Inhibition of mtor delayed but could not prevent experimental collapsing focal segmental glomerulosclerosis
Inhibition of mtor delayed but could not prevent experimental collapsing focal segmental glomerulosclerosis"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Anti-Thy1.1 transgenic mice develop glomerular lesions that mimic collapsing focal segmental glomerulosclerosis (FSGS) in humans with collapse of the glomerular tuft and marked
hyperplasia of the parietal epithelial cells (PECs). Immunostaining of phosphor-S6 ribosomal protein (pS6RP) revealed high mTOR activity in PECs of the FSGS lesions of these mice. In this
study we questioned whether the mTOR inhibitor rapamycin (sirolimus) could attenuate the development and progression of glomerulosclerotic lesions in the anti-Thy1.1 transgenic mice. We
observed reduced mTOR signalling and proliferation in human parietal epithelial cells after rapamycin treatment. Experiments with anti-Thy1.1. mice showed that early treatment with sirolimus
reduced the development of glomerular lesions and glomerular cell proliferation at day 4. Levels of albuminuria, podocyte injury and podocyte number were similar in the sirolimus and
vehicle treated groups. The initial beneficial effects of sirolimus treatment were not observed at day 7. Late sirolimus treatment did not reduce albuminuria or the progression of
glomerulosclerosis. Taken together, rapamycin attenuated PEC proliferation and the formation of early FSGS lesions in experimental FSGS and reduced human PEC proliferation _in vitro_.
However, the initial inhibition of PEC proliferation did not translate into a decline of albuminuria nor in a sustained reduction in sclerotic lesions. SIMILAR CONTENT BEING VIEWED BY OTHERS
MINNELIDE COMBINED WITH ANTI-ANGPTL3-FLD MONOCLONAL ANTIBODY COMPLETELY PROTECTS MICE WITH ADRIAMYCIN NEPHROPATHY BY PROMOTING AUTOPHAGY AND INHIBITING APOPTOSIS Article Open access 09
September 2023 SYSTEMIC GENE THERAPY WITH THYMOSIN Β4 ALLEVIATES GLOMERULAR INJURY IN MICE Article Open access 16 July 2022 ANTI-ANGPTL3-FLD MONOCLONAL ANTIBODY TREATMENT AMELIORATES
PODOCYTE LESIONS THROUGH ATTENUATING MITOCHONDRIAL DAMAGE Article Open access 13 October 2022 INTRODUCTION Focal segmental glomerulosclerosis (FSGS) is characterized by the formation of
sclerotic lesions in the glomeruli of the kidneys. FSGS is one of the most common glomerular disorders and the leading cause of end-stage renal disease (ESRD) in the United States1. Several
underlying conditions can lead to FSGS such as diabetes, hypertension and obesity. In addition, FSGS can be caused by genetic mutations affecting the function of essential glomerular cell
proteins or it can be idiopathic1,2. The diagnosis of FSGS is largely based on histopathological findings characterized by the adhesions of the Bowman’s capsule with the glomerular tuft, the
formation of focal and segmental sclerotic lesions, obliteration of glomerular capillaries and extracellular matrix accumulation3,4. Currently, the different histological patterns of FSGS
have been divided into five subvariants: the perihilar-, the tip -, the cellular-, the NOS (not otherwise specified)-, and the collapsing variant, latter is characterized by collapse of the
glomerular tuft and PEC hyperplasia2. The pathogenesis of FSGS is not completely unravelled. In the last decade we and others demonstrated that parietal epithelial cells (PECs) are crucially
involved in the formation of sclerotic lesions. Genetic tagging of podocytes or PECs in different models of FSGS showed that upon induction of glomerular injury PECs migrate to the
capillary tuft and induce the adhesion between Bowman’s capsule and the tuft5,6. These activated PECs invade the injured area and deposit extracellular matrix that results in the progression
of FSGS7,8. In addition, similar pathohistological patterns were observed in secondary FSGS that are independent of the underlying disease9. These findings strengthen the assumption that
common molecular pathways lead to PEC activation and subsequently to FSGS formation and progression. Hamatani and colleagues observed activation of the mammalian target of rapamycin (mTOR),
a main player in cell proliferation and survival, specifically in PECs in experimental models of glomerular diseases. They observed that a reduction of the stress-inducible protein sestrin-2
in PECs was associated with increased pS6RP expression as well as de _novo_ expression of the activation marker CD4410. The results from Hamatani _et al_. suggested that mTOR signalling
plays an important role in the switch from resting to activated PECs seen in progressive glomerular disease. Therefore, mTOR might be a pharmaceutical target to prevent or reduce PEC
activation and subsequently glomerulosclerosis formation. In the present study we investigated this hypothesis and studied the effects of mTOR inhibition on immortalized human parietal
epithelial cells and in anti-Thy1.1 transgenic mice, a model resembling collapsing FSGS in humans. RESULTS RAPAMYCIN REDUCES MTOR SIGNALLING AND CELL PROLIFERATION OF HUMAN PARIETAL
EPITHELIAL CELLS Since we hypothesized that mTOR signalling is important for PEC proliferation in FSGS, we investigated the effect of the mTOR inhibitor rapamycin on cultured human
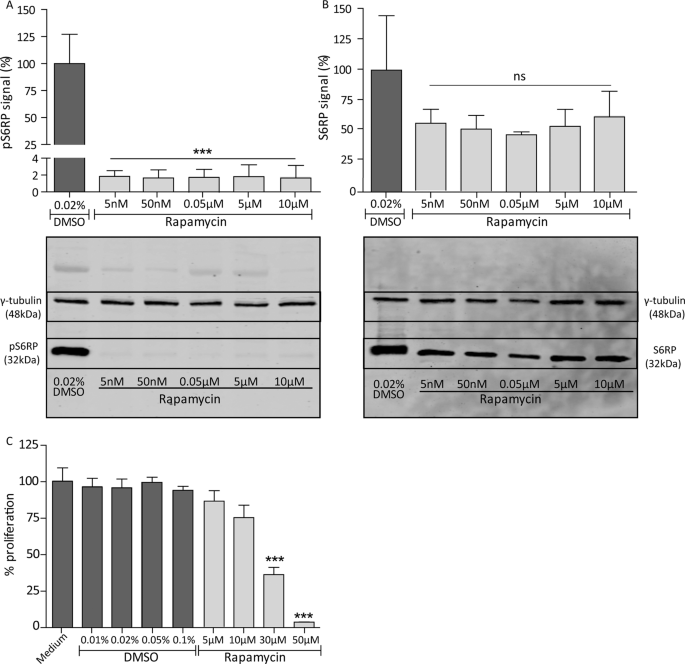
immortalized PECs. The expression of pS6RP as readout for mTOR signalling and PEC proliferation was studied, when exposed to different concentrations of rapamycin or DMSO as control. Already
at a concentration of 5 nM rapamycin the expression of the pS6RP was reduced (Fig. 1A, p < 0.001). The non-phosphorylated protein was not significantly affected (Fig. 1B). In addition,
higher concentrations of rapamycin (≥30 µM) reduced PEC proliferation (Fig. 1C, p < 0.001). These findings show that rapamycin can target PECs directly _in vitro_. As PEC proliferation is
described to be related to CD44 expression11, the effect of rapamycin on CD44 gene and protein expression was studied (Supplementary Fig. S1). The results indicate that the inhibition of
PEC proliferation after rapamycin treatment is independent of the expression level of CD44. MTOR ACTIVITY IS INCREASED IN SCLEROTIC LESIONS To study the effect of mTOR inhibition in PECs _in
vivo_ we used the transgenic anti-Thy1.1 mouse, an experimental model for collapsing FSGS. Injection of the anti-Thy1.1 antibody in the transgenic Thy1.1 mice results in an almost immediate
development of albuminuria that peaks at day 1 after injection12. Within 7 days the FSGS lesions, that resemble collapsing FSGS and are associated with hyperplasia of the PECs5,12,13, are
formed. Early stages of the lesions can already be observed after 4 days13. Immunostaining of the podocyte marker synaptopodin, the PEC marker SSeCKS14 and pS6RP as marker for mTOR
signalling revealed an increased pS6RP expression in PECs in the affected glomeruli of the anti-Thy1.1 mice, while in morphologically normal glomeruli only few cells showed mTOR signalling
(Fig. 2). These results suggest that increased mTOR activity may play a role in PEC activation and disease progression in the anti-Thy1.1. mice. SIROLIMUS TREATMENT DOES NOT REDUCE
ALBUMINURIA AND PODOCYTE DAMAGE We evaluated in the anti-Thy1.1 mouse model the effect of sirolimus on the development of the first visible glomerular lesions at day 4 and the fully
developed lesions at day 7. Following sirolimus treatment, albuminuria was not reduced at day 4 (Fig. 3A) and day 7 (Fig. 3B), while binding of the anti-Thy1.1 antibody was equal in the
sirolimus and phosal (vehicle) treated mice (data not shown). Immunofluorescent staining for synaptopodin and the podocyte injury marker desmin (Fig. 3C–E) showed no differences in the
amount of podocyte injury between the phosal and sirolimus treated mice at day 4 (Fig. 3F). In addition, immunofluorescent staining for DACH1 in combination with synaptopodin (Fig. 3G–I)
revealed that the number of podocytes was similar in sirolimus and phosal treated mice (Fig. 3J). These results indicate that early mTOR inhibition did not have an effect on the degree of
podocyte injury, podocyte number and proteinuria in the anti-Thy1.1 mouse model. SIROLIMUS REDUCES GLOMERULAR DAMAGE AND CELL PROLIFERATION AT DAY 4 Despite similar levels of albuminuria and
podocyte injury between the phosal and sirolimus treated mice, we observed less affected glomeruli in mice treated with sirolimus compared to the control mice at day 4 (Fig. 4A). Glomeruli
were considered affected when they showed one of the following histological changes: vacuolization of the epithelial cells, hyalinosis or epithelial cell hyperplasia (Supplementary Fig. S2).
The majority of phosal treated mice had a more severe histological phenotype showing more protein casts and affected glomeruli (Fig. 4C). The affected glomeruli of sirolimus treated mice
mainly appeared with vacuolization and mild cell proliferation. However, the greatest part of glomeruli was unaffected (Fig. 4E). In addition, we detected fewer proliferating (ki-67
positive) cells in the Bowman’s space in sirolimus treated mice, indicating less PEC proliferation (Fig. 4B, D, F). The sirolimus treated mice also showed fewer proliferating cells in the
glomerular tuft (Fig. 4B). As proliferating endothelial cells were identified in the mice (Supplementary Fig. S3), their proliferation was probably also reduced due to sirolimus treatment.
_In vitro_ we showed that rapamycin indeed has a direct effect on the glomerular endothelial cell proliferation (Supplementary Fig. S4). SIROLIMUS DOES NOT REDUCE GLOMERULAR DAMAGE AND CELL
PROLIFERATION AT DAY 7 As sirolimus reduced PEC proliferation and the formation of lesions during the early proliferative phases of FSGS development, we next investigated whether early
sirolimus treatment sustained reduction of sclerotic lesions. However, the number of affected glomeruli at day 7 in the phosal and sirolimus treated groups was similar (Fig. 5A).
Histological analysis revealed that hyalinosis was clearly visible in the glomeruli and that tubular protein casts could be observed in both the phosal and sirolimus treated mice. In
addition, the majority of affected glomeruli showed extensive PEC proliferation (Fig. 5C–F). Notably, the sirolimus treated mice showed the highest number of ki-67 positive cells in Bowman’s
space (Fig. 5B). SIROLIMUS TREATMENT AFTER GLOMERULOSCLEROSIS DEVELOPMENT DOES NOT REDUCE ALBUMINURIA AND SCLEROSIS To evaluate whether mTOR inhibition can attenuate the progression of
glomerulosclerosis in a later phase of disease development, we performed an experiment treating anti-Thy1.1 mice daily with sirolimus or phosal for a period of 20 days. Knowing that
sclerotic lesions are formed within the first 7 days after anti-Thy1.1 antibody injection13, we started sirolimus and phosal treatment at day 11. Comparing the albuminuria levels of the
sirolimus and phosal treated mice, no significant differences could be observed (Fig. 6A). Furthermore, the number of sclerotic glomeruli was similar (Fig. 6B). In general, the kidney tissue
of all mice showed influx of immune cells in the glomeruli and the interstitium. Hyalinosis and collapsing tufts could be detected in the glomeruli (Fig. 6C, D). Some glomeruli still
displayed some PEC proliferation. The results indicate that mTOR inhibition did not have an effect on the glomerular pathology once the glomerular lesions are formed. In general, our
findings indicate that sirolimus reduces PEC proliferation and formation of lesions in the early proliferative phase of FSGS development but cannot prevent the further development of
glomerulosclerosis. DISCUSSION In this study, we tested the hypothesis that mTOR signalling regulates PEC activation and that mTOR inhibition may prevent PEC proliferation and subsequently
glomerulosclerosis in the anti-Thy1.1 mouse model, an experimental model of collapsing FSGS. We observed an increased mTOR activity, expressed as the phosphorylation of S6RP, in affected
glomeruli of the anti-Thy1.1 mice. Especially PECs showed a striking increase in pS6RP expression. Sirolimus treatment reduced the number of affected glomeruli, which was associated with
less PEC proliferation, in the early lesions seen at day 4. The reduced PEC proliferation could be a direct effect of rapamycin on PECs, since also rapamycin treated PECs in culture showed
reduced pS6RP levels and reduced proliferation. In addition, the effects on PECs were independent of podocytes as the sirolimus treatment did not have an effect on albuminuria, the number of
podocytes or podocyte injury. However, despite the marked effects of rapamycin in culture and in the development of the early lesions in the mice, continuation of the treatment with
sirolimus could not prevent glomerulosclerosis at day 7. Also sirolimus treatment after sclerosis development could not reduce lesion formation. Recent studies indicated that in the
formation of lesions in FSGS and crescentic glomerulonephritis the PECs become activated5,9. The normal quiescent flat cells become enlarged and show different marker expression, i.e. a _de
novo_ expression of CD448,11,15. Although, we and others have established the involvement of PECs in the development of glomerular lesions, still little is known about the molecular
processes driving PEC activation16. Several studies have described a role for mTOR in glomerular disease models. Although, most of these studies focused on mTOR signalling in
podocytes17,18,19,20,21, a few studies have studied the pathway in PECs10,22,23. These studies indicated that mTOR signalling might be involved in PEC activation and therefore the
development of sclerotic lesions. This study shows a link between mTOR signalling and PEC activation as well. Inhibiting mTOR in the anti-Thy1.1 mice reduced PEC proliferation and sclerosis
formation at day 4. However, it was striking that the beneficial effects of mTOR signalling were not seen in later phases of the disease. These contradictory findings can also be seen in
other studies that investigated mTOR signalling in PECs. In general, all studies, including this work, showed that the effects of mTOR inhibition are dependent on the dose of the mTOR
inhibitor, the animal model used, the route of administration and on the onset and duration of treatment. Similar to our observations, Kurayama _et al_. showed that the outcome of mTOR
inhibition is dependent on the onset and duration of the mTOR inhibition. The study demonstrated that in a rat model of crescentic nephritis early treatment (day of disease induction) with
everolimus led to increased cellular necrosis. However, later treatment (7 days after disease induction) reduced glomerular crescent formation22. Since the anti-Thy1.1 model and the
nephritis model are different models of treatment strategies and time, the observed effects are difficult to compare to our findings. Nevertheless, both studies show that the timing of
treatment is critical. Besides the timing, also the dose of rapamycin is of importance for the treatment results. From seminal studies performed by Gödel and co-workers it has become clear
that mTOR signalling is tightly regulated and that an imbalance in mTOR activity may lead to adverse effects, facilitating glomerular disease17. In their study Gödel and colleagues deleted
the regulatory-associated protein (Raptor), an essential adaptor molecule of mTOR, in podocytes of diabetic mice. They demonstrated that complete reduction of mTORC1 in RaptorΔpodocyte mice
resulted in proteinuria and the progression of diabetic nephropathy, while deletion of only one allele of Raptor (RaptorHet podocyte) inhibited mTOR activation, leading to decreased
proteinuria and glomerulosclerosis17. Hence, it was demonstrated that complete reduction of mTOR activity can be responsible for the progression of glomerular injury. This means that the
reduction of mTOR activity to prevent disease progression should be done with care, as a too strong reduction can have deleterious effects. This conclusion can also be drawn from a similar
study performed by Zschiedrich _et al_. They showed that a homozygous podocyte specific knockout (KO) of Raptor in an adriamycin induced nephropathy mouse model caused massive proteinuria
and sclerosis, while a heterozygous KO resulted in a reduction of proteinuria and sclerosis21. They also showed that a high dose of administered rapamycin has a similar effect as a complete
Raptor KO, while low rapamycin concentrations resembled the beneficial outcome of the heterozygous KO, again stressing the tight regulation of mTOR in the glomerulus. The possible decrease
in endothelial proliferation due to mTOR inhibition might have contributed to the recurrent sclerosis formation observed in our experiment, as reduced endothelial cell proliferation
presumably impairs endothelial repair of capillaries that normally is required after glomerular damage due to anti-Thy1.1 injection24. Due to the narrow therapeutic window of sirolimus, and
in the murine studies described problems of treatment onset and duration, the implementation of sirolimus into the clinic to treat glomerulosclerosis will be challenging. Sirolimus and other
rapamycin analogues (rapalogous) are approved by the Food and Drug administration (FDA) for the treatment of cancers, transplant rejection, lymphangioleiomyomatosis and tuberous sclerosis
complex25. Therapeutic effects of sirolimus or rapalogous are often hampered due to recurrence of the disease assumably caused by negative feedback loops. To prevent this, treatments
combining sirolimus with drugs targeting the pathways involved in the negative feedback (e.g. Akt pathway), seem more promising25. Also, ATP-competitive mTOR inhibitors are developed that
prevent the feedback-mediated Akt activation26. Although the treatment with mTOR inhibitors can have beneficial effects, it is often accompanied with severe side effects such as
haematological abnormalities, nephrotoxicity, dermatological diseases, impaired wound healing and metabolic disorders27, which makes long lasting treatment difficult. In conclusion, this
study shows that rapamycin can have a direct effect on parietal epithelial cells, which _in vivo_ can lead to a short-lasting reduction of PEC proliferation and sclerotic lesions. Our
findings point out that an elevated mTOR expression in parietal epithelial cells might contribute to PEC activation and the formation of glomerulosclerosis. However, literature and this
study show that within the glomerulus mTOR signalling is tightly regulated, resulting in a narrow therapeutic window of rapamycin. When not used in optimal concentrations, as well as in the
favourable time window for treatment, mTOR inhibition can have no beneficial or even deleterious effects. In addition, systemic administration of the inhibitor may have non-specific effects,
decreasing mTOR inhibition in other cells resulting in for instance prevention of normal kidney repair functions. Whether ATP-competitive mTOR inhibitors or combination treatments of
sirolimus and drugs targeting pathways involved in negative feedback loops could decrease sclerosis formation permanently evoking tolerating side effects has to be investigated. MATERIAL AND
METHODS CELL CULTURE Immortalized polyclonal human parietal epithelial cells (hPECs) were cultured as described in Kietzmann _et al_.28. HPECs were differentiated for 2 weeks at 37 °C, 5%
(v/v) CO2. Conditionally immortalized glomerular endothelial cells (ciGENCs, clone 1D4) were kindly provided by Simon C. Satchell29. Details about the culture conditions can be found online
in the supplementary materials. ANIMAL STUDIES All experiments were performed using 6–8 weeks old male Thy1.1 transgenic mice from own breeding. These mice have been backcrossed into the
C57BL/6 J for 10 generations. The animal experiments were approved by the Animal Ethics Committee of the Radboud University Nijmegen and performed in accordance with the European Communities
Council Directive (86/609/EEC). The setup of the animal experiments is shown in Fig. 7. More details can be found online in the supplementary materials. ALBUMIN/CREATININE RATIO (ACR) Urine
albumin of the mice was determined using the Mancini immunodiffusion method. The creatinine values were determined at the Radboudumc laboratory of diagnostics (RLD), Radboudumc, Nijmegen,
the Netherlands, using a modulation analyser Cobas C8000-1/-2 (Roche Diagnostics). WESTERN BLOTTING RIPA buffer (Cell signaling, #9806) supplemented with 1 mM protease inhibitor
phenylmethylsulfonyl fluoride (PMSF) was used for protein extraction of hPECs and ciGENCs. Protein concentrations were determined using the Pierce™ BCA Protein Assay Kit (Thermo Fischer
Scientific). Samples were prepared using 4x Laemmli buffer (Bio-Rad) supplemented with beta-mercaptoethanol. Per sample, 25 µg protein was loaded on a 12% (v/v) Sodium Dodecyl
Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) gel and transferred to a nitrocellulose membrane. Antibodies used to detect the pS6RP, the S6RP and the housekeeping gene γ-tubulin are
shown in Table 1. These antibodies are well established and commonly used18,19,21,30,31,32,33. The fluorescent signal was analysed with the Odyssey Imaging System (LI-COR Biosciences) and
Odyssey Image studio Software (version 4.0). BROMODEOXYURIDINE (BRDU) ASSAY We performed the BrdU proliferation ELISA, according to manufacturer’s protocol (BrdU Cell Proliferation ELISA
Kit, Abcam, ab126556). Cells were treated with different rapamycin concentrations and their respective DMSO controls for 24 hours. The BrdU antibody was incubated for 15 hours. The hPECs
were seeded in a density of 20,000 cells/cm2 two days prior to rapamycin or DMSO exposure, ciGENCs were seeded in a density of 20,000 cells/cm2 4 days before starting the experiment. TISSUE
STAINING All stainings were performed on 4 µm formalin-fixed paraffin-embedded (FFPE) kidney sections. Antigen retrieval was performed in citrate buffer (pH 6) or Tris-EDTA buffer (pH 9) at
boiling point for 13 minutes. Details about primary- and matching secondary antibodies used are described in Table 1. Fluorescent stained slices were mounted in DAPI Fluoromount-G®
(SouthernBiotech). Co-staining of synaptopodin and desmin was performed to detect injured podocytes. The synaptopodin antibody used was detected with Alexa Fluor donkey anti-goat 546
followed by a 10% (v/v) goat serum in PBS blocking step. Next, the secondary antibody against desmin was applied. To assess pS6RP in podocytes and PECs a triple staining was performed. The
P-S6 Ribosomal Protein (S235/236) antibody was visualized using the Tyramide Signal Amplification (TSA) kit #16 (Molecular probes, life technologies, T20926). Subsequently podocytes and PECs
were stained with antibodies against synaptopodin and SSeCKS, respectively. To detect proliferating endothelial cells, sections were incubated with CD34 and ki-67 antibodies. Secondary
antibodies were used as shown in Table 1. For the detection of podocyte nuclei, sections were incubated with the nuclear marker DACH1 combined with the podocyte maker synaptopodin. The tuft
area was measured using CaseViewer software (3DHISTECH Ltd.) and DACH1 positive cells in the glomeruli were counted. A minimum of 48 glomeruli and a maximum of 110 glomeruli per mouse were
scored. From each scored glomerulus we calculated the ratio of DACH1 positive podocytes per tuft area (mm2) and took the average of these ratios for each mouse. For detecting ki-67 positive
cells immunohistochemically, endogenous peroxidases as well as endogenous biotin, biotin receptors, and avidin were blocked using 3% (v/v) hydrogen peroxide and the Avidin/Biotin blocking
Kit (Vector laboratories SP-2001). As primary antibody anti-ki-67 antibody (Table 1) was used, which was detected using a biotinylated Goat Anti-Rabbit IgG Antibody (Vector Laboratories,
BA-1000) and the VECTASTAIN® Elite®ABC-HRP Kit (Vector laboratories, PK-6100). The 3,3′-diaminobenzidine (DAB) horseradish peroxidase was used as substrate. Periodic acid–Schiff (PAS)
staining without haematoxylin stain was performed afterwards. Per mouse, the ki-67 positive cells were counted in a minimum of 57 and a maximum of 114 glomeruli. A PAS staining was performed
to identify affected glomeruli. A minimum of 58 glomeruli and a maximum of 122 glomeruli per mouse were scored. STATISTICS The differences between sirolimus and phosal treated mice
concerning podocyte count, glomerular proliferation, the percentage of affected glomeruli and desmin expression was statistically tested using a two-tailed Mann-Whitney U test with a
confidence interval of 95%. Differences in the albumin/creatinine ratio was tested using a 2-way ANOVA including a Bonferroni post-test for the first experiment and for the second experiment
a two-tailed Mann-Whitney U test was used. For the _in vitro_ results we used a One-way ANOVA with a Bonferroni’s Multiple Comparison Test. A P value below 0.05 was considered as
significant. DATA AVAILABILITY The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request. Additional
information and data is available from the corresponding author on reasonable request. REFERENCES * Rosenberg, A. Z. & Kopp, J. B. Focal Segmental Glomerulosclerosis. _Clin J Am Soc
Nephrol_ 12, 502–517, https://doi.org/10.2215/CJN.05960616 (2017). Article CAS PubMed PubMed Central Google Scholar * D’Agati, V. D., Fogo, A. B., Bruijn, J. A. & Jennette, J. C.
Pathologic classification of focal segmental glomerulosclerosis: A working proposal. _American Journal of Kidney Diseases_ 43, 368–382, https://doi.org/10.1053/j.ajkd.2003.10.024 (2004).
Article PubMed Google Scholar * Fogo, A. B. Causes and pathogenesis of focal segmental glomerulosclerosis. _Nat Rev Nephrol_ 11, 76–87, https://doi.org/10.1038/nrneph.2014.216 (2015).
Article CAS PubMed Google Scholar * Meliambro, K., Schwartzman, M., Cravedi, P. & Campbell, K. N. The Impact of Histologic Variants on FSGS Outcomes. _Int Sch Res Notices_ 2014,
913690, https://doi.org/10.1155/2014/913690 (2014). Article PubMed PubMed Central Google Scholar * Smeets, B. _et al_. Tracing the origin of glomerular extracapillary lesions from
parietal epithelial cells. _J Am Soc Nephrol_ 20, 2604–2615, https://doi.org/10.1681/ASN.2009010122 (2009). Article PubMed PubMed Central Google Scholar * Suzuki, T. _et al_. Genetic
podocyte lineage reveals progressive podocytopenia with parietal cell hyperplasia in a murine model of cellular/collapsing focal segmental glomerulosclerosis. _Am J Pathol_ 174, 1675–1682,
https://doi.org/10.2353/ajpath.2009.080789 (2009). Article PubMed PubMed Central Google Scholar * Dijkman, H., Smeets, B., van der Laak, J., Steenbergen, E. & Wetzels, J. The
parietal epithelial cell is crucially involved in human idiopathic focal segmental glomerulosclerosis. _Kidney Int_ 68, 1562–1572, https://doi.org/10.1111/j.1523-1755.2005.00568.x (2005).
Article PubMed Google Scholar * Smeets, B. _et al_. Parietal epithelial cells participate in the formation of sclerotic lesions in focal segmental glomerulosclerosis. _J Am Soc Nephrol_
22, 1262–1274, https://doi.org/10.1681/ASN.2010090970 (2011). Article CAS PubMed PubMed Central Google Scholar * Kuppe, C. _et al_. Common histological patterns in glomerular epithelial
cells in secondary focal segmental glomerulosclerosis. _Kidney Int_ 88, 990–998, https://doi.org/10.1038/ki.2015.116 (2015). Article CAS PubMed Google Scholar * Hamatani, H. _et al_.
Expression of a novel stress-inducible protein, sestrin 2, in rat glomerular parietal epithelial cells. _Am J Physiol Renal Physiol_ 307, F708–717,
https://doi.org/10.1152/ajprenal.00625.2013 (2014). Article CAS PubMed PubMed Central Google Scholar * Eymael, J. _et al_. CD44 is required for the pathogenesis of experimental
crescentic glomerulonephritis and collapsing focal segmental glomerulosclerosis. _Kidney Int_ 93, 626–642, https://doi.org/10.1016/j.kint.2017.09.020 (2018). Article CAS PubMed Google
Scholar * Smeets, B. _et al_. Podocyte changes upon induction of albuminuria in Thy-1.1 transgenic mice. _Nephrol Dial Transplant_ 18, 2524–2533 (2003). Article CAS Google Scholar *
Smeets, B. _et al_. The parietal epithelial cell: a key player in the pathogenesis of focal segmental glomerulosclerosis in Thy-1.1 transgenic mice. _J Am Soc Nephrol_ 15, 928–939 (2004).
Article Google Scholar * Burnworth, B. _et al_. SSeCKS sequesters cyclin D1 in glomerular parietal epithelial cells and influences proliferative injury in the glomerulus. _Lab Invest_ 92,
499–510, https://doi.org/10.1038/labinvest.2011.199 (2012). Article CAS PubMed Google Scholar * Fatima, H. _et al_. Parietal epithelial cell activation marker in early recurrence of FSGS
in the transplant. _Clin J Am Soc Nephrol_ 7, 1852–1858, https://doi.org/10.2215/CJN.10571011 (2012). Article PubMed PubMed Central Google Scholar * Miesen, L., Steenbergen, E. &
Smeets, B. Parietal cells-new perspectives in glomerular disease. _Cell Tissue Res_ 369, 237–244, https://doi.org/10.1007/s00441-017-2600-5 (2017). Article PubMed PubMed Central Google
Scholar * Godel, M. _et al_. Role of mTOR in podocyte function and diabetic nephropathy in humans and mice. _J Clin Invest_ 121, 2197–2209, https://doi.org/10.1172/JCI44774 (2011). Article
CAS PubMed PubMed Central Google Scholar * Inoki, K. _et al_. mTORC1 activation in podocytes is a critical step in the development of diabetic nephropathy in mice. _J Clin Invest_ 121,
2181–2196, https://doi.org/10.1172/JCI44771 (2011). Article CAS PubMed PubMed Central Google Scholar * Ito, N. _et al_. mTORC1 activation triggers the unfolded protein response in
podocytes and leads to nephrotic syndrome. _Lab Invest_ 91, 1584–1595, https://doi.org/10.1038/labinvest.2011.135 (2011). Article CAS PubMed Google Scholar * Mao, J. _et al_. Mammalian
target of rapamycin complex 1 activation in podocytes promotes cellular crescent formation. _Am J Physiol Renal Physiol_ 307, F1023–1032, https://doi.org/10.1152/ajprenal.00018.2014 (2014).
Article CAS PubMed Google Scholar * Zschiedrich, S. _et al_. Targeting mTOR Signaling Can Prevent the Progression of FSGS. _J Am Soc Nephrol_ 28, 2144–2157,
https://doi.org/10.1681/ASN.2016050519 (2017). Article CAS PubMed PubMed Central Google Scholar * Kurayama, R. _et al_. Role of amino acid transporter LAT2 in the activation of mTORC1
pathway and the pathogenesis of crescentic glomerulonephritis. _Lab Invest_ 91, 992–1006, https://doi.org/10.1038/labinvest.2011.43 (2011). Article CAS PubMed Google Scholar *
McNicholas, B. A. _et al_. Reducing mTOR augments parietal epithelial cell density in a model of acute podocyte depletion and in aged kidneys. _Am J Physiol Renal Physiol_ 311, F626–639,
https://doi.org/10.1152/ajprenal.00196.2016 (2016). Article CAS PubMed PubMed Central Google Scholar * Iruela-Arispe, L. _et al_. Participation of glomerular endothelial cells in the
capillary repair of glomerulonephritis. _Am J Pathol_ 147, 1715–1727 (1995). CAS PubMed PubMed Central Google Scholar * Li, J., Kim, S. G. & Blenis, J. Rapamycin: one drug, many
effects. _Cell Metab_ 19, 373–379, https://doi.org/10.1016/j.cmet.2014.01.001 (2014). Article CAS PubMed PubMed Central Google Scholar * Benjamin, D., Colombi, M., Moroni, C. &
Hall, M. N. Rapamycin passes the torch: a new generation of mTOR inhibitors. _Nat Rev Drug Discov_ 10, 868–880, https://doi.org/10.1038/nrd3531 (2011). Article CAS PubMed Google Scholar
* Pallet, N. & Legendre, C. Adverse events associated with mTOR inhibitors. _Expert Opin Drug Saf_ 12, 177–186, https://doi.org/10.1517/14740338.2013.752814 (2013). Article CAS PubMed
Google Scholar * Kietzmann, L. _et al_. MicroRNA-193a Regulates the Transdifferentiation of Human Parietal Epithelial Cells toward a Podocyte Phenotype. _J Am Soc Nephrol_ 26, 1389–1401,
https://doi.org/10.1681/ASN.2014020190 (2015). Article CAS PubMed Google Scholar * Satchell, S. C. _et al_. Conditionally immortalized human glomerular endothelial cells expressing
fenestrations in response to VEGF. _Kidney Int_ 69, 1633–1640, https://doi.org/10.1038/sj.ki.5000277 (2006). Article CAS PubMed Google Scholar * Daher, B. _et al_. Genetic Ablation of
the Cystine Transporter xCT in PDAC Cells Inhibits mTORC1, Growth, Survival, and Tumor Formation via Nutrient and Oxidative Stresses. _Cancer Res_ 79, 3877–3890,
https://doi.org/10.1158/0008-5472.CAN-18-3855 (2019). Article CAS PubMed Google Scholar * Mathieu, J. _et al_. Folliculin regulates mTORC1/2 and WNT pathways in early human pluripotency.
_Nat Commun_ 10, 632, https://doi.org/10.1038/s41467-018-08020-0 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Sunamura, N., Iwashita, S., Enomoto, K., Kadoshima, T.
& Isono, F. Loss of the fragile X mental retardation protein causes aberrant differentiation in human neural progenitor cells. _Sci Rep_ 8, 11585,
https://doi.org/10.1038/s41598-018-30025-4 (2018). Article ADS CAS PubMed PubMed Central Google Scholar * Ohashi, T., Yamamoto, T., Yamanashi, Y. & Ohsugi, M. Human TUBG2 gene is
expressed as two splice variant mRNA and involved in cell growth. _FEBS Lett_ 590, 1053–1063, https://doi.org/10.1002/1873-3468.12163 (2016). Article CAS PubMed Google Scholar Download
references ACKNOWLEDGEMENTS The authors wish to thank Rutger Maas, PhD, MD (Department of Nephrology, Radboudumc, Nijmegen) for the technical support with the ciGENC model. In addition we
kindly thank Simon C. Satchell, PhD (Academic Renal Unit, University of Bristol, UK) providing us with the conditionally immortalized glomerular endothelial cell line. The study was funded
by the Dutch Kidney foundation (grant 14A3D104) and The Netherlands Organization for Scientific Research (NWO VIDI grant: 016.156.363). AUTHOR INFORMATION Author notes * These authors
contributed equally: Johan van der Vlag and Bart Smeets. AUTHORS AND AFFILIATIONS * Department of pathology, Radboud Institute for Molecular Life Sciences, Radboud university medical center,
Nijmegen, The Netherlands Laura Miesen, Jennifer Eymael, Brigith Willemsen, Fieke Mooren, Henry Dijkman, Jitske Jansen & Bart Smeets * School of Biomedical Sciences, University of
Plymouth, Plymouth, UK Shagun Sharma * Department of nephrology, Radboud Institute for Molecular Life Sciences, Radboud Institute for Health Sciences, Radboud university medical center,
Nijmegen, The Netherlands Shagun Sharma, Markus A. Loeven, Marinka Bakker-van Bebber, Jack F. M. Wetzels & Johan van der Vlag * Institute of Cellular and Integrative Physiology, Center
for Experimental Medicine, University Medical Center Hamburg -Eppendorf, Hamburg, Germany Catherine Meyer-Schwesinger * Department of pediatric nephrology, Radboud Institute for Molecular
Life Sciences, Radboud university medical center, Amalia Children’s Hospital, Nijmegen, The Netherlands Jitske Jansen Authors * Laura Miesen View author publications You can also search for
this author inPubMed Google Scholar * Jennifer Eymael View author publications You can also search for this author inPubMed Google Scholar * Shagun Sharma View author publications You can
also search for this author inPubMed Google Scholar * Markus A. Loeven View author publications You can also search for this author inPubMed Google Scholar * Brigith Willemsen View author
publications You can also search for this author inPubMed Google Scholar * Marinka Bakker-van Bebber View author publications You can also search for this author inPubMed Google Scholar *
Fieke Mooren View author publications You can also search for this author inPubMed Google Scholar * Catherine Meyer-Schwesinger View author publications You can also search for this author
inPubMed Google Scholar * Henry Dijkman View author publications You can also search for this author inPubMed Google Scholar * Jack F. M. Wetzels View author publications You can also search
for this author inPubMed Google Scholar * Jitske Jansen View author publications You can also search for this author inPubMed Google Scholar * Johan van der Vlag View author publications
You can also search for this author inPubMed Google Scholar * Bart Smeets View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS L.M. carried out
the experiments and was supported by J.E., S.S., B.W., F.M., H.D., M.B.v.B. and M.A.L., C.M.S. provided the used PEC cell line. L.M wrote the manuscript with support of J.E., H.D., C.M.S,
J.F.M.W., J.J., J.v.d.V. and B.S. J.F.M.W, J.v.d.V and B.S. developed the theory and supervised the project. CORRESPONDING AUTHOR Correspondence to Bart Smeets. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIALS. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Miesen, L., Eymael, J., Sharma, S. _et al._ Inhibition of mTOR delayed but could
not prevent experimental collapsing focal segmental glomerulosclerosis. _Sci Rep_ 10, 8580 (2020). https://doi.org/10.1038/s41598-020-65352-y Download citation * Received: 20 September 2019
* Accepted: 30 April 2020 * Published: 22 May 2020 * DOI: https://doi.org/10.1038/s41598-020-65352-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Attention Required! | CloudflarePlease enable cookies. Sorry, you have been blocked You are unable to access portaltemponovo.com.br Why have I been bloc...
Page Not FoundPage Not Found The content that you're looking for is unavailable. You might find what you are looking for by using the ...
‘shark tank’ star daymond john signs first-look deal with audible‘Shark Tank’ Star Daymond John Signs First-Look Deal With Audible You will be redirected back to your article in seconds...
Maggi controversy: priety zinta breaks her silence on the fiascoThe actress was sued for doing the Maggi endorsement 12 years back. Priety took to Twitter to break her silence on the e...
Cannon's investors advised to press for its liquidation : analyst cites debt and sec scrutiny of german deal, but film maker's chief remains upbeat, tDebt-laden Cannon Group is under new pressure from a small New York investment firm, which Friday mailed a report to cli...
Latests News
Inhibition of mtor delayed but could not prevent experimental collapsing focal segmental glomerulosclerosisABSTRACT Anti-Thy1.1 transgenic mice develop glomerular lesions that mimic collapsing focal segmental glomerulosclerosis...
‘the crown’s josh o’connor says creator peter morgan “doesn’t go for the stereotype” – contenders tv: the nomineesPeter Morgan’s _The Crown_ showed that the fourth time might yet be a charm for the Netflix series, which tied with Disn...
‘chicago med’: yaya dacosta & torrey devitto exit nbc drama, remaining original cast members to returnEXCLUSIVE: As NBC‘s _Chicago Med_ is heading into its seventh season, the medical drama is bidding farewell to two origi...
Did 'liberals' set up a gofundme campaign for murder suspect cristhian rivera?Claim: In August 2018, "liberals" started a GoFundMe campaign to raise murder suspect Cristhian Rivera's ...
America's highest uninsured rate is in... Dfw?Last Friday in Dallas’ Oak Lawn neighborhood, a former college football player, a global expert on walkable cities, a 57...