Usefulness of selective arterial calcium injection tests for functional pancreatic neuroendocrine tumors
Usefulness of selective arterial calcium injection tests for functional pancreatic neuroendocrine tumors"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The selective arterial calcium injection (SACI) test is useful for patients with functional pancreatic neuroendocrine tumors (F-PNETs). This study evaluated which patients with
F-PNETs would benefit from the SACI test. We retrospectively analyzed the preoperative findings of patients on computed tomography (CT), magnetic resonance imaging (MRI), CT angiography
(CTA), and the SACI test. Fourteen patients who underwent pancreatectomy between January 1997 and September 2016 for F-PNETs were evaluated. We classified these patients into groups A, B,
and C; group A, one tumor detected by either CT or MRI; group B, multiple tumors detected; and group C, the tumor location was accordant on CT, MRI, and CTA, but the SACI test revealed
another tumor. In group A, the tumor was also detected by CTA and the SACI test was positive on calcium injection. In group B, the focus tumor among the multiple tumors was detected by the
SACI test. In group C, another tumor was identified by the SACI test, whose location was different from that detected using CT and MRI. The SACI test is more useful for multiple F-PNETs on
CT or MRI. If CT or MRI detects a single tumor, the SACI test or CTA may be unnecessary. SIMILAR CONTENT BEING VIEWED BY OTHERS USEFULNESS OF ENDOSCOPIC ULTRASONOGRAPHY FOR DIFFERENTIATING
BETWEEN NON-FUNCTIONAL PANCREATIC NEUROENDOCRINE NEOPLASM AND INTRAPANCREATIC ACCESSORY SPLEEN Article Open access 21 February 2025 ADULT PANCREATOBLASTOMA: CLINICAL FEATURES AND IMAGING
FINDINGS Article Open access 09 July 2020 IMAGING FEATURES OF INTRADUCTAL TUBULOPAPILLARY NEOPLASM OF THE PANCREAS AND ITS DIFFERENTIATION FROM CONVENTIONAL PANCREATIC DUCTAL ADENOCARCINOMA
Article Open access 16 September 2022 INTRODUCTION The use of the selective arterial calcium injection (SACI) test has resulted in the increased performance of curative resection surgery for
patients with functional pancreatic neuroendocrine tumors (F-PNETs), such as insulinomas and gastrinomas1,2. In 1991, Doppman et al.2 reported a method of localizing insulinomas using 0.025
mEq/kg calcium gluconate as the secretagogue. Moreover, the SACI test has a high diagnostic performance for the accurate localization of F-PNETs for curative resection when F-PNETs such as
insulinomas and gastrinomas are usually small, solitary, and intrapancreatic3,4,5. Other imaging studies, such as computed tomography (CT), magnetic resonance imaging (MRI), and CT
angiography (CTA), can also be used to visualize endocrine tumors as hypervascular tumors surrounding pancreatoduodenal lesions. Although the specificity and sensitivity of these methods are
both less than 70–80% for pancreatic tumors6, CT is the initial modality of choice to evaluate pancreatic lesions while MRI has a major role in pancreatic tumor characterization7. In
contrast, compared to CT or MRI, the SACI test and CTA are relatively invasive examinations for patients with F-PNETs; therefore, these invasive examinations should not be performed if
possible. Moreover, it is unclear which patients with F-PNETs would benefit from the SACI test, and only a few studies have determined the value of the SACI test compared to preoperative
findings of CT, MRI, and CTA5,8. Therefore, in this study, we aimed to investigate the preoperative findings of the SACI test, CT, MRI, and CTA, and to determine the type of patients with
F-PNETs in whom the SACI test would be very effective. PATIENTS AND METHODS PATIENTS Patients who underwent the SACI test and curative pancreatectomy for F-PNETs at our institution between
January 1997 and September 2016 were retrospectively reviewed. All patients were histologically confirmed as having F-PNETs such as insulinomas or gastrinomas. Along with the SACI test, all
patients underwent other preoperative tumor localization procedures such as dynamic CT, dynamic MRI, and CTA. The results of the SACI test were compared with the findings on these
procedures, as well as the surgical and pathological results. We conducted this retrospective study using the “opt-out” method of our hospital. The study was approved by the Ethics Committee
of Keio University School of Medicine, which waived the need of informed consent for our study, and conducted in accordance with the Helsinki Declaration of 1975. THE SACI TEST Before
angiography, a 5-Fr shepherd hook catheter (Hanako Medical, Terumo, Japan) was placed in the right or middle hepatic vein through the right femoral vein. In addition, a 5-Fr catheter was
placed in the celiac artery and superior mesenteric artery [SMA] through the right femoral artery followed by insertion of a microcatheter (Progreat ∑, Terumo, Japan) into an artery feeding
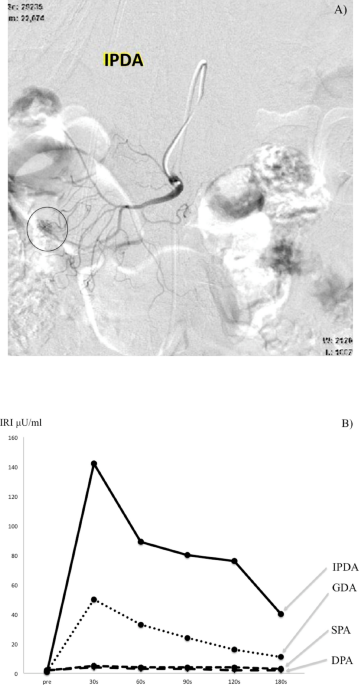
the pancreas (e.g., the common hepatic artery [CHA], gastroduodenal artery [GDA], splenic artery [SPA], distal pancreatic artery [DPA], or inferior pancreaticoduodenal artery [IPDA]).
Calcium gluconate at a dose of 0.025 mEq/kg was diluted to a 4 mL bolus, and injected rapidly into each artery. Blood samples for insulin or gastrin were obtained from the right hepatic vein
at 30, 60, 90, 120, and 150 or 180 s after the calcium injection. The results were considered positive for an insulinoma if the maximum increase in serum levels of immunoreactive insulin
(IRI) was twice at 30–60 s compared to the basal serum IRI level2. In contrast, for gastrinomas, the results were considered positive when serum levels of immunoreactive gastrin (IRG) were
> 80 pg/ml at 40 s after the calcium injection9, and IRG was > 20% above the basal serum IRG at 40 s after the calcium injection9. When a positive response was observed in the SPA and
DPA, the tumor was considered to be localized to the body or tail of the pancreas; when it was identified in the CHA, SMA, GDA, and IPDA, the tumor was considered localized to the pancreatic
head (Fig. 1). SURGICAL RESECTION AND PATHOLOGY Surgical procedures included enucleation, partial pancreatectomy, laparoscopic or open distal pancreatectomy, and pancreaticoduodenectomy.
Pathological staging was performed according to the Union for International Cancer Control Tumor-Node-Metastasis (UICC-TNM) Classification of Malignant Tumors (8th edition), and tumors were
classified as PanNET G1, PanNET G2, PanNET G3, and PanNEC (G3) according to the 4th edition of the WHO Classification of Tumors of Endocrine Organs. Immunostaining was performed for
chromogranin A, synaptophysin, CD56, insulin, gastrin, somatostatin, glucagon, and pancreatic polypeptide. Moreover, pancreatic neuroendocrine microadenomas, which are benign non-functioning
neuroendocrine tumors < 0.5 cm with a low proliferation rate, were also counted. POSTOPERATIVE ASSESSMENT AND FOLLOW-UP We evaluated the postoperative symptoms and the IRI or IRG levels,
and all the patients were followed-up at 1, 3, 6, and 12 months after surgery. Patients also underwent semiannual reviews. In addition, clinical examinations, laboratory investigations, and
abdominal computed tomography (to detect tumor recurrence) were performed. INFORMED CONSENT STATEMENT Patients were not required to give informed consent to the study because the analysis
used anonymous clinical data that were obtained after each patient agreed to treatment by written consent. This statement was approved by the Human Experimentation Committee of Keio
University School of Medicine. RESULTS DEMOGRAPHIC AND PERIOPERATIVE CHARACTERISTICS A total of 14 patients underwent the SACI test and curative resection for F-PNETs between January 1997
and September 2016. The patient characteristics are summarized in Table 1. There were 7 men and 7 women, with a median age of 55 years (range, 18–75 years). The most common clinical
presentations were hypoglycemia (n = 11, 78.6%), followed by melena (n = 1, 7.1%) and elevation in glycated hemoglobin levels (n = 1, 7.1%); 1 patient (7.1%) was asymptomatic. The diagnosis
was insulinoma in 11 patients (78.6%) and gastrinoma in 3 (21.4%). Five patients (35.7%) underwent open or laparoscopic distal pancreatectomy, 4 (35.7%) underwent partial pancreatectomy, 4
(35.7%) underwent open or laparoscopic enucleation, and 1 (7.1%) underwent pancreaticoduodenectomy. Three patients (no. 3, 4, and 7; 21.4%) were diagnosed with multiple endocrine neoplasia
type 1 (MEN-I). After surgery, all patients showed no symptoms, and the levels of IRI and IRG improved. Patient no. 4 had bone metastasis as well as metastases in the lymph nodes of the
aorta; the patient died approximately 5 years after surgery. The results of preoperative imaging studies in 14 patients (dynamic CT, MRI, CTA, and the SACI test) are shown in Table 2. The
detection rate of F-PNET was 78.6% for CT, 91.7% for MRI, 100% for CT and MRI, and 100% for CTA. We classified these patients into groups A, B, and C; group A, one tumor detected by either
CT or MRI; group B, multiple tumors detected; and group C, the tumor location was accordant on CT, MRI, and CTA, but the SACI test revealed another tumor. In group A consisting of patient
no. 1, 2, 5, 6, 8, 9, 10, 11, 12, and 13, the tumor was also detected by CTA and the SACI test was positive on calcium injection. In group B consisting of patient no. 3, 4, and 7, the focus
tumor among the multiple tumors was detected by the SACI test. Moreover, all patients in group B had MEN-I. In group C consisting of patient no. 14, another tumor was identified by the SACI
test, whose location was different from that detected using CT, MRI, and CTA. PATHOLOGICAL FINDINGS The pathological findings of the tumors are shown in Table 3. The UICC stage was 1A in 10
patients (71.4%) and 1B in 4 (28.6%), and PanNET G1 was detected in 9 patients (64.3%) and PanNET G2 in 5 (35.7%). Patients in group B had a tendency of having pancreatic neuroendocrine
microadenomas. DISCUSSION The study findings demonstrated that the SACI test should be performed when multiple tumors are detected on CT or MRI with inheritable characteristics such as
MEN-I; this will help in localizing the causative tumor among multiple tumors as well as in deciding the indications for the extent of surgery to resect the tumors in the pancreas. However,
the SACI test or CTA should not be performed if a single tumor is detected via CT and MRI because the SACI test is relatively invasive compared to CT or MRI. Nevertheless, we need to be
careful about false-positive results or occult tumors, such as those observed in no. 14 in which the tumor location revealed on the SACI test differed from that detected on CT and MRI.
According to international guidelines such as the European Neuroendocrine Tumor Society (ENETS)10, the North American Neuroendocrine Tumor Society (NANETS)11 and the National Comprehensive
Cancer Network (NCCN)12, the SACI tests should be considered in patients with F-PNETs when other imaging tests, such as CT or MRI, are equivocal or negative. Furthermore, most experts
recommend this test only for patients with persistent or recurrent insulin-mediated hypoglycemia. According to the ENETS guideline10, the SACI tests should be performed in patients with
multiple lesions or MEN-I, and excision should be performed based on the results of the SACI tests. Our results were consistent with the ENETS guideline, but not other guidelines. Therefore,
this study supported the adaptation of the SACI tests mentioned in the ENETS consensus guidelines. The SACI test is a method for the identification and differentiation of insulinomas or
gastrinomas among multiple NETs that are essentially impossible to identify on imaging techniques alone, and it is useful for planning the surgical strategy preoperatively3,4,5. In patient
no. 3 and 7 in group B, the SACI test revealed accurate localization among multiple NETs, and we performed the surgical procedure according to the result of the SACI test. However, in no. 4
in group B, although the SACI test revealed the tumor in the pancreatic head, we performed distal pancreatectomy and not pancreaticoduodenectomy. Although we planned pancreaticoduodenectomy
and enucleation for the tumor in the pancreatic body in no. 4 preoperatively, there was a main pancreatic duct injury while performing enucleation. We could not perform total pancreatectomy
per the patient’s wishes, so we had no choice but to perform distal pancreatectomy. However, fortunately, the symptoms of hypercalcemia and postoperative IRI improved after surgery;
therefore, we suspected that the tumor in patient no. 4 was in the pancreatic body, and that the SACI test did not always reveal the correct localization. In fact, Doppman et al.2 found that
elevated hepatic venous insulin levels were not always correlated with the correct localization: unequivocal localization was reported in only 5 of 9 patients (56%) but a gradient in venous
insulin levels was observed in all 9 patients. EUS plays a specific role in PNETs, but the necessity of EUS is a little different according to the international guidelines from ENETS10,
NANETS11, and NCCN12. These guidelines recommended that EUS can be used as appropriate if there are equivocal CT or MRI findings, or if the tumors were not detected by them. Furthermore, EUS
is recommended if pancreatic resection is considered for assessing and localizing tumors preoperatively. The NANETS guideline11 suggested that EUS should be performed when there is a
question of multifocality, such as in patients with MEN1, and EUS can be useful when some F-PNETs are too small to be imaged with CT or MRI. EUS-FNA can also be considered to confirm the
diagnosis and tumor grade, although tumor heterogeneity may preclude accurate assessment; thus, EUS or EUS-FNA can also be considered as additional imaging tools instead of CT or MRI in the
above specific situations11. Recently various studies10,11,12,13,14 have reported that positron emission tomography with CT (PET/CT) with 68 Ga-labeled somatostatin analogues (68 Ga-PET/CT)
has the highest sensitivity for localizing not also NF-PNETs but also FP-NETs, and is also a cornerstone tool for both diagnosis and staging for PNETs. The sensitivity varies from 86–100%,
and the specificity from 79–100% for all p-NETs. Thus, the SACI test, which is relatively invasive, could be omitted in patients with F-PNETs, especially those with gastrinomas. Furthermore,
international guidelines10,11,12 have recommended 68 Ga-PET/CT as the first-line diagnostic imaging method for staging in patients with gastrinoma. However, 68 Ga-PET/CT is not useful for
insulinoma. Shorma P et al. revealed that its sensitivity is only 25%11. Although the adaptation and usefulness of the SACI test has been limited because other non-invasive functional
imaging techniques, such as 68 Ga-PET/CT, are being used instead to determine surgical planning, staging, and medical management, the SACI tests for insulinoma may still useful under special
conditions for patients with FP-NETs (especially insulinoma) with multiple lesions or patients with MEN-I where CT or MRI are equivocal or negative. If F-PNETs are not detected on
preoperative imaging studies, i.e. if they are “occult tumors,” blind distal, subtotal, or total pancreatectomy is now obsolete. These blind pancreatic resections are no longer indicated and
should not be performed because of their short-term (e.g., pancreatitis, pancreatic fistula, and pancreatic abscess) and long-term morbidities (e.g., exocrine pancreatic insufficiency and
diabetes mellitus)6,15. Indications for occult insulinomas and gastrinomas have been reported in ENTES or NCCN guidelines10,12. First, for occult insulinoma, observation and surgical
exploration are options for occult tumors. Few studies have advocate intraoperative localization through palpation, and the addition of intraoperative ultrasound may be more sensitive than
invasive preoperative methods1,16,17. Second, for gastrinomas, exploratory surgery including duodenotomy or local resection/enucleation of tumors, periduodenal node dissection, and
intraoperative ultrasound have been considered in a previous report12. In patient no. 14 in group C, the tumor location revealed on the SACI test was different from the location observed on
CT, MRI, and CTA. Based on the result of the SACI test, the tumor might have been located in the pancreatic head although the tumor was not detected on CT, MRI, and CTA; therefore, the tumor
might have been an occult tumor. Another alternative could be that the result of the SACI test might have been a false-positive result and that there was no tumor there. After we informed
the patient of these two possibilities, we performed distal pancreatectomy, instead of blind pancreaticoduodenectomy, considering the postoperative surgical complications. Although we
performed intraoperative ultrasonography for the pancreatic head, a tumor was not detected. In addition, during the semiannual clinical examinations, laboratory investigations, and abdominal
computed tomography, a new tumor was not detected in the remnant pancreas and the IRG level decrease to 170 pg/ml. Therefore, in this case, we suspect that the result of the SACI test might
be a false-positive result and that there was no tumor at that location. According to the NCCN guidelines12, we may consider exploratory surgery including intraoperative ultrasound in the
future for patient #14 in our study if the patient’s IRG level continue to increase. We suggest that the SACI test and CTA do not need to be performed if a single tumor is detected on CT and
MRI. The preoperative detection rates of CT and MRI for PNETs depended on the patient age and type of study7,18,19, although the development in the technology of scanners has resulted in a
marked improvement in the sensitivity of CT and MRI6,7. In this study, the detection rate of F-PNETs was 100% for a combination of dynamic CT and MRI, and if a single tumor was observed, the
SACI test was not necessary for checking the localization of the single tumor to administer the calcium injection because we should not perform blind pancreatectomy for occult PNETs. CTA,
an invasive localization modality, is considered the “gold standard” for tumor detection, and the detection rates varied from 29 to 100% depending on the experience of the operator and the
center6,9,20,21. In our institution, CTA has often been performed for not only F-PNETs but also NF-PNETs, and as shown in Table 2, CTA may be useful to detect multiple small tumors with
hereditary disease, such as MEN-I. This study has a few limitations. First, this was a retrospective study conducted at a single institution with a relatively small number of patients.
However, as the number of patients with F-PNETs is small and most previous studies were case series22,23,24, a multi-center study with many patients is needed to further evaluate the
usefulness of the SACI test. Second, the time span of this study (from 1997 and 2016) is very long considering the technical improvements of both CT and MRI. In this study, two patients
underwent surgery in 1997 and 1999, and 12 patients had undergone pancreatic surgery since 2007, in which multi-detector row CT or dynamic MRI had a mechanical efficacy similar to the recent
CT or MRI technology. Although we considered excluding these two patients, there are only a few patients that undergo the SACI tests and this study already had a small number of patients;
thus, we included these two patients and the time span became long. Finally, among 14 patients, 8 patients (57.1%) underwent EUS10,11,12,25, 4 (28.6%) underwent 18F-FDG PET/CT26, and 2
(14.3%) underwent somatostatin receptor scintigraphy (SRS)27. We have been performing EUS on patients with pancreatic neuroendocrine tumors (PNETs) since 2007, and now we perform EUS more
actively in most patients with PNETs, future prospective research studies are needed to confirm and evaluate these preliminary findings, by including EUS, 18F-FDG PET/CT, and 68 Ga-PET/CT,
all of which are useful to evaluate the location of PNETs. In conclusion, the SACI test should be performed for F-PNETS with multiple tumors detected on CT or MRI, especially for those with
inheritable characteristics such as MEN-I. This will help in determining the tumor localization among multiple tumors and deciding the appropriate surgical method for the treatment of the
multiple tumors in the pancreas. Moreover, the SACI test or CTA should not be performed if a single tumor is detected via CT and MRI, because we should not perform blind pancreatectomy
against occult F-PNETs. REFERENCES * Imamura, M. _et al._ Usefulness of selective arterial secretin injection test for localization of gastrinoma in the Zollinger-Ellison syndrome. _Ann.
Surg._ 205, 230–239 (1987). Article CAS Google Scholar * Doppman, J. L. _et al._ Insulinomas: localization with selective intraarterial injection of calcium. _Radiology_ 178, 237–241
(1991). Article CAS Google Scholar * Imamura, M. _et al._ Biochemically curative surgery for gastrinoma in multiple endocrine neoplasia type 1 patients. _World J Gastroenterol._ 17,
1343–1353 (2011). Article Google Scholar * Tonelli, F. _et al._ Pancreatectomy in multiple endocrine neoplasia type 1-related gastrinomas and pancreatic endocrine neoplasias. _Ann. Surg._
244, 61–70 (2006). Article Google Scholar * Goh, B. K. _et al._ Accurate preoperative localization of insulinomas avoids the need for blind resection and reoperation: analysis of a single
institution experience with 17 surgically treated tumors over 19 years. _J. Gastrointest. Surg._ 13, 1071–1077 (2009). Article Google Scholar * Nikfarjam, M. _et al._ Improved contemporary
surgical management of insulinomas A 25-year experience at the Massachusetts General Hospital. _Ann. Surg._ 247, 165–172 (2008). Article Google Scholar * Canellas, R., Lo, G., Bhowmik,
S., Ferrone, C. & Sahani, D. Pancreatic neuroendocrine tumor: correlations between MRI features, tumor biology, and clinical outcome after surgery. _J. Magn. Reson. Imaging._ 47, 425–432
(2018). Article Google Scholar * Sung, Y. M. _et al._ Selective intra-arterial calcium stimulation with hepatic venous sampling for preoperative localization of insulinomas. _Korean J.
Radiol._ 4, 101–108 (2003). Article Google Scholar * Imamura, M. Recent standardization of treatment strategy for pancreatic neuroendocrine tumors. _World J. Gastroenterol._ 16, 4519–4525
(2010). Article Google Scholar * VelanFalconi, M. _et al._ ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and
non-functional pancreatic neuroendocrine tumors. _Neuroendocrinol. Neuroendocrinol._ 103, 153–1571 (2016). Article Google Scholar * Howe, J. R. _et al._ The North American Neuroendocrine
Tumor Society Consensus paper on the surgical management of pancreatic neuroendocrine tumors. _Pancreas_ 49, 1–33 (2020). Article Google Scholar * Shah, M. H. _et al._ NCCN guidelines
insights: Neuroendocrine and adrenal tumors. _J. Natl. Compr. CancrNetw._ 16, 693–702 (2018). Article CAS Google Scholar * Naswa, N. _et al._ Peptide receptor radioligand therapy is an
effective treatment for the long-term stabilization of malignant gastrinomas. _Cancer_ 117, 1377–1385 (2011). Article Google Scholar * Sharm, P. _et al._ Evaluation of Ga-DOTANOC PET/CT
imaging in a large exclusive population of pancreatic neuroendocrine tumors. _Abdom. Imaging._ 40, 299–309 (2015). Article Google Scholar * Tucker, O. N., Crotty, P. L. & Conlon, K. C.
The management of insulinoma. _Br. J. Surg._ 93, 264–275 (2006). Article CAS Google Scholar * Burns, A. R. & Dackiw, A. P. B. Insulinoma. _Curr. Treat. Opt. Oncol._ 4, 309–317
(2003). Article Google Scholar * Okabayashi, T. _et al._ Diagnosis and management of insulinoma. _World J. Gastroenterol._ 19, 829–837 (2013). Article Google Scholar * Lo, C. Y. _et al._
Pancreatic insulinomas: a 15-year experience. _Arch. Surg._ 132, 926–930 (1997). Article CAS Google Scholar * Ravi, K. & Britton, B. J. Surgical approach to insulinomas: are
preoperative localization tests necessary?. _Ann. R Coll. Surg. Engl._ 89, 212–217 (2007). Article CAS Google Scholar * Paul, T. V. _et al._ Management of insulinomas: analysis from a
tertiary care referral center in India. _World J. Surg._ 32, 576–582 (2008). Article Google Scholar * Geoghegan, J. G. _et al._ Localization and surgical management of insulinoma. _Br. J.
Surg._ 81, 1025–1028 (1994). Article CAS Google Scholar * Morera, J. _et al._ Preoperative localization of an insulinoma: selective arterial calcium stimulation test performance. _J.
Endocrinol. Invest._ 39, 455–463 (2016). Article CAS Google Scholar * Kajiwara, K. _et al._ New diagnostic criteria for the localization of insulinomas with the selective arterial calcium
injection test: decision tree analysis. _J. Vasc. Interv. Radiol._ 29, 1749–1753 (2018). Article Google Scholar * Hayashi, R. _et al._ Diagnostic accuracy of selective arterial calcium
injection test for localization of gastrinoma. _Endocr. J._ 67, 305–351 (2020). Article CAS Google Scholar * Wang, H., Ba, Y., Xing, Q. & Du, J. L. Diagnostic value of endoscopic
ultrasound for insulinoma localization: a systematic review and meta-analysis. _PLoS ONE_ 13, e0206099 (2018). Article Google Scholar * Matsumoto, T. _et al._ Clinical role of
fludeoxyglucose (18F) positron emission tomography/computed tomography ((18)F-FDG PET/CT) in patients with pancreatic neuroendocrine tumors. _Surg. Today._ 49, 21–26 (2019). Article CAS
Google Scholar * Hasegawa, S. _et al._ Clinical usefulness of somatostatin receptor scintigraphy in japanese patients with gastroenteropancreatic neuroendocrine tumors. _Digestion._ 96,
13–20 (2017). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We wish like to thank Editage (www.editage.jp) for English language editing. FUNDING The authors declare no
source of funding. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Surgery, Keio University School of Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo, 160-8582, Japan Yutaka
Nakano, Minoru Kitago, Masahiro Shinoda, Hiroshi Yagi, Yuta Abe, Go Oshima, Shutaro Hori, Takahiro Yokose & Yuko Kitagawa * Department of Diagnostic Radiology, Keio University School of
Medicine, 35 Shinanomachi, Shinjuku-ku, Tokyo, 160-8582, Japan Seishi Nakatsuka * Department of Nephrology, Endocrinology and Metabolism, Keio University School of Medicine, 35 Shinanomachi,
Shinjuku-ku, Tokyo, 160-8582, Japan Isao Kurihara Authors * Yutaka Nakano View author publications You can also search for this author inPubMed Google Scholar * Minoru Kitago View author
publications You can also search for this author inPubMed Google Scholar * Masahiro Shinoda View author publications You can also search for this author inPubMed Google Scholar * Seishi
Nakatsuka View author publications You can also search for this author inPubMed Google Scholar * Isao Kurihara View author publications You can also search for this author inPubMed Google
Scholar * Hiroshi Yagi View author publications You can also search for this author inPubMed Google Scholar * Yuta Abe View author publications You can also search for this author inPubMed
Google Scholar * Go Oshima View author publications You can also search for this author inPubMed Google Scholar * Shutaro Hori View author publications You can also search for this author
inPubMed Google Scholar * Takahiro Yokose View author publications You can also search for this author inPubMed Google Scholar * Yuko Kitagawa View author publications You can also search
for this author inPubMed Google Scholar CONTRIBUTIONS All authors helped conduct the research; N.Y. manuscript writing and data analysis; K.M., S.M., Y.H., A.Y., O.G. and H.S. data analysis;
N.S., K.I., Y.T. and K.Y. data analysis, drafting conception, and design; All authors read and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Minoru Kitago. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits
use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless
indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory
regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Nakano, Y., Kitago, M., Shinoda, M. _et al._ Usefulness of selective arterial
calcium injection tests for functional pancreatic neuroendocrine tumors. _Sci Rep_ 11, 235 (2021). https://doi.org/10.1038/s41598-020-80538-0 Download citation * Received: 03 May 2020 *
Accepted: 15 December 2020 * Published: 08 January 2021 * DOI: https://doi.org/10.1038/s41598-020-80538-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read
this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
The macrophage tetraspan MS4A4A enhances dectin-1-dependent NK cell–mediated resistance to metastasisThe plasma membrane tetraspan molecule MS4A4A is selectively expressed by macrophage-lineage cells, but its function is ...
Smoking, drinking and eating: public health should not be all about the individualDiseases linked to smoking tobacco, a lack of exercise, drinking alcohol and eating unhealthily are on the rise, even th...
Watch Live Sports TV - News & Live Sports Channels on SkyWatch Sky Sports On now Back Live Retro C'Ship 10: B'pool v Cardiff Live Live Fight Night: Simpson v Zucco Live Newcastl...
Network pharmacology suggests biochemical rationale for treating COVID-19 symptoms with a Traditional Chinese MedicineChinese herbal formulas including the lung-cleaning and toxicity-excluding (LCTE) soup have played an important role in ...
Thousands of fish dead at high rock lakehttp://66.225.205.104/JR20091028.mp3 A large number of fish have turned up dead on a cove at High Rock Lake northeast of...
Latests News
Usefulness of selective arterial calcium injection tests for functional pancreatic neuroendocrine tumorsABSTRACT The selective arterial calcium injection (SACI) test is useful for patients with functional pancreatic neuroend...
State of the states news, research and analysis - the conversationMay 4, 2025 David Clune, _University of Sydney_; Narelle Miragliotta, _Murdoch University_; Paul Williams, _Griffith Uni...
French couple innovate horse hoof health with plastic shoesIT MAY MEAN THE END OF THE ‘CLIP CLOP’ SOUND BUT THE POLYURETHANE HORSESHOE IS SAID TO BE A HEALTHIER ALTERNATIVE TO MET...
Iron age commenced from tamil landscape, says stalin citing asi findings - the statesmanArmed with recent research findings, Chief Minister M K Stalin on Thursday claimed iron age had commenced from the Tamil...
Govt does not want to interfere in the affairs of mutts: law ministerThe News Minute | December 22, 2014 | 4.05 pm ISTThe ruling Congress government’s introduction of a bill to amend the Hi...