Leaching and solvent extraction purification of zinc from mehdiabad complex oxide ore
Leaching and solvent extraction purification of zinc from mehdiabad complex oxide ore"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT An integrated hydrometallurgical process was used for the zinc leaching and purification from a zinc ore containing 9.75 wt% zinc. The zinc minerals in the ore were hemimorphite,
willemite, and calcophanite. Main gangue minerals were quartz, goethite, hematite, and calcite. Central composite design (CCD) method was used to design leaching experiments and the optimum
conditions were found as follows: 30% of solid fraction, 22.05% sulphuric acid concentration, and the leaching temperature of 45 °C. The PLS containing 35.07 g/L zinc, 3.16 g/L iron, and
4.58 g/L manganese impurities was produced. A special purification process including Fe precipitation and Zn solvent extraction was implemented. The results showed that after precipitation
of iron, Zn extraction of 88.5% was obtained with the 2 stages extraction system composed of 30 vol% D2EHPA as extractant. The overall Zn recovery from the ore was 71.44%. Therefore, an
appropriate solution containing 16.6 g/L Zn, 0.05 g/L Fe, and 0.11 g/L Mn was prepared for the electro-winning unit without using the roasting and calcination steps (conventional method),
which result in environmental pollution. SIMILAR CONTENT BEING VIEWED BY OTHERS STEPWISE EXTRACTION OF ZINC, INDIUM AND LEAD FROM SECONDARY ZINC OXIDE DUSTS EXPERIMENTAL STUDY Article Open
access 03 December 2024 CONTROLLABLE MECHANISM OF HAZARDOUS JAROSITE TRANSFORMATION INTO RECYCLABLE HEMATITE IN THE LEACHING SOLUTION OF SECONDARY ZINC OXIDE POWDER Article Open access 18
October 2024 PRODUCTION OF SYNTHETIC RUTILE FROM TIN ORE BENEFICIATION BYPRODUCT THROUGH PREOXIDATION AND REDUCTIVE LEACHING IN HYDROCHLORIC ACID Article Open access 31 May 2022 INTRODUCTION
The importance of zinc oxide ores processing such as carbonate and silicates minerals have increased because of the depletion of zinc sulphide ores and the restriction on sulphur emissions
during their processing1,2,3,4,5,6. The zinc oxide ores usually contain several different minerals including zincite (ZnO), smithsonite (ZnCO3), hydrozincite (2ZnCO3·3Zn(OH)), hemimorphite
(Zn4Si2O7(OH)2·H2O), and willemite (Zn2SiO4)2. Producing a concentrate from zinc oxide ores can be performed using flotation or gravity methods7. Afterwards, hydrometallurgical or
pyrometallurgical routes are used for processing of the concentrate8. Using of pyrometallurgical routes is not suitable for processing of the low grade oxide ore due to environmental
pollution, high capital investment, and significant energy consumption9. The main impurities associated with zinc oxide ores are calcium, magnesium, and silicon dioxide carbonates6. In
acidic leaching, presence of soluble silicon dioxide in the oxide ore causes the formation of silica gel in the solution6. Different methods including using flocculent chemicals10,11,
improvement of SiO2 precipitation condition12, SiO2 precipitation from colloid solutions with pH adjustment13, quick leaching14, using of microwave irradiation to quick leach1,15 have been
successfully used and it was proved that the direct leaching methods are better than conventional roasting-leaching methods. A number of research studies have been conducted on the alkaline
leaching of zinc oxide ores. Frenay16 found that alkaline media is better than acidic media. The optimum alkaline leaching conditions of 5% NaOH, temperature 90 to 95 °C in 90 min was
obtained for leaching zinc from an oxide ore17. The column leaching of low grade zinc oxide ore containing 5.2% zinc using ammonium sulphate was carried out and 92.2% of zinc was dissolved6.
Chen et al.8 discovered that when the zinc oxide ores of 65–76 µm size were leached for 120 min at 358 K in the presence of 5 mol/L sodium hydroxide and liquid/solid 10:1, the leaching
recovery of zinc was more than 73%. The main advantages of alkaline leaching are lower iron dissolution and lack of silica gel formation16. However, this method does not have the ability for
dissolving of hemimorphite mineral8. The Skorpion zinc processing method demonstrated the viability of the production of zinc from zinc oxide ores, using hydrometallurgical route including
the leaching, solvent extraction, and electro-winning process18,19. However, it is difficult to separate iron from organic solution in the stripping process and SiO2 can be precipitated
using CaCO38. Therefore, if the solvent extraction method is used to purify the pregnant leaching solution (PLS), the iron should be removed using a suitable method before the solvent
extraction stage. In hydrometallurgical processes, iron can be removed from the leaching solution in the form of hematite, goethite and various types of Jarosite20,21,22. The obtained PLS in
the leaching of oxide ores contains a number of impurities such as iron, Mn, Mg, Ca, SiO2, etc. Presence of iron in electrolyte can reduce zinc production and reducing current efficiency in
the electro-wining unit23. Presence of excess levels of manganese in the electrowinning process can significantly decrease the current efficiency. Therefore, it has to be removed before
entering the electrowinning process24. The maximum limit of Fe and Mn impurities to enter in the zinc electro-wining unit is 5 and 2000 mg/L, respectively25. Solvent extraction process is a
common way for selective separation or concentration of different metal ions. Purification and concentration of zinc using SX, by different organic solutions and extractants has been
reported26,27,28,29. Extraction mechanisms of zinc using D2EHPA in an aliphatic diluent can be written as follow30: $${Zn}^{2+}+2{(R-H)}_{org}\leftrightarrow {(Zn{R}_{2})}_{org}+2{H}^{+}$$
(1) The (RH) is organic agent which forms organic-metal ZnR2 molecule using H+ ion. In the Eq. (1), it was assumed that the reaction takes place at organic/aqueous phases interface and water
was not entered into organic phase. The zinc stripping reaction can be written as follow18: $${(Zn{R}_{2})}_{org}+2{H}^{+}\leftrightarrow 2{(RH)}_{org}+{Zn}^{2+}$$ (2) The present
investigation is focused on zinc leaching from a zinc ore followed by iron precipitation and Zn solvent extraction and stripping. The optimum conditions for the zinc oxide leaching, solvent
extraction, and stripping were determined. It should be noted that these processes alone are not novel, but the integration of these processes for the zinc oxide ores is considered as the
novelty of the current research study. MATERIALS AND METHODS SAMPLE PREPARATION AND CHARACTERIZATION A 1000 kg zinc oxide ore from the Mehdiabad mine (Yazd Province, Iran) was prepared and
it was divided into two equal portions after crushing to 1-inch size using a laboratory jaw crusher and homogenization. One portion was crushed to − 2 mm particles using jaw and roller
crushers. The sample was riffled to prepare representative sub-samples by using a Jones Riffler. Representative samples were milled by using a laboratory ball mill and prepared for leaching
experiments. A representative sub-sample was prepared for characterization. The elemental composition of the solution and the ore containing zinc, iron, and manganese was measured using
atomic absorption spectroscopy (AAS), a Varian Spectra AA 10. Other components were analysed by x-ray fluorescence (XRF) spectroscopy technique using a PW1480 instrument (Philips Company).
In the XRF method, samples were ground into a fine powder (< 75 μm), mixed with a binding aid and pressed to produce homogeneous sample pellets. Si and Al elements in the solution and ore
were analysed by inductively coupled plasma-optical emission spectrometry (ICP-OES, 730-ES Varian) method. For the case of Si and Al in solid samples, alkaline fusion using lithium borate
and leaching with nitric acid were performed before ICP analysis. The ore sample were analysed by Bruker D5000 x-ray powder diffraction (XRD) system and optical mineralogy by the thin as
well as polished sections. In addition, scanning electron microscope (SEM) was used to investigate smithsonite (carbonate), hemimorphite (silicate), and calcophanite (oxide) minerals. ZINC
LEACHING EXPERIMENTS The leaching tests were conducted in a stirred-tank reactor by H2SO4 and de-ionized water as leaching solution with agitation rate of 500 rpm, pH of less than 2 and
particle size (d80) of 300 µm5. In each test, the amount of 100 g ore was added into the reactor and temperature was set at the desired temperature, then the leaching was performed for 2 h.
The pH of solution was measured every 30 min. At the end of each test, the pulp was filtered, and the residue was washed a number of times by water at high temperature, then, it was dried at
temperature of 105 °C. Combination of the pregnant leach solution (PLS) and wash solutions was used for the determination of zinc, iron, and manganese. All leaching samples were analysed to
determine the leaching efficiency in %, as shown in Eq. (3)31: $$R=\frac{V\times {C}_{s}}{F\times {C}_{f}}\times 100$$ (3) where V is the PLS volume (L), Cs is metal concentration in the
PLS (mg/L), F is the feed weight (t), and Cf is the grade of metal in the ore (mg/t). NEUTRALIZATION AND IMPURITIES PRECIPITATION EXPERIMENTS The maximum Fe and Mn impurities concentration
in the solution entering the electrowinning unit need to be 0.005 and 0.2 g/L, respectively. Therefore, it is required to conduct neutralization and impurities precipitation experiments to
reduce impurities concentration in the solution. In order to conduct the iron precipitation stage, lime milk was added to the leaching pulp to increase pH value from about 2 to 4–4.5 for 2 h
at 50 °C. SOLVENT EXTRACTION AND STRIPPING Aqueous solution containing zinc, iron, and manganese for the solvent extraction process was obtained from leaching experiments. The organic phase
was prepared by dissolving determined amounts of D2EHPA as extractant in kerosene as diluent. The effect of initial pH (2, 2.5, 3), vol/vol percent of extractant to diluent (10%, 20%, 30%),
aqueous solution to organic (A:O) phase ratio (0.5, 1, and 2) at constant temperature, mixing time of 50 min, and agitation rate = 250 rpm were investigated. The stripping process was
performed using a solution containing 220 g/L H2SO4 with the A:O phase ratio of 1 at pH 1. RESULTS AND DISCUSSION MINERALOGY THE ORE ELEMENTAL COMPOSITION The ore elemental composition was
given in Table 1. It can be observed that the ore contains 9.75% Zn, 20.77% Fe, 20.3% SiO2, 5.65% Mn, and 5.1% Al2O3. MINERALOGICAL STUDIES The XRD results showed that the dominant minerals
in the ore are quartz (SiO2), hematite (Fe2O3), goethite (FeO(OH)), willemite (Zn2SiO4), hemimorphite (Zn4Si2O7(OH)2·H2O), dolomite (CaMg(CO3)2), cryptomelane (K(Na)Mn8O16), and calcite
(CaCO3). In order to investigation of highly important minerals and their association with the gangue minerals, the polished and thin sections were studied. It was observed that metallic
minerals in the polished sections are iron minerals mainly as hematite and goethite, manganese-zinc minerals mainly as calcophanite and hetaerolite (ZnMn2O4), manganese mineral as
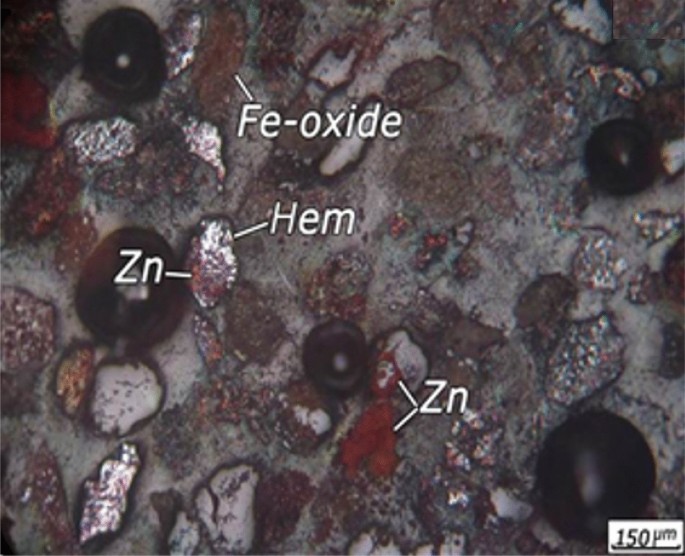
pyrplozite, and zinc minerals mainly as willemite and hemimorphite. An optical microscopic image from + 90 µm size fraction is shown in Fig. 1. It is evident from Fig. 1 that zinc minerals
are mainly associated with iron oxides and manganese minerals. The willemite and hemimorphite minerals were not visible in the sections and zinc zap solution was used for the identification
of these minerals. Zinc non-sulphide minerals were become red and identifiable after using the zinc zap solution. Iron minerals are usually in the form of free crystalline phases (Fig. 2b),
associated with carbonate minerals (Fig. 2a) manganese oxides, and manganese-zinc oxide minerals. The iron oxide and hydroxide minerals is observed in red colour and in the form boxwork
texture in Fig. 2. The iron oxides veinlets are visible between carbonate minerals (Fig. 2). MINERALS DEGREE OF FREEDOM Minerals degree of freedom in a size fraction was defined as the ratio
of a valuable mineral in the form of free particles to total area of the valuable mineral in the section. Table 2 shows the degree of freedom for different size fractions. It can be
observed that the zinc minerals are significantly associated with other minerals. It should be mentioned that it is not necessary to get all valuable mineral free from gangue minerals in the
hydrometallurgical processes. For a valuable mineral to be leached, the contact of leaching solution with part of the mineral is enough. Therefore, leaching experiments are usually
performed on different sizes to select the optimal size so that the target metal is selectively leached from other metals. The zinc minerals association with other minerals was investigated
using SEM technique. The results showed that hemimorphite and smithsonite minerals are mostly close to each other. These minerals association with iron oxide minerals can be seen in Fig. 3.
The calcophanite mineral was observed in different grain sizes including small and large grains. Small grains had higher association with other minerals and the grains were locked with iron
minerals. However, large grains are mostly free. The large grains are divided into two groups including pure calcophanite large grains and grains containing high amount of iron. MINERALS
COMPOSITION The ore mineral composition was approximately determined based on XRF, XRD, microscopic image, and SEM. The minerals composition of the ore is presented in Table 3. Based on the
Table 3, 28.37 to 30.41%, 46.70 to 46.97%, 22.62 to 24.93% of the zinc are in the calcophanite, hemimorphite, and smithsonite minerals, respectively. DIRECT LEACHING OF THE ORE The solid
fraction (20%, 30%, and 40%), H2SO4 concentration (12%, 18%, and 24%), and temperature (45, 60, and 75 °C) were considered as variable parameters and the particles size, leaching time, and
agitation rate was kept constant in all leaching experiments. A central composite design (CCD) of experiments was carried out by Demo version of Design-Expert software version 7.0.0
(STAT-EASE Inc., Minneapolis, USA)32 in order to evaluate the operating parameters influence on the system performance. The preliminary experiments were conducted to find the range of
changes in variable parameters. THE INFLUENCE OF SOLID FRACTION Effect of increasing in the solid fraction on the zinc leaching efficiency is presented in Fig. 4a. It was observed that the
zinc leaching efficiency decreases from 82.22% to 69.5% with increasing the solid fraction from 30% (S/L = 3/7) to 40% (S/L = 2/3) at acid concentration of 18%. Tiechui et al.33 reached
maximum Zn extraction of 85.69% at S/L = 1/12 (g/L) using ammonium hydroxide and ammonium chloride agents. Hua et al.15 investigated the quick H2SO4 leaching of a Zn silicate ore at a
constant S/L = 1/10 in the presence of microwave irradiation and reached the maximum Zn extraction of 99.08%. Rao et al.34 studied the influence of S/L in the range of 1/10 to 1/40 g/mL and
found that decreasing the ratio from 1/10 to 1/40 g/mL increased the leaching efficiency of zinc by 10%. It could be concluded that the decrease of S/L increases the mole ratio of the
leaching agent to the target mineral and increases the leaching efficiency of zinc. It could be emphasized that in the aforementioned cases, high Zn leaching efficiency is obtained in the
condition of small solid to liquid ratio. _THE INFLUENCE OF H_ _2_ _SO_ _4_ _ CONCENTRATION_ Figure 4b presents the influence of H2SO4 concentration on the zinc leaching efficiency. It was
observed that the enhancement of acid concentration increased the zinc leaching efficiency. Similar results by11 have shown that increasing the acid concentration to 4.5 M increased the zinc
leaching efficiency from hemimorphite and smithsonite up to 95%. However, it should be noted that dissolution of impurities including iron and manganese was also increased with too much
increasing of acid concentration. Therefore, the acid concentration plays a key role in the leaching of zinc from oxide ores. The minimum and maximum zinc leaching efficiencies were obtained
72.18% and 79.84% at 12 wt% and 24 wt% acid concentrations (35% solid fraction), respectively.
$${Zn}_{4}{Si}_{2}{O}_{7}{(OH)}_{2}.{H}_{2}O+4{H}_{2}{SO}_{4}=4ZnS{O}_{4}+{{Si}_{2}O(OH)}_{6}+3{H}_{2}O$$ (4) $${ZnCO}_{3}+{H}_{2}{SO}_{4}=ZnS{O}_{4}+{H}_{2}O+{CO}_{2(g)}$$ (5) In the
reactions (4) and (5), ZnSO4 is soluble in water solution. But, Si2O(OH)6, known as disilicic acid is polymerized to polysilicic acid and produces a gelatinous phase in acidic medium. This
gelatinous phase can reduce the zinc leaching efficiency in addition to causing the filtration problem by creating an inactive layer on the zinc mineral particles35. As mentioned in the
“Minerals composition”, hemimorphite mineral contains 46.70 to 46.97% of the total zinc in the ore. According to Table 3, if the hemimorphite mineral is completely dissolved, 1.99 to 2.24%
of the total SiO2 in the ore enters the solution. As shown in Table 5, SiO2 leaching efficiency was 4.7%. Grinding the ore to d80 = 300 micron (80% finer than 300 micron) and leaching at pH
around 2 could be at least part of the reason why SiO2 leaching efficiency is minimum. It has been reported that maintaining the pH between 1.8 and 2, maximises the stability of colloidal
silica18. INFLUENCE OF TEMPERATURE The operating temperature for the zinc leaching efficiency was selected in the range of 45 to 75 °C. Preliminary results showed considerable reduction in
the zinc leaching efficiency at temperatures less than 45 °C. Souza et al.36, showed that the leaching efficiency of hemimorphite increased with increasing of temperature from 30 to 60 °C.
Abdel-Aal37 showed that the enhancement of the temperature from 40 to 70 °C, the Zn leaching efficiency increased from 70 to about 95%. Previous work conducted by the authors showed that
increasing the temperature above 45 °C, increases the leaching efficiency of impurities5. Therefore, during the leaching experiments, the temperature was set at 45 °C. OPTIMIZATION OF
LEACHING EXPERIMENTS The suggested experiment conditions by the software was shown in Table 4. The zinc and impurities concentrations in the PLS and their leaching efficiencies were given in
Table 5. Maximum Zn leaching efficiency reached 83.81% at 30% solid, 22.05wt% sulphuric acid concentration, 45 °C, leaching time = 2 h as well as particle size of 300 µm (d80). In this
condition, the leaching efficiency of Fe, Si and Al was 3.55%, 4.7%, and 4.2%, respectively. Mn leaching efficiency was 18.9% and considerable amount of Mn entered the PLS. The main reason
for the low leaching efficiency of Fe, Si, Al, and Mn elements is the larger particle size of the leaching feed (d80 = 300 µm). In addition, the leaching temperature was set at 45 °C to
minimize the leaching efficiency of impurities. The effect of particle size and temperature have been studied in another study by the authors5. IMPURITIES PRECIPITATION The obtained PLS from
the optimum leaching experiment requires to be prepared for electro-wining unit by removing impurities that could unselectively be extracted from the solution by D2EHPA. Therefore, firstly,
the precipitation process was used to remove or decrease impurities from solution. The lime milk was added to the pulp to increase the pH of the PLS from less than 2 to about 4–4.5.
Increasing the pH of the PLS could precipitate Fe(III) and metal ions as their hydroxide form. Although the precipitate produced by hydroxide precipitation method is more difficult to filter
than precipitate produced in hematite, goethite and jarosite methods, but its implementation on an industrial scale has advantages such as lower temperature, less residual iron in solution
(less than 0.1 g/L) and stronger ability to absorb cations and anions present in the solution. It can be observed from Table 6 that 99.45% Fe, 18.12% Mn, 3.09% Zn, 90% Al, and 75% Si were
precipitated, respectively. As shown in Fig. 5, Iron and aluminium precipitation occurs at lower pH values in comparison to Mn and Zn. Therefore, it should be pointed out that the
precipitation process is remarkably effective to remove Fe impurity from the PLS while there is still significant amount of manganese in the solution. Therefore, another purification process
needs to be used to remove manganese impurity from the solution. ZINC SOLVENT EXTRACTION A number of solvent extraction experiments at different operating conditions were designed using
Design-Expert 7.0 software32. The influence of different operating conditions including pH (2, 2.5, and 3), O/A phase ratio (0.5, 1, and 2), and the extractant concentration (10, 20, and
30%) on the Zn extraction from aqueous solution was given in Fig. 6. By increasing the pH from 2 to 2.5, Zn extraction increased and further increasing the pH had no considerable effect on
the zinc extraction (Fig. 6a). The reason for the decrease in zinc extraction at pH values below 2.5 is the protonation of D2EHPA39. In fact, as pH decreases, reaction 1 proceeds from right
to left and H+ competes with zinc to react with organic matter. As shown in Fig. 6b, Zn extraction enhanced from 48.1 to 51.35% with increasing D2EHPA concentration from 10 to 30%. In
addition, by increasing the organic to aqueous volume ratio from 0.5 to 2 at 20% extractant concentration, ambient temperature, and pH 2.5, zinc extraction increased from 49 to 50.8% (Fig.
6c). The maximum extraction of 52.92% was obtained under optimum operating conditions. SOLVENT EXTRACTION EXPERIMENTS AT OPTIMUM CONDITION The amount of zinc recovery was obtained 52.66% at
optimum conditions. The iron and manganese recovery was found to be 66.52% and, 2.63%. As it can be seen, the iron extraction is also high. The D2EHPA extracted both zinc and iron at pH 2.5.
However, this problem was solved in the stripping process because iron was remained in the organic phase. The extraction isotherm was determined with combination of the neutralized solution
and the organic phase at different organic phase to aqueous solution ratios from 0.2 to 5, initial pH 2.5 and 30% vol./vol. D2EHPA concentration. The extraction distribution isotherms of
zinc using different O:A phase ratios is presented in Fig. 7. From Fig. 7, it was found that 2 the theoretical extraction stages are required, when organic phase to aqueous solution ratio is
2. Increasing the organic phase to aqueous solution ratio can increase the extraction concentration in the system and unnecessary enhancement of its concentration with reduce its absorption
ability. Therefore, O:A ratio of 2 was selected as the best option. To verify this, A batch-continuous zinc extraction test at two stages was performed and the results are provided in Table
8. As presented in Fig. 7 and Table 7, the obtained results for the two-stage SX case show the good agreement with the predicted results using McCabe–Thiele plot. The amount of 90.91% zinc
extraction was found using 30% D2EHPA concentration at two stages solvent extraction. 94.11% iron and 7.61% manganese were also extracted (Table 7), indicating the selectivity of the
extraction process to manganese impurity. It has been reported that the order of D2EHPA selectivity is18: * Fe(III) < Zinc < Calcium < Al(III) < Manganese < Cd ~ Copper <
Magnesium < Cobalt < Nickel. STRIPPING OF ZINC The aim of this section was to evaluate the optimum operating conditions for the stripping of zinc from the organic solution containing
zinc. The zinc and impurities concentration for obtained electrolyte from the stripping process at operating conditions of A:O = 1, pH 1, and 220 g/L sulphuric acid concentration was
demonstrated in Table 8. The concentration of impurities is lower than the maximum limit. Therefore, it is suitable for electro-wining unit. In addition, it was observed that the H2SO4 is an
appropriate strip solution for the selective separation of zinc with 93% recovery from organic solution. Final PLS must contain 50–60 g/L of zinc and no more than 100–150 g/L of sulphuric
acid. The solution obtained in the present work after re-extraction had a zinc concentration of only 16.6 g/L (Table 8) which is not suitable for electrowinning process. Therefore, it is
necessary to conduct the experiments on the extraction and re-extraction of zinc to obtain a solution with a higher concentration of zinc and a lower concentration of sulphuric acid.
CONCLUSION An integrated hydrometallurgical process for Zn leaching and solvent extraction purification from a complex zinc oxide ore was suggested. Mineralogical analyses showed that the
ore contains 9.75 w.% zinc and impurities of iron (20.77 wt%) and manganese (5.65 wt%). The Maximum Zn leaching efficiency reached 83.81% at 30% solid fraction, leaching temperature of 45
°C, acid concentration of 22.05%, leaching time of 2 h, and particles size of 300 µm. One interesting result of the present work is the obtaining of pregnant leaching solution (PLS) with a
high concentration of Zn (35.07 g/L) and a relatively low concentration of Fe (3.16 g/L). The PLS was purified from iron and the loss of zinc in the Fe (III) hydroxide precipitate was low
(only 3%). Afterwards, 90.91% of Zn was selectively extracted over manganese by solvent extraction. An electrolyte solution with the 16.6 g/L zinc concentration was obtained in the stripping
process. Presence of impurities in the solution were in the range of acceptable level for entering electro-winning unit (16.6 g/L Zn, 0.05 g/L Fe, and 0.11 g/L Mn). The overall Zn recovery
from the ore was 71.44% (83.81% Zn leaching efficiency, 3.09% loss in Fe precipitation process, and 88.5% extraction-stripping efficiency). Results showed that the whole process can
successfully be applied to the zinc oxide ores containing considerable amounts of Fe and Mn impurities. REFERENCES * Yang, K. _et al._ Microwave roasting and leaching of an oxide-sulphide
zinc ore. _Hydrometallurgy_ 166, 243–251. https://doi.org/10.1016/j.hydromet.2016.07.012 (2016). Article CAS Google Scholar * Kashani, A. N. & Rashchi, F. Separation of oxidized zinc
minerals from tailings: Influence of flotation reagents. _Miner. Eng._ 21, 967–972 (2008). Article Google Scholar * Yang, K. _et al._ Role of sodium citrate in leaching of low-grade and
multiphase zinc oxide ore in ammonia–ammonium sulfate solution. _Hydrometallurgy_ 169, 534–541. https://doi.org/10.1016/j.hydromet.2017.03.013 (2017). Article CAS Google Scholar * Yang,
T. _et al._ Leaching of low grade zinc oxide ores in nitrilotriacetic acid solutions. _Hydrometallurgy_ 161, 107–111. https://doi.org/10.1016/j.hydromet.2016.01.024 (2016). Article CAS
Google Scholar * Soltani, F. _et al._ Direct leaching of low-grade zinc oxide ore containing high amounts of iron and manganese. _Trans. Indian Inst. Met._ 72, 1371–1380 (2019). Article
CAS Google Scholar * Feng, L., Yang, X., Shen, Q., Xu, M. & Jin, B. Pelletizing and alkaline leaching of powdery low grade zinc oxide ores. _Hydrometallurgy_ 89, 305–310 (2007).
Article CAS Google Scholar * Bulatovic, S. M. _Handbook of Flotation Reagents: Flotation of Gold, PGM and Oxide Minerals_ (Elsevier Science, Amsterdam, 2010). Google Scholar * Chen, A.
_et al._ Alkaline leaching Zn and its concomitant metals from refractory hemimorphite zinc oxide ore. _Hydrometallurgy_ 97, 228–232 (2009). Article CAS Google Scholar * Choi, W., Torma,
A., Ohline, R. & Ghali, E. Electrochemical aspects of zinc sulphide leaching by Thiobacillus ferrooxidans. _Hydrometallurgy_ 33, 137–152 (1993). Article CAS Google Scholar * Perry, W.
Refining zinc silicate ore by special leaching technique. _Chem. Eng._ 73, 182–184 (1966). CAS Google Scholar * Bodas, M. Hydrometallurgical treatment of zinc silicate ore from Thailand.
_Hydrometallurgy_ 40, 37–49 (1996). Article CAS Google Scholar * Ikenobu, S. Method for processing siliceous zinc ores. _Lead-Zinc_ 2000, 427–436 (2000). Google Scholar * Matthew, I. G.
& Elsner, D. The hydrometallurgical treatment of zinc silicate ores. _Metall. Trans. B_ 8, 73–83 (1977). Article Google Scholar * Dufresne, R. E. Quick leaching of siliceous zinc ore.
_J. Metals_ 28, 8–12 (1976). CAS Google Scholar * Hua, Y., Lin, Z. & Yan, Z. Application of microwave irradiation to quick leach of zinc silicate ore. _Miner. Eng._ 15, 451–456 (2002).
Article CAS Google Scholar * Frenay, J. Leaching of oxidized zinc ores in various media. _Hydrometallurgy_ 15, 243–253 (1985). Article CAS Google Scholar * Zhao, Y. & Stanforth,
R. Production of Zn powder by alkaline treatment of smithsonite Zn–Pb ores. _Hydrometallurgy_ 56, 237–249 (2000). Article CAS Google Scholar * Gnoinski, J. Skorpion Zinc: optimization and
innovation. _J. S. Afr. Inst. Min. Metall._ 107, 657–662 (2007). CAS Google Scholar * De Wet, J. & Singleton, J. Development of a viable process for the recovery of zinc from oxide
ores. _J. S. Afr. Inst. Min. Metall._ 108, 253–259 (2008). Google Scholar * Salinas, E., Roca, A., Cruells, M., Patiño, F. & Córdoba, D. J. H. Characterization and alkaline
decomposition–cyanidation kinetics of industrial ammonium jarosite in NaOH media. _Hydrometallurgy_ 60, 237–246 (2001). Article CAS Google Scholar * Rodríguez, E. S. _et al._ Kinetics of
alkaline decomposition and cyaniding of Argentian rubidium jarosite in NaOH medium. _Metall. Mater. Trans. B_ 43, 1027–1033 (2012). Article Google Scholar * Perez-Labra, M. _et al._
Synthesis, thermodynamic, and kinetics of rubidium jarosite decomposition in calcium hydroxide solutions. _Metall. Mater. Trans. B_ 43, 773–780 (2012). Article CAS Google Scholar * Adhia,
J. _Effect and Control of Impurities in Electrolytic Zinc Production_ (Springer, New York, 1969). Google Scholar * Zhang, W. & Cheng, C. Y. Manganese metallurgy review. Part III:
Manganese control in zinc and copper electrolytes. _Hydrometallurgy_ 89, 178–188. https://doi.org/10.1016/j.hydromet.2007.08.011 (2007). Article CAS Google Scholar * Cole, P. M. &
Sole, K. C. Zinc solvent extraction in the process industries. _Miner. Process. Extr. Metall. Rev._ 24, 91–137 (2003). Article CAS Google Scholar * Balesini-Aghdam, A.-A., Yoozbashizadeh,
H. & Moghaddam, J. Simple separation method of Zn(II) and Cd(II) from brine solution of zinc plant residue and synthetic chloride media using solvent extraction. _Chin. J. Chem. Eng._
28, 1055–1061. https://doi.org/10.1016/j.cjche.2019.12.003 (2020). Article Google Scholar * Shah, K., Gupta, K. & Sengupta, B. Selective separation of copper and zinc from spent
chloride brass pickle liquors using solvent extraction and metal recovery by precipitation-stripping. _J. Envir. Chem. Eng._ 5, 5260–5269. https://doi.org/10.1016/j.jece.2017.09.061 (2017).
Article CAS Google Scholar * Mądrzak-Litwa, I. & Borowiak-Resterna, A. Solvent extraction of zinc from chloride solutions using dialkyl derivatives of 2,2′-bibenzimidazole as
extractants. _Hydrometallurgy_ 182, 8–20. https://doi.org/10.1016/j.hydromet.2018.10.005 (2018). Article CAS Google Scholar * Jafari, H., Abdollahi, H., Gharabaghi, M. & Balesini, A.
A. Solvent extraction of zinc from synthetic Zn-Cd-Mn chloride solution using D2EHPA: Optimization and thermodynamic studies. _Sep. Purif. Technol._ 197, 210–219.
https://doi.org/10.1016/j.seppur.2018.01.020 (2018). Article CAS Google Scholar * Martï, F. Extending zinc production possibilities through solvent extraction. _J. S. Afr. Inst. Min.
Metall._ 102, 463–467 (2002). Google Scholar * Banihashemi, S. R., Taheri, B., Razavian, S. M. & Soltani, F. Selective nitric acid leaching of rare-earth elements from calcium and
phosphate in fluorapatite concentrate. _JOM_ 71, 4578–4587. https://doi.org/10.1007/s11837-019-03605-6 (2019). Article ADS CAS Google Scholar * Inc., S.-E. _Design-Expert version 7.0.0_,
www.statease.com/software/design-expert/ (2007). * Tiechui, Y., Qinyuan, C. & Jie, L. Effects of mechanical activation on physicochemical properties and alkaline leaching of
hemimorphite. _Hydrometallurgy_ 104, 136–141. https://doi.org/10.1016/j.hydromet.2010.05.008 (2010). Article CAS Google Scholar * Rao, S. _et al._ Leaching of low grade zinc oxide ores in
NH4Cl–NH3 solutions with nitrilotriacetic acid as complexing agents. _Hydrometallurgy_ 158, 101–106. https://doi.org/10.1016/j.hydromet.2015.10.013 (2015). Article CAS Google Scholar *
Safari, V., Arzpeyma, G., Rashchi, F. & Mostoufi, N. A shrinking particle—shrinking core model for leaching of a zinc ore containing silica. _Int. J. Miner. Process._ 93, 79–83.
https://doi.org/10.1016/j.minpro.2009.06.003 (2009). Article CAS Google Scholar * Souza, A. D., Pina, P. S., Lima, E. V. O., da Silva, C. A. & Leão, V. A. Kinetics of sulphuric acid
leaching of a zinc silicate calcine. _Hydrometallurgy_ 89, 337–345. https://doi.org/10.1016/j.hydromet.2007.08.005 (2007). Article CAS Google Scholar * Abdel-Aal, E. A. Kinetics of
sulfuric acid leaching of low-grade zinc silicate ore. _Hydrometallurgy_ 55, 247–254. https://doi.org/10.1016/S0304-386X(00)00059-1 (2000). Article CAS Google Scholar * Monhemius, A. J.
Precipitation diagrams for metal hydroxides, sulphides, arsenates and phosphates. _J-GLOBAL_ 68, 202–206 (1977). Google Scholar * Asadi, T., Azizi, A., Lee, J.-C. & Jahani, M. Solvent
extraction of zinc from sulphate leaching solution of a sulphide-oxide sample using D2EHPA and Cyanex 272. _J. Dispers. Sci. Technol._ 39, 1328–1334.
https://doi.org/10.1080/01932691.2017.1402338 (2018). Article CAS Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Mining Engineering Department, Arak
University of Technology, Arak, Iran Faraz Soltani * Mining Engineering Department, Tarbiat Modares University, Tehran, Iran Hossna Darabi * Academic Center for Education, Culture and
Research (ACECR) On TMU, Tehran, Iran Reza Aram * Institute of Research and Development, Duy Tan University, Da Nang, 550000, Viet Nam Mahdi Ghadiri * The Faculty of Environment and Chemical
Engineering, Duy Tan University, Da Nang, 550000, Viet Nam Mahdi Ghadiri Authors * Faraz Soltani View author publications You can also search for this author inPubMed Google Scholar *
Hossna Darabi View author publications You can also search for this author inPubMed Google Scholar * Reza Aram View author publications You can also search for this author inPubMed Google
Scholar * Mahdi Ghadiri View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.D. and F.S: Conceptualization, Methodology, Experimental, Formal
analysis, Writing, Original draft preparation R.A.: Conceptualization, Resources, Reviewing and Editing, Data curation M.G.: Conceptualization, Resources, Reviewing and Editing.
CORRESPONDING AUTHOR Correspondence to Mahdi Ghadiri. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are
included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons
licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of
this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Soltani, F., Darabi, H., Aram, R. _et al._ Leaching and solvent
extraction purification of zinc from Mehdiabad complex oxide ore. _Sci Rep_ 11, 1566 (2021). https://doi.org/10.1038/s41598-021-81141-7 Download citation * Received: 01 September 2020 *
Accepted: 04 January 2021 * Published: 15 January 2021 * DOI: https://doi.org/10.1038/s41598-021-81141-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Disney and Universal Sue Midjourney for AI Copyright Infringement in Major Hollywood Legal Challenge – channelnewsDisney and NBCUniversal have filed a joint federal lawsuit against AI image generator Midjourney, alleging widespread co...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
Oreo Maker Mondelēz Sues Aldi US Over Alleged Copycat Packaging of Famous Snack Brands – channelnewsMondelēz International, the corporation behind Oreo cookies, has filed a lawsuit against discount supermarket chain Aldi...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
Latests News
Leaching and solvent extraction purification of zinc from mehdiabad complex oxide oreABSTRACT An integrated hydrometallurgical process was used for the zinc leaching and purification from a zinc ore contai...
Stereochemistry in the disorder–order continuum of protein interactionsABSTRACT Intrinsically disordered proteins can bind via the formation of highly disordered protein complexes without the...
Page not found - HW News EnglishNationalGermany Calls Indian Students Amid US CrackdownNews DeskJune 10, 2025June 10, 2025040The German Embassy in India...
Crashes and pollution prompt calls to close L.A. airport - Los Angeles TimesEva Avalos, far right, whose Pacoima home she shares with her daughter Margarita Lopez and grandson Ethan Vasquez, says ...
Big area calls for big tank - farmers weekly13 MARCH 1998 ------------------------- BIG AREA CALLS FOR BIG TANK Switching to a 160hp forward control sprayer has mea...