Bacterial inducible expression of plant cell wall-binding protein yeso through conflict between glycine max and saprophytic bacillus subtilis
Bacterial inducible expression of plant cell wall-binding protein yeso through conflict between glycine max and saprophytic bacillus subtilis"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Saprophytic bacteria and plants compete for limited nutrient sources. _Bacillus subtilis_ grows well on steamed soybeans _Glycine max_ to produce the fermented food, _natto_. Here
we focus on bacterial responses in conflict between _B. subtilis_ and _G. max_. _B. subtilis_ cells maintained high growth rates specifically on non-germinating, dead soybean seeds. On the
other hand, viable soybean seeds with germinating capability attenuated the initial growth of _B. subtilis_. Thus, _B. subtilis_ cells may trigger saprophytic growth in response to the
physiological status of _G. max_. Scanning electron microscope observation indicated that _B. subtilis_ cells on steamed soybeans undergo morphological changes to form apertures,
demonstrating cell remodeling during saprophytic growth. Further, transcriptomic analysis of _B. subtilis_ revealed upregulation of the gene cluster, _yesOPQR_, in colonies growing on
steamed soybeans. Recombinant YesO protein, a putative, solute-binding protein for the ATP-binding cassette transporter system, exhibited an affinity for pectin-derived oligosaccharide from
plant cell wall. The crystal structure of YesO, in complex with the pectin oligosaccharide, was determined at 1.58 Å resolution. This study expands our knowledge of defensive and offensive
strategies in interspecies competition, which may be promising targets for crop protection and fermented food production. SIMILAR CONTENT BEING VIEWED BY OTHERS THE _BRADYRHIZOBIUM
DIAZOEFFICIENS_ TYPE III EFFECTOR NOPE MODULATES THE REGULATION OF PLANT HORMONES TOWARDS NODULATION IN _VIGNA RADIATA_ Article Open access 16 August 2021 PHYSIOCHEMICAL INTERACTION BETWEEN
OSMOTIC STRESS AND A BACTERIAL EXOMETABOLITE PROMOTES PLANT DISEASE Article Open access 28 May 2024 THE EXTRACYTOPLASMIC SIGMA FACTOR ΣX SUPPORTS BIOFILM FORMATION AND INCREASES BIOCONTROL
EFFICACY IN _BACILLUS VELEZENSIS_ 118 Article Open access 13 February 2025 INTRODUCTION Plant seeds, each consisting of an embryo, storage tissues, and protective outer coat, remain dormant,
waiting for favorable environmental conditions for germination. Stored nutrient sources, such as carbohydrates, lipids, and proteins, are available to the germinating embryo, and also to
seed microbiota, a community of bacteria and fungi surrounding the seeds1,2. Plants have evolved sophisticated mechanisms to prevent phytopathogen colonization and infection. However, once
ungerminated seeds undergo senescence, decreased antimicrobial activity of dead seeds and, thus, increased accessibility to nutrients may permit microbial growth using seed decomposition
products, i.e., saprophytic growth3,4,5. The molecular basis for recognition of seed physiological status is required to understand the competition between microorganisms and plants.
_Bacillus subtilis_ is a Gram-positive, spore-forming, saprophytic bacterium ubiquitously isolated from soil, water, air, decaying plant materials, and fermented foods6,7,8,9. During
production of _natto_, a traditional fermented soybean food in Japan, soybean seeds are soaked and steamed before inoculation with _B. subtilis_. This process, which kills seeds, is regarded
as essential for enhancing _B. subtilis_ growth. Therefore, _B. subtilis_ cells growing on soybean seeds may be a useful model to study cell responses and molecular recognition at
saprophytic growth onset. _B. subtilis_ is widely used in emerging research fields as a Gram-positive model organism10,11; however, its physiological responses and behaviors on soybean seeds
remain unclear. Saprophytic growth requires nutrient uptake by decomposer organisms from decaying plant material. Bacterial ATP-binding cassette (ABC)-family transporters translocate a
variety of substrates into cells, using energy from ATP hydrolysis12,13,14. A typical ABC transporter consists of three components: integral membrane proteins that form pores,
nucleotide-binding proteins (NBPs) that bind and hydrolyze ATP, and solute-binding proteins (SBPs) that recognize and deliver target compounds into cells. SBPs are critical elements for
substrate specificity and high affinity of ABC transporter systems15,16,17,18. For instance, a conformational change of SBP in pathogenic bacteria is critical for selective recognition and
import of mammalian host glycosaminoglycans19,20. Pathogenic and symbiotic microorganisms secrete numerous enzymes to penetrate and degrade plant cell walls to provide
nutrition21,22,23,24,25. Thus, cell wall decomposition products are potential targets for saprophytic bacteria, which may use them to monitor plant physiological status. Primary plant cell
walls consist of a cellulose-hemicellulose framework embedded in an inter-fibrillar matrix of pectins26,27. Complex and diverse pectic polysaccharides include homogalacturonan (HG),
rhamnogalacturonan type I (RG-I), and rhamnogalacturonan type II (RG-II). HG is a linear polymer of α-1,4-linked galacturonic acid. RG-I is composed of a backbone, based on
disaccharide-repeating units of rhamnose and galacturonic acid, with side chains of galactans, arabinans, and arabinogalactans. RG-II contains HG as a backbone, with complex side chains
composed of a variety of sugars. Although studies on HG degradation have progressed28,29,30, bacterial decomposition of RG-I and RG-II has been reported in a limited number of papers so
far31,32,33. This report is the first on the competition between saprophytic _B. subtilis_ and soybean _Glycine max_ on the surfaces of soybean seeds. This article further focuses on the
physiological and transcriptomic traits of _B. subtilis_, and provides structural insight into SBP-dependent recognition of RG-I-derived trisaccharide during the saprophytic growth. RESULTS
AND DISCUSSION GROWTH OF _B. SUBTILIS_ ON LIVE AND DEAD SOYBEANS _Natto_, a traditional Japanese fermented food, is manufactured from steamed soybean seeds7,9. Saprophytic _B. subtilis_
cells grow on dead soybeans, using decomposition products as nutrients. Steaming may cause not only loss of viability but also denaturation of proteins; it may alter other soybean components
as well. Therefore, what exactly triggers saprophytic growth is an open question. Heat-related impacts to soybean components were reduced by adopting physiological senescence, induced at
different temperatures, by prolonged seed storage. Six-month storage at 4 °C maintained viability, but six-month storage at an ambient temperature severely limited seed germination
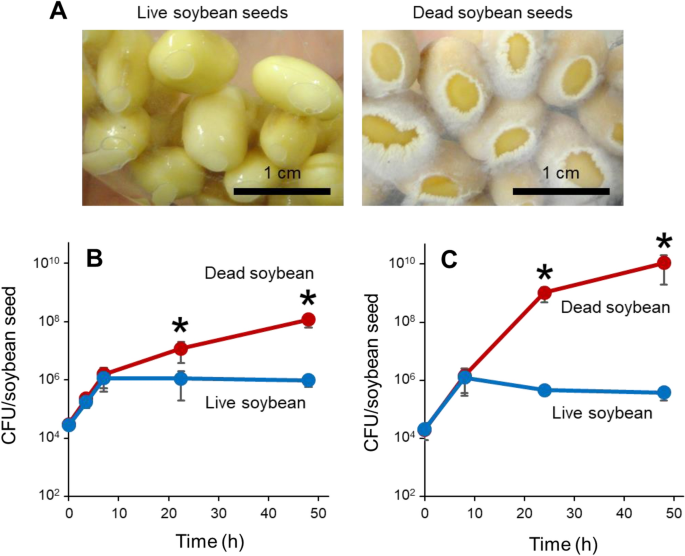
(Supplementary Fig. S1A). Thus, seeds stored under the former and the latter conditions were used as live and dead soybeans, respectively. _Bacillus subtilis_ cells were found to grow
vigorously on non-germinating dead soybean seeds, but not on live seeds (Supplementary Video S1, Fig. 1A). Thus, seed germination and bacterial growth inhibition may be closely linked. We
first discovered this phenomenon using _B. subtilis natto_-producing strain NBRC 1644934. Quantitative analysis of colony-forming units (CFUs) indicated that _B. subtilis_ initially
proliferated on both live and dead soybeans. However, growth attenuated specifically on live soybeans when bacterial cell numbers reached 106 CFUs per seed (Fig. 1B). _B. subtilis_
laboratory standard strain 16835 exhibited a similar growth profile on live and dead seeds (Fig. 1C). Thus, live soybeans can severely limit _B. subtilis_ growth on their surfaces. Invading
bacteria and germinating plant embryos may compete for nutrients in soybean seeds. _B. subtilis_ cells gain access to seed nutrients when the seeds lose germination ability. _B. subtilis_
growth on soybean seeds is a promising model for bacterial-plant interactions in seed microbiota. MORPHOLOGICAL TRAITS OF _B. SUBTILIS_ DURING SAPROPHYTIC GROWTH To understand the strategy
of _B. subtilis_ for saprophytic growth on dead soybeans during _natto_ production, morphological and transcriptional responses were examined. Scanning electron microscope (SEM) analysis
revealed cell morphological changes of _B. subtilis_ NBRC 16449 during saprophytic growth on steamed seeds (Fig. 2A–C). The early logarithmic growth phase, 5 h after inoculation, was
characterized by elongated, rod-shaped cells, 6–10 μm long. As the growth progressed, most cells displayed short-rod forms, approximately 2 μm long, with distinctive aperture-like structures
(Fig. 2C). _B. subtilis_ NBRC 16449 (Supplementary Fig. S2) and 168 (Fig. 2D–F) strains exhibited apertures on soybean seed-mimicking solid medium (Supplementary Fig. S1B). No apertures
were observed in liquid medium (Fig. 2G–I), suggesting that a solid substrate is needed for this culture-specific response. Similar aperture-like structures are previously reported in
_Clostridium sporogenes_ during formation of endospores36. _B. subtilis_ cell membranes are subject to engulfment during spore development under nutrient-depleted conditions37,38, and
saprophytically growing _B. subtilis_ may undergo similar morphological remodeling. How solid-state culture stimulates the starvation-triggered sporulation pathway is of great interest from
a signal transduction viewpoint. TRANSCRIPTOMIC PROFILE OF _B. SUBTILIS_ DURING SAPROPHYTIC GROWTH Comprehensive understanding of saprophytism-specific recognition and responses was sought
using the transcriptomic profiles of _B. subtilis_ NBRC 16449 cells from steamed soybeans and from soybean seed-mimicking solid medium, using RNA-Seq analysis. Reads were mapped to the
genomic sequence of _B. subtilis_ subsp. _natto_ BEST195 strain39,40. Expression of 93 and 107 genes among a total of 4271 analyzed genes was over tenfold upregulated and downregulated,
respectively, in _B. subtilis_ grown on steamed soybeans. Upregulated transcripts included several ABC transporter components and their neighboring genes (Fig. 3A). The gene cluster
_yesOPQR_ was highly expressed, as previously reported in _B. subtilis_ 168 cells grown on RG-I, a component of plant cell wall pectin32. Based on sequence homology, _yesO_ and _yesPQ_ genes
encode a putative SBP and transmembrane permeases for the ABC-family sugar transport system, respectively. Deleting _yesOPQ_ has a negative impact on utilization of RG-I as a carbon
source41. YesR is a cytosolic hydrolase that acts on unsaturated rhamnogalacturonan disaccharide derived from RG-I31. Another upregulated gene, _yurJ_, may encode a functional NBP for YesOPQ
and other ABC transporters41,42. Thus, _B. subtilis_ may recognize and use plant cell wall-derived RG-I as a carbon source by inducing _yesOPQR_ and _yurJ_ genes during saprophytic growth
(Fig. 3B). AFFINITY BETWEEN YESO AND RG-I OLIGOSACCHARIDES YesO is a putative SBP involved in RG-I assimilation, but its binding substrate is unknown. Recombinant YesO protein and the
decomposition product of RG-I backbones (oligo-RG-I) were prepared to address this knowledge gap. Comparisons between YesO of _B. subtilis_ laboratory strain 168 and its orthologs from
_Bacillus sonorensis_, _Bacillus licheniformis_ DSM 13, _Paenibacillus_ sp. Aloe-11, and _Paenibacillus macerans_ revealed low conservation of the N-terminus (Supplementary Fig. S3). Thus,
N-terminus-deleted YesO, of approximately 45 kDa, was overexpressed in _Escherichia coli_, and purified to homogeneity (Fig. 4A,B). To obtain oligo-RG-I, RG-I backbones were treated with
exolyase YesX (Fig. 4C). Thin-layer chromatography (TLC) analysis of reaction products showed that released oligosaccharides consisted mainly of unsaturated rhamnogalacturonan disaccharide,
as reported previously32. The interaction between YesO and oligo-RG-I was analyzed by measuring YesO fluorescent intensity in the presence or absence of oligo-RG-I (Fig. 4D). Adding
oligo-RG-I decreased the fluorescent intensity from tryptophan residues of YesO by 3–7%. Based on the plot, the dissociation constant (_K_d) was 1.6 μM. No significant decrease of
fluorescent intensity was observed when other disaccharides, such as sucrose, maltose, cellobiose, chitobiose, and digalacturonic acid, were used as a ligand (Supplementary Fig. S4). YesO
appears to recognize oligo-RG-I as a substrate selectively. BINDING MODE OF YESO TO OLIGO-RG-I Oligo-RG-I recognition by YesO was analyzed with X-ray crystallography. The crystal structures
of YesO and its complex form with oligo-RG-I (YesO/oligo-RG-I) were determined at 1.97 Å and 1.58 Å resolution, respectively. Data collection and refinement statistics are summarized in
Supplementary Table S1. Ligand-free YesO structure (Fig. 4E) was similar to a previously determined structure (PDB ID 4R6K). YesO is an α/β protein with 15 α helices (residues 23–39, 52–65,
76–86, 92–96, 111–113, 135–140, 152–166, 177–187, 205–219, 253–262, 312–318, 328–336, 340–354, 368–383, and 381–405) and 8 β sheets (residues 15–20, 45–55, 71–73, 122–126, 130–133, 248–252,
267–270, and 288–290). Note that amino acid residue numbers of our YesO are 20 fewer than those of the full-length YesO to adjust to PDB ID 4R6K. The substrate-binding cleft is formed
between two globular N- and C-domains (Fig. 4F), as typically observed among SBPs of ABC transport systems15,16,17,18. YesO is categorized as a Class II SBP, based on a β2–β1–β3–β8–β4
topology of the β sheet core in the N-domain. N- and C-domains are connected through two short hinges (residues Val126-Leu129 and Leu281-Lys284), assigning YesO to Cluster D SBPs. Comparison
of ligand-free YesO with YesO/oligo-RG-I revealed a 26.2°-closed conformation upon binding of a ligand molecule (Fig. 4G), suggesting a Venus-flytrap conformational change43. Although our
prepared oligo-RG-I was mostly disaccharide (Fig. 4C), X-ray crystallography of YesO/oligo-RG-I indicated an interaction between YesO and the trisaccharide, ΔGalUA-Rha-Gal, composed of
unsaturated galacturonic acid and rhamnose from the main chain and galactose from the side chain (Fig. 4H). This result reproduced another independent YesO/oligo-RG-I crystal structure. The
molecular docking simulation suggested that rhamnogalacturonan tetrasaccharide is too large to fit in the ligand-binding cleft (Supplementary Fig. S5). Thus, YesO may selectively recognize
ΔGalUA-Rha-Gal trisaccharide as a ligand. ΔGalUA-Rha-Gal binds to the YesO cleft via hydrogen bonds and C–C contacts (Table 1). There were 19 hydrogen bonds (ΔGalUA, 10; Rha, 2; Gal, 7) and
44 C–C contacts (ΔGalUA, 22; Rha, 17; Gal, 5). ΔGalUA-interacting residues, Arg25, Asn127, Asn254, and Ser286, are highly conserved among the known YesO orthologs, highlighting the
importance of ΔGalUA in the YesO ligand. RECOGNITION OF ΔGALUA-RHA-GAL DURING SAPROPHYTIC GROWTH In the present study, _B. subtilis yesOPQR_ was identified as an upregulated gene cluster
during saprophytic growth on dead soybean surfaces (Fig. 3B). YesO was found to act as an SBP for uptake of RG-I-derived ΔGalUA-Rha-Gal trisaccharide. YesPQ are putative transmembrane
permeases, and YesR catalyzes intracellular degradation of unsaturated rhamnogalacturonan disaccharide31. Expression of the _yurJ_ gene, encoding a putative NBP for YesOPQ40, was also
induced on dead soybeans. Thus, YesOPQR/YurJ-dependent assimilation of ΔGalUA-Rha-Gal is likely to play a major role in saprophytic growth. Assuming that plant cell wall degradation is
closely associated with wounding, pathogen attack, or cell death, induction of the _yesOPQR_ and _yurJ_ genes might be one of the initial responses for saprophytic growth on dead plant
surfaces. Our hypothesis is that ΔGalUA-Rha-Gal is used as a nutrient or signaling molecule, or both. ΔGalUA-Rha-Gal is degraded into assimilable monosaccharides by an unidentified
intracellular β-1,4-galactosidase and YesR. Since the soybean surface is a low-nutrient environment, pectin-derived oligosaccharides may be the carbon source that supports saprophytic
growth. Further, ΔGalUA-Rha-Gal may also serve as a signaling molecule to trigger saprophytism. Some bacterial two-component regulatory systems adopt SBPs for signal recognition18. Our
recent study demonstrated that SPH1118, an SBP in _Sphingomonas_ sp. strain A1, is responsible not only for pectin assimilation but also for chemotaxis toward pectin44. Assuming such
alternative functions of YesO, recognizing ΔGalUA-Rha-Gal may trigger signal transduction for proliferation or adaptation under saprophytic conditions. Based on the YesO/oligo-RG-I
three-dimensional structure, YesO traps ΔGalUA-Rha-Gal trisaccharide, although most of the oligo-RG-I used in this study was disaccharide. How is ΔGalUA-Rha-Gal generated via pectin
degradation? _B. subtilis_ cells secrete a series of enzymes for RG-I degradation, such as YesWX lyases to cleave α-1,4-glycosidic linkages between rhamnose and galacturonic acid residues in
the RG-I backbone32, GanAB galactosidases to degrade galactan branches45, and AbfA and Xsa arabinofuranosidases to catalyze hydrolysis of terminal arabinofuranoside bonds of arabinogalactan
or arabinan46. YesWX-dependent degradation of RG-I backbones, with a single galactose residue at each branch point, may release ΔGalUA-Rha-Gal trisaccharide (see red areas in Fig. 3B). In
conclusion, this report is the first to describe the competition between saprophytic _B. subtilis_ and soybean _G. max_ on soybean seeds surfaces. Live soybeans prevent bacterial invasion,
while _B. subtilis_ YesO recognizes pectin-derived ΔGalUA-Rha-Gal trisaccharide to establish saprophytic growth on dead soybean seeds. This study will help elucidate the diversity of
bacterial-plant interactions, particularly for seed microbiota. MATERIALS AND METHODS MATERIALS AND MICROORGANISMS Soybean seeds of Suzumaru (_G. max_) were purchased from Mamehei (Japan).
RG-I (from potatoes) was purchased from Megazyme (Ireland). _B. subtilis_ laboratory standard strain 168 was obtained from the National Bio Resource Project (NBRP), Japan. _B. subtilis_
strain NBRC 16449 was obtained from the NITE Biological Research Center (NBRC), Japan. _E. coli_ strain BL21-Gold(DE3) (Agilent, USA) was used for recombinant protein overexpression. _E.
coli_ strain DH5α was used for cloning and plasmid maintenance. Bacterial cells were routinely grown in LB medium (1% tryptone, 0.5% yeast extract, 1% sodium chloride, pH 7.2) at 37 °C.
GROWTH TEST Soybean seeds were stored at 4 °C and at room temperature for 6 months to prepare live and dead soybeans, respectively. The seeds were washed and soaked in pure water at room
temperature for 3.5 h. The seed surfaces were sterilized by 20-min ultraviolet exposure. This exposure did not produce severe effects on living soybean seeds’ germination capability
(Supplementary Fig. S1A). Soybean seed-mimicking solid LB medium blocks (solidified by 3% agar, 300 μl each; Supplementary Fig. S1B) were prepared aseptically in microtubes. Thirty seeds or
blocks were used for each growth test. _Bacillus subtilis_ was precultured at 37 °C in liquid LB medium overnight, washed, and resuspended in 0.85% sodium chloride. Approximately 104 cells
per seed or solid medium block were inoculated and incubated at 37 °C. To assay CFUs, _B. subtilis_ cells on the sampled seeds or blocks were collected in 0.85% sodium chloride, spread on LB
agar plates, and incubated at 37 °C overnight. SEM ANALYSIS _Bacillus subtilis_ cells were grown on steamed soybean seeds, on soybean mimicking solid LB medium blocks, and in liquid LB
medium at 37 °C. Steamed soybeans were prepared by washing and soaking live seeds with pure water at room temperature for 3.5 h, followed by autoclaving at 121 °C for 20 min. For protein
fixation, samples (soybean seeds or collected cells) were mixed with 4% formaldehyde and incubated for 1 h. Subsequently, for lipid fixation, samples were washed with 10 mM potassium
phosphate buffer or pure water three times and immersed in 1% osmium oxide for 1–2 h. Fixed samples were washed with 10 mM potassium phosphate buffer or pure water three times, dehydrated
sequentially with 50%, 70%, 90%, and 100% ethanol, and immersed in _t_-butyl alcohol at 4 °C. Freeze-dried samples were coated with a platinum-palladium layer under argon gas and observed
with a field emission (FE)-SEM SU8230 (Hitachi, Japan) using a 1.5 kV electron beam. RNA-SEQ ANALYSIS _Bacillus subtilis_ NBRC 16449 cells were grown on steamed soybean seeds or
soybean-mimicking solid LB medium blocks to the logarithmic phase. Cells on the seeds or blocks were collected in 0.85% sodium chloride, treated with RNAprotect bacteria reagent (Qiagen,
Netherlands), and frozen immediately in liquid nitrogen. Total RNA was prepared using the hot phenol method47 by Nihon Gene Research Laboratories, Inc. (Japan). RNA-Seq analysis was
performed by Macrogen Japan Corp. (Japan). In brief, paired-end sequencing of the constructed library was performed using HiSeq 2500 (Illumina, USA). Raw data were filtered using Trimmomatic
v0.3248 to remove adapter sequences and bases with a Phred score below 30. Trimmed reads were aligned against the _B. subtilis_ subsp. _natto_ BEST195 reference genome (DDBJ accession no.
DRA000001)39,40, using Bowtie as the alignment algorithm49. Differential expression analysis was conducted using HTSeq50. RNA-Seq data, generated from this study, were deposited in the NCBI
Gene Expression Omnibus (GEO) and are accessible through GEO series accession number GSE109523. EXPRESSION AND PURIFICATION OF RECOMBINANT YESO PROTEIN To construct an expression system for
YesO that lacks the weakly conserved N-terminus (Supplementary Fig. S3), the _yesO_ gene of _B. subtilis_ strain 168 was amplified by genomic PCR, using primers
5′-GGCATATGACACTCAGAATCGCGTGGTGGGGC-3′ and 5′GGCTCGAGTCAATTATTCCTCTCTAATATCTCATT-3′ (with underlined restriction sites), and cloned into the NdeI-XhoI site of pET21b(+) (Novagen, USA). The
resultant pET21b(+)-_yesO_ plasmid was introduced into _E. coli_ BL21-Gold(DE3) cells. _Escherichia coli_ cells harboring the plasmid were cultured in LB liquid medium with 100 μg ml−1
ampicillin at 30 °C. When optical density at 600 nm (OD600) reached 0.3, isopropyl-β-d-thiogalactopyranoside (IPTG) was added to the culture at a final concentration of 0.1 mM. After further
incubation at 16 °C for 2 days, cells were washed, suspended in 20 mM Tris–HCl (pH 7.5), and disrupted with an ultrasonicator 201 M (Kubota, Japan). The supernatant obtained by
centrifugation (20,000 × _g_, 4 °C, 20 min) was used for YesO purification. Cell extracts were applied to an anion exchange chromatography system with a TOYOPEARL DEAE-650M column (Tosoh,
Japan). Proteins were eluted using a linear gradient of sodium chloride (0–250 mM) in 20 mM Tris–HCl (pH 7.5). After separation with 12.5% SDS-PAGE, fractions containing the target protein
were combined and purified by gel filtration chromatography using HiLoad 16/60 Superdex 200 pg resin (GE Healthcare, USA) equilibrated with 200 mM sodium chloride in 20 mM Tris–HCl (pH 7.5).
After separation with 12.5% SDS-PAGE, fractions containing the target protein were combined. PREPARATION OF OLIGO-RG-I Substrate RG-I backbones were prepared from commercially available
RG-I, as previously reported32. A reaction mixture, consisting of 1% RG-I backbone, 50 mM Tris–HCl (pH 7.5), 20 mM manganese(II) chloride, and lysates of YesX-expressing _E. coli_ cells32,
was incubated at 30 °C for 3 h. The mixture was boiled for 5 min and centrifuged (7200 × _g_, room temperature, 5 min). The supernatant was passed through a Centriprep centrifugal filter (3
kDa NMWL; Merck Millipore, USA) to obtain decomposition products. Resultant oligo-RG-I was detected by TLC analysis, as reported previously32. FLUORESCENCE SPECTRUM ANALYSIS Fluorescence of
protein, with a peak around 340 nm, is partially quenched by adding a binding ligand51. Fluorescent intensities of YesO, with increasing concentrations of oligo-RG-I, were measured by
spectrophotometer FP-6500 (JASCO, Japan). Measurement parameters during reactions were: excitation band width, 1 nm; emission band width, 10 nm; response, 2 s; sensitivity, high; excitation
wavelength, 280 nm; start to end emission wavelength, 300–500 nm; data pitch, 1 nm; and scan speed, 100 nm min−1. The reaction mixture contained 0.14 μM YesO, 20 mM Tris–HCl (pH 7.5), and
0–40 μM ligands. Decreases in fluorescent intensity by adding ligands were plotted, and the dissociation constant (_K_d) was determined based on nonlinear regression with one site-specific
binding model51. X-RAY CRYSTALLOGRAPHY Ligand-free YesO and YesO/oligo-RG-I were crystallized by sitting drop vapor diffusion, using the JBScreen crystallization kit (Jena Bioscience,
Germany). In 96-well sitting drop plates, 1 μl of 15 mg ml−1 YesO (without or with 1 mM oligo-RG-I) and 1 μl of reservoir solution were mixed. The mixture was kept at 20 °C until crystals
grew sufficiently. Ligand-free YesO was crystallized in the drop, consisting of 100 mM malonate-imidazole-boric acid (MIB) buffer (pH 6.0), 25% polyethyleneglycol (PEG)-1500, and 1 mM
digalacturonate. YesO/oligo-RG-I was crystallized in the drop, consisting of 100 mM Tris–HCl (pH 8.5), 20% PEG-1000, and 25% glycerol. Each single crystal was picked up using a nylon loop
from the drop, soaked in a reservoir solution containing 20% ethylene glycol, and instantly frozen using cold nitrogen gas. Synchroton radiation X-ray irradiated the crystals at 1.00 Å
wavelength, and X-ray diffraction data were collected using a MAR225HE CCD detector (Rayonix, USA) at the BL-26B1 and BL-38B1 beamlines in SPring-8 (Japan). Diffraction data were processed
using the HKL-2000 program52. The crystal structures of YesO and YesO/oligo-RG-I were determined through molecular replacement with the Molrep program53 in the CCP4Interface package, using
the ligand-free YesO structure (PDB ID 4R6K) as a reference model. Structure refinement was conducted using the phenix.refine54 and REFMAC553 programs. At each refinement cycle, the model
was adjusted manually with the WinCoot program55. Images of protein structures were prepared using the PyMOL molecular graphics system (Schrödinger, USA). The coordinates and structure
factors (accession codes 5Z6C for ligand-free YesO and 5Z6B for YesO/oligo-RG-I) have been deposited in the PDB. CODE AVAILABILITY _Accession codes_ RNA-Seq data were deposited in the NCBI
GEO and are accessible through GEO series accession number GSE109523. Protein structures of YesO and YesO/oligo-RG-I were deposited in the PDB under accession codes 5Z6C and 5Z6B,
respectively. REFERENCES * Nelson, E. B. Microbial dynamics and interactions in the spermosphere. _Annu. Rev. Phytopathol._ 42, 271–309 (2004). Article CAS PubMed Google Scholar * Shade,
A., Jacques, M. A. & Barret, M. Ecological patterns of seed microbiome diversity, transmission, and assembly. _Curr. Opin. Microbiol._ 37, 15–22 (2017). Article PubMed Google Scholar
* Prusky, D., Alkan, N., Mengiste, T. & Fluhr, R. Quiescent and necrotrophic lifestyle choice during postharvest disease development. _Annu. Rev. Phytopathol._ 51, 155–176 (2013).
Article CAS PubMed Google Scholar * Fatima, U. & Senthil-Kumar, M. Plant and pathogen nutrient acquisition strategies. _Front. Plant Sci._ 6, 750 (2015). Article PubMed PubMed
Central Google Scholar * Poole, P., Ramachandran, V. & Terpolilli, J. Rhizobia: From saprophytes to endosymbionts. _Nat. Rev. Microbiol._ 16, 291–303 (2018). Article CAS PubMed
Google Scholar * Earl, A. M., Losick, R. & Kolter, R. Ecology and genomics of _Bacillus subtilis_. _Trends Microbiol._ 16, 269–275 (2008). Article CAS PubMed PubMed Central Google
Scholar * Murooka, Y. & Yamashita, M. Traditional healthful fermented products of Japan. _J. Ind. Microbiol. Biotechnol._ 35, 791–798 (2008). Article CAS PubMed Google Scholar *
Fira, D., Dimkić, I., Berić, T., Lozo, J. & Stanković, S. Biological control of plant pathogens by _Bacillus_ species. _J. Biotechnol._ 285, 44–55 (2018). Article CAS PubMed Google
Scholar * Kimura, K. & Yokoyama, S. Trends in the application of _Bacillus_ in fermented foods. _Curr. Opin. Biotechnol._ 56, 36–42 (2019). Article CAS PubMed Google Scholar *
Kalamara, M., Spacapan, M., Mandic-Mulec, I. & Stanley-Wall, N. R. Social behaviours by _Bacillus subtilis_: Quorum sensing, kin discrimination and beyond. _Mol. Microbiol._ 110, 863–878
(2018). Article CAS PubMed PubMed Central Google Scholar * Liu, Y., Liu, L., Li, J., Du, G. & Chen, J. Synthetic biology toolbox and chassis development in _Bacillus subtilis_.
_Trends Biotechnol._ 37, 548–562 (2019). Article CAS PubMed Google Scholar * Davidson, A. L., Dassa, E., Orelle, C. & Chen, J. Structure, function, and evolution of bacterial
ATP-binding cassette systems. _Microbiol. Mol. Biol. Rev._ 72, 317–364 (2008). Article CAS PubMed PubMed Central Google Scholar * Murata, K., Kawai, S., Mikami, B. & Hashimoto, W.
Superchannel of bacteria: Biological significance and new horizons. _Biosci. Biotechnol. Biochem._ 72, 265–277 (2008). Article CAS PubMed Google Scholar * Locher, K. P. Mechanistic
diversity in ATP-binding cassette (ABC) transporters. _Nat. Struct. Mol. Biol._ 23, 487–493 (2016). Article CAS PubMed Google Scholar * Fukami-Kobayashi, K., Tateno, Y. & Nishikawa,
K. Domain dislocation: A change of core structure in periplasmic binding proteins in their evolutionary history. _J. Mol. Biol._ 286, 279–290 (1999). Article CAS PubMed Google Scholar *
Berntsson, R. P., Smits, S. H., Schmitt, L., Slotboom, D. J. & Poolman, B. A structural classification of substrate-binding proteins. _FEBS Lett._ 584, 2606–2617 (2010). Article CAS
PubMed Google Scholar * Maqbool, A. _et al._ The substrate-binding protein in bacterial ABC transporters: Dissecting roles in the evolution of substrate specificity. _Biochem. Soc. Trans._
43, 1011–1017 (2015). Article CAS PubMed Google Scholar * Scheepers, G. H., Nijeholt, J. A. L. & Poolman, B. An updated structural classification of substrate-binding proteins.
_FEBS Lett._ 590, 4393–4401 (2016). Article CAS PubMed Google Scholar * Oiki, S., Mikami, B., Maruyama, Y., Murata, K. & Hashimoto, W. A bacterial ABC transporter enables import of
mammalian host glycosaminoglycans. _Sci. Rep._ 7, 1069 (2017). Article ADS PubMed PubMed Central CAS Google Scholar * Oiki, S., Kamochi, R., Mikami, B., Murata, K. & Hashimoto, W.
Alternative substrate-bound conformation of bacterial solute-binding protein involved in the import of mammalian host glycosaminoglycans. _Sci. Rep._ 7, 17005 (2017). Article ADS PubMed
PubMed Central CAS Google Scholar * Juge, N. Plant protein inhibitors of cell wall degrading enzymes. _Trends Plant Sci._ 11, 359–367 (2006). Article CAS PubMed Google Scholar *
Oldroyd, G. E., Murray, J. D., Poole, P. S. & Downie, J. A. The rules of engagement in the legume-rhizobial symbiosis. _Annu. Rev. Genet._ 45, 119–144 (2011). Article CAS PubMed
Google Scholar * Glass, N. L., Schmoll, M., Cate, J. H. & Coradetti, S. Plant cell wall deconstruction by ascomycete fungi. _Annu. Rev. Microbiol._ 67, 477–498 (2013). Article CAS
PubMed Google Scholar * Kubicek, C. P., Starr, T. L. & Glass, N. L. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. _Annu. Rev. Phytopathol._ 52,
427–451 (2014). Article PubMed CAS Google Scholar * Garron, M. L. & Cygler, M. Uronic polysaccharide degrading enzymes. _Curr. Opin. Struct. Biol._ 28, 87–95 (2014). Article CAS
PubMed Google Scholar * Caffall, K. H. & Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. _Carbohydr. Res._ 344, 1879–1900 (2009).
Article CAS PubMed Google Scholar * Lampugnani, E. R., Khan, G. A., Somssich, M. & Persson, S. Building a plant cell wall at a glance. _J. Cell Sci._ 131, jcs207373 (2018). Article
PubMed CAS Google Scholar * Charnock, S. J., Brown, I. E., Turkenburg, J. P., Black, G. W. & Davies, G. J. Convergent evolution sheds light on the anti-β-elimination mechanism common
to family 1 and 10 polysaccharide lyases. _Proc. Natl. Acad. Sci. USA_ 99, 12067–12072 (2002). Article ADS CAS PubMed PubMed Central Google Scholar * Abbott, D. W. & Boraston, A.
B. Structural biology of pectin degradation by _Enterobacteriaceae_. _Microbiol. Mol. Biol. Rev._ 72, 301–316 (2008). Article CAS PubMed PubMed Central Google Scholar *
Hugouvieux-Cotte-Pattat, N., Condemine, G. & Shevchik, V. E. Bacterial pectate lyases, structural and functional diversity. _Environ. Microbiol. Rep._ 6, 427–440 (2014). Article CAS
PubMed Google Scholar * Itoh, T., Ochiai, A., Mikami, B., Hashimoto, W. & Murata, K. A novel glycoside hydrolase family 105: The structure of family 105 unsaturated rhamnogalacturonyl
hydrolase complexed with a disaccharide in comparison with family 88 enzyme complexed with the disaccharide. _J. Mol. Biol._ 360, 573–585 (2006). Article CAS PubMed Google Scholar *
Ochiai, A., Itoh, T., Kawamata, A., Hashimoto, W. & Murata, K. Plant cell wall degradation by saprophytic _Bacillus subtilis_ strains: Gene clusters responsible for rhamnogalacturonan
depolymerization. _Appl. Environ. Microbiol._ 73, 3803–3813 (2007). Article CAS PubMed PubMed Central Google Scholar * Kunishige, Y. _et al._ Crystal structure of exo-rhamnogalacturonan
lyase from _Penicillium chrysogenum_ as a member of polysaccharide lyase family 26. _FEBS Lett._ 592, 1378–1388 (2018). Article CAS PubMed Google Scholar * Ogawa, Y., Hosoyama, H.,
Hamano, M. & Motai, H. Purification and properties of γ-glutamyltranspeptidase from _Bacillus subtilis_ (_natto_). _Agric. Biol. Chem._ 55, 2971–2977 (1991). CAS PubMed Google Scholar
* Kunst, F. _et al._ The complete genome sequence of the gram-positive bacterium _Bacillus subtilis_. _Nature_ 390, 249–256 (1997). Article ADS CAS PubMed Google Scholar * Brunt, J.,
Cross, K. L. & Peck, M. W. Apertures in the _Clostridium sporogenes_ spore coat and exosporium align to facilitate emergence of the vegetative cell. _Food Microbiol._ 51, 45–50 (2015).
Article PubMed PubMed Central Google Scholar * Higgins, D. & Dworkin, J. Recent progress in _Bacillus subtilis_ sporulation. _FEMS Microbiol. Rev._ 36, 131–148 (2012). Article CAS
PubMed Google Scholar * Dworkin, J. Protein targeting during _Bacillus subtilis_ sporulation. _Microbiol. Spectr._ 2, TBS-0006-2012 (2014). Article PubMed CAS Google Scholar * Itaya,
M. & Matsui, K. Conversion of _Bacillus subtilis_ 168: _Natto_ producing _Bacillus subtilis_ with mosaic genomes. _Biosci. Biotechnol. Biochem._ 63, 2034–2037 (1999). Article CAS
Google Scholar * Nishito, Y. _et al._ Whole genome assembly of a _natto_ production strain _Bacillus subtilis natto_ from very short read data. _BMC Genomics_ 11, 243 (2010). Article
PubMed PubMed Central CAS Google Scholar * Ferreira, M. J., Mendes, A. L. & de Sá-Nogueira, I. The MsmX ATPase plays a crucial role in pectin mobilization by _Bacillus subtilis_.
_PLoS ONE_ 12, e0189483 (2017). Article PubMed PubMed Central CAS Google Scholar * Quentin, Y., Fichant, G. & Denizot, F. Inventory, assembly and analysis of _Bacillus subtilis_ ABC
transport systems. _J. Mol. Biol._ 287, 467–484 (1999). Article CAS PubMed Google Scholar * Mao, B., Pear, M. R., McCammon, J. A. & Quiocho, F. A. Hinge-bending in
l-arabinose-binding protein. The “Venus’s-flytrap” model. _J. Biol. Chem._ 257, 1131–1133 (1982). Article CAS PubMed Google Scholar * Konishi, H., Hio, M., Kobayashi, M., Takase, R.
& Hashimoto, W. Bacterial chemotaxis towards polysaccharide pectin by pectin-binding protein. _Sci. Rep._ 10, 3977 (2020). Article ADS CAS PubMed PubMed Central Google Scholar *
Watzlawick, H., Heravi, K. M. & Altenbuchner, J. Role of the _ganSPQAB_ operon in degradation of galactan by _Bacillus subtilis_. _J. Bacteriol._ 198, 2887–2896 (2016). Article CAS
PubMed PubMed Central Google Scholar * Inácio, J. M., Correia, I. L. & de Sá-Nogueira, I. Two distinct arabinofuranosidases contribute to arabino-oligosaccharide degradation in
_Bacillus subtilis_. _Microbiology_ 154, 2719–2729 (2008). Article PubMed CAS Google Scholar * Damm, K. _et al._ Impact of RNA isolation protocols on RNA detection by Northern blotting.
_Methods Mol. Biol._ 1296, 29–38 (2015). Article CAS PubMed Google Scholar * Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data.
_Bioinformatics_ 30, 2114–2120 (2014). Article CAS PubMed PubMed Central Google Scholar * Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient
alignment of short DNA sequences to the human genome. _Genome Biol._ 10, R25 (2009). Article PubMed PubMed Central CAS Google Scholar * Anders, S., Pyl, P. T. & Huber, W. HTSeq—A
Python framework to work with high-throughput sequencing data. _Bioinformatics_ 31, 166–169 (2015). Article CAS PubMed Google Scholar * Ohnishi, M., Yamashita, T. & Hiromi, K. Static
and kinetic studies by fluorometry on the interaction between gluconolactone and glucoamylase from _Rh. niveus_. _J. Biochem._ 81, 99–105 (1977). CAS PubMed Google Scholar * Otwinowski,
Z. & Minor, W. Processing of X-ray diffraction data collected in oscillation mode. _Methods Enzymol._ 276, 307–326 (1997). Article CAS PubMed Google Scholar * Murshudov, G. N.,
Vagin, A. A. & Dodson, E. J. Refinement of macromolecular structures by the maximum-likelihood method. _Acta Crystallogr. D. Biol. Crystallogr._ 53, 240–255 (1997). Article CAS PubMed
Google Scholar * Adams, P. D. _et al._ PHENIX: A comprehensive Python-based system for macromolecular structure solution. _Acta Crystallogr. D. Biol. Crystallogr._ 66, 213–221 (2010).
Article CAS PubMed PubMed Central Google Scholar * Emsley, P. & Cowtan, K. Coot: Model-building tools for molecular graphics. _Acta Crystallogr. D. Biol. Crystallogr._ 60, 2126–2132
(2004). Article PubMed CAS Google Scholar Download references ACKNOWLEDGEMENTS Diffraction data were collected at the BL26B1 and BL38B1 stations of SPring-8 (Hyogo, Japan) with the
approval of JASRI (proposal nos. 2016A2574, 2016B2574, 2017B2547, and 2017B2592). This work was supported in part by Grants-in-Aid for Scientific Research from the Japan Society for the
Promotion of Science (to W.H.), and Research Grant (to W.H.) from Fuji Foundation for Protein Research. The authors would like to thank Enago (https://www.enago.com) for the English language
review. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Laboratory of Basic and Applied Molecular Biotechnology, Division of Food Science and Biotechnology, Graduate School of Agriculture,
Kyoto University, Uji, Kyoto, 611-0011, Japan Haruka Sugiura, Ayumi Nagase, Sayoko Oiki, Daisuke Watanabe & Wataru Hashimoto * Laboratory of Applied Structural Biology, Division of
Applied Life Sciences, Graduate School of Agriculture, Kyoto University, Uji, Kyoto, 611-0011, Japan Bunzo Mikami Authors * Haruka Sugiura View author publications You can also search for
this author inPubMed Google Scholar * Ayumi Nagase View author publications You can also search for this author inPubMed Google Scholar * Sayoko Oiki View author publications You can also
search for this author inPubMed Google Scholar * Bunzo Mikami View author publications You can also search for this author inPubMed Google Scholar * Daisuke Watanabe View author publications
You can also search for this author inPubMed Google Scholar * Wataru Hashimoto View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS W.H.
designed the study; H.S., A.N., S.O., B.M., and W.H. performed the experiments; H.S., A.N., S.O., B.M., D.W., and W.H. analyzed the data; H.S., D.W., and W.H. wrote the manuscript.
CORRESPONDING AUTHOR Correspondence to Wataru Hashimoto. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION 1. Supplementary
Video 1. Supplementary Video S1. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise
in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the
permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sugiura, H., Nagase, A., Oiki, S. _et al._ Bacterial inducible expression of plant cell wall-binding protein YesO through conflict between
_Glycine max_ and saprophytic _Bacillus subtilis_. _Sci Rep_ 10, 18691 (2020). https://doi.org/10.1038/s41598-020-75359-0 Download citation * Received: 11 July 2020 * Accepted: 07 October
2020 * Published: 29 October 2020 * DOI: https://doi.org/10.1038/s41598-020-75359-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
This Morning viewers ask 'what was that' as Holly Willoughby and Phillip Schofield 'replaced'This Morning viewers ask 'what was that' as Holly Willoughby and Phillip Schofield 'replaced'All eyes have been on the d...
Avengers endgame, spider-man far from home trailers tease mcu future?If you watch the Avengers Endgame trailer and then Spider-Man Far From Home’s they’re certainly very different. We know ...
Page not found – SmmsurgeThe page can’t be found.It looks like nothing was found at this location....
Susan calman health: her mental health is no laughing matterAppearing on BBC Radio 4's The News Quiz and I'm Sorry I Haven't a Clue, Susan Calman is well-versed in w...
Enthusiasts commemorate the ride of captain jack, celebrate 'meck dec day'“Meck Dec Day,” a holiday in Charlotte and Mecklenburg County that commemorates the signing of the Mecklenburg Declarati...
Latests News
Bacterial inducible expression of plant cell wall-binding protein yeso through conflict between glycine max and saprophytic bacillus subtilisABSTRACT Saprophytic bacteria and plants compete for limited nutrient sources. _Bacillus subtilis_ grows well on steamed...
COMING ATTRACTIONS - Los Angeles Times<i> Arts and entertainment reports from The Times, national and international news services and the nation's ...
Is your house protected? Twenty five per cent of brits have no home seEarlier this week Sara Thornton - the head of the new National Police Chiefs' Council - revealed budget cuts and th...
Walker's $47,300 leads in torrance fund-raising raceDevelopers, public employee groups and companies doing business with the city made major contributions to Torrance City ...
The nba teams that leveled up — and found bargains — in free agencyNBA THE NBA TEAMS THAT LEVELED UP — AND FOUND BARGAINS — IN FREE AGENCY By Jared Dubin Jul. 7, 2022, at 6:00 AM On Wedne...