Three-dimensional observation and analysis of remineralization in dentinal caries lesions
Three-dimensional observation and analysis of remineralization in dentinal caries lesions"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The remineralization mechanism in dental caries lesions is not completely understood. This study reports on ultrastructural and chemical changes observed within arrested caries
lesions. Carious human teeth were observed using scanning electron microscopy (SEM) and focused-ion-beam (FIB)-SEM. The crystals detected in the caries lesions were characterized by
transmission electron microscopy (TEM), along with chemical element mapping using energy-dispersive spectroscopy (EDS)-STEM. FIB-SEM 3D reconstructions revealed a severely damaged dentin
surface abundantly covered by bacteria. Although the dentin tubules were clogged up to a depth of 100 μm, bacterial invasion into dentin tubules was not observed. TEM crystal analysis and
EDS-STEM revealed the presence of Ca and P, as well as of Mg within the HAp crystals deposited inside the dentin tubules. It was concluded that extensive remineralization with deposition of
Mg-HAp crystals had occurred in dentin tubules of caries-arrested dentin. Understanding the natural remineralization process is thought to be helpful for developing clinical biomimetic
remineralization protocols. SIMILAR CONTENT BEING VIEWED BY OTHERS COMPARISON OF CALCIUM-BASED TECHNOLOGIES TO REMINERALISE ENAMEL SUBSURFACE LESIONS USING MICRORADIOGRAPHY AND MICROHARDNESS
Article Open access 14 June 2022 INFLUENCE OF HYDROXYAPATITE NANOPARTICLES ON THE FORMATION OF CALCIUM FLUORIDE SURFACE LAYER ON ENAMEL AND DENTINE IN VITRO Article Open access 20 October
2022 REMINERALIZATION OF EARLY ENAMEL LESIONS WITH A NOVEL PREPARED TRICALCIUM SILICATE PASTE Article Open access 15 June 2022 INTRODUCTION Dental caries is the most prevalent health
condition in the world1,2. This disease is a dysbiosis caused by undisturbed bacterial accumulations, which can be up regulated by the presence of fermentable carbohydrates and down
regulated by fluoride. Although salivary flow rate has its role in the process, the general and main etiologic cause is undoubtedly undisturbed biofilm accumulations with release of
metabolic acids leading to substantial mineral loss from the tooth structure3,4. When the pH of the oral cavity is neutral and saliva is sufficiently saturated with calcium and phosphate,
remineralization by mineral deposition will occur5. The caries process is well documented to progress when the balance between demineralization and remineralization is disturbed6. However,
the process of caries initiation is not yet fully described and understood. Remineralization of carious dentin has been investigated before. Lesters and Boyde6 documented the crystal
formation and described the crystal structure of enamel and dentinal caries using SEM and TEM. Ogawa _et al_. investigated the transparent and sub-transparent layers of caries lesions by SEM
and measured the different layer’s surface hardness. They found that apatite was deposited in the tubules of the transparent and sub-transparent caries layers7. Frank and Voegel8 also
observed partially and completely calcified dentin tubules in the area between the translucent zone of carious dentin and inner dentin. However, they did not report the presence of
remineralized dentin in the immediate surrounding of the carious lesion. Later, several researchers documented the crystal structure of demineralized dentin using TEM8,9,10,11. Zavgorodny
_et al_.10,11 observed a transparent zone close to the demineralized caries area. This transparent zone constitutes carious dentinal tissue with intratubular mineralization. In their study,
remineralization occurred only partially and did not completely clog the dentinal tubules. Although many of these researches involved nano-scale observations of remineralization, the 3D
structural organization of naturally remineralized dentin was not completely disclosed. Counteracting caries development, active bio-remineralization using calcium phosphate compounds or
bioactive glasses has thoroughly been studied12. Casein phosphate is for instance known to inhibit demineralization of enamel _in vitro_; clinical evidence of remineralization of enamel and
dentin by casein phosphate is however today still lacking13. Likewise, bioactive glass was shown to have remineralization potential _in vitro_, while this effect has so far not been proven
_in vivo_14. Another remineralization strategy concerns biomimetic analogs of noncollagenous proteins2,15. Investigating remineralization, most studies have employed SEM and TEM to study _in
vitro_ remineralization in 2D, while the actual 3D process of natural _in vivo_ remineralization has not been completely described thus far. Hence, an in-depth _in/ex vivo_ study of caries
and the involved de- and remineralization processes in human teeth may be very helpful to develop clinical strategies to achieve efficient tooth remineralization. To better understand the
lateral extent and thickness of remineralization, 3D Focused-ion-beam Scanning Electron Microscopy (FIB-SEM) imaging was conducted in this study. FIB-SEM is a research technique enabling to
study the structure of 3D volumes by wide-area high-resolution 3D reconstruction of consecutively taken SEM photomicrographs. In the biological field, FIB-SEM has for instance been applied
to image nano-structures of cells such as at the tendon-bone interface16 and periodontal ligament17, and also to observe specific tooth-related aspects such as dentin cracks18 and
adhesive-tooth interfaces19. In addition, FIB-SEM is a useful tool for preparing TEM specimens of precisely selected areas20. TEM can provide high-resolution structural information along
with site-specific chemical analytic data when combined with Energy-Dispersive X-ray Spectrometry (EDS), as is commonly used in scanning TEM or STEM mode, and with crystallographic
information when combined with Selected Area Electron Diffraction (SAED). The primary purpose of this exploratory study was to characterize actual _in-vivo_ remineralization within dentinal
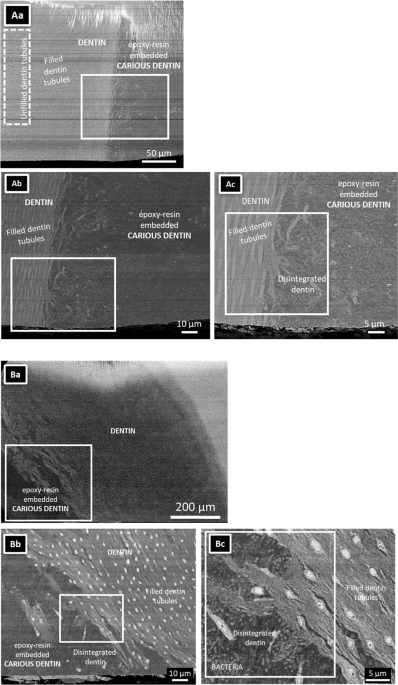
caries, correlatively utilizing structural FIB-SEM imaging with EDS-STEM chemical and SAED crystal analysis. RESULTS Low-magnification SEM disclosed an invasive dentinal caries lesion (Fig.
1Aa–c). Open dentin tubules were observed in dentin far away from the carious tissue (left side of the caries lesion), while filled tubules were observed near the caries lesion (Fig. 1Aa).
Higher-magnification SEM revealed a destroyed dentin structure at the caries lesion. Disintegrated dentin along with a considerable number of bacteria were embedded in epoxy resin (Fig.
1Ab). The dentin tubules near the caries lesion were filled with a high-density substance (Fig. 1Ac). SEM of the arrested caries lesion in the second tooth revealed a similar structure (Fig.
1Ba–c). Severely damaged dentin contained voids and bacteria, while the dentinal tubules were filled with a dense substance (Fig. 1Bc). 3D structural information was obtained by FIB-SEM,
showing that the dentinal tissue was severely damaged by caries (Fig. 2A1–8,B1–8). The white substance within the dentin tubules formed a rigid structure and extended clearly within each
dentin tubule. At the interface of dentin with the caries lesion, the dentinal tubule structure was fragmented, while the white tubule-filling substance remained nearly intact. Bacteria were
observed within dentin but did not penetrate into the dentin tubules (Supplemental Figure). EDS-STEM elemental mapping at high magnification revealed Ca, O, P and Mg within the highly
intratubular substance (Fig. 3A,B: arrows); this substance appeared much denser than intratubular dentin. The Ca/P ratio at sites 1 to 5 in Fig. 3A (arrows) was 1.70, 1.66, 1.70, 1.66 and
1.63, respectively, and that in Fig. 3B (arrow) was 1.61. This difference in density was confirmed by TEM (Fig. 4A,B). SAED confirmed the presence of hydroxyapatite (HAp) for all spots
marked (Fig. 4Aa–d,Ba–c)21,22,23. TEM confirmed the high density of the intratubular substance in contrast to the low density of dentin. SAED confirmed the presence of HAp in both the
intertubular dentin remnants and the intratubular substance21,22,23. DISCUSSION This study investigated the process of caries arrest due to remineralization within caries lesions by
correlative ultra-structural characterization using SEM, FIB-SEM and (S)TEM, combined with chemical analysis using EDS-STEM and SAED. First, we observed relatively large areas using SEM,
upon which we defined the area for FIB-SEM 3D structural analysis using a serial sectioning method20. Finally, we prepared TEM specimens for crystal identification, also using FIB-SEM.
FIB-SEM is often used for preparing TEM specimens, because areas with a spatial accuracy of ~10 nm can be selected20. In conventional FIB-SEM, the SEM and FIB optical axes intersect one
another at ~60°. When imaging serial-sectioned slice planes, proper alignment is crucial to reconstruct the specimen volume in 3D. Using orthogonal FIB-SEM, the incident secondary electron
beam is always perpendicular to the FIB-sliced plane. Hence, the contrast of the base plane becomes uniform and the entire dynamic range of the contrast can be used to observe the structure
itself, thereby enabling imaging with extremely high contrast24. All teeth exhibiting arrested caries lesions revealed severely destroyed dentin. Despite this dentin destruction, the
dentinal tubules near the interface with the caries lesion were filled with a highly dense substance (Fig. 1). Several researchers observed crystal-filled dentin tubules in a vertical
cross-section of the tubules25,26. 3D FIB-SEM reconstructions in this study clearly revealed precipitate growth in the dentin tubules, even when the surrounding dentin was severely destroyed
by caries. Moreover, these precipitate clusters kept their structure, even when separated from dentin due to the caries progression. Surprisingly, bacteria did not penetrate into the dentin
tubules, most likely because the tubules were filled at the surface with precipitates, this even though abundant bacteria were present in the dentinal caries. This finding agrees with the
observation by Daculsi _et al_.9. The progression of an arrested dentin caries has been reported to be slow26,27. This slow progression is probably related to the fact that bacteria are not
able to infiltrate the dentin tubules. The hardness of chronic/arrested caries is known to be larger than that of acute/active caries, although the hardness of chronic/arrested caries
remains below that of sound dentin28. This high hardness of chronic/arrested carious dentin is most likely caused by partial remineralization and crystal formation in the carious dentin.
Nucleation and growth of calcium phosphate are important to induce remineralization, which depends on the supersaturation and ionic strength of saliva or dentinal fluid. We could not observe
caries lesions and remineralization over time, so we cannot show direct mechanistic evidence of the remineralization process. Nevertheless, two possible mechanisms of intratubular
precipitate/crystal formation can be proposed. The first possible mechanism is that remineralization is produced by the odontoblast process or a physicochemical process activated by
odontoblast-bacterial interaction9. Frank and Voegel have demonstrated remineralization around odontoblast processes8. Essential for this mechanism is the presence of the odontoblast process
in the dentin tubule. Different studies have discussed the length of the odontoblast process within dentin tubules. Some researchers have reported that odontoblast processes solely remained
within the inner side of dentin, less than 1 mm from the pulp27, while other researchers have reported that the odontoblast process extends to the outer layer of dentin, even up to the
dentin-enamel junction29. In our study, odontoblast processes were not observed in the neighborhood of the precipitates. However, it is not known if odontoblast processes are solely
effective in their immediate environment with which they make contact or if they can also induce effects at some distance. Daculsi’s proposal also mentioned the additional contributing
effect of bacteria; surrounding bacteria may influence remineralization. A second possible mechanism concerns apatite present in peritubular dentin. Peritubular dentin is highly
mineralized30. In the current study, carious dentin was partially demineralized with apatite remaining in dentin, especially at peritubular dentin. This residual mineral may serve as
nucleus, on which calcium-phosphate crystals grow within the dentin tubules. Hayakawa _et al_.31 proposed a mechanism of apatite nucleation and growth, based on an experiment conducted with
dry titanium oxide in a narrow-confined space filled with Kokubo’s simulated body fluid (SBF), so to confine the composing ions of apatite32. Since precursors of embryos and embryos of
apatite remained close to the titanium oxide surface in the confined space, they were thought to contribute to the nucleation and growth of apatite crystallites while consuming Ca and P ions
from SBF. Relating these observations to our study, a dentin tubule provides a narrow space and peritubular dentin can act as a nucleation site. Dentinal fluid contains Ca and P ions and
flows in the dentin tubules. Therefore, our observation supports the mechanism that the nucleation and growth of calcium phosphate crystals can be initiated at peritubular dentin in the
dentin tubules. To identify the crystal structure of the intratubular precipitates, EDS elemental mapping and SAED were performed. EDS-STEM element mapping revealed a high Ca and P content
within the dentinal tubules. In addition, also Na and Mg were detected within the precipitates (Fig. 3). SAED crystal analysis identified the presence of HAp in the intratubular precipitate
(Fig. 4), but Mg-ß-TCP was not found despite Mg was detected by EDS-STEM. In literature, the crystals formed during remineralization in dentinal caries have been categorized as Mg-containing
ß-tricalcium phosphate (Mg-β-TCP or whitlockite) and Mg-containing HAp (Mg-HAp). Daculsi _et al_. have reported whitlockite and apatite in dentin26. At the early stage of remineralization,
Mg-ß-TCP (whitlockite) and Mg-poor apatite are formed. Later Mg-ß-TCP is partially converted into Mg-poor apatite. Zavgordniy _et al_.10,11 also analyzed the crystals in dentinal caries in
two different studies. They first reported the presence of Mg-β-TCP in the carious transparent zone10, but later only mentioned the presence of HAp11. In our observation, the Ca/P ratio of
the intratubular participates varied around 1.6 to 1.7 (Fig. 3). This measured Ca/P ratio supports the formation of HAp10,33. Several specific ions influence the process of remineralization
and the actual crystal formation and which type of crystal is formed34. Not only our data but also previous studies have revealed the presence of Mg in precipitates or crystals35. Abbona _et
al_.36 reported that HAp can be formed in a low Mg-containing solution (Mg/Ca ratio in solution below 0.4). A higher amount of Mg, such as 0.4 < Mg/Ca in solution < 4.0, forms stable
amorphous calcium phosphate or Mg-ß-TCP (whitlockite). On the other hand, HAp is generally formed in SBF solution with a Mg/Ca ratio of 0.6 and pH of 7.432. Mg is typically found in 0.72,
0.44, 1.23 wt% in bone, enamel and dentin, respectively37,38; saliva contains about 0.345 mmol/l Mg. The concentration of Mg and Ca ions in saliva depends on the human and physical
conditions39. EDS revealed a Mg/Ca ratio varying from 0.01 to 0.08 within the intratubular precipitates in our study. The Mg/Ca ratio of Mg-whitlockite or Ca18Mg2H2(PO4)14 is 0.1140. Hence,
the Mg concentration detected in the intratubular precipitates must have been too low to form Mg-whitlockite and may therefore have resulted into HAp formation in our study. In this respect,
the Mg-whitlockite observed by Daculsi _et al_.9 must have been produced in a high Mg-concentrated medium. On the other hand, Zavgorodniy _et al_.10 proposed the effect of Si. Silicon is
known as an essential element for bone remineralization; Si was shown to induce precipitation of HAp in bone34. Silicon may also influence the type of crystal formed during remineralization.
Zavgordniy _et al_.10 in fact detected Mg, Si and sometimes Cl in addition to Ca and P in the remineralization crystals. The Ca/P ratio of remineralization spots they analyzed was 1.6 and
1.25. The 1.25 Ca/P ratio was much lower than the Ca/P ratio of pure HAp. They explained that Si and Cl may have been inserted into the crystals and that Si may have substituted Mg to form
Si-substituted Mg-β-TCP crystals in absence of sufficient Mg. In our observation, Si was not detected. Differences in presence and concentrations of specific ions may influence
remineralization and which crystal type is formed in different regions of the caries lesion. In our study, the conditions in the immediate environment around the intratubular precipitates
within the arrested caries lesions may have been suitable for HAp formation but not for whitlockite formation. Meanwhile, Mg is known to inhibit HAp-crystal growth35,39. SAED confirmed HAp
formation, but TEM revealed that the crystal size of the precipitates in the dentin tubules was less than 100 nm. We cannot deny the co-existence of amorphous calcium phosphates in addition
to HAp crystals in the intratubular precipitates. Magnesium may possible be present in both the crystal and amorphous calcium phosphates10,35. Magnesium in saliva and Mg released from tooth
tissue may have inhibited crystal growth of HAp and formed amorphous carbonate apatite instead. At a later stage, amorphous calcium phosphate can convert to HAp41. However, Mg ions adsorbed
on the surface of amorphous calcium phosphate retard the transformation to HAp35. In the caries-remineralization process investigated in this study, we could not observe the early stage of
precipitation. 3D imaging by FIB-SEM indicated that the precipitates formed a tight cluster of HAp crystals and amorphous calcium phosphate, and clogged the dentin tubules. Based on this
observation, we suggest that small HAp crystals precipitated and that amorphous calcium phosphate was formed in the dentin tubules due to the salivary environment where Mg exists. The
intratubular precipitation may grow with some transformation into additional HAp crystals from the amorphous calcium phosphates. Many _in vitro_ studies reported on crystal formation in
Mg-containing solutions35,38,41. On the other hand, in the mouth several ions exist in saliva and are provided with drinks. When dentinal HAp is dissolved by caries, in addition to several
ions, proteins are released and will also influence the remineralization process. It is difficult to observe crystal formation and its growth _in vivo_, although crystal growth is a key
element in the process of tooth remineralization. In-depth investigation of the mechanism of nuclei formation and precipitates’ growth in dental caries or the oral environment is needed to
understand _in vivo_ remineralization. Furthermore, F has often been reported to play a role in remineralization12. Fluoride is well known to prevent dental caries by inhibiting enamel and
dentin demineralization; hence, a high F concentration around crystals may possibly protect the crystals against dissolution42. A small F amount will on the other hand promote formation of
fluoridated HAp43. In this study, F was clearly detected (Fig. 3). A high fluoride concentration was recorded in intratubular dentin and less in the intratubular precipitates. Fluoride was
strongly found around a cluster of crystals in one specimen (Fig. 3B). Peritubular dentin is commonly mineralized up to a higher degree than intertubular dentin44; in intertubular dentin F
can be detected in a higher amount45. Compared to the intratubular precipitates, peritubular and intratubular dentin are longer exposed to the oral environment, by which these tissues may
absorb more F than the precipitates. In addition, the fluoride uptake in peritubular dentin may make it more resistant against caries demineralization as well as better protect the
precipitates in the dentin tubules. From our observations, we found that the dentin tubules in caries were extensively and deeply remineralized with precipitation of HAp and formation of
amorphous calcium phosphate. The surrounding dentin was severely demineralized by the progressing caries. Especially 3D FIB-SEM imaging clearly disclosed intratubular precipitation, filling
the dentin tubules with dense mineral deposits. This zone of filled dentin tubules inhibits bacterial penetration, thereby retarding caries progression. Several ions in the oral environment,
such as Mg and F, may influence the process of nucleation and crystal growth during remineralization. To counteract caries as disease, clinically effective and biocompatible materials with
remineralization capability should be developed12. A better understanding of the natural process of remineralization within carious dentin is essential, as it must be useful to develop
bioactive materials that can effectively induce biomimetic remineralization. METHODS TEETH PREPARATION Two extracted pre-molar teeth exhibiting deep dentinal caries from different persons
were selected. Due to periodontal reasons or severe caries, dentists decided to extracted teeth with patients’ agreement. Informed consent was obtained from both participants according the
guideline Ethical Guidelines for Medical and Health Research Involving Human Subjects. The protocol of this research was approved by the Commission for Medical Ethics of Okayama University
Hospital, which in accordance with the guideline Ethical Guidelines for Medical and Health Research Involving Human Subjects (Approval number: No. 1606-020). Teeth were fixed using 2.5%
glutaraldehyde (Sigma Aldrich, St. Louis, MO, USA), stained with 4% osmium tetroxide (Sigma Aldrich), gradually dehydrated using ascending ethanol concentrations and finally embedded in
epoxy resin (Sigma Aldrich). SEM AND FIB-SEM The teeth were cut into 0.8 × 4 × 4 mm squares including carious dentin using an auto cut-off machine (Accutom, Struers, Ballerup, Denmark) prior
to having the cross-sections mechanically polished using diamond lapping films (3 M, St. Paul, MN, USA) and argon-ion polishing (SM-090101 Cross-Section Polisher, JEOL, Tokyo, Japan) (Fig.
5). Subsequently, a thin layer of carbon was coated on the surface (JEE-420T Vacuum Evaporator, JEOL), after which the specimens were examined using a field-emission-gun SEM (Feg-SEM;
JSM-6701F, JEOL) operated at 5 kV using an annular semiconductor detector. Among the five carious teeth preliminary examined, two teeth revealed an area of remineralized dentin immediately
adjacent of the caries lesion, which was further characterized using FIB-SEM and EDS-STEM. Orthogonal FIB-SEM (SMF-1000, HITACHI High-Tech Science, Tokyo, Japan) imaging was conducted for 3D
structural characterization. The FIB and SEM ion/electron beams were orthogonally aligned, instead of being set at the standard angle of ∼60°, for obtaining high-spatial resolution and
high-contrast SEM photomicrographs24. Lucis Pro (Microtechnics, CA, USA) and Stacker and Visualizer-Kai software (System In Frontier, Tokyo, Japan) packages were utilized for 3D
reconstruction. SEM observation was performed at an accelerating voltage of 0.5 kV. Images were taken by a mixture of two detectors; an annular in-lens secondary electron detector and an
annular in-lens energy-selected backscattered electron detector. The observation area was 30 × 30 μm with 1000 pixels each. Serial-sectioning observations were carried out with a slice pitch
of 30 nm for 1000 sheets, i.e. one voxel size is 30 nm. EDS-STEM AND SAED After serial sectioning by FIB-SEM, the successive plane of the remaining specimens was processed for EDS-STEM
(Hitachi HD-2700, HITACHI High-Tech Science) operated at 200 kV and equipped with a silicon-drift type x-ray detector (AMETEK EDAX Octane 100 mm2, AMETEK EDAX, Mahwah, NJ, USA). TEM images
and electron diffraction patterns were recorded with a JEM-2010F (JEOL) TEM operated at 200 kV. REFERENCES * Kassebaum, N. J. _et al_. Global burden of untreated caries: a systematic review
and metaregression. _J. Dent. Res._ 94, 650–658 (2015). Article CAS PubMed Google Scholar * Kind, L. _et al_. Biomimetic Remineralization of Carious Lesions by Self-Assembling Peptide.
_J. Dent. Res._ 96, 790–797 (2017). Article CAS PubMed Google Scholar * Pitts, N. B. _et al_. A. Dental caries. _Nat. Rev. Dis. Primers_ 25, 17030 (2017). Article Google Scholar *
Lamont, R. J., Koo, H. & Hajishengallis, G. The oral microbiota: dynamic communities and host interactions. _Nat. Rev. Microbiol._ 16, 745–759 (2018). Article CAS PubMed PubMed
Central Google Scholar * Featherstone, J. D. The continuum of dental caries - evidence for a dynamic disease process. _J. Dent. Res._ 83, 39–42 (2004). Article Google Scholar * Lester,
K. S. & Boyde, A. Some preliminary observations on caries (remineralization) crystals in enamel and dentin by surface electron microscopy. _Virchows. Arch. Pathpl. Anat. Physiol. Klin.
Med._ 344, 196–212 (1967). Article CAS Google Scholar * Ogawa, K., Yamashita, Y., Ichijo, T. & Fusayama, T. The ultrastructure and hardness of the transparent layer of human carious
dentin. _J. Dent. Res._ 62, 7–10 (1983). Article CAS PubMed Google Scholar * Frank, R. M. & Voegel, J. C. Ultrastructure of the human odontoblast process and its mineralization
during dental caries. _Caries. Res._ 14, 367–380 (1980). Article CAS PubMed Google Scholar * Daculsi, G., LeGeros, R. Z., Jean, A. & Kerebel, B. Possible physico-chemical processes
in human dentin caries. _J. Dent. Res._ 66, 1356–1359 (1978). Article Google Scholar * Zavgorodniy, A. V., Rohanizadeh, R., Bulcock, S. & Swain, M. V. Ultrastructural observations and
growth of occluding crystals in carious dentin. _Acta Biomater._ 4, 1427–1439 (2008). Article CAS PubMed Google Scholar * Zavgorodniy, A. V., Rohanizadeh, R. & Swain, M. V.
Ultrastructure of dentine carious lesions. _Arch. Oral. Biol._ 53, 124–132 (2008). Article PubMed Google Scholar * Li, X., Wang, J., Joiner, A. & Chang, J. The remineralisation of
enamel: a review of the literature. _J. Dent._ 242, 12–20 (2014). Article CAS Google Scholar * Peters, M. C. _et al_. _In vivo_ dentin remineralization by calcium-phosphate cement. _J.
Dent. Res._ 89, 286–291 (2010). Article CAS PubMed Google Scholar * Fernando, D. _et al_. Bioactive glass for dentin remineralization: A systematic review. Mater. _Sci. Eng. C. Mater.
Biol Appl._ 76, 1369–1377 (2017). Article CAS Google Scholar * Niu, L. N. _et al_. Biomimetic remineralization of dentin. _Dent. Mater._ 30, 77–96 (2014). Article CAS PubMed Google
Scholar * Kanazawa, T. _et al_. Histomorphometric and ultrastructural analysis of the tendon-bone interface after rotator cuff repair in a rat model. _Sci. Rep._ 20, 33800,
https://doi.org/10.1038/srep33800 (2016). Article ADS CAS Google Scholar * Hirashima, S. _et al_. Three-dimensional ultrastructural analysis of cells in the periodontal ligament using
focused ion beam/scanning electron microscope tomography. _Sci. Rep._ 20, 39435, https://doi.org/10.1038/srep39435 (2016). Article ADS CAS Google Scholar * Nalla, R. K. _et al_.
Ultrastructural examination of dentin using focused ion-beam cross-sectioning and transmission electron microscopy. _Micron._ 36, 672–680 (2005). Article CAS PubMed Google Scholar *
Coutinho, E. _et al_. Ultrastructural characterization of tooth-biomaterial interfaces prepared with broad and focused ion beams. _Dent. Mater._ 25, 1325–1337 (2009). Article CAS PubMed
Google Scholar * Hara, T. _et al_. A. Application of orthogonally arranged FIB-SEM for precise microstructure analysis of materials. _J. Alloy. Com._ 577S, 717–721,
https://doi.org/10.1093/jmicro/dfu077 (2013). Article Google Scholar * Posner, A. S., Perloff, A. & Diorio, A. F. Refinement of the hydroxyapatite structure. _Acta. Cryst._ 11, 308–309
(1958). Article CAS Google Scholar * Kay, M. I., Young, R. A. & Posner, A. S. Crystal structure of hydroxyapatite. _Nature_ 12, 1050–1052 (1964). Article ADS Google Scholar * Li,
Y. & Aparicio, C. Discerning the subfibrillar structure of mineralized collagen fibrils: a model for the ultrastructure of bone. _PLoS One 23_ 8, e76782,
https://doi.org/10.1371/journal.pone.0076782 (2013). Article CAS Google Scholar * Wirth, R. Focused Ion Beam (FIB) combined with SEM and TEM: Advanced analytical tools for studies of
chemical composition, microstructure and crystal structure in geomaterials on a nanometer scale. _Chemical Geology_ 261, 217–229 (2009). Article ADS CAS Google Scholar * Sarnat, B. &
Massler, M. Microstructure of active and arrested dentinal caries. _Adv. Dent. Res._ 44, 1389–1401 (1965). Article Google Scholar * Daculsi, G., Kerebel, B., Le Cabellec, M. T. &
Kerebel, L. M. Qualitative and quantitative data on arrested caries in dentine. _Caries Res._ 13, 190–202 (1979). Article CAS PubMed Google Scholar * Yoshiba, K., Yoshiba, N., Ejiri, S.,
Iwaku, M. & Ozawa, H. Odontoblast processes in human dentin revealed by fluorescence labeling and transmission electron microscopy. _Histochem. Cell. Biol._ 118, 205–212 (2002). Article
CAS PubMed Google Scholar * Zheng, L., Hilton, J. F., Habelitz, S., Marshall, S. J. & Marshall, G. W. Dentin caries activity status related to hardness and elasticity. _Eur. J.
Oral. Sci._ 111, 243–252 (2003). Article PubMed Google Scholar * Thomas, H. F. The extent of the odontoblast process in human dentin. _J. Dent. Res._ 58, 2207–2218 (1979). Article CAS
PubMed Google Scholar * Zhang, Y. R., Du, W., Zhou, X. D. & Yu, H. Y. Review of research on the mechanical properties of the human tooth. _Int. J. Oral. Sci._ 6, 61–69 (2014). Article
PubMed PubMed Central Google Scholar * Hayakawa, S. _et al_. Calcium phosphate crystallization on titania in a flowing Kokubo solution. _J. Mater. Sci. Mater. Med._ 26, 222–235 (2015).
Article PubMed CAS Google Scholar * Kokubo, T. & Takadama, H. How useful is SBF in predicting _in vivo_ bone bioactivity? _Biomaterials_ 27, 2907–2915 (2006). Article CAS PubMed
Google Scholar * Champion, E. Sintering of calcium phosphate bioceramics. _Acta Biomater._ 9, 5855–5875 (2013). Article CAS PubMed Google Scholar * Hoppe, A., Güldal, N. S. &
Boccaccini, A. R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. _Biomaterials_ 32, 2757–2774 (2011). Article CAS PubMed
Google Scholar * Ding, H., Pan, H., Xu, X. & Tang, R. Toward a detailed understanding of magnesium ions on hydroxyapatite crystallization inhibition. _Cryst. Growth Des._ 14, 763–769
(2014). Article CAS Google Scholar * Abbona, F. & Franchini-Angela, M. Crystallization of calcium and magnesium phosphates from solutions of low concentration. _J. Crystal Growth_
104, 661–671 (1990). Article ADS CAS Google Scholar * Laurencin, D. _et al_. Magnesium incorporation into hydroxyapatite. _Biomaterials_ 32, 1826–1837 (2011). Article CAS PubMed
Google Scholar * Ren, F., Leng, Y., Xin, R. & Ge, X. Synthesis, characterization and ab initio simulation of magnesium-substituted hydroxyapatite. _Acta Biomater._ 6, 2787–96 (2010).
Article CAS PubMed Google Scholar * Su, Y. X. _et al_. Increased calcium and decreased magnesium and citrate concentrations of submandibular/sublingual saliva in sialolithiasis. _Arch.
Oral Biol._ 55, 15–20 (2010). Article CAS PubMed Google Scholar * Gopal, R., Calvo, C., Ito, J. & Sabine, W. K. Crystal Structure of Synthetic Mg-Whitlockite, Ca18Mg2H2(PO4)14. _Can.
J. Chem._ 52, 1155–1164 (1974). Article CAS Google Scholar * Combes, C. & Rey, C. Amorphous calcium phosphates: synthesis, properties and uses in biomaterials. _Acta Biomater._ 6,
3362–3378 (2010). Article CAS PubMed Google Scholar * Brauer, D. S., Karpukhina, N., O’Donnell, M. D., Law, R. V. & Hill, R. G. Fluoride-containing bioactive glasses: effect of glass
design and structure on degradation, pH and apatite formation in simulated body fluid. _Acta Biomater._ 6, 3275–3282 (2010). Article CAS PubMed Google Scholar * Fan, Y., Sun, Z. &
Moradian-Oldak, J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. _Biomaterials_ 30, 478–483 (2009). Article CAS PubMed Google Scholar * Kinney, J. H.,
Balooch, M., Marshall, S. J., Marshall, G. W. Jr. & Weihs, T. P. Hardness and Young’s modulus of human peritubular and intertubular dentine. _Arch. Oral Biol._ 41, 9–13 (1996). Article
CAS PubMed Google Scholar * Figures, K. H., Ellis, B. & Lamb, D. J. Fluoride penetration into dentine abutments _in vitro_. _Caries Res._ 24, 301–305 (1990). Article CAS PubMed
Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the NIMS microstructural characterization platform as a program of “Nanotechnology Platform” of the Ministry
of Education, Culture, Sports, Science and Technology (MEXT) in Japan. This study was supported by JSPS KAKENHI [grant number JP 16K2045508, 18K17068]. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * National Institute of Advanced Industrial Science and Technology (AIST), Health Research Institute, 2217-14 Hayashi-Cho, Takamaysu, Kagawa, 761-0395, Japan Kumiko Yoshihara *
Okayama University, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Department of Pathology & Experimental Medicine, 2-5-1 Shikata-cho, Kita-ku, Okayama, 700-8558,
Japan Kumiko Yoshihara * Okayama University Dental School, Advanced Research Center for Oral and Craniofacial Sciences, 2-5-1 Shikata-cho, Kita-ku, Okayama, 700-8558, Japan Noriyuki Nagaoka
* National Institute for Materials Science, 1-2-1 Sengen, Tsukuba, Ibaraki, 305-0047, Japan Akiko Nakamura & Toru Hara * Okayama University, Graduate School of Interdisciplinary Science
and Engineering in Health Systems, Biomaterials Laboratory, 3-1-1, Tsushima-naka, Kita-ku, Okayama, 700-8530, Japan Satoshi Hayakawa * Hokkaido University, Faculty of Dental Medicine,
Department of Biomaterials and Bioengineering, Kita 13, Nishi 7, Kita-ku, Sapporo, Hokkaido, 060-8586, Japan Yasuhiro Yoshida * KU Leuven (University of Leuven), Department of Oral Health
Research, BIOMAT & UZ Leuven (University Hospitals Leuven), Dentistry, Kapucijnenvoer 7, 3000, Leuven, Belgium Bart Van Meerbeek Authors * Kumiko Yoshihara View author publications You
can also search for this author inPubMed Google Scholar * Noriyuki Nagaoka View author publications You can also search for this author inPubMed Google Scholar * Akiko Nakamura View author
publications You can also search for this author inPubMed Google Scholar * Toru Hara View author publications You can also search for this author inPubMed Google Scholar * Satoshi Hayakawa
View author publications You can also search for this author inPubMed Google Scholar * Yasuhiro Yoshida View author publications You can also search for this author inPubMed Google Scholar *
Bart Van Meerbeek View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS K. Yoshihara, S. Hayakawa and B. Van Meerbeek wrote the main manuscript
text. K. Yoshihara and Y. Yoshida conceived and planned the experiments. K. Yoshihara and N. Nagaoka prepared the specimens documented in Fig. 1. K. Yoshihara, N. Nagaoka and A. Nakamura
observed and prepared the specimens for Fig. 2 and the Appendix. K. Yoshihara, N. Nagaoka and Toru Hara observed and prepared the specimens documented in Figs. 3 and 4. All authors provided
critical feedback and helped to shape the research, analysis and manuscript. CORRESPONDING AUTHOR Correspondence to Kumiko Yoshihara. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTAL INFORMATION. SUPPLEMENTAL VIDEO. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yoshihara, K., Nagaoka, N., Nakamura, A. _et al._ Three-dimensional observation and
analysis of remineralization in dentinal caries lesions. _Sci Rep_ 10, 4387 (2020). https://doi.org/10.1038/s41598-020-61111-1 Download citation * Received: 26 March 2019 * Accepted: 10
February 2020 * Published: 09 March 2020 * DOI: https://doi.org/10.1038/s41598-020-61111-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Danny Amendola Seemingly Takes a Shot at Zedd After Reports the DJ Might Be Dating Olivia CulpoDanny Amendola Seemingly Takes a Shot at Zedd After Reports the DJ Might Be Dating Olivia Culpo Culpo and Zedd recently ...
Extreme Enterprises | TechCrunchExtreme EnterprisesHeadlines Only Load MoreIf Ambition Were All It Took, YourNight Would Be Sitting On GoldJan 9, 2009Ju...
Heartsong Choir sings at famous Carnegie HallAdNewsLatest NewsNewsLatest NewsNews HomeGood evening, Your ContentNewslettersAccount & SupportMy AccountContact UsHelp ...
What climate change means for central america, with paul j. AngeloLINDSAY: Welcome to The President's Inbox, a CFR podcast about the foreign policy challenges facing the United Stat...
Kids in their element at 2017 Bemboka Show | PHOTOS, VIDEOSAdNewsLatest NewsNewsLatest NewsNews HomeGood evening, Your ContentNewslettersAccount & SupportMy AccountContact UsHelp ...
Latests News
Three-dimensional observation and analysis of remineralization in dentinal caries lesionsABSTRACT The remineralization mechanism in dental caries lesions is not completely understood. This study reports on ult...
Learning french: when and why do we say arriver à bon port?A PHRASE TO USE UPON REACHING YOUR DESTINATION, SAFE AND SOUND Je suis arrivé à bon port ! We look at how to use this ha...
Suze Orman on: Saving for Retirement — AARP3:21 AARP Videos Money Suze Orman on: Saving for Retirement — AARP Suze Orman takes us into a secret vault to answer que...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
Up elections 2017: bjp leaders upset by rss leader's ill-timed anti-reservation commentThe quota row touched off by a senior RSS functionary's remarks has sparked concerns in the BJP over its fallout in...