Psychological factors that influence decision-making regarding trauma-related pain in adolescents with temporomandibular disorder
Psychological factors that influence decision-making regarding trauma-related pain in adolescents with temporomandibular disorder"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT We evaluated the clinical, magnetic resonance imaging (MRI), and psychological characteristics of adolescents with temporomandibular disorder (TMD) and compared facial macrotrauma
effects between young and older adolescents. This case–control study included 70 randomly selected patients (35 young adolescents aged 12–16 years and 35 older adolescents aged 17–19 years)
who had been diagnosed with TMD. Each age group was further subdivided according to the presence (T1) or absence (T0) of a macrotrauma history. All patients completed questionnaires on
temporomandibular joint (TMJ) pain and dysfunction. We analyzed TMD severity symptoms using TMD-related indexes and the physical changes of TMJ using TMJ MR images. The Symptom
Checklist-90-Revised was used to evaluate the patients’ psychological status. Anterior disc displacement was the most frequently observed MRI finding, occurring in a significant proportion
of young (47 joints, 67.1%) and older adolescents (40 joints, 57.1%). The prevalence of all the MRI findings (disc displacement, disc deformity, condylar degeneration, and effusion) did not
differ between the T0 and T1 subgroups among young and older adolescents. Conversely, the psychological factors differed significantly between the subgroups. Among young adolescents, the
mean scores of somatization, obsessive-compulsiveness, hostility, phobic ideation, and psychosis were significantly higher in the T1 subgroup than in the T0 subgroup (all p < 0.05).
Furthermore, these increased psychological scores positively correlated with TMD indexes. Clinicians should consider that a weakened psychological status could be an aggravating factor in
young adolescents with TMD and should consider the implications in future assessment of such patients. SIMILAR CONTENT BEING VIEWED BY OTHERS TEMPOROMANDIBULAR INVOLVEMENT IN CHILDREN AND
ADOLESCENTS WITH JUVENILE IDIOPATHIC ARTHRITIS: A 2-YEAR PROSPECTIVE COHORT STUDY Article Open access 06 March 2024 WOMEN ARE WORSE OFF IN DEVELOPING AND RECOVERING FROM TEMPOROMANDIBULAR
DISORDER SYMPTOMS Article Open access 08 February 2025 CLASSIFICATION OF TEMPOROMANDIBULAR JOINT INTERNAL DERANGEMENT BASED ON MAGNETIC RESONANCE IMAGING AND CLINICAL FINDINGS OF 435
PATIENTS CONTRIBUTING TO A NONSURGICAL TREATMENT PROTOCOL Article Open access 22 October 2021 INTRODUCTION Temporomandibular disorder (TMD) is an umbrella term that covers heterogeneous
clinical problems involving the temporomandibular joint (TMJ), masticatory muscles, or both. The most frequent symptoms are pain in the masticatory muscles and/or in the TMJ, joint sounds,
and limitation or deviation of jaw motion1,2. TMD is the most common orofacial pain condition of non-dental origin, even though its actual prevalence is a matter of debate3. The reported
prevalence of TMD ranges from 1% to 75% for objective signs and from 5% to 33% for subjective symptoms4,5. Female predominance of TMD has been reported; however, a male predominance of TMD
was also reported among patients with a traumatic etiology6,7. TMD has numerous causes, including microtrauma due to parafunctional habits and malocclusion, macrotrauma, and stressful
conditions2. The causes of adolescent TMD have been poorly studied; macrotrauma and psychological impairment are two representative causes8, and both should be considered in adolescent TMD,
since successful management of TMD is dependent on identifying and controlling the key etiological factors2. The first onset of TMD and development of chronic TMD are fundamentally related
to the patient’s age as well as sex. Thus, identification of age-related patterns of TMD symptoms is important. Adolescence is defined as the distinct period covering the transition from
childhood to adulthood, and historically, this typically spans from 12 to 19 years of age9,10. In accordance with the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD)
guidelines and the DC/TMD guidelines, which is the evidence-based new version of the RDC/TMD, patients with TMD under 18 years of age were excluded in many previous studies, owing to the
lack of reliability, which has been verified in adult populations6,11. Thus, numerous previous TMD studies have focused on adults. A large case−cohort project, named Orofacial Pain:
Prospective Evaluation and Risk Assessment (OPPERA), was undertaken to identify the genetic, physiological, psychosocial, and clinical characteristics that influence the development of
TMD12,13, but was conducted on adults aged over 18 years14. However, adolescence is a unique period in which physical, psychological, and socioemotional development occurs simultaneously,
and these factors interact with each other in complex ways9,15. Although macrotrauma plays an important role in the development, maintenance, and worsening of TMD symptoms, it has rarely
been investigated in adolescents with TMD. Traumatic injuries due to motor vehicle accidents, forceful intubation, third molar extraction, physical abuse, and sports are reported to be
etiological factors in the development of TMD2. Some recent studies have been performed on adolescent TMD. Among children and adolescents, TMD was more prevalent among girls than boys, with
a lower prevalence than in adults14. TMD in adolescents can lead to permanent complications involving joint damage or deficits in mandibular growth, resulting in micrognathia, posterior
rotation of the mandible, and malocclusion16. However, significant knowledge gaps remain in case of adolescent TMD. TMD pain in adolescents may be multifactorial, involving a complex growth
trajectory from the biopsychosocial model17. Older girls aged between 16 and 19 years had significantly higher pain scores than did younger boys aged between 12 and 15 years18, as well as
higher analgesic consumption and school absences than did older boys19. In addition, highly anxious adolescents tend to function poorly, regardless of the level of pain20. That is, the pain
experience has different aspects depending on the sex, and it may change or be affected by the psychological state. Somatic complaints and headache have been strongly associated with TMD
pain in a previous study of a population-based sample of 12- to 19-year-olds10. In previous studies on adult patients with TMD, anxiety correlated with clinical signs of TMD and muscle
tenderness21. With regard to a macrotrauma history, patients with whiplash trauma had higher scores of muscle pain and psychological distress, as well as a poorer prognosis, than did those
without a whiplash history22. According to a review of the literature, adolescents with traumatic experiences had comorbid conditions, including anxiety, sleep problems, and attention and
learning problems23. Although macrotrauma plays an important role in the development, maintenance, and worsening of TMD symptoms, it has rarely been investigated in adolescents with TMD.
Magnetic resonance imaging (MRI) is considered the gold standard for evaluating physical and/or structural abnormalities of the TMJ and adjacent structures in patients with TMD. MRI provides
high tissue contrast, while being noninvasive and radiation-free24. In previous MRI studies, anterior disc displacement was most prevalent in adolescents with TMD, while bone changes were
more prevalent in the elderly25,26. Disc displacement was reported to precede disc degeneration, joint effusion, and degenerative osseous changes of the condyle and temporal bone24. However,
few MRI studies have been performed in adolescents with TMD. Moreover, findings regarding the effects of trauma on the symptoms and signs of TMD in the young have been inconsistent, and no
comparison has been made between the MRI findings of patients with TMD, with or without trauma. Therefore, the aim of the present study was to evaluate the clinical, MRI, and psychological
characteristics of TMD signs and symptoms, and their relationships in adolescents, as well as to compare these findings between young and older adolescent patients, in the context of the
relevant available literature. Additionally, we aimed to determine whether psychological impairments generally considered predictive of TMD are associated with increased pain intensity and a
history of trauma. METHODS PATIENT SELECTION We retrospectively analyzed the data of patients aged between 12 and 19 years who visited the orofacial pain clinic at Kyung Hee University
Dental Hospital between January 2013 and January 2019 because of TMD symptoms. They were diagnosed with TMD according to the RDC/TMD6 and underwent bilateral TMJ-MRI, including closed- and
open-mouth views, during the first visit. Among them, 35 patients were randomly selected, using a simple random sampling procedure employing a random number table for each of two age groups:
those aged 12–16 years were designated as young adolescents, and those aged 17–19 years were designated as older adolescents. Thus, the present study included 70 adolescents with TMD (31
female and 39 male patients; mean age: 16.46 ± 2.36 years). Patients in both groups were sub-divided according to the presence (T1) or absence (T0) of a trauma history and were further
analyzed. The exclusion criteria were as follows: subjects with a history of facial fracture injury, ongoing orthodontic treatment that could interfere with osteoarthritis, systemic
osteoarthritis, and those with juvenile idiopathic arthritis. Written informed consent was obtained from all study patients. In patients under the age of 18 years, informed consent was
obtained from a parent and/or legal guardian. The study design was approved by the appropriate ethics review boards of Kyung Hee University Dental Hospital. MRI ACQUISITION AND ANALYSIS
TMJ-MRI was performed using a 1.5 T MRI system (Signa Genesis; GE Healthcare, Chicago, IL, USA) employing a 6 × 8-cm-diameter surface coil. The protocol for TMJ examination included
T2-weighted imaging (T2WI), T1-weighted imaging (T1WI), and proton density (PD) imaging of both TMJs in the coronal and sagittal oblique planes by using thin sections of 3 mm or less, with a
15 cm field of view and a 256 × 224 matrix. T2W, T1W, and PD images were acquired at 2650/82, 650/14, and 2650/82 repetition time/echo time sequences. Two experienced head and neck
radiologists, blinded to the patients’ clinical information, performed visual analyses of the MR images. An initial analysis was first carried out to estimate the agreement between the two
radiologist’s opinions by using dichotomous levels to reflect a clinically significant change with Kappa statistics. The Kappa coefficient was 0.93, indicating a high level of agreement
between the two radiologists. After the TMJ images were acquired, the TMJ was evaluated using an Infinitt Picture Archiving and Communication System (Infinitt Corp., Seoul, Korea). Images of
the TMJ in the sagittal and coronal planes were acquired to determine the presence of internal TMJ derangement, including disc displacement with and without reduction, effusion, disc
deformity, and condylar degeneration. Disc position was determined in the closed- and open-mouth positions in the oblique sagittal plane. ASSESSMENT A clinical examination was performed in
accordance with the RDC/TMD, and maximum unassisted pain-free jaw opening as well as mandibular movement capacity and associated pain were measured in millimeters, with a ruler, between the
maxillary and mandibular central incisors. The presence of joint sounds and palpatory pain of the temporomandibular muscles and joints were also assessed. Thus, the following multiple
diagnoses could be made: myofascial pain, disc displacement, and/or arthralgia/osteoarthrosis. Two examiners (L.Y.H. and H.J.P.), trained and specialized in orofacial pain and TMD, with over
5 years of experience, were calibrated for diagnosing TMD based on the RDC/TMD criteria6. The Kappa coefficient for inter-examiner diagnostic agreement was 0.95. The acceptable reliability
of the questionnaire, clinical examination, and diagnosis has been previously reported27. In the questionnaire, the patients reported the intensity, frequency, duration, and location of
TMD-related symptoms and jaw function. In terms of the factors contributing to TMD, the patients were asked if they had been told or whether they themselves had noticed that they had each
contributing factor, and their responses were recorded as “yes” or “no.” The T1 subgroup comprised patients with TMD in whom TMD symptoms occurred after macrotrauma to their neck and facial
areas. The types of trauma included violent attacks (n = 10), car accidents (n = 6), falling-down injury (n = 3), and being hit by something (n = 11). Psychological characteristics were
evaluated using the Symptom Checklist-90-Revised (SCL-90-R). The SCL-90-R comprises nine symptom subscales, including somatization, obsessive-compulsiveness, interpersonal sensitivity,
depression, anxiety, hostility, phobic anxiety, paranoid ideation, and psychosis, and three global indices of functioning, including the global severity index (GSI), positive symptom
distress index, and positive symptom total (PST). CLINICAL AND MRI DATA COLLECTION All patients completed questionnaires regarding TMD pain and dysfunction, and all were assessed according
to RDC/TMD Axis I27. The adolescents rated their subjective TMD-related pain intensity on a 0–10 visual analogue scale (VAS). The severity of TMD was measured using TMD indexes, including a
palpation index (PI), dysfunction index (DI), and craniomandibular index (CMI)28. In addition, mandibular movement in the centric position (CMO, comfortable mouth opening without pain; and
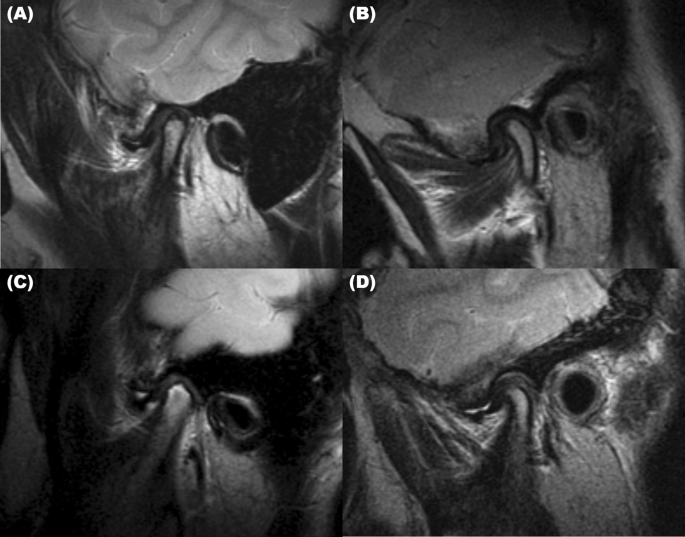
MMO, unassisted maximum mouth opening with pain) and eccentric positions (protrusion and lateral movement) were investigated. We used the MRI findings to investigate the presence of disc
displacement (Fig. 1A), disc deformity (Fig. 1B), condylar degeneration (Fig. 1C), and effusion (Fig. 1D). To determine whether differences between the measurements were statistically
significant, masticatory muscle values in each MR sequence of the right and left sides of the same patients were compared. STATISTICAL METHODS Descriptive statistics were used to present
percentages, means, and standard deviations (SDs) for continuous variables. Student’s t-test, for non-normally distributed variables, and chi-square test, for normally distributed variables,
were used to compare the young and older adolescents, and patients with trauma vs. patients without trauma in each adolescent group. Differences in the means of continuous variables between
the independent groups were examined using Student’s t-test. Fisher’s exact test was used to determine the equality of proportions. For analyzing the bivariate correlations between
categorical and continuous variables, the chi-square test and Pearson’s correlation test were used. The Kappa statistics was used to measure the agreement degree (Kappa coefficient) between
the two examiners who evaluated and rated the same subjects. Logistic regression analysis was performed, with each psychological factor as a dependent variable and the presence of a trauma
history as an independent variable. The regression coefficient value (B), standard error (SE), p-values, and odds ratios (ORs) with 95% confidence intervals (95% CIs) were investigated.
Spearman’s correlation analysis was performed to investigate the relationship between psychological factors and the TMD indexes. A two-tailed p-value < 0.05 was considered statistically
significant. Data were analyzed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY, USA). ETHICS APPROVAL AND CONSENT TO PARTICIPATE Procedures on human subjects were
performed in accordance with the ethical standards of the Committee on Human Experimentation of our institution as well as the Helsinki Declaration of 1975. In addition, the study design
was approved by the appropriate ethics review boards of Kyung Hee University Dental Hospital. RESULTS DEMOGRAPHIC CHARACTERISTICS OF PATIENTS AND PAIN INTENSITY Patients’ characteristics are
given in Table 1. The mean age of young adolescents was 14.54 ± 1.80 years, and that of older adolescents was 18.37 ± 0.69 years. The symptom duration of young adolescents was 149.63 ±
217.24 days (4.1 ± 7.6 months), which was significantly shorter than that of older adolescents 467.23 ± 500.28 days (15.6 ± 16.7 months) (p = 0.001). The ratio of girls to boys was
significantly higher among young adolescents (65.7%) than among older adolescents (22.9%) (p = 0.006). The distribution of symptoms reported by the patients, the side affected by
osteoarthritis, and the prevalence rate of osteoarthritis were not significantly different between the two TMD groups. TMJ pain was the most prevalent symptom among young adolescents, while
TMJ noise was the most prevalent among older adolescents. In terms of the range of mandibular movements, the mean CMO and of MMO values were significantly smaller among young adolescents
than among older adolescents. The mouth opening range usually increases with age until 14 years of age and peaks in individuals between 14 and 30 years of age29,30. Although reduced mouth
opening has been known to have diagnostic value for assessing the TMD status and the function of the TMJ, reports of measuring the mouth opening range in adolescents with TMD are lacking. As
compared to our previous results31, it is reasonable to assume that these levels are affected by both TMD status and body growth, as only the CMO value is smaller in young adolescents than
in adults with TMD (32.31 ± 10.42 vs. 34.56 ± 11.20 mm, p = 0.003). The VAS score, which shows the degree of subjective pain, was higher in young adolescents, but the difference was not
significant (p = 0.794). The PI, which shows the severity of muscle pain, was significantly higher among young adolescents than older adolescents (p = 0.012). DISTRIBUTION OF CLINICAL
SYMPTOMS AND CONTRIBUTING FACTORS The distribution of TMD symptoms and contributing factors was significantly different between the young and older adolescents (Table 2). The presence of
tinnitus (8.6% vs. 28.6%), headache (40.0% vs. 60.0%), history of maxillary orthodontic treatment (0.0% vs. 22.9%), and sleep problems (0.0% vs. 14.3%) was significantly lower among young
adolescents than among older adolescents. Conversely, the prevalence of a preference for hard food (37.1% vs. 14.3%), excessive talking (37.1% vs. 11.4%), and a history of trauma (54.3% vs.
31.4%) was significantly higher among young adolescents than among older adolescents. The most prevalent contributing factor in young adolescents was a history of trauma, and that among
older adolescents was unilateral chewing (34.3%). DISTRIBUTION OF MRI FINDINGS ACCORDING TO THE ADOLESCENT GROUPS We investigated the presence of disc displacement, disc deformity, condylar
degeneration, and effusion on MR images of both TMJs (Table 3). Only anterior displacement, and not posterior displacement of the TMJ disc, was observed. Anterior disc displacement was
observed at a substantial rate among both young and older adolescents. The prevalence of disc displacement (80% vs. 54.3%) and effusion (60% vs. 28.6%) of the right TMJ was significantly
higher among young adolescents than older adolescents. The most frequently observed MRI findings did not differ between the age groups; that is, in both young and older adolescents, disc
displacement was the most common MRI finding, followed by disc deformity, effusion, and condylar degeneration. Distribution of these MRI findings on the left side was not significantly
different between the groups. Interestingly, when we analyzed the data according to the presence or absence of a history of trauma, no significant differences were observed in the MRI
variables in either of the adolescent groups. PSYCHOLOGICAL FACTORS AND TRAUMA HISTORY No significant difference was observed in the SCL-90-R subscales between young and older adolescents.
The mean T-score of somatization was the highest among the nine psychological subscales. In young adolescents with TMD, a significant difference was observed in the T-scores of the SCL-90-R
subscales between those with (T1) and those without (T0) a history of trauma. The T1 subgroup had significantly higher T-scores in five of the symptom subscales of the SCL-90-R, including
somatization (50.5 vs. 41.9), obsessive-compulsivity (45.5 vs. 37.9), hostility (46.3 vs. 41.7), phobic ideation (49.4 vs. 42.9), and psychosis (47.7 vs. 42.3) than did the T0 subgroup. The
mean difference in the anxiety T-score between the T0 and T1 subgroups (45.0 vs. 40.3) was borderline significant (p = 0.063). Furthermore, among young adolescents, the T1 subgroup presented
with higher global indices of GSI and PST than did the T0 subgroup. In contrast, among older adolescents, no significant differences were observed in the SCL-90-R subscales between T0 and
T1. LOGISTIC REGRESSION ANALYSIS RESULTS Logistic regression analysis yielded noteworthy results only among young adolescents. The presence of a trauma history statistically significantly
increased the scores of six subscales: somatization, obsessive-compulsiveness, anxiety, hostility, phobic ideation, and psychosis, by 8.589, 7.651, 4.697, 4.576, 6.546, and 5.171,
respectively (Fig. 2). This was consistent with the results shown in Table 4, and a history of trauma resulted in the greatest increase in the logistic regression coefficient value for
somatization. In contrast, the presence of trauma did not significantly increase the score of any of the SCL-90-R subscales among older adolescents (Fig. 3). CORRELATIONS BETWEEN THE TMD
INDEXES AND SCL-90-R SUBSCALES Overall, the scores of the TMD indexes and T-scores of the SCL-90-R subscales were positively correlated (Table 5). The specific correlations of each TMD group
and subgroup showed some similarities and differences. The PI was positively correlated with somatization in both the TMD groups and their subgroups. In the T1 subgroup of both the TMD
groups, the PI displayed a significant positive correlation with hostility; the DI showed a positive correlation with somatization, obsessive-compulsiveness, anxiety, psychosis, and the
three global indices; and the CMI displayed a positive correlation with obsessive-compulsiveness. However, conflicting results were observed in the T0 subgroups regarding the relationship
between the PI and anxiety and between the CMI and depression; these were positively correlated in the T0 subgroup of young adolescents, whereas these were negatively correlated in the T0
subgroup of older adolescents. Among young adolescents, additional significant correlations were found. In the T0 subgroup of young adolescents, the PI was positively correlated with
depression and psychosis, but this was not observed in the T0 subgroup of older adolescents. In the T1 subgroup of young adolescents, the PI was positively correlated with
obsessive-compulsiveness and anxiety, but this was not observed in the T1 subgroup of older adolescents. DISCUSSION To our knowledge, no previous study has comprehensively investigated the
relationship between a history of macrotrauma and clinical TMD symptoms, MRI findings, and psychological factors in adolescents with TMD. Interestingly, the TMD in young adolescents is
unique, and clinicians should be more concerned about increased muscle pain and the relationship between psychological characteristics and a history of trauma. Although patients aged younger
than 18 years have been commonly excluded from TMD studies32, TMD research in adolescents is indispensable. Approximately 4% of adolescents aged 12–19 years had TMD pain, 8–38% had
headache, and 4–40% had musculoskeletal pain18,33. The lack of an identifiable etiology along with the complex biopsychosocial nature of adolescent TMD leads to delayed treatment that can
exacerbate existing symptoms. Therefore, the results of this study provide clinical and imaging characteristics of adolescents with TMD and the use of these characteristics in making
treatment decisions. We emphasize that the two age-divided adolescent TMD groups have different clinical characteristics. First, this study showed that young adolescents with TMD presented
higher PI scores (0.461 ± 0.354 vs. 0.275 ± 0.237) and a higher prevalence of a history of trauma (54.3% vs. 31.4%) than older adolescents. According to Kim _et al_., the PI scores increase
significantly with a history of trauma in patients with TMD34. TMD pain was reported to increase with increasing age in adolescents aged 12–19 years18, which was consistent with our results.
Nilsson _et al_. analyzed the overall level of TMD pain, whereas we investigated muscle- and joint-origin pain. As the PI measures the level of muscle tenderness in the stomatognathic
system, this index separates joint problems from muscle problems28. Conversely, older adolescents with TMD presented with a higher prevalence of headache (40.0% vs. 62.9%). Headaches appear
to be strongly associated with TMD in adolescents, with headaches most commonly accompanied by TMD pain10. Additional studies are needed to clarify the relationship between headache and
aging in adolescents with TMD. Young adolescents with TMD presented a shorter symptom duration than did older adolescents (149.63 ± 217.24 days vs. 467.23 ± 500.28 days). The role of
parental influence in the significantly shorter symptom duration among young adolescents may be considered, because they visited accompanied by parents at a higher frequency than did older
adolescents. Parental influence is relatively more important in developmental changes during early adolescence; thereafter, it slowly decreases, as peer influence increases throughout
adolescence35. Parental influence can affect both the child’s self-esteem and self-efficacy, as well as pain perception, pain experience, and pain behavior36. With regard to the presence of
parafunctional habits and contributing factors, significant differences were observed between the adolescent TMD groups. Parafunctional habits, including bruxism, clenching, and other
repetitive habitual behaviors are associated with psychological distress and are thought to contribute to the development of TMD via joint overload, which leads to cartilage breakdown,
synovial fluid changes, and other changes within the joint2,37. However, the paucity of research on adolescent TMD is too great to conclude that a relationship exists between parafunctional
habits and TMD. Taken together, we hypothesized that the etiology of the two adolescent TMD groups would be different. Regarding the presence of a trauma history on MRI analysis, our results
showed that the prevalence of TMJ abnormalities was not different between the groups; this may imply a weak correlation between a history of trauma and structural changes to the TMJ in
adolescents with TMD. Three MRI studies performed on patients who developed TMJ symptoms after macrotrauma, such as whiplash injury, revealed the presence of disc displacement in 56%38,
87%39, and 40%40 of the joints. In each study the prevalence of disc displacement was higher than that in the control group without trauma. However, in one previous prospective study the
prevalence of disc displacement on MRI did not differ significantly between patients with and without whiplash trauma at either of the two follow-up MRI examinations at 1 year and 15
years41. We also found that anterior disc displacement was a prominent MRI finding in both age-divided subgroups; moreover, all disc displacement was anterior. Vogl _et al_. reported that
anterior disc displacement was observed in 35% of adults clinically diagnosed with TMD and posterior disc displacement was observed in only 3%24. According to Su _et al_., disc changes were
more prevalent in adolescents with TMD than in older patients with TMD, and disc displacement was the most common finding25, which is consistent with our results. Joint effusion was observed
in 44.2% of all adolescents with TMD and in 53.3% of individuals with microtrauma. The prevalence of joint effusion varied in previous studies; Pressman _et al_. reported effusion in 65% of
the joints in patients with TMD and macrotrauma38, whereas others reported this in only 6% of TMJs in the whiplash trauma patient group40. In our study no significant difference was
observed in the MRI findings according to age or a macrotrauma history. Knowledge regarding the relationship between macrotrauma and physical changes observed on MRI in adolescents with TMD
has remained limited and few studies have been conducted to allow us to draw clear conclusions. Nevertheless, the proximate relationship between the presence of trauma and aggravation of
psychological factors was clear. It was noteworthy that somatization, obsessive-compulsiveness, hostility, phobic ideation, psychosis scores, and GSI were significantly higher in young
adolescents with a history of trauma. That is, unlike in older adolescents, a macrotrauma history increases psychological distress in young adolescents. The development of autonomy from
parents is an important developmental task for adolescents42. Sense of autonomy increased across ages 13 to 19 and rose sharply between ages 15 and 1743, indicating that steep increases
occur in late adolescence. In addition, impaired autonomy is related to chronic pain, pain-related disability, and psychological distress44,45. These findings may be the basis for explaining
how TMD clinical characteristics differ according to the age of adolescents. Patients with TMD and a history of trauma displayed higher TMD indexes and had a longer duration of symptoms, as
well as greater somatization, depression, anxiety, phobic anxiety, and paranoid ideation than did those without a history of trauma34. In addition, adolescents with posttraumatic stress
disorder demonstrated increased somatization, interpersonal sensitivity, obsessive-compulsiveness, depression, anxiety, and phobic anxiety over time46. Using linear regression analysis, this
relationship was further quantified in our study; a higher score for these factors was associated with the presence of a history of trauma in young adolescents. Psychological factors have
been widely recognized to be involved in the pain perception process in children and adolescents47. Although the etiology of TMD according to age in adolescents is unclear, psychological
factors have been implicated in the predisposition, initiation, and perpetuation of TMD48,49. In general, individuals with TMD pain exhibit greater psychological maladjustment than do
healthy controls46. Moderate to severe somatization was observed in approximately 60% of patients with TMD50. The individuals who were unable to cope well with TMD demonstrate higher rates
of somatization and depression51. Unfortunately, our study did not include a comparison of healthy adolescents and adolescents with TMD. Nevertheless, we explored the psychological factors
playing a role in adolescents with TMD and a history of trauma, which has rarely been done previously. Thus, a history of trauma can exacerbate psychological factors associated with TMD in
young adolescents. To understand the psychological factors related to pain and trauma in young adolescents, we must consider the multidimensional nature of adolescent TMD, within the context
of the biopsychosocial model, because adolescents are vulnerable in terms of functional TMJ pain derived from the interplay between organic dysfunction and psychosocial factors52,53.
Psychological stress resulting from events occurring at school and in the family, and the related muscle hyperactivity and muscle fatigue, as well as oral habits, have been suggested as
etiological factors2. Clinically, hypervigilance and hypersensitivity often cause heightened awareness of pain in young adolescents54. Therefore, young adolescents describe more pain on
muscle palpation and have a heightened fear of being touched. Further research is needed to understand the complex biobehavioral processes involved in adolescent TMD. In addition to our
strengths, our research has several limitations. First, this study is fragmentary with cross-sectional observational study design. With this design, it was not possible to clarify the change
over time of the variable or the causality between the variables, only to examine the fragmentation state or correlations. Second, although randomly selected patients were included, the
sample size was small (n = 70). Due to the nature of clinical studies with MRI-based diagnosis, there was a practical limitation that the number of samples could not be as large as LeResche
_et al_.’s cohort studies on predictors for TMD pain in adolescents with outstanding and important results55,56. Conversely, we performed direct observation of the patient rather than
conducting telephonic interviews, and the patient was diagnosed by experienced dentists and radiologists, and not hygienists. Furthermore, we conducted statistical power analyses using
G*Power 3.157, and obtained reliable power values of the main variables, at above 80%. Finally, we investigated many variables for the limited number of patients. Some variables were
related, and the hypotheses for each test were not completely independent in our study, possibly increasing statistical type I errors. We also simply compared the values of two TMD groups,
not more than three. Thus, the Bonferroni corrections, known as a strict and conservative method of reducing type I errors but increasing type II errors58, was not implemented. Nevertheless,
further longitudinal cohort studies with large samples are needed to confirm and extend our findings. CONCLUSIONS No previous studies have simultaneously investigated the characteristics of
clinical, psychological, and MRI findings in adolescents with TMD and their comparison according to the presence and absence of trauma. The multidimensional nature of adolescent TMD can be
best considered within the context of the biopsychosocial model. Our study emphasized that a history of trauma may be a key factor for increased psychological dimensions in this condition.
Furthermore, our findings provide evidence that measuring psychological functioning in the presence of a history of trauma in young adolescents with TMD may be beneficial in its treatment.
REFERENCES * Okeson, J. P. & de Leeuw, R. Differential diagnosis of temporomandibular disorders and other orofacial pain disorders. _Dental clinics of North America_ 55, 105–120,
https://doi.org/10.1016/j.cden.2010.08.007 (2011). Article PubMed Google Scholar * Sharma, S., Gupta, D. S., Pal, U. S. & Jurel, S. K. Etiological factors of temporomandibular joint
disorders. _National Journal of Maxillofacial Surgery_ 2, 116–119, https://doi.org/10.4103/0975-5950.94463 (2011). Article PubMed PubMed Central Google Scholar * Burakoff, R. P. &
Kaplan, A. S. Temporomandibular disorders: current concepts of epidemiology, classification, and treatment. _Journal of pain and symptom management_ 8, 165–172 (1993). Article CAS PubMed
Google Scholar * LeResche, L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. _Critical reviews in oral biology and medicine: an
official publication of the American Association of Oral Biologists_ 8, 291–305 (1997). Article CAS Google Scholar * Dworkin, S. F. _et al_. Epidemiology of signs and symptoms in
temporomandibular disorders: clinical signs in cases and controls. _Journal of the American Dental Association (1939)_ 120, 273–281 (1990). Article CAS Google Scholar * Dworkin, S. F.
& LeResche, L. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. _Journal of craniomandibular disorders: facial
& oral pain_ 6, 301–355 (1992). CAS Google Scholar * Steed, P. A. & Wexler, G. B. Temporomandibular disorders–traumatic etiology vs. nontraumatic etiology: a clinical and
methodological inquiry into symptomatology and treatment outcomes. _Cranio: the journal of craniomandibular practice_ 19, 188–194 (2001). Article CAS PubMed Google Scholar * Gage, J. P.
Collagen biosynthesis related to temporomandibular joint clicking in childhood. _The Journal of prosthetic dentistry_ 53, 714–717 (1985). Article CAS PubMed Google Scholar * Jaworska, N.
& MacQueen, G. Adolescence as a unique developmental period. _Journal of psychiatry & neuroscience: JPN_ 40, 291–293 (2015). Google Scholar * Nilsson, I. M., List, T. &
Drangsholt, M. Headache and co-morbid pains associated with TMD pain in adolescents. _Journal of dental research_ 92, 802–807, https://doi.org/10.1177/0022034513496255 (2013). Article
PubMed Google Scholar * Schiffman, E. _et al_. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: recommendations of the International
RDC/TMD Consortium Network* and Orofacial Pain Special Interest Groupdagger. _Journal of oral & facial pain and headache_ 28, 6–27, https://doi.org/10.11607/jop.1151 (2014). Article
Google Scholar * Slade, G. D. _et al_. Study methods, recruitment, sociodemographic findings, and demographic representativeness in the OPPERA study. _J Pain_ 12, T12–26,
https://doi.org/10.1016/j.jpain.2011.08.001 (2011). Article PubMed PubMed Central Google Scholar * Fillingim, R. B. _et al_. Potential psychosocial risk factors for chronic TMD:
descriptive data and empirically identified domains from the OPPERA case-control study. _J Pain_ 12, T46–60, https://doi.org/10.1016/j.jpain.2011.08.007 (2011). Article PubMed PubMed
Central Google Scholar * Østensjø, V., Moen, K., Storesund, T. & Rosén, A. Prevalence of Painful Temporomandibular Disorders and Correlation to Lifestyle Factors among Adolescents in
Norway. _Pain Research & Management_ 2017, 2164825, https://doi.org/10.1155/2017/2164825 (2017). Article Google Scholar * Casey, B. J., Jones, R. M. & Hare, T. A. The adolescent
brain. _Annals of the New York Academy of Sciences_ 1124, 111–126, https://doi.org/10.1196/annals.1440.010 (2008). Article CAS ADS PubMed PubMed Central Google Scholar * Gorska, A. _et
al_. Temporomandibular joint dysfunction and disorders in the development of the mandible in patients with juvenile idiopathic arthritis - preliminary study. _Advances in clinical and
experimental medicine: official organ Wroclaw Medical University_ 23, 797–804 (2014). Article Google Scholar * Shaw, L., Morozova, M. & Abu-Arafeh, I. Chronic post-traumatic headache
in children and adolescents: systematic review of prevalence and headache features. _Pain management_ 8, 57–64, https://doi.org/10.2217/pmt-2017-0019 (2018). Article PubMed Google Scholar
* Nilsson, I. M. Reliability, validity, incidence and impact of temporormandibular pain disorders in adolescents. _Swedish dental journal. Supplement_, 7–86 (2007). * Nilsson, I. M.,
Drangsholt, M. & List, T. Impact of temporomandibular disorder pain in adolescents: differences by age and gender. _Journal of orofacial pain_ 23, 115–122 (2009). PubMed Google Scholar
* Cohen, L. L., Vowles, K. E. & Eccleston, C. The impact of adolescent chronic pain on functioning: disentangling the complex role of anxiety. _J Pain_ 11, 1039–1046,
https://doi.org/10.1016/j.jpain.2009.09.009 (2010). Article PubMed Google Scholar * Bonjardim, L. R., Gaviao, M. B., Pereira, L. J. & Castelo, P. M. Anxiety and depression in
adolescents and their relationship with signs and symptoms of temporomandibular disorders. _The International journal of prosthodontics_ 18, 347–352 (2005). PubMed Google Scholar *
Krogstad, B. S., Jokstad, A., Dahl, B. L. & Soboleva, U. Somatic complaints, psychologic distress, and treatment outcome in two groups of TMD patients, one previously subjected to
whiplash injury. _Journal of orofacial pain_ 12, 136–144 (1998). CAS PubMed Google Scholar * Caffo, E. & Belaise, C. Psychological aspects of traumatic injury in children and
adolescents. _Child and adolescent psychiatric clinics of North America_ 12, 493–535 (2003). Article PubMed Google Scholar * Vogl, T. J. _et al_. The value of MRI in patients with
temporomandibular joint dysfunction: Correlation of MRI and clinical findings. _European journal of radiology_ 85, 714–719, https://doi.org/10.1016/j.ejrad.2016.02.001 (2016). Article
PubMed Google Scholar * Su, N., Poon, R., Friedman, L., Darling, M. & Grushka, M. TMJ Changes in Adolescent TMD Patients Seen on MRI in Clinical Setting. _The New York state dental
journal_ 81, 27–30 (2015). PubMed Google Scholar * Whyte, A. M., McNamara, D., Rosenberg, I. & Whyte, A. W. Magnetic resonance imaging in the evaluation of temporomandibular joint disc
displacement–a review of 144 cases. _International journal of oral and maxillofacial surgery_ 35, 696–703, https://doi.org/10.1016/j.ijom.2005.12.005 (2006). Article CAS PubMed Google
Scholar * Look, J. O., Schiffman, E. L., Truelove, E. L. & Ahmad, M. Reliability and Validity of Axis I of the Research Diagnostic Criteria for Temporomandibular Disorders (RDC/TMD)
with Proposed Revisions. _Journal of oral rehabilitation_ 37, 744–759, https://doi.org/10.1111/j.1365-2842.2010.02121.x (2010). Article CAS PubMed PubMed Central Google Scholar *
Fricton, J. R. & Schiffman, E. L. Reliability of a craniomandibular index. _Journal of dental research_ 65, 1359–1364, https://doi.org/10.1177/00220345860650111701 (1986). Article CAS
PubMed Google Scholar * Muller, L., van Waes, H., Langerweger, C., Molinari, L. & Saurenmann, R. K. Maximal mouth opening capacity: percentiles for healthy children 4-17 years of age.
_Pediatric rheumatology online journal_ 11, 17, https://doi.org/10.1186/1546-0096-11-17 (2013). Article PubMed PubMed Central Google Scholar * Al-Dlaigan, Y. H. & Asiry, M. A.
Maximum mouth opening in saudi adolescents. _J Int Oral Health_ 6, 45–49 (2014). PubMed PubMed Central Google Scholar * Lee, Y.-H., Lee, K. M., Auh, Q. S. & Hong, J.-P. Magnetic
Resonance Imaging-Based Prediction of the Relationship between Whiplash Injury and Temporomandibular Disorders. _Front Neurol_ 8, 725–725, https://doi.org/10.3389/fneur.2017.00725 (2018).
Article PubMed PubMed Central Google Scholar * Cavalcanti, R. F., Studart, L. M., Kosminsky, M. & de Goes, P. S. A. Validation of the multimedia version of the RDC/TMD axis II
questionnaire in Portuguese. _Journal of Applied Oral Science_ 18, 231–236, https://doi.org/10.1590/S1678-77572010000300006 (2010). Article PubMed PubMed Central Google Scholar * King,
S. _et al_. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. _Pain_ 152, 2729–2738, https://doi.org/10.1016/j.pain.2011.07.016 (2011). Article
PubMed Google Scholar * Kim, H. I., Lee, J. Y., Kim, Y. K. & Kho, H. S. Clinical and psychological characteristics of TMD patients with trauma history. _Oral diseases_ 16, 188–192,
https://doi.org/10.1111/j.1601-0825.2009.01626.x (2010). Article PubMed Google Scholar * Meeus, W. & Dekoviic, M. Identity development, parental and peer support in adolescence:
results of a national Dutch survey. _Adolescence_ 30, 931–944 (1995). CAS PubMed Google Scholar * Palermo, T. M. & Eccleston, C. Parents of children and adolescents with chronic pain.
_Pain_ 146, 15–17, https://doi.org/10.1016/j.pain.2009.05.009 (2009). Article PubMed PubMed Central Google Scholar * Rainbow, R., Ren, W. & Zeng, L. Inflammation and Joint Tissue
Interactions in OA: Implications for Potential Therapeutic Approaches. _Arthritis_ 2012, 741582, https://doi.org/10.1155/2012/741582 (2012). Article PubMed PubMed Central Google Scholar
* Pressman, B. D., Shellock, F. G., Schames, J. & Schames, M. MR imaging of temporomandibular joint abnormalities associated with cervical hyperextension/hyperflexion (whiplash)
injuries. _Journal of magnetic resonance imaging: JMRI_ 2, 569–574 (1992). Article CAS PubMed Google Scholar * Garcia, R. Jr. & Arrington, J. A. The relationship between cervical
whiplash and temporomandibular joint injuries: an MRI study. _Cranio: the journal of craniomandibular practice_ 14, 233–239 (1996). Article PubMed Google Scholar * Bergman, H., Andersson,
F. & Isberg, A. Incidence of temporomandibular joint changes after whiplash trauma: a prospective study using MR imaging. _AJR. American journal of roentgenology_ 171, 1237–1243,
https://doi.org/10.2214/ajr.171.5.9798853 (1998). Article CAS PubMed Google Scholar * Sale, H., Bryndahl, F. & Isberg, A. A 15-year follow-up of temporomandibular joint symptoms and
magnetic resonance imaging findings in whiplash patients: a prospective, controlled study. _Oral surgery, oral medicine, oral pathology and oral radiology_ 117, 522–532,
https://doi.org/10.1016/j.oooo.2014.01.020 (2014). Article PubMed Google Scholar * Riggenbach, A., Goubert, L., Van Petegem, S. & Amouroux, R. Topical Review: Basic Psychological
Needs in Adolescents with Chronic Pain-A Self-Determination Perspective. _Pain Res Manag_ 2019, 8629581, https://doi.org/10.1155/2019/8629581 (2019). Article PubMed PubMed Central Google
Scholar * Gutman, L. M. & Eccles, J. S. Stage-environment fit during adolescence: trajectories of family relations and adolescent outcomes. _Developmental psychology_ 43, 522–537,
https://doi.org/10.1037/0012-1649.43.2.522 (2007). Article PubMed Google Scholar * Palermo, T. M., Putnam, J., Armstrong, G. & Daily, S. Adolescent autonomy and family functioning are
associated with headache-related disability. _The Clinical journal of pain_ 23, 458–465, https://doi.org/10.1097/AJP.0b013e31805f70e2 (2007). Article PubMed Google Scholar * Chow, E. T.,
Otis, J. D. & Simons, L. E. The Longitudinal Impact of Parent Distress and Behavior on Functional Outcomes Among Youth With Chronic Pain. _The journal of pain: official journal of the
American Pain Society_ 17, 729–738, https://doi.org/10.1016/j.jpain.2016.02.014 (2016). Article Google Scholar * Fillingim, R. B. _et al_. Psychological Factors Associated with Development
of TMD: the OPPERA Prospective Cohort Study. _The journal of pain: official journal of the American Pain Society_ 14, https://doi.org/10.1016/j.jpain.2013.1006.1009 (2013). * Zernikow, B.
& Hechler, T. Pain Therapy in Children and Adolescents. _Deutsches Ärzteblatt International_ 105, 511–522, https://doi.org/10.3238/arztebl.2008.0511 (2008). Article PubMed PubMed
Central Google Scholar * Sipila, K. _et al_. Association between symptoms of temporomandibular disorders and depression: an epidemiological study of the Northern Finland 1966 Birth Cohort.
_Cranio: the journal of craniomandibular practice_ 19, 183–187 (2001). Article CAS PubMed Google Scholar * Rudy, T. E., Turk, D. C., Kubinski, J. A. & Zaki, H. S. Differential
treatment responses of TMD patients as a function of psychological characteristics. _Pain_ 61, 103–112 (1995). Article CAS PubMed Google Scholar * List, T. & Dworkin, S. F. Comparing
TMD diagnoses and clinical findings at Swedish and US TMD centers using research diagnostic criteria for temporomandibular disorders. _Journal of orofacial pain_ 10, 240–253 (1996). CAS
PubMed Google Scholar * Dworkin, S. F. & Massoth, D. L. Temporomandibular disorders and chronic pain: disease or illness? _The Journal of prosthetic dentistry_ 72, 29–38 (1994).
Article CAS PubMed Google Scholar * Adams, L. M. & Turk, D. C. Psychosocial Factors and Central Sensitivity Syndromes. _Current rheumatology reviews_ 11, 96–108 (2015). Article
PubMed PubMed Central Google Scholar * Vetter, T. R., McGwin, G., Bridgewater, C. L., Madan-Swain, A. & Ascherman, L. I. Validation and Clinical Application of a Biopsychosocial Model
of Pain Intensity and Functional Disability in Patients with a Pediatric Chronic Pain Condition Referred to a Subspecialty Clinic. _Pain Research and Treatment_ 2013, 143292,
https://doi.org/10.1155/2013/143292 (2013). Article PubMed PubMed Central Google Scholar * Clinch, J. & Eccleston, C. Chronic musculoskeletal pain in children: assessment and
management. _Rheumatology (Oxford, England)_ 48, 466–474, https://doi.org/10.1093/rheumatology/kep001 (2009). Article Google Scholar * LeResche, L., Mancl, L. A., Drangsholt, M. T.,
Saunders, K. & Von Korff, M. Relationship of pain and symptoms to pubertal development in adolescents. _Pain_ 118, 201–209, https://doi.org/10.1016/j.pain.2005.08.011 (2005). Article
PubMed Google Scholar * LeResche, L., Mancl, L. A., Drangsholt, M. T., Huang, G. & Von Korff, M. Predictors of onset of facial pain and temporomandibular disorders in early
adolescence. _Pain_ 129, 269–278, https://doi.org/10.1016/j.pain.2006.10.012 (2007). Article PubMed Google Scholar * Faul, F., Erdfelder, E., Buchner, A. & Lang, A. G. Statistical
power analyses using G*Power 3.1: tests for correlation and regression analyses. _Behav Res Methods_ 41, 1149–1160, https://doi.org/10.3758/brm.41.4.1149 (2009). Article PubMed Google
Scholar * Armstrong, R. A. When to use the Bonferroni correction. _Ophthalmic & physiological optics: the journal of the British College of Ophthalmic Opticians (Optometrists)_ 34,
502–508, https://doi.org/10.1111/opo.12131 (2014). Article Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank Sujin Jung, a statistician at Kyung Hee University,
for her statistical support, which greatly improved the manuscript. This work was supported by the Basic Science Research Program through the Ministry of Education of the Republic of Korea
(NRF-2016R1D1A1B03933173) and by the National Research Foundation of Korea (NRF) grant funded by the Korea Government (MSIP) (NRF-2017R1E1A2A0207113). AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Orofacial Pain and Oral Medicine, Kyung Hee University Dental Hospital, #26 Kyunghee-daero, Dongdaemun-gu, Seoul, 02447, South Korea Yeon-Hee Lee & Jung-Pyo
Hong * Department of Radiology, Kyung Hee University College of Medicine, Kyung Hee University Hospital, #26 Kyunghee-daero, Dongdaemun-gu, Seoul, 02447, South Korea Kyung Mi Lee *
Department of Biomedical Science and Engineering, Gwangju Institute of Science and Technology, #123 Cheomdangwagi-ro, Buk-gu, Gwangju, 61005, South Korea Tae Kim Authors * Yeon-Hee Lee View
author publications You can also search for this author inPubMed Google Scholar * Kyung Mi Lee View author publications You can also search for this author inPubMed Google Scholar * Tae Kim
View author publications You can also search for this author inPubMed Google Scholar * Jung-Pyo Hong View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS Yeon-Hee Lee contributed substantially to the conception and design of the study, the acquisition of data, the analysis and interpretation of data, the preparation of all
figures, and critical writing of main manuscript text and revising the content. Kyung Mi Lee contributed substantially to concept and design, and interpretation of data. Tae Kim contributed
substantially to concept and design, and interpretation of data. Jung-Pyo Hong drafted and provided critical revision of the article. CORRESPONDING AUTHOR Correspondence to Yeon-Hee Lee.
ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION CONSORT CHECKLIST RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lee, YH., Lee, K.M., Kim, T. _et al._ Psychological Factors that Influence
Decision-Making Regarding Trauma-Related Pain in Adolescents with Temporomandibular Disorder. _Sci Rep_ 9, 18728 (2019). https://doi.org/10.1038/s41598-019-55274-9 Download citation *
Received: 18 July 2019 * Accepted: 26 November 2019 * Published: 10 December 2019 * DOI: https://doi.org/10.1038/s41598-019-55274-9 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
Trending News
Something went wrong, sorry. :(Sobe para 3 o número de mortos em Israel após ataques do Irã; há mais de 80 feridos Exército israelense diz ter matado n...
Mutation analysis of the cystic fibrosis transmembrane conductance regulator (CFTR) gene, the cationic trypsinogen (PRSS1) gene, and the serine proteaSusceptibility to alcoholic chronic pancreatitis (ACP) could be genetically determined. Mutations in cationic trypsinoge...
Something went wrong, sorry. :(Haberler - Sağlık Abone Ol Böbrek taşı nedir? Böbrek taşı neden olur? Belirtileri ve tedavisi... Erkeklerde kadınlara gö...
Westgold breathes new life into historic cue mineWestgold Resources appears to have been vindicated in its strategy of going deeper in search of greater mineralisation a...
Tumor-specific exhaustion | Nature ImmunologyAccess through your institution Buy or subscribe T cell exhaustion is driven by persistent antigen and stress signaling....
Latests News
Psychological factors that influence decision-making regarding trauma-related pain in adolescents with temporomandibular disorderABSTRACT We evaluated the clinical, magnetic resonance imaging (MRI), and psychological characteristics of adolescents w...
Something went wrong, sorry. :(Haberler - Sağlık Abone Ol Böbrek taşı nedir? Böbrek taşı neden olur? Belirtileri ve tedavisi... Erkeklerde kadınlara gö...
Westgold breathes new life into historic cue mineWestgold Resources appears to have been vindicated in its strategy of going deeper in search of greater mineralisation a...
Tumor-specific exhaustion | Nature ImmunologyAccess through your institution Buy or subscribe T cell exhaustion is driven by persistent antigen and stress signaling....
Something went wrong, sorry. :(A demanda do setor de embalagens aumentou durante a pandemia do novo coronavírus (Covid-19). Porém, no caso das indústri...