al._ Sirolimus-eluting biodegradable poly-l-lactide stent for peripheral vascular application: A preliminary study in porcine
carotid arteries. _J. Surg. Res._ 139, 77–82. https://doi.org/10.1016/j.jss.2006.07.035 (2007). Article CAS PubMed Google Scholar * Farooq, V. _et al._ Intracoronary optical coherence
tomography and histology of overlapping everolimus-eluting bioresorbable vascular scaffolds in a porcine coronary artery model: The potential implications for clinical practice. _JACC
Cardiovasc. Interv._ 6, 523–532. https://doi.org/10.1016/j.jcin.2012.12.131 (2013). Article PubMed Google Scholar * Kim, K. Y. _et al._ Sirolimus-eluting biodegradable poly-l-lactic acid
stent to suppress granulation tissue formation in the rat urethra. _Radiology_ 286, 140–148. https://doi.org/10.1148/radiol.2017170414 (2018). Article PubMed Google Scholar * Wang, X. _et
al._ Inflammatory cytokines IL-17 and TNF-α up-regulate PD-L1 expression in human prostate and colon cancer cells. _Immunol. Lett._ 184, 7–14. https://doi.org/10.1016/j.imlet.2017.02.006
(2017). Article CAS PubMed PubMed Central Google Scholar * Youn, Y. J. _et al._ Safety and efficacy of a new ultrathin sirolimus-eluting stent with abluminal biodegradable polymer in
real-world practice. _Korean Circ. J._ 50, 317–327. https://doi.org/10.4070/kcj.2019.0258 (2020). Article PubMed Google Scholar * Park, J. H. _et al._ Balloon-expandable biodegradable
stents versus self-expandable metallic stents: A comparison study of stent-induced tissue hyperplasia in the rat urethra. _Cardiovasc. Interv. Radiol._ 42, 1343–1351.
https://doi.org/10.1007/s00270-019-02239-0 (2019). Article Google Scholar * Lee, J. H. _et al._ Comparison of the utility of covered metal stents versus uncovered metal stents in the
management of malignant biliary strictures in 749 patients. _Gastrointest. Endosc._ 78, 312–324. https://doi.org/10.1016/j.gie.2013.02.032 (2013). Article PubMed ADS Google Scholar *
Knyrim, K., Wagner, H. J., Bethge, N., Keymling, M. & Vakil, N. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. _N.
Engl. J. Med._ 329, 1302–1307. https://doi.org/10.1056/nejm199310283291803 (1993). Article CAS PubMed Google Scholar * Chung, F. T. _et al._ Factors leading to tracheobronchial
self-expandable metallic stent fracture. _J. Thorac. Cardiovasc. Surg._ 136, 1328–1335. https://doi.org/10.1016/j.jtcvs.2008.05.039 (2008). Article PubMed Google Scholar * No, J. H. _et
al._ Long-term outcome of palliative therapy for gastric outlet obstruction caused by unresectable gastric cancer in patients with good performance status: Endoscopic stenting versus
surgery. _Gastrointest. Endosc._ 78, 55–62. https://doi.org/10.1016/j.gie.2013.01.041 (2013). Article PubMed ADS Google Scholar * van Hooft, J. E. _et al._ Safety and efficacy of a new
non-foreshortening nitinol stent in malignant gastric outlet obstruction (DUONITI study): A prospective, multicenter study. _Endoscopy_ 43, 671–675. https://doi.org/10.1055/s-0030-1256383
(2011). Article PubMed Google Scholar * Moon, S., Yang, S. G. & Na, K. An acetylated polysaccharide-PTFE membrane-covered stent for the delivery of gemcitabine for treatment of
gastrointestinal cancer and related stenosis. _Biomaterials_ 32, 3603–3610. https://doi.org/10.1016/j.biomaterials.2011.01.070 (2011). Article CAS PubMed Google Scholar * Liu, X., De
Scheerder, I. & Desmet, W. Dexamethasone-eluting stent: An anti-inflammatory approach to inhibit coronary restenosis. _Expert Rev. Cardiovasc. Ther._ 2, 653–660.
https://doi.org/10.1586/14779072.2.5.653 (2004). Article CAS PubMed Google Scholar * Tsauo, J. _et al._ EW-7197, an oral transforming growth factor β type I receptor kinase inhibitor,
for preventing peritoneal adhesion formation in a rat model. _Surgery_ 164, 1100–1108. https://doi.org/10.1016/j.surg.2018.07.005 (2018). Article PubMed Google Scholar * Park, J. H. _et
al._ Local heat treatment for suppressing gastroduodenal stent-induced tissue hyperplasia using nanofunctionalized self-expandable metallic stent in rat gastric outlet model. _ACS Biomater.
Sci. Eng._ 6, 2450–2458. https://doi.org/10.1021/acsbiomaterials.0c00307 (2020). Article CAS PubMed Google Scholar * Park, W. _et al._ Metallic stent mesh coated with silver
nanoparticles suppresses stent-induced tissue hyperplasia and biliary sludge in the rabbit extrahepatic bile duct. _Pharmaceutics_ 12, 563. https://doi.org/10.3390/pharmaceutics12060563
(2020). Article CAS PubMed Central Google Scholar * Cho, Y. C. _et al._ Photothermal therapy via a gold nanoparticle-coated stent for treating stent-induced granulation tissue formation
in the rat esophagus. _Sci. Rep._ 11, 10558. https://doi.org/10.1038/s41598-021-90182-x (2021). Article CAS PubMed PubMed Central ADS Google Scholar * Bell, E. _et al._ Rapamycin has a
deleterious effect on MIN-6 cells and rat and human islets. _Diabetes_ 52, 2731–2739. https://doi.org/10.2337/diabetes.52.11.2731 (2003). Article CAS PubMed Google Scholar * Sarkar, S.,
Ravikumar, B., Floto, R. A. & Rubinsztein, D. C. Rapamycin and mTOR-independent autophagy inducers ameliorate toxicity of polyglutamine-expanded huntingtin and related proteinopathies.
_Cell Death Differ._ 16, 46–56. https://doi.org/10.1038/cdd.2008.110 (2009). Article CAS PubMed Google Scholar * Mori, H. _et al._ Very Late pathological responses to cobalt-chromium
everolimus-eluting, stainless steel sirolimus-eluting, and cobalt-chromium bare metal stents in humans. _J. Am. Heart Assoc._ 6, e007244. https://doi.org/10.1161/jaha.117.007244 (2017).
Article PubMed PubMed Central Google Scholar * Dyet, J. F., Watts, W. G., Ettles, D. F. & Nicholson, A. A. Mechanical properties of metallic stents: How do these properties influence
the choice of stent for specific lesions?. _Cardiovasc. Interv. Radiol._ 23, 47–54. https://doi.org/10.1007/s002709910007 (2000). Article CAS Google Scholar * Sone, M., Kato, T. &
Nakashima, T. Current concepts of otitis media in adults as a reflux-related disease. _Otol. Neurotol._ 34, 1013–1017. https://doi.org/10.1097/MAO.0b013e318299aa52 (2013). Article PubMed
Google Scholar * Pau, H. W. Eustachian tube and middle ear mechanics. _HNO_ 59, 953–963. https://doi.org/10.1007/s00106-011-2368-y (2011). Article CAS PubMed Google Scholar * Williams,
B., Taylor, B. A., Clifton, N. & Bance, M. Balloon dilation of the Eustachian tube: A tympanometric outcomes analysis. _J. Otolaryngol. Head Neck Surg._ 45, 13.
https://doi.org/10.1186/s40463-016-0126-6 (2016). Article PubMed PubMed Central Google Scholar * McCoul, E. D. & Anand, V. K. Eustachian tube balloon dilation surgery. _Int. Forum
Allergy Rhinol._ 2, 191–198. https://doi.org/10.1002/alr.21007 (2012). Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS This study was supported by a National Research
Foundation of Korea (NRF) grant funded by the Korean government (MSIT; Ministry of Science, ICT; no. 2020R1F1A1049412). AUTHOR INFORMATION Author notes * These authors contributed equally:
Jeon Min Kang and Song Hee Kim. AUTHORS AND AFFILIATIONS * Biomedical Engineering Research Center, Asan Institute for Life Sciences, Asan Medical Center, 88 Olympic-ro 43-gil, Songpa-gu,
Seoul, 05505, Republic of Korea Jeon Min Kang, Song Hee Kim, Yubeen Park, Dae Sung Ryu & Jung-Hoon Park * Department of Otorhinolaryngology-Head and Neck Surgery, Asan Medical Center,
University of Ulsan College of Medicine, 88 Olympic-ro 43-gil, Songpa-gu, Seoul, 05505, Republic of Korea Yeon Joo Choi, Woo Seok Kang & Hong Ju Park Authors * Jeon Min Kang View author
publications You can also search for this author inPubMed Google Scholar * Song Hee Kim View author publications You can also search for this author inPubMed Google Scholar * Yeon Joo Choi
View author publications You can also search for this author inPubMed Google Scholar * Yubeen Park View author publications You can also search for this author inPubMed Google Scholar * Dae
Sung Ryu View author publications You can also search for this author inPubMed Google Scholar * Woo Seok Kang View author publications You can also search for this author inPubMed Google
Scholar * Jung-Hoon Park View author publications You can also search for this author inPubMed Google Scholar * Hong Ju Park View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS J.-H.P. and H.J.P. conceptualized the project and designed the experiments. J.M.K., S.H.K., Y.J.C., Y.P., D.S.R., and W.S.K. conducted the experimental
work. J.M.K., S.H.K., Y.J.C, Y.P., D.S.R., and J.-H.P. collected and analysed the data and prepared the manuscript. J.M.K., S.H.K., W.S.K., J.-H.P., and H.J.P. wrote the manuscript. The
final manuscript was reviewed and approved by all authors. CORRESPONDING AUTHORS Correspondence to Jung-Hoon Park or Hong Ju Park. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare
no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless
indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory
regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kang, J.M., Kim, S.H., Choi, Y.J. _et al._ Sirolimus-eluting cobalt–chrome alloy
stent suppresses stent-induced tissue hyperplasia in a porcine Eustachian tube model. _Sci Rep_ 12, 3436 (2022). https://doi.org/10.1038/s41598-022-07471-2 Download citation * Received: 29
October 2021 * Accepted: 11 February 2022 * Published: 02 March 2022 * DOI: https://doi.org/10.1038/s41598-022-07471-2 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
Trending News
How tragic photographer masahisa fukase examined himself through picturing others...In 1992, just before his 60th birthday, Hokkaido-born photographer Masahisa Fukase was leaving his favourite bar when he...
Sulfonated poly(aryl ether ketone) random copolymers having crosslinking structure for proton exchange membrane of fuel cellABSTRACT We have succeeded in developing a new methodology for proton exchange membrane (PEM) of direct methanol fuel ce...
This desi dad’s reaction to his daughter hiring people from matrimonial sites is just hilarious - scoopwhoopThis post on Twitter can be best described as an onion. So many layers! A super cute dad, a boss lady with extreme baddi...
Fitch upgrades pakistan’s sovereign rating to ccc after imf dealFitch upgrades Pakistan’s sovereign rating to CCC after IMF deal | WTVB | 1590 AM · 95.5 FM | The Voice of Branch County...
Georgia’s presidential primary: biden and sanders on housingNext week, Georgia voters finally will get to choose their preferred candidate for the Democratic presidential nominatio...
Latests News
Sirolimus-eluting cobalt–chrome alloy stent suppresses stent-induced tissue hyperplasia in a porcine eustachian tube modelABSTRACT Various preclinical studies with developed Eustachian tube (ET) stents are in progress but have not yet been cl...
Countywide : board boosts fees to drill, deepen wellsVentura County supervisors approved without comment Tuesday a new water-well ordinance that substantially increases coun...
My mother passed away. A cousin thinks she’s entitled to an inheritance | members onlyLet’s start with the fact that you’re in the process of grieving and, understandably, in an emotionally fragile state. C...
China rages at 'provocative' uk visit as mps travel to taiwanMPs from the Foreign Affairs Committee are visiting Taiwan, which China claims as its territory, until Saturday and meet...
Impound dangerous stray dogs, cull rabid and diseased ones: kerala hc's guideline to govtGovernment and local bodies can impound all stray dogs posing danger to the public and cull the ones that are rabid and ...