Temporal and spatial variations in the bacterial community composition in lake bosten, a large, brackish lake in china
Temporal and spatial variations in the bacterial community composition in lake bosten, a large, brackish lake in china"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The bacteria inhabiting brackish lake environments in arid or semi-arid regions have not been thoroughly identified. In this study, the 454 pyrosequencing method was used to study
the sedimentary bacterial community composition (BCC) and diversity in Lake Bosten, which is located in the arid regions of northwestern China. A total of 210,233 high-quality sequence reads
and 8,427 operational taxonomic units (OTUs) were successfully obtained from 20 selected sediment samples. The samples were quantitatively dominated by members of _Proteobacteria_ (34.1% ±
11.0%), _Firmicutes_ (21.8% ± 21.9%) and _Chloroflexi_ (13.8% ± 5.2%), which accounted for more than 69% of the bacterial sequences. The results showed that (i) Lake Bosten had significant
spatial heterogeneity, and TOC(total organic carbon), TN(total nitrogen) and TP(total phosphorus) were the most important contributors to bacterial diversity; (ii) there was lower taxonomic
richness in Lake Bosten, which is located in an arid region, than in reference lakes in eutrophic floodplains and marine systems; and (iii) there was a low percentage of dominant species in
the BCC and a high percentage of unidentified bacteria. Our data help to better describe the diversity and distribution of bacterial communities in contaminated brackish lakes in arid
regions and how microbes respond to environmental changes in these stable inland waters in arid or semi-arid regions. SIMILAR CONTENT BEING VIEWED BY OTHERS BACTERIAL DIVERSITY IN SURFACE
SEDIMENTS OF COLLAPSED LAKES IN HUAIBEI, CHINA Article Open access 22 September 2022 MICROBIAL COMMUNITY OF SODA LAKE VAN AS OBTAINED FROM DIRECT AND ENRICHED WATER, SEDIMENT AND FISH
SAMPLES Article Open access 15 September 2021 THE SPATIO-TEMPORAL DISTRIBUTION OF ALKALINE PHOSPHATASE ACTIVITY AND _PHOD_ GENE ABUNDANCE AND DIVERSITY IN SEDIMENT OF SANCHA LAKE Article
Open access 22 February 2023 INTRODUCTION Heterotrophic bacteria are major constituents of pelagic aquatic ecosystems, where they play a prominent role in the breakdown of organic compounds
and the remineralization of nutrients as well as in trophic coupling to eukaryote predators1. Recently, lakes, especially lakes in arid and semi-arid regions, have been described as early
indicators of both regional and global environmental change. Microorganisms are some of the most important factors in the nutrient cycling and decomposition of organic matter in lake
sediments2, and bacteria are thought to be a sensitive sentinel of those environmental changes3. Sediments are some of the most diverse microbial habitats and play a key role in the
biogeochemical cycles of organic matter decomposition and of major elements, including carbon, nitrogen and phosphorus. Sediment is now an major topic in the investigation of bacterial
communities because analysis of the community in sediments can provide clues to understanding benthic ecosystem processes, particularly in saline and eutrophic aquatic environments. Sediment
bacteria, due to their high population density (>108 cells/g), play an important ecological role in the biogeochemical cycle in lake ecosystems4. The biomass and community structure of
sediment bacteria change due to changes in the physical, chemical and biological factors in water and sediment, so they can also be considered representative organisms indicating
environmental changes5. The combination of abiotic factors and microorganisms in sediments has an important effect on nutrient balance in water. Arid and semi-arid regions account for almost
one-third of the world’s land area6, and lakes in these areas provide sparse but valuable water resources for fragile environments and humans. However, these water resources are also
inevitably affected by human activities. Studies have shown that many water resources in arid and semi-arid regions have been contaminated by eutrophication or salinity7,8,9. Lake Bosten is
the largest inland brackish lake in the arid regions of northwestern China, and it plays an important role in the surrounding terrestrial ecosystems. Surprisingly, the lake was a freshwater
lake before the 1960s, but climate change and human activities over the past 50 years have developed it into a brackish lake with moderate nutrient levels10,11. Salinization and
eutrophication continue to affect Lake Bosten. Therefore, Lake Bosten provides us with an exclusive opportunity to carefully study the potential response mechanisms of bacterial communities
in the early stages of salinization and eutrophication. At the same time, due to anthropogenic agricultural and tourism activities, it also forms a nutritional gradient from malnutrition to
eutrophication12, and this complex ecosystem is an ideal model for studying the response of bacterial communities to salinity and nutrient gradients13. This work is particularly important
for the restoration of polluted dry lakes, as changes in microbial communities may be some of the more rapid agents of biological change. Although a small number of studies have reported
temporal changes in community composition in the sediments of the Lake Bosten, these studies have concentrated on a single sample from a single season14. There is little research on the
variations over the four seasons across the whole lake. In this study, we used high-throughput sequencing technology and redundancy analysis to explore the association between
spatial-temporal changes in Lake Bosten bacterial communities and environmental factors. The research objectives are to identify (1) Whether there is spatial-temporal heterogeneity in the
bacterial communities of lake sediments under the dual effects of eutrophication and salinity and (2) Which environmental factors control the assembly of bacterial communities in the
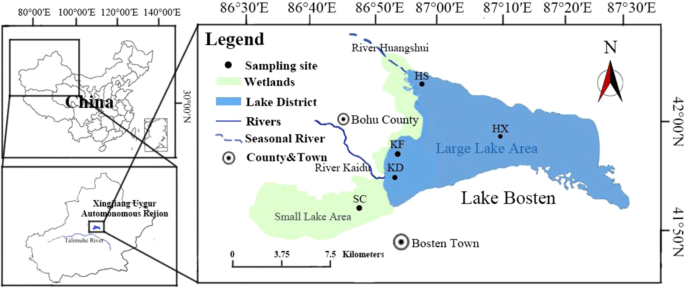
sediments of Lake Bosten. MATERIALS AND METHODS STUDY AREA AND SAMPLING Lake Bosten (86°19′~87°28′E, 41°46′~42°08′N, 1046 m above sea level) is located in the southern part of Bohu County,
Xinjiang Province, in the lower reaches of the Kaidu River. The basin is located in the center of Eurasia. The combination of sunlight and heat, the annual average precipitation is 64.3 mm,
and the annual average evaporation is 1,881.2 mm, resulting in an inland desert climate15. The lake covers an area of approximately 1,100 km2 16 with an average water depth of 7 meters and a
maximum depth of more than 16 meters3. The annual average temperature is about 7.9 °C, and the coldest temperature occurs in January (−12.7 °C). There is a four-month freeze season at Lake
Bosten from December to March every year15. Mountain Tian in Xinjiang, which is the lowest point of the Basin Yan. Additionally, the lake covers a vast area of more than 1,100 km2 and has a
maximum water depth of more than 16 m. Originating from the snow-covered Mountain Tian, the Kaidu River, with an annual average runoff volume of 34 × 108 m3, supplies 85% of the water volume
of Lake Bosten17. Moreover, as Lake Bosten is located in the centre of Eurasia, a good level of light and heat provide extremely favourable natural conditions for the development of the
lake, allowing the basin to be rich in animal and plant resources. The Kaidu River is divided into two branches at the Baolang Sumu Water Diversion Project, which are respectively injected
into two lakes of Lake Bosten. The Large Lake Area is 55 km from east to west and 20 km wide from north to south. At an altitude of 1,048.75 m, the water surface area is 1,002. 4 km2, volume
is 88 × 108 m3, average water depth is 7.38 m, the maximum depth is 16 m. The centre of lake area (HX) is located in the centre of the Large Lake Area and is highly disturbed by
hydrodynamic forces, but is less affected by external influences. Huangshuigou area (HS) located in the northwest of the Large Lake Area and is enriched with nutrients (nitrogen and
phosphorus) and salinity from agricultural irrigation and industrial wastewater discharged from the surrounding rivers. There are the highest nutrients and salinity in the area of Lake
Bosten4. The Large Lake Area is regularly monitored by the Environmental Protection Bureau of Bayinguoleng Mongolian Autonomous Prefecture and has been continuously monitored for over ten
years. Small Lake Area, also known as macrophyte-dominated area (SC), covers an area of about 300 km2, and is an important reed production base in China. From 8 May 2013 to 18 January 2014,
20 samples were collected from the centre of Lake area (HX, 87°08′00″E, 42°00′00″N), Huangshuigou area (HS, 86°50′30″E, 42°06′00″N), estuary of the Kaidu River (KD, 86°44′30″E, 41°53′40″N)
and its nearby area (KF, 86°46′20″E, 41°57′00″N) and macrophyte-dominated area (SC, 86°38′5″E, 41°47′24″N). We avoided extreme weather when sampling and sampled when it was fine. Undisturbed
duplicate sediments (0–5 cm deep) were collected seasonally using a Petersen grab sampler from 8 May 2013 to 18 January 2014 (Fig. 1). The sediment samples were transferred into sterile
plastic containers, and immediately frozen at −80 °C for storage until analysis. PHYSICOCHEMICAL ANALYSIS The sediment samples were dried to constant weight using a freeze dryer (Alpha 1–2
LD, Martin Christ Instrument Co, Germany). Total nitrogen (TN), total phosphorus (TP) and total organic carbon (TOC) content in the sediments were determined according to standard methods
(Jin and Tu 1990). The temperature, salinity, dissolved oxygen (DO), Salinity (TDS), and pH of the lowest water layer covering the sediment were measured using a multi-parameter water
quality detector (YSI 6600V2, USA). After DAPI (4′, 6-diamidino-2-phenylindole) staining direct counting, the bacterial abundance in the deposited samples was determined by epifluorescence
microscopy12,15. We used one-way ANOVA to test the correlation between environmental factors. DNA EXTRACTION, PURIFICATION AND PCR AMPLIFICATION Total DNA was extracted from twenty 0.25 g
freeze-dried pellets using the Soil Rapid DNA Rotation Kit (MP Biomedicals, Fountain Pkwy. Solon, OH, USA). Genomic DNA concentration and purity were determined by an ultraviolet
spectrophotometer. According to the concentration test results, the integrity of the DNA sample was detected by 0.8% agarose gel electrophoresis. The test conditions were as follows: voltage
120 V, electrophoresis time was about 20 minutes. The extracted DNA stock was diluted to 20 ng.μl−1 and used as a PCR template. The V1–V3 region of the bacterial 16S rRNA gene was amplified
using PCR amplification of universal primers 8 F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 533 R (5′-TTACCGCGGCTGCTGGCAC-3′)18. PCR amplification was carried out using a 50 μL reaction system under
the following conditions: predenaturation at 94 °C for 5 minutes, and denaturation at 50 °C. Anneal at 94 °C for 30 seconds, 55 °C for 45 seconds, 72 °C for 1 minute, 27 cycles, 72 °C for 8
minutes, and stored at 4 °C, the experiment was repeated 3 times. The PCR product was purified and concentrated using E.Z.N.A. ®Cycle-Pure Kit. The PCR products from each sample were then
mixed in a single tube in equimolar ratios. 454 PYROSEQUENCING AND DATA ANALYSIS Amplicon pyrosequencing was performed on a Roche Genome Sequencer GS FLX Titanium platform using a 454/Roche
A sequencing primer kit. Each read file produced by a pyrosequencing run is associated with one quality file, which contains the quality score for each base. After sequencing, the QIIME
software package19 was used to exclude sequences with a length of less than 200 bases, an average mass fraction of less than 25, or no primers and barcode sequences. The rest of the
sequences were trimmed and compared against the Bacterial Silva database (SILVA version 106; http://www.arb-silva.de/documentation/background/release-106/) via QIIME. Similar sequences were
clustered into Operational Taxonomic Units (OTUs) using a minimum identity of 97% by UCLUST software20. Good’s coverage, abundance-based coverage estimator (ACE), Chao 1 richness estimator,
Shannon and Simpson diversity indices were also calculated by QIIME pipeline. RESULTS PHYSICAL AND CHEMICAL FACTORS The environmental characteristics of the studied lake are presented in
Fig. S1. The overlying water temperatures showed a characteristic annual cycle, with a higher temperature in the HS sample (26.9 °C, summer) and lower temperatures in the KF and SC samples
(1.0 °C, winter). In the overlying water, the concentration of TDS increased from 278 mg L−1 in the KD sample (winter) to 2,238 mg L−1 in the HS sample (spring). The average concentrations
of TN and TP in the seasonal sediment samples remained stable at approximately 1.74 (KD, winter) to 4.08 (SC, winter) mg·g−1 and 0.15 (KD, winter) to 0.52 (HS, winter) mg·g−1, respectively.
The average concentration of TOC among the seasonal sediment samples ranged from 3.07 (HX, summer) to 12.4 (HS, summer) mg·g−1. BACTERIAL DIVERSITY The seasonal variation in the bacterial
community structure in Lake Bosten was determined using 454 pyrosequencing technology. From all sediment samples, sequencing analysis yielded a total of 210,233 high-quality sequence reads
with an average length of approximately 400 bp effective sequence, 8,427 OTUs, and 97% sequence identity threshold. The effective sequence length for each sediment sample ranged from 8,729
to 12,909, and the number of OTUs ranged from 1,319 to 2,209 (Table 1). The sparse curve with a cluster distance of 0.03 was still not saturated and increased (Fig. 2), indicating that new
bacterial populations may continue to appear after 10,000 reads and sequencing. However, the values of the Shannon index (4.68–6.64) for all sediment samples in this study stabilized,
suggesting that more sequencing may be required, but most of the bacterial diversity of the sample has been captured. In addition, good reports showed that these libraries represent the
majority of bacterial 16S rRNA sequences present in each sediment sample, ranging from 0.902 to 0.951 (Table 1). BACTERIAL COMMUNITY COMPOSITION In the filtered high-quality sequences, the
dominant bacterial phylum or sub-phylum in each sediment sample was identified by the SILVA database using the QIIME algorithm (the top 10 most abundant phyla or sub-phyla in each sample).
The most abundant bacterial phyla were _Proteobacteria_ (34.1% ± 11.0%), _Firmicutes_ (21.8% ± 21.9%) and _Chloroflexi_ (13.8% ± 5.2%), representing more than 69.0% of the bacterial
sequences (Fig. 3). In addition, a large number of bacteria were detected at lower numbers (<4% relative abundance per phyla) in the sediment samples, such as _Planctomycetes_, _TA06_,
_Candidate division WS3_, _Bacteroidetes, Spirochaetae_, _Chlorobi_, _OD1_, _OP8_ and _Deferribacteres_ (Fig. 3). _Firmicutes_ (10.9%–65.7%) dominated in the spring and summer samples. In
contrast, the autumn and winter samples were mainly dominated by _Deltaproteobacteria_ (0.19%–27.2%) and _Chloroflexi_ (0.18%–22.1%) (Fig. 3). Figure 4 shows the 56 dominant genera in the
sediment samples. At the genus level, the differences between the sediment samples are more pronounced. Microorganisms from the genera Uncultured _Neisseriaceae_ (21.62%) and
_Lysinibacillus_ (22.63%) predominated in the BS1 and BS2 samples but were less abundant in the other samples. The genus _Clostridium_ (27.94%) and unclassified _Peptostreptococcaceae_
(16.47%) had higher proportions in the HS (summer) sample. Unclassified _Anaerolineaceae_ (2.42%–16.38%) and _Sva0485_ (0.77%–16.33%) were more abundant in SC (spring) and KD (summer)
samples. Therefore, the main bacterial populations in the twenty sediment samples are slightly different. REDUNDANCY ANALYSIS OF SEDIMENT BACTERIA AND ENVIRONMENTAL FACTORS Microorganisms
are highly sensitive to environmental changes, and the relationship between different environmental factors and the microflora structure can be determined according to the size of the angle
between bacterial and environmental factors and the length of the connection in a redundancy analysis (Fig. 5). _Betaproteobacteria_, _Firmicutes_, and _Gammaproteobacteria_ were negatively
correlated with TDS and DO, whereas _Chloroflexi_ and _Bacteroidetes_ were positively correlated with TDS and DO. _Deltaproteobacteria_ was positively correlated with each indicator. In the
RDA analysis diagram, combining the sediments showed that the levels of the physical and chemical indicators phylum flora structure, TN, TOC and TP were the main factors that influenced the
growth of bacteria, but other environmental factors also had certain effects. DISCUSSION The five sampling points selected from the Large Lake area and Small Lake area in Lake Bosten are
representative areas for studying the sediment bacterial community structure of Lake Bosten. Based on the results of the physical and chemical analyses, the physical and chemical indicators
of the 20 samples showed certain similarities and differences due to factors such as time and geographical location. There was no significant change in the pH value among the 20 samples, but
the other indicators had significant differences (Fig. S1). Most obviously, the values of TDS at the Huangshuigou and central lake areas were significantly higher than those at the other
sampling points (P < 0.05). According to previous research, agricultural production around Lake Bosten discharges a large amount of farmland drainage with high salinity that includes
chloride ions, sulfate ions, ammonia nitrogen and so on. This discharge was the main reason for the increased salt content in Lake Bosten, as 71% and 29% of the lake water flows into the
Large Lake Area and Small Lake Area, respectively21. In addition, the TDS of the summer and autumn samples from the estuary of Lake Bosten and nearby areas was higher than the TDS in other
seasons. Weberscannell _et al_.22 noted that changes in the concentrations of TDS in natural waters are often attributed to the discharge of industrial effluent, increased precipitation, or
saltwater intrusion. In the summer and autumn, the Kaidu River glaciers melted and created abundant snow and ice water resources, which caused the water volume to increase rapidly23, leading
to a significant increase in TDS content. However, the specific causes of this difference in TDS could be complex, and other potential reasons for this difference still deserve further
investigation. Interestingly, the contents of TD, TP and TOC in the Huangshuigou and macrophyte-dominated area were higher in winter than in summer (Fig. S1), which may be because the
continuous low temperature in winter inhibits microbial activity and reduces the effect of microorganisms on the removal of nitrogen and phosphorus24. On the other hand, TN and TP
concentrations were reduced due to dilution and biological blocking25. In addition, a large number of aquatic plants in the aquatic grass area can enrich N and P and desalinize water by
adsorbing, decomposing, oxidizing and precipitating the water, salt, and N and P26. This also explained why the value of pollution indicators in the macrophyte-dominated area was higher than
that in the Huangshuigou area. A large number of studies have shown that temperature is an important factor affecting the structure of bacterial communities27. High temperatures can
increase the metabolic rate, thus causing the cycle of organic matter to increase in speed; a drop in temperature will lower the growth rate of bacteria and lower productivity27. This trend
has also been confirmed in the laboratory28. Therefore, the bacterial community composition of the samples in autumn and winter was more complicated than that in the samples in spring and
summer (Fig. 3). This may be due to the relative abundance of the dominant bacteria _Firmicutes_ in spring and summer decreasing greatly under the influence of temperature. Temperature
changes improve the competitive advantage of other bacteria and increase the relative abundance of other bacteria, leading to a higher diversity of bacteria in autumn and winter. The
competitive advantage between microorganisms changes under conditions such as temperature changes29,30, and some studies on bacteria also illustrate this point. In aquatic ecosystems, in
addition to temperature, the most relevant factor to bacterial growth and reproduction is inorganic salt nutrition (such as TN, TP) and algae biomass31. Shiah′s study also suggests that in
the Chesapeake at temperatures below 20 °C, temperature, more than nutrient levels, functions as the main control factor of planktonic bacteria. At temperatures above 20 °C, the nutrient
control became dominant5. In the whole bacterial community, the three most abundant phyla were _Firmicutes_ (21.8% ± 21.9%), _Proteobacteria_ (_Deltaproteobacteria_ and
_Gammaproteobacteria_) (34.1% ± 11.0%) and _Chloroflexi_ (13.8% ± 5.2%), accounting for more than 69.0% of the bacterial community. This community structure is largely in accordance with
those found in other sediments and soils worldwide32,33 (Fig. 3). _Firmicutes_ (10.9%–65.7%) dominated sediment samples in spring and summer but was present at less than 1% abundance in
autumn and winter. The RDA suggested that the members of _Firmicutes_ were not particularly dependent on the nutrients in the seasonal samples (Fig. 5). This finding was consistent with that
from a previous study in Lake Dongping, a shallow lake34. Under anaerobic conditions, nitrate can be used to achieve denitrification35. DO in spring and summer was lower than that in autumn
and winter, suggesting that the increase in DO would restrain the growth of _Firmicutes_. Related studies have shown that DO controls the _Firmicutes_ community structure more than other
main factors, which is consistent with the results of RDA analysis (Fig. 5). The genus _Clostridium_ are specialized anaerobic bacteria that mainly exist in anaerobic environments and have
strong degradation ability and high metabolic activity36. The genus _Bacillus_ was the primary component of _Firmicutes_, and the group was abundant in the spring and summer samples (Fig.
4). A previous study observed significant variations in the composition of the genus _Bacillus_ in different estuary sediments from Lake Taihu37, and their presence caused the production of
high amounts of ammonia, nitrite and other hazardous substances38. The genus _Lysinibacillus_, which includes facultative bacteria, can reduce macromolecular organic matter to low molecular
weight acids, dissolve nutrients in the soil sediment39, degrade crude compounds40, fix nitrogen from the air41 and prevent plant diseases and insect pests39, and therefore has certain
environmental benefits. _Proteobacteria_ (13.5%–55.0%) were the dominant bacteria in all sediment samples. However, in samples from different seasons, the relative abundances of different
kinds of _Proteobacteria_ were very different (Fig. 3). In this study, _Deltaproteobacteria_ were the main group of _Proteobacteria_, followed by _Gammaproteobacteria_ and
_Betaproteobacteria_ (Fig. 3). In the sediment samples from autumn and winter, _Deltaproteobacteria_ dominated. In freshwater sediments, _Deltaproteobacteria_ were also abundant6,42.
_Deltaproteobacteria_ was mainly represented by unclassified _Desulfarculaceae_ in the present study (Fig. 4). As sulfate-reducing bacteria, the family _Desulfarculaceae_ has been detected
as being dominant in the salt marsh sediments of the North Atlantic and on the East Coast of the US. These bacteria are important participants in the sulfur biogeochemical cycle43,44 and can
degrade organic matter. The resultant compensatory nutrition and energy sources play an important ecological role, especially in the anaerobic degradation of organic matter and the
conversion process45,46. In the past 20 years, the chemicals in Lake Bosten have come to include high levels of sodium sulfate47; a high concentration of SO42− and TDS provide good nutrient
conditions, thereby promoting the growth of bacteria. Therefore, its dominant position in the sample may be related to nutrients in the lake. As a second group of _Proteobacteria_48,49,
_Gammaproteobacteria_ play an important role in the degradation and absorption of organic compounds50, ammonia51 and sulfide52,53 (Fig. 3). The relative abundance of _Gammaproteobacteria_
(1.27–16.40%) in the estuary and its vicinity was significantly higher than that in the other samples. Several previous studies have revealed that _Gammaproteobacteria_ usually exist in
sediments in areas contaminated with agricultural pollution or organic matter15,54. The agricultural activities around Lake Bosten have increased the amounts of organic pollutants in the
estuary55, and the organic content of the sediments has increased56. Therefore, it was not surprising that _Gammaproteobacteria_ were abundant in the estuarine sediments of Lake Bosten, and
a high relative abundance of _Gammaproteobacteria_ has also been observed in other aquatic systems57,58. Additionally, the genus _Pseudomonas_ was found to be representative of
_Gammaproteobacteria_. The group appeared as the dominant genus in the estuarine sediment samples, especially in the spring sample (Fig. 4). A high relative abundance of the genus
_Pseudomonas_ has been observed in the estuarine sediments of Lake Poyang59. In addition, the genus _Acinetobacter_ was a minor proportion of the _Gammaproteobacteria_ (Fig. 4). A previous
study indicated that the genus _Acinetobacter_ is commonly detected as phosphorus-accumulating microorganisms in sediments60 and that the presence of the genus _Acinetobacter_ might explain
the phosphorus-accumulating microorganism concentration in the phosphorus-containing sediment samples from summer (Fig. S1)59. In this study, _Betaproteobacteria_ appeared as the predominant
phylum in all sediment samples (Fig. 3). This finding was consistent with that of a previous study in which _Betaproteobacteria_ was found to be the predominant group in the upper sediments
of Lake Taihu61. _Betaproteobacteria_ (0.87–40.56%) are a dominant bacterial group widely distributed in freshwater lakes around the world. They are present in surface waters and are one of
the most studied groups to date62, but they are rarely found on the open seas or in salt lakes2,63. The presence and success of _Betaproteobacteria_ in freshwater lakes are related to their
ability to respond quickly to changes in bioavailable nutrient content, e.g., they have a rapid response to dissolved organic carbon64. However, the results of the RDA analysis in this
study showed that nutrients such as TOC, TP and TN did not exhibit positive correlations with the relative abundance of _Betaproteobacteria_ (Fig. 5). Interestingly, the most abundant OTU in
_Betaproteobacteria_ was the genus _Thiobacillus_. The genus _Thiobacillus_ is a dominant genus in habitats polluted with mine wastewater65, and they are very abundant in all four seasons.
_Thiobacillus_ was the dominant genus in the HS samples. This indicates that there may be substantial mineral pollution in the HS area. _Chloroflexi_ (3.94–22.13%) was evenly distributed
among the various sediment samples. A previous study indicated that members of _Chloroflexi_ are active not only in deep subsurface sediments on the ocean floor66 but also in various
wastewater treatment systems67. In this study, the RDA showed a positive correlation between the concentration of TOC and the relative abundance of _Chloroflexi_ (Fig. 5). This finding was
consistent with that of a previous study that reported on riparian sedimentary ecosystems34. _Chloroflexi_ can biodegrade organic pollutants68, giving it a better growth advantage in
eutrophic environments69. In addition, _Chloroflexi_ efficiently degrades chlorides. Zanaroli _et al_.70 found that the genus does not produce O2 by photosynthesis and cannot fix nitrogen
but has a dechlorination function. Unclassified _Anaerolineaceae_ was dominant in _Chloroflexi_, and the group dominated in all seasonal sediment samples (Fig. 4). The family
_Anaerolineaceae_ has previously been found to be dominant in the estuarine sediments of Lake Taihu69, and members of this family are often involved in the anaerobic degradation process of
alkanes71,72, as well as in the biodegradation process of organic pollutants59,73. Bacteroidetes were dominant in samples from all seasons (Fig. 3). A previous study revealed that
Bacteroidetes has the capacity to decompose complex molecules in freshwater sediments74. Furthermore, our results indicated that the genus Flavobacterium and unclassified vadinHA17 were the
two primary components of Bacteroidetes, of which the genus Flavobacterium was the most abundant OTU. A similar study revealed that the genus Flavobacterium functions in heterotrophic
nitrification and metabolizes refractory organic compounds75. Therefore, the enrichment of the genus Flavobacterium may indicate widespread organic pollution in Lake Bosten. It is noteworthy
that there was a substantial percentage of unclassified bacterial groups detected in these seasonal samples (Fig. 4). In addition to those mentioned above, there were still several dominant
unclassified bacteria found in the samples, such as unclassified _Caldilineaceae_, unclassified _KD4–96_, and some incertae cedis bacteria (Fig. 4). Numerous studies have observed the
abundance of unclassified bacteria in various lake systems76,77, especially in sulfur-rich and anoxic environments18,78. The existence of abundant unclassified species in the estuarine
sediments of Lake Bosten indicates that new bacterial communities inhabit Lake Bosten and that the bacterial diversity, taxonomy and phylogenetics of the location deserve further attention.
CONCLUSION In this study, 454 pyrosequencing technology was used to study the bacterial community structure of the sediments in Lake Bosten. We found that the bacterial community of Lake
Bosten sediments is spatially specific. For example, the genus _Thiobacillus_, which is mainly found in mine wastewater and had a relatively high abundance in other areas, was found in the
HS area; enrichment in the HS area may indicate that the area is rich in minerals. In addition, there are obvious seasonal changes in the bacterial community of sediments. For example, the
relative abundance of the Firmicutes in the SC samples in the spring sampling was 53.3%, but only 0.14% in winter. The discharge of agricultural sewage caused the TN and TP of the HS area to
be higher than those of other samples, and the adsorption and decomposition of aquatic plants led to the enrichment of pollutants in the SC area. Furthermore, temperature and nutrient
status are key factors driving the bacterial community structure in sediments. We have discovered nitrifying bacteria with a large amount of degradable pollutants in Bosten Lake, and our
research provides a reference for preventing and remediating lake pollution. REFERENCES * Azam, F. Microbial control of oceanic carbon flux: the plot thickens. _Science_ 280, 694–696 (1998).
Article CAS Google Scholar * Tamaki, H., Sekiguchi, Y. & Hanada, S. Comparative analysis of bacterial diversity in freshwater sediment of a shallow eutrophic lake by molecular and
improved cultivation-based techniques. _Appl. Environ. Microb._ 71, 2162–2169 (2005). Article CAS Google Scholar * Newton, R. J. _et al_. A guide to the natural history of freshwater lake
bacteria. _Microbiol. Mol. Biol. R._ 75, 14–49 (2011). Article CAS Google Scholar * Nealson, K. H. Sediment bacteria: who’s there, what are they doing, and what’s new? _Annu. Rev. Earth
Pl. Sc._ 25, 403–434 (1997). Article ADS CAS Google Scholar * Zhang, R., Thiyagarajan, V. & Qian, P. Y. Evaluation of terminal-restriction fragment length polymorphism analysis in
contrasting marine environments. _FEMS Microbiol. Ecol._ 65, 169–178 (2008). Article CAS PubMed Google Scholar * Wang, H. Optimized estimation and its uncertainties of gross primary
production over oasis-desert ecosystems in an arid region of China. _Agu. Fall. Meeting_. (2017). * Huo, S. _et al_. Establishing water quality reference conditions for nutrients,
chlorophyll a and Secchi depth for 7 typical lakes in arid and semiarid ecoregion, China. _Environ. Earth Sci._ 73(8), 4739–4748 (2015). Article CAS Google Scholar * Cardona, A. _et al_.
Salinization in coastal aquifers of arid zones: an example from Santo Domingo, Baja California Sur, Mexico[J]. _Environ. Geol._ 45(3), 350–366 (2004). Article CAS Google Scholar * Liu, H.
_et al_. Disappearing Lakes in Semiarid Northern China: Drivers and Environmental Impact. _Environ. Sci. & Technol._ 47(21), 12107–12114 (2013). Article ADS CAS Google Scholar *
Sai, B. _et al_. Response of planktonic bacterial abundance to eutrophication and salinization in Lake Bosten, Xinjiang. _J. Lake Sci._ 23, 934–941 (2011). Article CAS Google Scholar *
Tang, X. _et al_. Influence of salinity on bacterial community composition in Lake Bosten, a large oligosaline lake in the arid northwestern China. _Appl. Env. Microb._ 78, 4748–4751 (2012).
Article CAS Google Scholar * Xie, G. _et al_. Spatio-temporal heterogeneity of water quality and succession patterns in Lake Bosten during the past 50 years. _J. Lake Sci._ 23, 988–998
(2011). Google Scholar * Tang, X. _et al_. Influence of salinity on the bacterial community composition in Lake Bosten, a large oligosaline lake in arid northwestern China. _Appl. Environ.
Microbiol._ 78(13), 4748–4751 (2012). Article CAS PubMed PubMed Central Google Scholar * Shi, Z. Characterization of the underwater light field and the affecting factors in Bosten Lake
in summer. _Acta Scientiae Circumstantiae_ 32(12), 2969–2977 (2012). Google Scholar * Zhang, L. _et al_. Pyrosequencing analysis of bacterial communities in Lake Bosten, a large brackish
inland lake in the arid northwest of China. _Can. J. Microbiol._ 62, 455–463 (2016). Article CAS PubMed Google Scholar * Mischke, S. & Wünnemann, B. The Holocene salinity history of
Bosten Lake (Xinjiang, China) inferred from ostracod species assemblages and shell chemistry: possible palaeoclimatic implications. _Quatern Int._ 154, 100–112 (2006). Article Google
Scholar * Xie, G. _et al_. Spatio-temporal heterogeneity of water quality and succession patterns in Lake Bosten during the past 50 years. _Lake Sci._ 23, 837–846 (2011). Article CAS
Google Scholar * Bai, Y. _et al_. Bacterial communities in the sediments of Dianchi Lake, a partitioned eutrophic waterbody in China. _PLoS one_ 7, e37796 (2012). Article ADS CAS PubMed
PubMed Central Google Scholar * Caporaso, J. G. _et al_. QIIME allows analysis of high-throughput community sequencing data. _Nat. Methods_ 7, 335–336 (2010). Article CAS PubMed
PubMed Central Google Scholar * Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. _Bioinformatics_ 26, 2460–2461 (2010). Article CAS PubMed Google Scholar *
Sai, B., Chen, M. P. & Feng, L. Agricultural non-point source pollution of Bosten Lake basin (in Chinese). _Water Resour. Prot._ 28, 25–29 (2012). Google Scholar * Weberscannell, P. K.
& Duffy, L. K. Effects of Total Dissolved Solids on Aquatic Organisms: A Review of Literature and Recommendation for Salmonid Species. _Ameri. J. Env. Sci._ 3, 1–6 (2007). Article CAS
Google Scholar * Wu, J., Ma, L. & Zeng, H. Analysis on water quality and water quantity of Lake Bosten in xinjiang and its evolution characteristics. _J. Geogr. sci._ 33, 231–237
(2013). Google Scholar * Liu, T. T. The seasonally change and output of carbon, nitrogen and phosphorus in the waterbody of Jialing River (In Chinese). Chongqing, Southwest Universe (2009).
* Guoming, Z., Yang, G. & Zhaojun, L. Study on non point resource dynamic transport of Lanhe watershed located in Fenhe River s upstream. _J. Soil. Water Conserv._ 22, 102 (2008).
Google Scholar * Chen, M. X. _et al_. N, P and salt contents in hydrophytes in the Bostern Lake, Xinjiang (in Chinese). _Arid. Zone Res._ 31, 844–849 (2014). Google Scholar * White, P. A.
_et al_. The effect of temperature and algal biomass on bacterial production and specific growth rate in freshwater and marine habitats. _Microb. Ecol._ 21, 99–118 (1991). Article CAS
PubMed Google Scholar * Kirchman, D. L., Rich, J. H. & Barber, R. T. Biomass and biomass production of heterotrophic bacteria along 140 W in the equatorial Pacific: effect of
temperature on the microbial loop. _Deep-Sea Res. Pt II_ 42, 603–619 (1995). Article ADS Google Scholar * Eduok, S. _et al_. Aged-engineered nanoparticles effect on sludge anaerobic
digestion performance and associated microbial communities. _Sci. Total. Environ._ 609, 232–241 (2017). Article ADS CAS PubMed Google Scholar * He, S. _et al_. Effects of temperature on
anammox performance and community structure. _Bioresour. Technol._ 260, 186–195 (2018). Article CAS PubMed Google Scholar * Smith, R. Limnology—Inland water ecosystems. _J. N. Am.
Benthol. Soc._ 21, 346–347 (2002). Article Google Scholar * Shao, K. _et al_. Comparing sediment bacterial communities in the macrophyte-dominated and algae-dominated areas of eutrophic
Lake Taihu, China. _Can. J. Microbiol._ 57, 263–272 (2011). Article CAS PubMed Google Scholar * Zaitseva, S. V. _et al_. Microbial community of the bottom sediments of the brackish Lake
Beloe (Transbaikal region). _Microbiology_ 83, 861–868 (2014). Article CAS Google Scholar * Song, H. _et al_. Bacterial communities in sediments of the shallow Lake Dongping in China. _J.
appl. microbiol._ 112, 79–89 (2012). Article CAS PubMed Google Scholar * Luo, J. _et al_. Microbial community structures in a closed raw water distribution system biofilm as revealed by
454-pyrosequencing analysis and the effect of microbial biofilm communities on raw water quality. _Bioresour. Technol._ 148, 189–195 (2013). Article CAS PubMed Google Scholar * Brown,
J. _et al_. Serum bone Gla-protein: A specific marker for bone formation in postmenopausal osteoporosis. _Lancet_ 323, 1091–1093 (1984). Article Google Scholar * Klepacceraj, V. _et al_.
High overall diversity and dominance of microdiverse relationships in salt marsh sulphate-reducing bacteria. _Env. Microbiol._ 6, 686–698 (2004). Article CAS Google Scholar * Angermeyer,
A., Crosby, S. C. & Huber, J. A. Decoupled distance–decay patterns between dsr A and 16S r RNA genes among salt marsh sulfate‐reducing bacteria. _Env. microbiol._ 18, 75–86 (2016).
Article CAS Google Scholar * Ferreira, J., Matthee, F. & Thomas, A. Biological control of eutypa lata on grapevine by an antagonistic strain of Bacillus subtilis. _Phytopathology_ 81,
283–287 (1991). Article Google Scholar * Yang, S. Q., Li, S. Y. & Li, F. Y. Selection of microflora and dominant bacteria degrading petroleum hydrocarbon in frozen soil in Shenfuzhi
irrigation area. _J. Meteorol. Environ._, 54–56 (2006). * Xu, Q. F., Huang, X. L. & Chen, T. W. Classification and determination of nitrogen fixation activity of eight strains of
Bacillus strains. _Microbiol. China_, 253–258 (1998). * Spring, S. _et al_. Identification and characterization of ecologically significant prokaryotes in the sediment of freshwater lakes:
molecular and cultivation studies. _FEMS Microbiol. Rev._ 24, 573–590 (2000). Article CAS PubMed Google Scholar * Porter, K. G. & Feig, Y. S. The use of DAPI for identifying and
counting aquatic microflora. _Limnol. Oceanogr._ 25, 943–948 (1980). Article ADS Google Scholar * Shibata, A. _et al_. Anaerobic biodegradation of 4-alkylphenols in a paddy soil microcosm
supplemented with nitrate. _Chemosphere_ 68, 2096–2103 (2007). Article ADS CAS PubMed Google Scholar * Bowman, J. P. & McCuaig, R. D. Biodiversity, community structural shifts, and
biogeography of prokaryotes within Antarctic continental shelf sediment. _Appl. Env. Microb._ 69, 2463–2483 (2003). Article CAS Google Scholar * Soltwedel, T. & Vopel, K. Bacterial
abundance and biomass in response to organism-generated habitat heterogeneity in deep-sea sediments. _Mar. Ecol- Prog. Ser._ 219, 291–298 (2001). Article ADS Google Scholar * Li, W. H.
& Yuan, L. Water and salt changes and their influencing factors in Lake Bosten, Xinjiang (In Chinese). _J. Lake sci._ 14, 233–227 (2002). Google Scholar * Taoka, A. _et al_.
Characterization of uncultured giant rod-shaped magnetotactic Gammaproteobacteria from a freshwater pond in Kanazawa, Japan. _Microbiology_ 160, 2226–2234 (2014). Article CAS PubMed
Google Scholar * Williams, K. P. _et al_. Phylogeny of gammaproteobacteria. _J. Bacteriol._ 192, 2305–2314 (2010). Article CAS PubMed PubMed Central Google Scholar * Disayathanoowat,
T. _et al_. Isolation and characterization of bacteria from the midgut of the Asian honey bee (Apis cerana indica). _J. Apic. Res._ 51, 312–319 (2012). Article CAS Google Scholar * Padhi,
S. K. _et al_. Characterisation of heterotrophic nitrifying and aerobic denitrifying Klebsiella pneumoniae CF-S9 strain for bioremediation of wastewater. _Int. Biodeter Biodegr_ 78, 67–73
(2013). Article CAS Google Scholar * Cai, J., Jiang, J. & Zheng, P. Isolation and identification of bacteria responsible for simultaneous anaerobic ammonium and sulfate removal. _Sci.
China Chem._ 53, 645–650 (2010). Article CAS Google Scholar * Marshall, K. T. & Morris, R. M. Isolation of an aerobic sulfur oxidizer from the SUP05/Arctic96BD-19 clade. _ISME J._ 7,
452 (2013). Article CAS PubMed Google Scholar * Beardsley, C. _et al_. Quantitative role of shrimp fecal bacteria in organic matter fluxes in a recirculating shrimp aquaculture system.
_FEMS Microbiol. Ecol._ 77, 134–145 (2011). Article CAS PubMed Google Scholar * Zheng, B. _et al_. Records of organic carbon and nitrogen stable isotopes of lake environment changes in
the past 200 years in Lake Bosten. 2012 32, 165–171 (2012). * Huang, W. _et al_. Characterization of sediment bacterial communities in plain lakes with different trophic statuses.
_MicrobiologyOpen_ 6, e00503 (2017). Article PubMed Central CAS Google Scholar * DeLong, E. F. Microbial community genomics in the ocean. _Nat. Rev. Microbiol._ 3, 459–469 (2005).
Article CAS PubMed Google Scholar * Friedline, C. _et al_. Bacterial assemblages of the eastern Atlantic Ocean reveal both vertical and latitudinal biogeographic signatures.
_Biogeosciences_ 9, 2177–2193 (2012). Article ADS Google Scholar * Sheng, P. _et al_. Bacterial diversity and distribution in seven different estuarine sediments of Poyang Lake, China.
_Env. Earth Sci._ 75, 479 (2016). Article Google Scholar * Martiny, J. B. H. _et al_. Microbial biogeography: putting microorganisms on the map. _Nat. Rev. Microbiol._ 4, 102 (2006).
Article CAS PubMed Google Scholar * Liu, L. _et al_. Vertical Structure of Bacterial and Archaeal Communities within the Sediment of a Eutrophic Lake as Revealed by Culture-Independent
Methods. _J. Freshw. Ecol._ 25, 565–573 (2010). Article Google Scholar * Tang, X. _et al_. Dynamics of organic‐aggregate‐associated bacterial communities and related environmental factors
in Lake Taihu, a large eutrophic shallow lake in China. _Limnol. Oceanogr._ 55, 469–480 (2010). Article ADS CAS Google Scholar * Barberán, A. & Casamayor, E. O. Global phylogenetic
community structure and β-diversity patterns in surface bacterioplankton metacommunities. _Aquat. Microb. Ecol._ 59, 1–10 (2010). Article Google Scholar * Newton, R. J. _et al_. Microbial
community dynamics in a humic lake: differential persistence of common freshwater phylotypes. _Env. Microbiol._ 8, 956–970 (2006). Article Google Scholar * Fortin, D., Davis, B. &
Beveridge, T. Role of Thiobacillus and sulfate‐reducing bacteria in iron biocycling in oxic and acidic mine tailings. _FEMS Microbiol. Ecol._ 21, 11–24 (1996). Article CAS Google Scholar
* Huber, J. A. _et al_. Microbial life in ridge flank crustal fluids. _Env. Microbiol._ 8, 88–99 (2006). Article CAS Google Scholar * Björnsson, L. _et al_. Filamentous Chloroflexi (green
non-sulfur bacteria) are abundant in wastewater treatment processes with biological nutrient removal. _Microb._ 148, 2309–2318 (2002). Article Google Scholar * Xue, Y. Q., Liu, F. &
Jiang, X. D. Diversity of winter sediment bacterial communities in different lake areas of Lake Taihu (In Chinese). _China Env. Sci._ 38, 719–728 (2008). Google Scholar * Wu, H. _et al_.
Sediment bacterial communities in a eutrophic lake influenced by multiple inflow-rivers. _Env. Sci. Pollut. R._ 24, 19795–19806 (2017). Article CAS Google Scholar * Zanaroli, G. _et al_.
A Chloroflexi bacterium dechlorinates polychlorinated biphenyls in marine sediments under _in situ_-like biogeochemical conditions. _J. Hazard. Mater._ 209, 449–457 (2012). Article PubMed
CAS Google Scholar * Chen, R. _et al_. Evolution of the microbial community of the biofilm in a methane-based membrane biofilm reactor reducing multiple electron acceptors. _Env. Sci.
Pollut. R._ 23, 9540–9548 (2016). Article CAS Google Scholar * Liang, B. _et al_. Anaerolineaceae and Methanosaeta turned to be the dominant microorganisms in alkanes-dependent
methanogenic culture after long-term of incubation. _AMB. Express_ 5, 37 (2015). Article PubMed Central CAS Google Scholar * Xiong, W. _et al_. Sources of organic matter affect
depth-related microbial community composition in sediments of Lake Erhai, Southwest China. _J. limnol._ 74 (2014). * Kirchman, D. L. The ecology of Cytophaga–Flavobacteria in aquatic
environments. _FEMS Microbiology Ecol._ 39(2), 91–100 (2002). CAS Google Scholar * Zhao, B. _et al_. Heterotrophic nitrogen removal by a newly isolated Acinetobacter calcoaceticus HNR.
_Bioresour. Technol._ 101(14), 5194–5200, https://doi.org/10.1016/j.biortech.2010.02.043 (2010). Article CAS PubMed Google Scholar * Peter, H. _et al_. Changes in bacterioplankton
community structure during early lake ontogeny resulting from the retreat of the Greenland Ice Sheet. _ISME J._ 12, 544–555 (2018). Article Google Scholar * Zhang, H. _et al_. Water
Bacterial and Fungal Community Compositions Associated with Urban Lakes, Xi’an, China. _Inter. J. Env. Res. Pu. Heal._ 15, 469 (2018). Article CAS Google Scholar * Klepacceraj, V. _et
al_. Microbial diversity under extreme euxinia: Mahoney Lake, Canada. _Geobiology_ 10, 223–235 (2012). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank staff at
the Environmental Monitoring Station of the Environmental Protection Bureau of Bayingolin Mongolia Autonomous Prefecture for helping with sample collection and water chemical analysis. This
work was supported by the National Science Foundation of China (41601573), the Key University Science Research Project of Anhui Province (KJ2019A0641), the Linkage Project of Anhui Public
Welfare Technology Application Research (1704f0804053) and the Science and the Technology Innovation Strategy and Soft Science Research Special Project of Anhui Province (1706a02020048).
Anonymous reviewers are acknowledged for their constructive comments and helpful suggestions. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Civil Engineering and Architecture,
Chuzhou University, Chuzhou, 239000, China Lei Zhang, Tingting Shen, Yu Cheng, Tingting Zhao & Li Li * State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing,
China Pengfei Qi Authors * Lei Zhang View author publications You can also search for this author inPubMed Google Scholar * Tingting Shen View author publications You can also search for
this author inPubMed Google Scholar * Yu Cheng View author publications You can also search for this author inPubMed Google Scholar * Tingting Zhao View author publications You can also
search for this author inPubMed Google Scholar * Li Li View author publications You can also search for this author inPubMed Google Scholar * Pengfei Qi View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS L.Z.: wrote the manuscript. T.T.S.: provided materials. Y.C.: proof read the manuscript. T.T.Z.: data analysis. L.L.: laboratory
analysis. F.P.Q.: collection of the sedmients samples. All authors read and approved the final manuscript. CORRESPONDING AUTHOR Correspondence to Lei Zhang. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION: ENVIRONMENTAL VARIABLES FROM TWENTY SEDIMENTS IN LAKE BOSTEN. RIGHTS AND PERMISSIONS OPEN ACCESS This article
is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you
give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material
in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, L., Shen, T., Cheng, Y. _et al._ Temporal and
spatial variations in the bacterial community composition in Lake Bosten, a large, brackish lake in China. _Sci Rep_ 10, 304 (2020). https://doi.org/10.1038/s41598-019-57238-5 Download
citation * Received: 17 April 2019 * Accepted: 26 December 2019 * Published: 15 January 2020 * DOI: https://doi.org/10.1038/s41598-019-57238-5 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative
Trending News
Michigan museum searching for women in wartime industryLast fall, the true identity of the woman who inspired the iconic Rosie the Riveter WWII posters was revealed as 95-year...
Channelnews : kaleidescape launches entry-level 4k strato e movie playerUS brand Kaleidescape has unveiled the Strato E, a new entry-level 4K movie player designed to make its premium home cin...
The marketwatch 50 - marketwatchHOW WE CAME UP WITH THE LIST: We asked our readers to submit nominations and the Marketwatch newsroom collected and revi...
Beat the chasers undergoes huge changes ahead of return to itvHost Bradley Walsh will once again be back on audiences’ screens as he welcomes all six Chasers to spin-off Beat The Cha...
U. S. To provide ukraine with $2. 6 billion more in military aidWASHINGTON — The U.S. will send Ukraine about $500 million in ammunition and equipment and spend more than $2 billion on...
Latests News
Temporal and spatial variations in the bacterial community composition in lake bosten, a large, brackish lake in chinaABSTRACT The bacteria inhabiting brackish lake environments in arid or semi-arid regions have not been thoroughly identi...
The page you were looking for doesn't exist.You may have mistyped the address or the page may have moved.By proceeding, you agree to our Terms & Conditions and our ...
Is lionel messi’s salary offside? | thearticleThe Barcelona footballer Lionel Messi has racked up a spectacular score sheet. A leak to Spanish media reveals that his ...
Smoke warning for motorists | The West AustralianDANIEL MERCERThe West Australian Motorists in the South-West are warned to take care this morning as heavy smoke is blan...
Burning Mouth Syndrome | International Journal of Oral ScienceABSTRACT Most clinicians dread seeing the patient presenting with a primary complaint of a burning pain on one or more o...