Arctic climate change and pollution impact little auk foraging and fitness across a decade
Arctic climate change and pollution impact little auk foraging and fitness across a decade"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Ongoing global changes apply drastic environmental forcing onto Arctic marine ecosystems, particularly through ocean warming, sea-ice shrinkage and enhanced pollution. To test
impacts on arctic marine ecological functioning, we used a 12-year integrative study of little auks (_Alle alle_), the most abundant seabird in the Atlantic Arctic. We monitored the foraging
ecology, reproduction, survival and body condition of breeding birds, and we tested linkages between these biological variables and a set of environmental parameters including sea-ice
concentration (SIC) and mercury contamination. Little auks showed substantial plasticity in response to SIC, with deeper and longer dives but less time spent underwater and more time flying
when SIC decreased. Their diet also contained less lipid-rich ice-associated prey when SIC decreased. Further, in contrast to former studies conducted at the annual scale, little auk fitness
proxies were impacted by environmental changes: Adult body condition and chick growth rate were negatively linked to SIC and mercury contamination. However, no trend was found for adult
survival despite high inter-annual variability. Our results suggest that potential benefits of milder climatic conditions in East Greenland may be offset by increasing pollution in the
Arctic. Overall, our study stresses the importance of long-term studies integrating ecology and ecotoxicology. SIMILAR CONTENT BEING VIEWED BY OTHERS POPULATION CHANGES IN A SOUTHERN OCEAN
KRILL PREDATOR POINT TOWARDS REGIONAL ANTARCTIC SEA ICE DECLINES Article Open access 28 October 2024 KEYSTONE SEABIRD MAY FACE THERMOREGULATORY CHALLENGES IN A WARMING ARCTIC Article Open
access 04 October 2023 LONG-DISTANCE WINTER MIGRATIONS OF CHINSTRAP PENGUINS AND ELEPHANT SEALS TO A PERSISTENT BLOOM AT THE EDGE OF THE ROSS GYRE Article Open access 21 March 2025
INTRODUCTION Arctic biotas are facing rapid environmental modifications. The Arctic is warming twice as fast as any other place on earth, with visible negative impacts on sea-ice
distribution, significantly changing wind regimes and precipitation levels1,2. The concurrent emergence of competitors3, parasites4 or pollutants5,6 poses additional new threats for Arctic
wildlife7. In this context, there is an urgent need for long-term monitoring programs to investigate Arctic species and ecosystem reactions to multiple environmental
modifications8,9,10,11,12. This is especially true in the North as arctic biomes are poorly studied compared to the rest of the world7. In this region, coastal ecosystems deserve particular
attention11; they host endemic seabirds which have been identified as powerful ecological indicators, and are emblematic for Arctic peoples9. Seabirds are subjected to a fair number of
long-term monitoring programs in polar regions8,13,14,15,16,17,18. Yet most of these studies focus on the sub-Antarctic and the Antarctic, and integrative, long-term studies of the impacts
of environmental changes on the foraging ecology and fitness proxies of arctic seabirds are rare (e.g.8,15,17,19). Those are however needed to fully apprehend the incidence of ecosystem
modifications on these vulnerable species. In this study, we focused on little auks (_Alle alle_), the most abundant seabird breeding in the Arctic (estimated 40 to 80 million
individuals20). Little auks are ecologically important in regional food webs21 and could be negatively impacted by ongoing environmental change22,23,24. Notably, little auks are
zooplanktivorous, and the distribution of their main prey, Calanoid copepods, is changing along with the warming of the North Atlantic. As a result, the range of the smallest and less
energy-rich species, _Calanus finmarchicus_, of Atlantic origin, is extending northwards25. It may result in replacement of the two larger and energetically favoured Arctic species, _C_.
_glacialis_ and _C_. _hyperboreus_, that are preferred by little auks26,27. Such a change could prevent birds from covering their energetic needs22,28. Although previous studies highlighted
that little auks from different colonies can demonstrate strong behavioural plasticity to foraging conditions and prey availability, these studies were performed at the scale of one or a few
breeding seasons29,30 and longer term impacts are unknown. Furthermore, the largest little auk populations rely on sea-ice and polynya31, which are likely to disappear soon from their
summer foraging grounds according to IPCC predictions1, and this could further modify bird foraging behaviour and reproduction32. Similarly, changes in wind regimes could directly affect
little auk energetics33 and their capacity to respond to the aforementioned changes. At a broader spatial scale, the North Atlantic Oscillation (NAO) index reflects climatic conditions and
is commonly used to test for the effects of climate on seabirds (e.g.34,35). The NAO index seems to be particularly well suited to studying the population dynamics of migrants that rely on
climatic clues36. As an example, survival of little auks breeding in Spitsbergen was linked to winter NAO with a time lag of 2 years, with negative effects on the birds being possibly
mediated through varying intakes of little auk prey23. In addition to these climatic and resource modifications, little auks could face large changes in the contamination of their
environment. For instance, mercury (Hg) concentrations measured in little auks from East Greenland and reflecting the contamination of their environment have increased by 3.4% per year over
the last decade37. Mercury is a powerful neurotoxin as well as an endocrine disruptor38 which could therefore have significant impacts on the reproduction of this arctic seabird
species39,40. High mercury concentrations could also act as an additional stress factor for adult birds and, in combination with other aforementioned environmental changes, indirectly impact
their body condition or foraging performances41. In this context, we propose to examine the multiannual behavioural plasticity of this species in response to environmental change and to
investigate impacts on bird fitness. More specifically, and based on recent findings for little auks and their prey, we tested the following hypotheses: (1) the proportion of ice-associated
prey in little auk chick diet is decreasing with decreasing sea-ice extent, and the proportion of _Calanus finmarchicus_ is increasing25. (2) Adult little auks modify their foraging
behaviour to cope with a changing environment during summer, to maintain their body condition and the provisioning of their chicks, thereby also maintaining chick growth rates29,42. (3)
Increasing Hg contamination of little auk environments directly impacts their breeding performances (hatching date, chick growth rate)39,40 and acts as an additional stress factor for
adults, affecting their body condition41. (4) Little auk inter-annual survival is impacted by environmental conditions, both during the breeding and the inter-breeding seasons23,43. To test
these hypotheses, we used the longest integrative time-series currently available with respect to little auk ecology and ecotoxicology, which we collected at the breeding colony of
Ukaleqarteq (East Greenland, Supplementary Fig. S1) during 12 consecutive summers (2004–2015). We investigated adult foraging behaviour, adult and chick diet, adult winter survival, chick
growth, hatching date, and tested the incidence of environmental conditions (sea-surface temperature, sea-ice concentration (SIC), wind force, North Atlantic Oscillation and Hg
concentrations inferred from levels measured in bird feathers). RESULTS A summary of sample sizes and biological parameters monitored annually is presented in Table 1. Despite a 12-year long
dataset, we were limited by gaps in some of our biological measures and environmental variables (Table 1). This prevented the use of a global approach with causal inference, such as path
analysis44, as well as the use of principal component analysis to reduce the number of environmental covariables45. For this reason, we decided to apply a hypothesis-based approach to target
specific questions. We therefore built independent regression models for each biological parameter studied, to test the effects of one or two environmental covariates at a time, to avoid
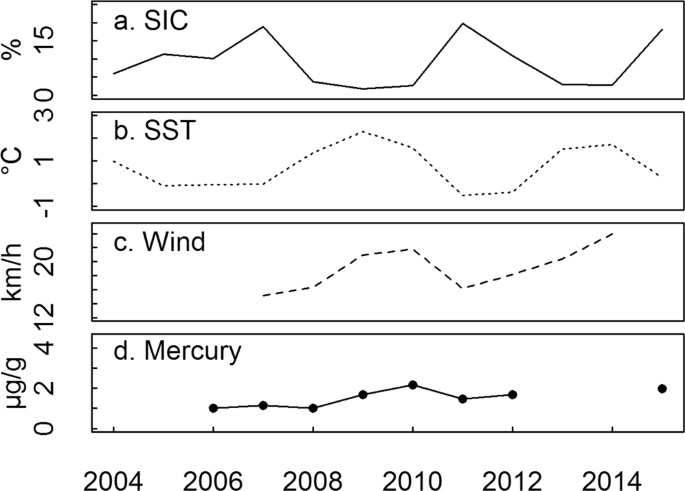
overfitting45. A summary of the results is presented in Table 2. ENVIRONMENTAL VARIABLES Over the period 2004–2015, mean summer SIC varied between 1.8 and 19.8% within the foraging range of
little auks (Fig. 1), allowing us to study the links between ice conditions and little auk ecology. SIC was negatively related to sea-surface temperature (SST, n = 12, R² = 0.74, p <
0.001, y = −6.1x + 13.6) and to wind speed (n = 8, R² = 0.57, p = 0.03, y = −1.3x + 42.9, Fig. 1). No temporal trend was found for summer SIC during the study period, however, a decrease in
SIC was found in the same area for the period 1979–201426. In addition, the range of SIC encountered in our study period was lower than the range of SIC for the period 1979–2000 (10 to
40%26). Summer Hg contamination of little auk environment (derived from body feathers, BF, see methods for details) did not vary with SIC (p > 0.1). VARIATIONS IN CHICK AND ADULT DIET We
found an inter-annual variability in chick diet (Figs 2 and 3, n = 298). Changes in the proportions of _Calanus finmarchicus_, _C_. _glacialis_ and other prey were best explained by GAMs
(Fig. 3a–c) while proportions of _Apherusa glacialis_ and _C_. _hyperboreus_ were best explained by linear models (Fig. 3d,e). The proportion of _Calanus finmarchicus_ increased at higher
SIC (N = 298, p = 0.001, R² = 0.05, Fig. 3a). The proportion of _Calanus glacialis_ was lower when SIC was >10% (N = 298, p < 0.0001, R² = 0.13, Fig. 3b). The proportion of _Apherusa
glacialis_ increased with SIC (N = 298, p = 0.01, R² = 0.04, y = 0.035x − 2.1, Fig. 3d). The proportion of _C_. _hyperboreus_ did not vary with SIC (N = 298, p = 0.25, R² = 0.005, Fig. 3e).
The proportion of other prey was higher when SIC was <5% (N = 298, p < 0.0001, R² = 0.28, Fig. 3c). Adult diet, as reflected by δ15N isotopic values, did not change along with SIC (n =
167, p > 0.1). However, adult foraging ecology changed over time (Fig. 4, trophic status δ15N: n = 167, R² = 0.32, p < 0.001, y = −138 + 0.07x, feeding habitat δ13C: n = 167, R² =
0.36, p < 0.001, y = 96.4 − 0.06x). FORAGING BEHAVIOUR Analysis of dive depths for all dives across the study period showed a bimodal pattern with two distinct peaks: one with dives <7
m and one with dives between 10 and 40 m (Fig. 5). Further investigations showed a link between diving behaviour and SIC (Fig. 6). Dives were deeper and the proportion of shallow dives
(<7 m) decreased when there was less ice (Fig. 6a,b; Maximum depth: n = 67, R² = 0.26, p < 0.001, y = −0.40x + 21.7, Proportion of shallow dives: n = 67, R² = 0.40, p < 0.001, y =
1.68x + 5.74). Dives were also longer and birds performed fewer dives per day when SIC decreased (Fig. 6c,d; Dive duration: n = 67, R² = 0.38, p < 0.001, y = −0.91x + 63.0, Number of
dives per 24 h: n = 61, R² = 0.26, p < 0.001, y = 9.6x + 226.8). Birds spent slightly less time underwater and more time flying when SIC was low (Fig. 6e,f; Time underwater: n = 61, R² =
0.09, p = 0.02, y = 0.31x + 17.0, Time spent flying: n = 53, R² = 0.12, p = 0.01, y = −0.62x + 38.0). There was no link between foraging trip duration and SIC (n = 53, R² = 0.004, p >
0.1). ADULT BODY CONDITION, CHICK GROWTH AND HATCHING DATE Hatching date was delayed when SIC increased (n = 303, R² = 0.08, p < 0.001, y = 16.7 + 0.15x, Fig. 7a) but was not linked to
female exposure to Hg during winter (n = 225, p > 0.1). Chicks hatched later over time (n = 303, R² = 0.11, p < 0.001, y = −660 + 0.34x). A decrease in chick growth rate (mass gained
per day) was observed when SIC increased (n = 252, R² = 0.03, p = 0.007, y = −0.037x + 6.79, Fig. 7b). Chick growth rate decreased when summer Hg increased (n = 165, R² = 0.03, p = 0.02, y =
−0.66x + 7.2, Fig. 7c) and chick growth rate tended to decrease over time (n = 252, R² = 0.02, p = 0.052, y = 103–0.05x). Adult body condition worsened with increasing summer Hg and
increasing SIC (n = 1051, R² = 0.04, p(Hg) < 0.001, p(SIC) < 0.001, y = 9.9–0.030x1–4.2x2–0.18x3, with x1 = day of year, x2 = Hg, x3 = SIC, Fig. 8), but there was no difference between
years (n = 1787, p > 0.1). SURVIVAL Results of the goodness-of-fit tests are detailed in the methods and in Table 3. A summary of the selection model process is presented in Table 4. We
constructed a model with capture heterogeneity due to the physical structure of the colony where some burrow entrances were harder to monitor46. Survival probabilities for the best model
(φ(t), p(het + t)) is presented in Fig. 9. No direct relationship was found between survival and environmental parameters (tested one by one: North-Atlantic Oscillation (current year,
previous year and two years before23), SST in their wintering area (current and previous year) and in their breeding area, SIC, wind conditions, summer and winter Hg). Survival probability
was lower for two years: 2006–2007 and 2012–2013 (Fig. 9). DISCUSSION Using a unique dataset of biological parameters from a 12-year long-term monitoring program in East Greenland, we found
that little auks are impacted by current environmental changes occurring in the Arctic. (1) As expected, the proportion of ice-associated species in chick diet was related to SIC, but the
proportion of _C_. _finmarchicus_ (copepod of Atlantic origin) did not decrease with increasing SIC. (2) Despite substantial plasticity in foraging behaviour and diet, adult body condition
and chick growth rates decreased when SIC increased. (3) Adult body condition and chick growth rate were negatively related to summer levels of mercury. (4) Despite these changes, adult
survival was not linked to environmental variables. METHODOLOGICAL CAVEATS Overall, we detected strong environmental impacts on little auks, but our work entails methodological limitations.
Indeed, organism answer to overall forcing is the integration of all environmental parameters, and disentangling the relative importance of each factor as well as their interactions requires
advanced statistical methods that we could not apply due to temporal gaps in our datasets (Table 1). Consequently, as we used only one or two environmental independent variables at a time
to explain one biological parameter with a hypothesis-based approach, the percentage of covariance explained by our significant relationships was low. It is therefore crucial to continue
long-term monitoring programs to increase sample sizes. In addition, we possibly missed important additional environmental factors. For instance, we know that the timing of breeding in
little auks is linked to the timing of snow melt in spring, which determines nest accessibility47. Spring snow melt can be approximated by spring temperature, but we did not have access to
this information at our study site. Also, other pollutants could, in addition to Hg, impact little auk reproduction and survival (e.g.48,49) but were not considered in the present study.
Concerning survival data, we could not take oil spills into account50, although it is known that little auks can be highly impacted during winter, with for instance an estimated 22,000
guillemots and little auks killed by hydrocarbon contamination in 2011–2012 off Newfoundland51. FORAGING PLASTICITY AS A BUFFER TO CLIMATE CHANGE Among all biological parameters
investigated, foraging behaviour was the most variable. While little auks foraged in the same areas at the shelf break with or without sea-ice26, we found that diving behaviour changed with
SIC: Birds performed shallower and shorter dives when SIC was the highest (Fig. 6). Thereby, dives <7 m (Fig. 5, Supplementary Fig. S2) probably reflected foraging directly underneath the
sea ice, to feed on sympagic species such as _Apherusa glacialis_, and indeed the proportion of this species in chick diet increased along with SIC (Fig. 3d). This was also supported by
preliminary results concerning birds for which diving behaviour and diet were collected simultaneously (n = 15, Amélineau _et al_. unpublished). Little auks seem capable of switching from
pelagic to below-ice feeding, and therefore to cope with a wide range of foraging conditions. Their energy expenditure as determined using the doubly-labelled water (DLW) technique thereby
seemed to remain unchanged29, yet additional studies combining 3D acceleration recordings of their actual foraging movements, and DLW are needed to fully test the impact of foraging
plasticity on energy balance52,53,54. Changes in little auk diet reflect their preferences, as well as prey availability in the environment. Recent studies suggest that little auks favour
larger and fattier species26,27 and, therefore, observed changes in prey proportions are likely to reflect the availability of larger prey species in the foraging range of birds. In the
North Atlantic Arctic, it is predicted that smaller _C_. _finmarchicus_ should be present during “warm” conditions (high SST, low SIC), and larger _C_. _glacialis_ and _C_. _hyperboreus_ in
“cold” conditions (low SST, high SIC)25,55,56. However, proportions of little auk main prey, the three species of _Calanus_, did not vary as expected: the proportion of _C_. _finmarchicus_
slightly increased at higher SIC, and the proportion of _C_. _glacialis_ decreased at higher SIC. Underlying mechanisms driving zooplankton abundance at a given place are complex and do not
depend solely on local summer SIC/SST, but might also vary with, for instance salinity and depth57 which were not included in this study. However, little auks have access to distant foraging
areas spread over dozens of kilometers where depth and salinity and thus prey availability might vary substantially27. In addition, it should be noted that although slightly increasing with
SIC, the proportion of _C_. _finmarchicus_ in chick diet remained low in comparison to other _Calanus_ species, whatever the SIC (Fig. 2). Interestingly, despite no clear pattern in chick
diet, adult diet shifted to a higher trophic level (higher δ15N values) and to more offshore feeding habitats (lower δ13C values) during the study period (Fig. 4). Such stable isotope
analyses are particularly integrative and could therefore reflect fine changes occurring in the longer term among the zooplankton community. Moreover, it is still unclear whether adults and
chicks feed on the exact same prey because the compositions of their diets have never been measured concomitantly. While stomach contents of adults are comparable to gular pouch
samples58,59, stable isotope studies suggest that there could be a difference60,61, as do our observations. More generally, little auks seem able to cope with different prey assemblages in
their environment27,30,62,63,64. DO LITTLE AUK LIVING CONDITIONS IMPROVE IN THE ABSENCE OF SEA-ICE? Little auks have a non-obligate affinity to sea-ice which varies according to location or
timing of the year. During the breeding season, they can forage at the marginal ice zone in Spitsbergen32,65 but can also thrive in the absence of sea-ice26,66. After the breeding season,
birds from different colonies migrate towards higher latitudes and the MIZ to moult67,68, before reaching their wintering grounds. At our study site, contrasting SIC from year to year
allowed to study impacts on little auk foraging and fitness proxies. Interestingly, adult body condition and chick growth rates were higher when SIC was low, meaning that less sea-ice and
higher SSTs provided better environmental conditions for breeding little auks in East Greenland. The link between environmental conditions and fitness proxies could be direct, as higher
temperatures reduce energy requirements for thermoregulation33, and energy gained could then be reallocated to body maintenance or chick rearing. Linkages could also be indirect, via trophic
interactions and bottom-up effects: lower SIC during summer reflects an earlier sea-ice breakup and a shorter lag between ice-algae bloom and pelagic phytoplankton bloom69. Our results
suggest that prey quality and/or availability would be better when sea-ice breakup occurs earlier. However, this is not in accordance with previous findings from Spitsbergen, where earlier
sea-ice breakup lead to a mismatch between algal blooms and copepod _C_. _glacialis_ phenology, and ultimately to a lower chick survival in little auks and Brünnich’s guillemots70,71.
Changes in the trophic interactions occurring in the Western Greenland Sea are probably more complex and reflects high variability of local conditions throughout the Arctic12. Lastly, SIC
encountered during the study period (2004–2015, 9.1 ± 6.8%) were already lower than SIC encountered during the period 1979–2003 (20.0 ± 10.4%)26 and may already feature suboptimal conditions
for little auks. Regarding SSTs, previous studies suggested that the most profitable foraging areas for little auks are located in the cold waters encountered in the Sørkapp Current (SW
Spitsbergen) and in the East Greenland Current that contain bigger _Calanus_ species27,72. Our results contrast with those from previous studies performed in Spitsbergen where higher SSTs
were associated with a higher proportion of _C_. _finmarchicus_ in the environment and in chick diet, and led to a lower chick survival or probability to fledge22,72, and to a lower adult
survival23. SSTs are higher in Western Spitsbergen than in East Greenland, and these contrasting findings suggest that little auk fitness could follow a quadratic relationship with SST,
peaking at intermediate SST. An increase in SST could then be beneficial at low SSTs (East Greenland, mean SST of 0.67 °C) but detrimental at high SSTs (Spitsbergen, mean SST of 1.81 °C at
Hornsund and 4.51 °C at Kongsfjorden). However, little auks did not seem impacted by observed changes in SST around Bjørnøya, where they foraged in warm waters (median SST of 6.6 °C) during
the period 2005–201266. Among pagophilic seabird species, reactions to variations in SIC are diverse and depend on species-specific sea-ice affinity. High SIC during the breeding season can
reduce access to prey and lead to lower breeding success for moderately pagophilic seabirds15,16,73. Species that are more dependent on sea-ice, on the contrary, have lower breeding success
and survival when SIC is reduced, and have to travel over longer distances to reach the MIZ74,75. At the larger temporal and spatial scales, changes in SIC likely lead to changes in species
range to the detriment of pagophilic species9,76. POLLUTANTS OFFSET OBSERVED BENEFITS FROM LOWER SIC Hg concentrations during summer increased in adult little auks, likely linked to an
increase in prey Hg concentrations over time37, but also to changes in diet towards prey that are higher in the food chain (biomagnifications)77, as reflected by the increase in δ15N in
adult blood (Fig. 4a). Although measured Hg concentrations were below toxicity thresholds38,78, they were negatively related to adult body condition and chick growth rate. These observed
effects suggest that Hg might act as a cumulative stressor which, in combination with other environmental constraints like the quality of their habitat or resource availability41, could
impact the condition of marine predators beyond its single effects. In addition, one should also bear in mind that little auks are exposed not only to Hg, but to a variety of pollutants
reaching the Arctic from northern mid-latitude industrial regions, some of them emerging but already of high concern in this sensitive region79. We specifically focused on Hg, which is known
to bioaccumulate in polar regions and severely impact marine top predators38,78, but these other pollutants may as well impact little auk metabolism and ultimately their body condition and
growth rate, such as organochlorine pesticides or PFASs80. In addition, Hg disrupts breeding behavior in black-legged kittiwakes and snow petrels40,81 and this mechanism could also explain
reduced chick growth when Hg concentrations are high in our study. However, no link was found between Hg and adult survival in our study, as well as in other seabird species48,82,83,84. Hg
levels in the Arctic are modified by ongoing environmental changes85. In particular, increasing Hg trends are expected with permafrost thawing, the warming of ocean water masses and
increasing human activities in the Arctic85,86,87. According to our results, negative effects of increasing Hg could offset observed positive effects of climate warming in East Greenland.
This stresses again the complexity of biological answers to environmental changes and the need for integrative approaches. CONCLUSION Understanding how animals will cope with environmental
changes is a topical challenge in ecology. Since the Arctic is warming twice as fast as the rest of the world it can be seen as a natural laboratory to anticipate changes occurring at a more
global scale. Unfortunately, logistical constraints, including year round access, limit fieldwork studies in this part of the world. In addition, biological responses are complex and
integrate environmental constraints which may be logistically difficult to evaluate at remote locations. Our results highlight the importance of pursuing long term monitoring programs in the
Arctic to improve dataset length and quality, and gain power to elaborate more complex models88. METHODS GENERAL FIELDWORK CONTEXT All field work in East Greenland was conducted in
accordance with guidelines for the use of animals89. Experiments were approved by the Danish Polar Center and the Government of Greenland, Ministry of Environment and Nature and Department
of Fisheries, Hunting and Agriculture (No. 512–240 (2005), No. 512-258 (2006), No. 07-501 (2007), No. 66.24/23 (2008), No. 66.01.13 (2009 and 2010), No. 2011-047447 (2011), No. 2012-065815
(2012), No. 2013- 083634 (2013), No. 2014-098814 (2014) No. 2015-115290 (2015). Little auks from Ukaleqarteq (Kap Höegh, East Greenland, 70°44′N, 21°35′W, Supplementary Fig. S1a) were
studied during the breeding season (mid-July/mid-August) from 2004 to 2015. Birds breed under rocks in steep boulder fields, where they raise a single offspring. Adult birds fly out to sea
where they forage on zooplankton, which they bring back to their chick in a sublingual pouch. During the inter-breeding period (Sept-May), birds migrate to wintering areas in the North
Atlantic, notably off Newfoundland90. Each summer, a set of biological parameters detailed below were monitored, and sample sizes are presented in Table 1. Adult birds were caught either in
their nest or on the surrounding rocks using a lasso or noose carpets. Breeding status was assessed by the presence of a chick, a full sublingual pouch or a brood patch. Handling time was
<10 min. For all sampling except for the survival study (see below), each year different individuals were studied. Therefore, our investigations were mainly conducted at the population –
and not at the individual – level. CHICK AND ADULT DIET Breeding adults were captured on arrival at the colony and the content of their sublingual pouch (chick diet) was removed and stored
either in 4% borax-buffered formaldehyde solution (2005 to 2007) or in 70% ethanol (2008 and beyond). Samples were identified to the lowest possible taxonomical level under a
stereomicroscope following groups presented in Harding _et al_.28. Adult diet was estimated from stable isotope analyses (δ15N and δ13C) performed on total blood samples61. δ15N isotopic
values reflect the relative trophic position of birds and are considered an indicator of their diet a couple of weeks before the sampling91. δ13C was also considered as an indicator of bird
foraging habitats with higher values representing more coastal habitats91. Blood samples (0.3 ml) were collected from bird brachial vein, stored in 70% ethanol and kept frozen at −20 °C.
Prior to analyses, blood samples were freeze-dried for 48 h and homogenized. Stable isotope analyses were then performed on ~0.5 mg subsamples of homogenized, non-lipid extracted whole blood
loaded into tin cups, and using an elemental analyzer (Flash EA 1112, Thermo Fisher) coupled in continuous flow mode to an isotope ratio mass spectrometer (Delta V Advantage, Thermo Fisher,
Bremen, Germany). Stable isotope abundances were expressed in δ notation as the deviation from standards in parts per thousand (‰) according to the equation: δX = [(Rsample/Rstandard) − 1]
× 1000 where X is 13C or 15N and R is the corresponding ratio 13C/12C or 15N/14N. Standard values were Vienna Pee Dee Belemnite (VPDB) for C and atmospheric N2 (air) for N. Replicate
measurements of internal laboratory standards (acetanilide) indicated that the measurement error was <0.2% for both δ15N and δ13C values. FORAGING BEHAVIOUR Numbers of equipped birds are
presented in Table 1. Breeding adults were equipped with temperature-depth recorders (TDRs) attached ventrally, recording at 0.2, 0.5 or 1 Hz for 2–5 d during the chick-rearing period.
Details on TDR types and attachment methods are presented in Supplementary Methods and Supplementary Table S1. Data were analyzed with MultiTrace™ to extract maximum dive depth, dive and
pause duration for each dive. Depth was corrected for Star Oddi devices because they showed a slight underestimation of depth according to a calibration made in Amélineau _et al_.26. We also
measured time spent flying and foraging trip duration following Welcker _et al_.92, and calculated the time spent underwater, and the number of dives per day. For each parameter, a mean
value per individual was calculated and used for statistical analyses. HATCHING DATE AND CHICK GROWTH Nests were controlled for hatching date and chicks were weighed every second day. For
each chick, we calculated the chick growth rate (g d−1) as the slope of the linear growth period26 (4–14 days). Due to logistical constraints usually preventing measurements after August 10,
we could not control all nests until fledging to measure fledging success (range of fledging age = 21–31 days93). ADULT BODY CONDITION AND MERCURY CONTAMINATION Each handled adult was
weighed (g), and wing and head-bill lengths were measured (mm). We constructed a body condition index, correcting adult body mass by wing length and head-bill length to take bird size into
account. The body condition index was calculated as the residual body mass from a regression of body mass on wing length and head-bill length43. Total Hg was measured on one complete back
cover feather (abbreviated BF hereafter) or one complete throat feather (abbreviated HF hereafter) using an advanced Hg analyzer spectrophotometer (Altec AMA 254) as described in Bustamante
_et al_.94. Hg in little auk BF reflect the amount of Hg accumulated by birds during the previous breeding season spent in East Greenland (year preceding the sample), while Hg in HF reflect
the amount of Hg accumulated during the previous winter (see Fort _et al_.37). Hence, BF collected from 2007 to 2016 were analyzed for comparison with the biological time-series. Previous
studies showed that >90% of Hg in seabird feathers is methyl-Hg (Me-Hg), the most toxic form of Hg95. Total Hg measured in feathers is thus considered as an indicator of bird exposure to
Me-Hg. Hg concentrations measured in bird feathers are also an indicator of the contamination level of the food chain on which birds feed during both seasons37. Analyses were repeated two or
three times (two or three feathers) for each bird and feather type until the relative standard deviation for two samples was <10%; samples not meeting this criterion were excluded from
the analysis. The mean Hg concentrations for those two or three measurements were then considered for statistical analyses. To ensure the accuracy of measurements, a certified reference
material (CRM) was used [Lobster Hepatopancreas Tort-2; NRC, Canada; Hg concentration of 0.27 ± 0.06 µg.g−1 of dry weight (dw)]. The CRM was measured every 10 samples and the average
measured value was 0.26 ± 0.01 µg.g−1 dw (n = 113). Additionally, blanks were run at the beginning of each sample set. The detection limit of the method was 0.005 µg.g−1 dw. SURVIVAL
ANALYSIS One plot of the colony was dedicated to a capture-mark-recapture experiment. Birds (n = 333) were marked with a unique code composed of 3 colour rings and one metal ring. Each
season, recapture sessions lasted 6 d with 7 h of continuous observation per day. Data were analyzed using a capture-recapture model96 with E-SURGE. We first built a structural model without
any external covariate. To define the structural model, we first did single state goodness-of-fit tests (GOF, Table 3) using U-CARE97. Only the 2.CT test was significant, indicating a
difference in the probability of being recaptured at i + 1 for birds seen and not seen at occasion i. In order to take into account recapture heterogeneity among marked birds, we used a
model with two classes of capture98 and defined three states: individuals with a high recapture probability, individuals with a low recapture probability and dead individuals. Changes of
state between high and low recapture probability were not permitted. Such a structure explained our data better than a model including trap-dependence or allowing changes of recapture
probability through time (if, for example, this was linked to breeding status). Biologically, recapture heterogeneity was due to the structure of the colony, where some birds nest in areas
where it is more difficult to see them enter and leave their burrows. Such a model with recapture heterogeneity was used for least auklets (_Aethia pusilla_) that also breed in burrows like
little auks46. The model selection was conducted with E-SURGE99. The general starting model was (φ(t), p(het.t)), where “het” denotes the heterogeneous effect on capture with two levels
(seen with a high or low probability), t denotes the time effect. Models were selected based on Akaike100 Information Criterion (AIC) corrected for sample sizes and overdispersion (QAICc).
During first step, we selected the best model with only time and state as explanatory variables. In step two, we included environmental variables (one or two at a time45, the absence of
correlation was verified when two environmental variables were included simultaneously) to the model with the best structure in the first step (Table 4). ENVIRONMENTAL DATA For the summer
period, environmental data were dealt with within a 160 × 200 km plot surrounding the colony, which also included little auk at-sea habitats as determined through GPS tracking26
(Supplementary Fig. S1b). Monthly SST came from the Multi-scale Ultra-high resolution (MUR) SST analysis from the NASA (v4.1, global 0.01° resolution, monthly) and were acquired from
http://coastwatch.pfeg.noaa.gov/erddap/index.html. Monthly wind data came from the Metop/ASCAT data set (0.25° resolution, monthly, starting in 2007) from CERSAT and were acquired from
http://cersat.ifremer.fr/. Daily sea-ice concentration (SIC, percentage of sea surface covered by ice in a given area) came from the Eumetsat OSI SAF and were acquired from
http://osisaf.met.no/, using the Global Sea Ice Concentration reprocessing dataset (0.25° resolution, daily). For wind and SST data, we used monthly values for July and we calculated the
mean annual value for the foraging area defined above. For SIC, we calculated a mean annual value from the daily SIC concentration in the area between 15th July and 15th August.
Overwintering locations of birds from Ukaleqarteq were known from birds equipped with geolocators90, and we defined the core wintering area of birds as the 50% kernel area of positions
between 1st November to 28th February obtained for 94 little auks equipped between 2009 and 2015101. Yearly winter environmental conditions (wind speed, SST) were calculated as a mean value
within the core wintering area from November to February from monthly values of the datasets mentioned above. In addition, we calculated the proportion of days with high winds (mean daily
wind speed > 40 km/h) during the same period, using daily wind speeds from the Metop/ASCAT data set (0.25° resolution). Data for the North Atlantic Oscillation (NAO) came from the UCAR
and were acquired from https://climatedataguide.ucar.edu/climate-data. Herein we used the winter NAO index23. Winter environmental parameters were used for the survival analyses only.
STATISTICAL ANALYSES All analyses were performed with the R software (v. 3.4.2; R core team 2017). We adopted an hypothesis-based approach to study the link between environmental variables
and biological parameters, i.e. we tested specific linear models with one or two explanatory variables at a time that are meaningful in a biological context, instead of testing all possible
combinations of factors. This ensured to reduce type 1 errors, and to avoid overfitting, as well as issues regarding autocorrelation among environmental variables45. In particular, summer
SIC, summer SST and summer wind were highly related (Fig. 1). Among these environmental variables, SIC was selected as the main environmental parameter to test, due to its strong and direct
influence on the foraging behaviour of little auks at our study site. Mean yearly Hg concentrations in head or body feathers were considered as environmental variables reflecting Hg found in
the environment in winter and in the previous summer, respectively37. Hg was either tested independently when a direct influence is expected (chick growth rate and hatching date), or tested
concomitantly with SIC when it is expected to be an additional stress factor (adult body condition). We also investigated temporal variations of biological parameters.”Day of year” was
included in the models with adult body condition as a response variable as body mass is slightly decreasing along the breeding season. For adult diet, foraging behaviour, adult body
condition and chick growth rate, we performed linear regressions to model the relationship between biological variables and environmental variables, when the assumptions were met. We did not
use mixed-effects models because each bird was only sampled one time. Results of the tests were considered significant when p-value was <0.05. For changes in prey proportion in chick
diet in relation to SIC, we first used Generalized Additive Models (GAMs) as no linear curve was expected. We performed a logit transformation of the proportions (p): log((p + a)/(1 − (p +
a)), and where _a_ is a constant (_a_ = 0.1) in order to get a logit for all proportions including null values. Each GAM was then compared with a linear model and the model with the lower
AIC was retained (all differences in AIC were greater than 2). DATA AVAILABILITY All data are available on request from the CEFE CNRS database accessible here:
http://www.cefe.cnrs.fr/fr/ressources/base-de-donnees/1114-puechdb-station-experimentale-de-puechabon-tour-a-flux-sp-23356. REFERENCES * IPCC. Climate Change 2013: The Physical Science
Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. [Stocker, T. F., D. Qin, G.-K. Plattner, M. Tignor, S. K. Allen, J.
Boschung, A. Nauels, Y. Xia, V. Bex and P. M. Midgley (eds)] Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, 1535 pp. (2013). * AMAP. Snow, water, ice and
permafrost. Summary for policy-makers. (Arctic Monitoring and Assessment Programme (AMAP) Oslo, 2017). * Gilg, O. _et al_. Climate change and the ecology and evolution of Arctic vertebrates.
_Ann. N. Y. Acad. Sci._ 1249, 166–190 (2012). Article PubMed ADS Google Scholar * Kutz, S. J., Hoberg, E. P., Polley, L. & Jenkins, E. J. Global warming is changing the dynamics of
Arctic host–parasite systems. _Proc. R. Soc. Lond. B Biol. Sci._ 272, 2571–2576 (2005). Article CAS Google Scholar * Amélineau, F. _et al_. Microplastic pollution in the Greenland Sea:
Background levels and selective contamination of planktivorous diving seabirds. _Environ. Pollut._ 219, 1131–1139 (2016). Article PubMed CAS Google Scholar * Vorkamp, K. _et al_. Novel
brominated flame retardants and dechlorane plus in Greenland air and biota. _Environ. Pollut._ 196, 284–291 (2015). Article CAS PubMed Google Scholar * Meltofte, H. _et al_. Arctic
Biodiversity Assesment. Synthesis. (Conservation of Arctic Flora and Fauna (CAFF), 2013). * Descamps, S. _et al_. Climate change impacts on wildlife in a High Arctic archipelago–Svalbard,
Norway. _Glob. Change Biol._ 23, 490–502 (2017). Article ADS Google Scholar * Eamer, J. _et al_. Life linked to ice. A guide to sea-ice associated biodiversity in this time of rapid
change. Conservation of Arctic Flora and Fauna (CAFF). Akureyri, Iceland. (Conservation of Arctic Flora and Fauna (CAFF), 2013). * Høye, T. T., Post, E., Meltofte, H., Schmidt, N. M. &
Forchhammer, M. C. Rapid advancement of spring in the High Arctic. _Curr. Biol._ 17, R449–R451 (2007). Article PubMed CAS Google Scholar * Post, E. _et al_. Ecological consequences of
sea-ice decline. _Science_ 341, 519–524 (2013). Article CAS PubMed ADS Google Scholar * Wassmann, P., Duarte, C. M., Agustí, S. & Sejr, M. K. Footprints of climate change in the
Arctic marine ecosystem. _Glob. Change Biol._ 17, 1235–1249 (2011). Article ADS Google Scholar * Bost, C. A. _et al_. Large-scale climatic anomalies affect marine predator foraging
behaviour and demography. _Nat. Commun._ 6, 8220 (2015). Article CAS PubMed Google Scholar * Croxall, J. P., Trathan, P. N. & Murphy, E. J. Environmental change and Antarctic seabird
populations. _Science_ 297, 1510–1514 (2002). Article CAS PubMed ADS Google Scholar * Gaston, A. J., Gilchrist, H. G. & Hipfner, J. M. Climate change, ice conditions and
reproduction in an Arctic nesting marine bird: Brunnich’s guillemot (_Uria lomvia L._). _J. Anim. Ecol._ 74, 832–841 (2005). Article Google Scholar * Lescroël, A., Ballard, G., Grémillet,
D., Authier, M. & Ainley, D. G. Antarctic climate change: extreme events disrupt plastic phenotypic response in Adélie penguins. _PloS One_ 9, e85291 (2014). Article PubMed PubMed
Central ADS CAS Google Scholar * Vihtakari, M. _et al_. Black-legged kittiwakes as messengers of Atlantification in the Arctic. _Sci. Rep._ 8, 1178 (2018). Article PubMed PubMed
Central ADS CAS Google Scholar * Weimerskirch, H., Louzao, M., de Grissac, S. & Delord, K. Changes in wind pattern alter albatross distribution and life-history traits. _Science_
335, 211–214 (2012). Article CAS PubMed ADS Google Scholar * Divoky, G. J., Lukacs, P. M. & Druckenmiller, M. L. Effects of recent decreases in arctic sea ice on an ice-associated
marine bird. _Prog. Oceanogr._ 136, 151–161 (2015). Article ADS Google Scholar * Egevang, C., Boertmann, D., Mosbech, A. & Tamstorf, M. P. Estimating colony area and population size
of little auks _Alle alle_ at Northumberland Island using aerial images. _Polar Biol._ 26, 8–13 (2003). Article Google Scholar * Karnovsky, N. J. & Hunt, G. L. Estimation of carbon
flux to dovekies (_Alle alle_) in the North Water. Deep-Sea Res. Part Ii-Top. _Stud. Oceanogr._ 49, 5117–5130 (2002). Article CAS Google Scholar * Kidawa, D. _et al_. Parental efforts of
an Arctic seabird, the little auk _Alle alle_, under variable foraging conditions. _Mar. Biol. Res._ 11, 349–360 (2015). Article Google Scholar * Hovinen, J. E. H. _et al_. Climate warming
decreases the survival of the little auk (_Alle alle_), a high Arctic avian predator. _Ecol. Evol._ 4, 3127–3138 (2014). Article PubMed PubMed Central Google Scholar * Jakubas, D.,
Wojczulanis-Jakubas, K., Iliszko, L. M., Strøm, H. & Stempniewicz, L. Habitat foraging niche of a High Arctic zooplanktivorous seabird in a changing environment. _Sci. Rep._ 7, 16203
(2017). Article PubMed PubMed Central ADS CAS Google Scholar * Reygondeau, G. & Beaugrand, G. Future climate-driven shifts in distribution of _Calanus finmarchicus_. _Glob. Change
Biol._ 17, 756–766 (2011). Article ADS Google Scholar * Amélineau, F., Grémillet, D., Bonnet, D., Bot, T. L. & Fort, J. Where to forage in the absence of sea ice? Bathymetry as a key
factor for an Arctic seabird. _PLOS ONE_ 11, e0157764 (2016). Article PubMed PubMed Central CAS Google Scholar * Karnovsky, N. _et al_. Foraging distributions of little auks _Alle alle_
across the Greenland Sea: implications of present and future Arctic climate change. _Mar. Ecol. Prog. Ser._ 415, 283–293 (2010). Article ADS Google Scholar * Harding, A. M. A. _et al_.
Estimating prey capture rates of a planktivorous seabird, the little auk (_Alle alle_), using diet, diving behaviour, and energy consumption. _Polar Biol._ 32, 785–796 (2009). Article
Google Scholar * Grémillet, D. _et al_. Little auks buffer the impact of current Arctic climate change. _Mar Ecol Prog Ser_ 454, 197–206 (2012). Article ADS Google Scholar * Karnovsky,
N. J. _et al_. Inter-colony comparison of diving behavior of an Arctic top predator: implications for warming in the Greenland Sea. _Mar Ecol Prog Ser_ 440, 229–240 (2011). Article ADS
Google Scholar * Mosbech, A. _et al_. On the crucial importance of a small bird: The ecosystem services of the little auk (_Alle alle_) population in Northwest Greenland in a long-term
perspective. _Ambio_ 47, 226–243 (2018). Article PubMed PubMed Central Google Scholar * Jakubas, D. _et al_. Foraging closer to the colony leads to faster growth in little auks. _Mar.
Ecol. Prog. Ser._ 489, 263–278 (2013). Article ADS Google Scholar * Fort, J., Porter, W. P. & Grémillet, D. Thermodynamic modelling predicts energetic bottleneck for seabirds
wintering in the northwest Atlantic. _J. Exp. Biol._ 212, 2483–2490 (2009). Article PubMed Google Scholar * Sandvik, H., Erikstad, K. E., Barrett, R. T. & Yoccoz, N. G. The effect of
climate on adult survival in five species of North Atlantic seabirds. _J. Anim. Ecol._ 74, 817–831 (2005). Article Google Scholar * Sandvik, H., Erikstad, K. E. & S\a ether, B.-E.
Climate affects seabird population dynamics both via reproduction and adult survival. _Mar. Ecol. Prog. Ser._ 454, 273–284 (2012). Article ADS Google Scholar * Frederiksen, M., Harris, M.
P., Daunt, F., Rothery, P. & Wanless, S. Scale-dependent climate signals drive breeding phenology of three seabird species. _Glob. Change Biol._ 10, 1214–1221 (2004). Article ADS
Google Scholar * Fort, J., Grémillet, D., Traisnel, G., Amélineau, F. & Bustamante, P. Does temporal variation of mercury levels in Arctic seabirds reflect changes in global
environmental contamination, or a modification of Arctic marine food web functioning? _Environ. Pollut._ 211, 382–388 (2016). Article CAS PubMed Google Scholar * Wolfe, M. F.,
Schwarzbach, S. & Sulaiman, R. A. Effects of mercury on wildlife: a comprehensive review. _Environ. Toxicol. Chem._ 17, 146–160 (1998). Article CAS Google Scholar * Tartu, S. _et al_.
To breed or not to breed: endocrine response to mercury contamination by an Arctic seabird. _Biol. Lett._ 9, 20130317 (2013). Article PubMed PubMed Central Google Scholar * Tartu, S.
_et al_. Mercury exposure, stress and prolactin secretion in an Arctic seabird: an experimental study. _Funct. Ecol._ 30, 596–604 (2016). Article Google Scholar * van Straalen, N. M.
Ecotoxicology becomes stress ecology. (ACS Publications, 2003). * Jakubas, D. _et al_. Intra-seasonal variation in zooplankton availability, chick diet and breeding performance of a high
Arctic planktivorous seabird. _Polar Biol_. 1–15, https://doi.org/10.1007/s00300-015-1880-z (2016). * Harding, A. M. _et al_. Adverse foraging conditions may impact body mass and survival of
a high Arctic seabird. _Oecologia_ 167, 49–59 (2011). Article PubMed ADS Google Scholar * Shipley, B. Cause and correlation in biology: a user’s guide to path analysis, structural
equations and causal inference with R. (Cambridge University Press, 2016). * Grosbois, V. _et al_. Assessing the impact of climate variation on survival in vertebrate populations. _Biol.
Rev._ 83, 357–399 (2008). Article CAS PubMed Google Scholar * Jones, I. L., Hunter, F. M. & Robertson, G. J. Annual adult survival of Least Auklets (Aves, Alcidae) varies with
large-scale climatic conditions of the North Pacific Ocean. _Oecologia_ 133, 38–44 (2002). Article PubMed ADS Google Scholar * Moe, B. _et al_. Climate change and phenological responses
of two seabird species breeding in the high-Arctic. _Mar. Ecol. Prog. Ser._ 393, 235–246 (2009). Article ADS Google Scholar * Goutte, A. _et al_. Survival rate and breeding outputs in a
high Arctic seabird exposed to legacy persistent organic pollutants and mercury. _Environ. Pollut._ 200, 1–9 (2015). Article CAS PubMed Google Scholar * Tartu, S. _et al_. Increased
adrenal responsiveness and delayed hatching date in relation to polychlorinated biphenyl exposure in Arctic-breeding black-legged kittiwakes (_Rissa tridactyla_). _Gen. Comp. Endocrinol._
219, 165–172 (2015). Article CAS PubMed Google Scholar * Votier, S. C. _et al_. Oil pollution and climate have wide-scale impacts on seabird demographics. _Ecol. Lett._ 8, 1157–1164
(2005). Article PubMed Google Scholar * Robertson, G. J., Wiese, F. K., Ryan, P. C. & Wilhelm, S. I. Updated numbers of murres and dovekies oiled in Newfoundland waters by chronic
ship-source oil pollution. In _Proceedings of the 37th AMOP Technical Seminar on Environmental Contamination and Response_ 265–275 (Environment Canada Ottawa, ON, 2014). * Woo, K. J.,
Elliott, K. H., Davidson, M., Gaston, A. J. & Davoren, G. K. Individual specialization in diet by a generalist marine predator reflects specialization in foraging behaviour. _J. Anim.
Ecol._ 77, 1082–1091 (2008). Article PubMed Google Scholar * Fort, J., Porter, W. P. & Grémillet, D. Energetic modelling: A comparison of the different approaches used in seabirds.
_Comp. Biochem. Physiol. A. Mol. Integr. Physiol._ 158, 358–365 (2011). Article PubMed CAS Google Scholar * Watanabe, Y. Y. & Takahashi, A. Linking animal-borne video to
accelerometers reveals prey capture variability. _Proc. Natl. Acad. Sci._ 110, 2199–2204 (2013). Article CAS PubMed ADS PubMed Central Google Scholar * Beaugrand, G., Luczak, C. &
Edwards, M. Rapid biogeographical plankton shifts in the North Atlantic Ocean. _Glob. Change Biol._ 15, 1790–1803 (2009). Article Google Scholar * Kjellerup, S. _et al_. Effects of a
future warmer ocean on the coexisting copepods _Calanus finmarchicus_ and _C. glacialis_ in Disko Bay, western Greenland. _Mar. Ecol. Prog. Ser._ 447, 87–108 (2012). Article CAS ADS
Google Scholar * Carstensen, J., Weydmann, A., Olszewska, A. & Kwaśniewski, S. Effects of environmental conditions on the biomass of _Calanus spp._ in the Nordic Seas. _J. Plankton
Res._ 34, 951–966 (2012). Article Google Scholar * Lønne, O. J. & Gabrielsen, G. W. Summer diet of seabirds feeding in sea-ice-covered waters near Svalbard. _Polar Biol._ 12, 685–692
(1992). Article Google Scholar * Mehlum, F. & Gabrielsen, G. W. The diet of high-arctic seabirds in coastal and ice-covered, pelagic areas near the Svalbard archipelago. _Polar Res._
12, 1–20 (1993). Article Google Scholar * Harding, A. M. _et al_. Can stable isotope (δ13C and δ15N) measurements of little auk (_Alle alle_) adults and chicks be used to track changes in
high-Arctic marine foodwebs? _Polar Biol._ 31, 725–733 (2008). Article Google Scholar * Fort, J. _et al_. Geographic and seasonal variability in the isotopic niche of little auks. _Mar.
Ecol. Prog. Ser._ 414, 293–302 (2010). Article ADS Google Scholar * Pedersen, C. E. & Falk, K. Chick diet of dovekies _Alle alle_ in Northwest Greenland. _Polar Biol._ 24, 53–58
(2001). Article Google Scholar * Rosing-Asvid, A., Hedeholm, R., Arendt, K. E., Fort, J. & Robertson, G. J. Winter diet of the little auk (_Alle alle_) in the Northwest Atlantic.
_Polar Biol._ 36, 1601–1608 (2013). Article Google Scholar * Kwasniewski, S. _et al_. The impact of different hydrographic conditions and zooplankton communities on provisioning Little
Auks along the West coast of Spitsbergen. _Prog. Oceanogr._ 87, 72–82 (2010). Article ADS Google Scholar * Jakubas, D., Iliszko, L., Wojczulanis-Jakubas, K. & Stempniewicz, L.
Foraging by little auks in the distant marginal sea ice zone during the chick-rearing period. _Polar Biol._ 35, 73–81 (2012). Article Google Scholar * Jakubas, D. _et al_. Foraging
behavior of a high-Arctic zooplanktivorous alcid, the little auk, at the southern edge of its breeding range. _J. Exp. Mar. Biol. Ecol._ 475, 89–99 (2016). Article Google Scholar *
Bradstreet, M. S. W. Pelagic feeding ecology of dovekies, _Alle alle_, in Lancaster Sound and Western Baffin Bay. _Arctic_ 35, 126–140 (1982). Google Scholar * Fort, J. _et al_. Multicolony
tracking reveals potential threats to little auks wintering in the North Atlantic from marine pollution and shrinking sea ice cover. _Divers. Distrib._ 19, 1322–1332 (2013). Article Google
Scholar * Ji, R., Jin, M. & Varpe, Ø. Sea ice phenology and timing of primary production pulses in the Arctic Ocean. _Glob. Change Biol._ 19, 734–741 (2013). Article ADS Google
Scholar * Søreide, J. E., Leu, E., Berge, J., Graeve, M. & Falk-Petersen, S. Timing of blooms, algal food quality and _Calanus glacialis_ reproduction and growth in a changing Arctic.
_Glob. Change Biol._ 16, 3154–3163 (2010). Google Scholar * Ramírez, F. _et al_. Sea ice phenology and primary productivity pulses shape breeding success in Arctic seabirds. _Sci. Rep_. 7
(2017). * Hovinen, J. E. H. _et al_. Fledging success of little auks in the high Arctic: do provisioning rates and the quality of foraging grounds matter? _Polar Biol._ 37, 665–674 (2014).
Article Google Scholar * Ropert-Coudert, Y. _et al_. A complete breeding failure in an Adélie penguin colony correlates with unusual and extreme environmental events. _Ecography_ 38,
111–113 (2015). Article Google Scholar * Jenouvrier, S., Péron, C. & Weimerskirch, H. Extreme climate events and individual heterogeneity shape life-history traits and population
dynamics. _Ecol. Monogr._ 85, 605–624 (2015). Article Google Scholar * Gilg, O. _et al_. Living on the edge of a shrinking habitat: the ivory gull,_ Pagophila eburnea_, an endangered
sea-ice specialist. _Biol. Lett._ 12, 20160277 (2016). Article PubMed PubMed Central Google Scholar * Gilg, O., Boertmann, D., Merkel, F., Aebischer, A. & Sabard, B. Status of the
endangered ivory gull, _Pagophila eburnea_, in Greenland. _Polar Biol._ 32, 1275–1286 (2009). Article Google Scholar * Dietz, R., Outridge, P. M. & Hobson, K. A. Anthropogenic
contributions to mercury levels in present-day Arctic animals—a review. _Sci. Total Environ._ 407, 6120–6131 (2009). Article CAS PubMed ADS Google Scholar * Dietz, R. _et al_. What are
the toxicological effects of mercury in Arctic biota? _Sci. Total Environ._ 443, 775–790 (2013). Article CAS PubMed ADS Google Scholar * AMAP. AMAP Assessment 2016: Chemicals of
Emerging Arctic Concern. xvi + 353pp (Arctic Monitoring and Assessment Programme (AMAP), 2017). * Blévin, P. _et al_. Contaminants and energy expenditure in an Arctic seabird: Organochlorine
pesticides and perfluoroalkyl substances are associated with metabolic rate in a contrasted manner. _Environ. Res._ 157, 118–126 (2017). Article PubMed CAS Google Scholar * Tartu, S.
_et al_. Corticosterone, prolactin and egg neglect behavior in relation to mercury and legacy POPs in a long-lived Antarctic bird. _Sci. Total Environ._ 505, 180–188 (2015). Article CAS
PubMed ADS Google Scholar * Thompson, D. R., Hamer, K. C. & Furness, R. W. Mercury accumulation in Great Skuas _Catharacta skua_ of known age and sex, and its effects upon breeding
and survival. _J. Appl. Ecol._ 28, 672–684 (1991). Article Google Scholar * Goutte, A. _et al_. Demographic consequences of heavy metals and persistent organic pollutants in a vulnerable
long-lived bird, the wandering albatross. _Proc. R. Soc. Lond. B Biol. Sci._ 281, 20133313 (2014). Article Google Scholar * Goutte, A. _et al_. Demographic responses to mercury exposure in
two closely related Antarctic top predators. _Ecology_ 95, 1075–1086 (2014). Article CAS PubMed Google Scholar * Stern, G. A. _et al_. How does climate change influence arctic mercury?
_Sci. Total Environ._ 414, 22–42 (2012). Article CAS PubMed ADS Google Scholar * Rydberg, J., Klaminder, J., Rosén, P. & Bindler, R. Climate driven release of carbon and mercury
from permafrost mires increases mercury loading to sub-arctic lakes. _Sci. Total Environ._ 408, 4778–4783 (2010). Article CAS PubMed ADS Google Scholar * Cossa, D. Marine
biogeochemistry: methylmercury manufacture. _Nat. Geosci._ 6, 810 (2013). Article CAS ADS Google Scholar * Rigét, F. _et al_. Twenty years of monitoring of persistent organic pollutants
in Greenland biota. A review. _Environ. Pollut._ 217, 114–123 (2016). Article PubMed CAS Google Scholar * Buchanan, K. _et al_. Guidelines for the treatment of animals in behavioural
research and teaching. _Anim. Behav._ 83, 301–309 (2012). Article Google Scholar * Fort, J., Beaugrand, G., Grémillet, D. & Phillips, R. A. Biologging, remotely-sensed oceanography and
the Continuous Plankton Recorder reveal the environmental determinants of a seabird wintering hotspot. _PLoS ONE_ 7, e41194 (2012). Article CAS PubMed PubMed Central ADS Google Scholar
* Kelly, J. F. Stable isotopes of carbon and nitrogen in the study of avian and mammalian trophic ecology. _Can. J. Zool._ 78, 1–27 (2000). Article Google Scholar * Welcker, J. _et al_.
Flexibility in the bimodal foraging strategy of a high Arctic alcid, the little auk _Alle alle_. _J. Avian Biol._ 40, 388–399 (2009). Article Google Scholar * Wojczulanis-Jakubas, K. &
Jakubas, D. When and why does my mother leave me? The question of brood desertion in the dovekie (_Alle alle_). _The Auk_ 129, 632–637 (2012). Article Google Scholar * Bustamante, P.,
Lahaye, V., Durnez, C., Churlaud, C. & Caurant, F. Total and organic Hg concentrations in cephalopods from the North Eastern Atlantic waters: influence of geographical origin and feeding
ecology. _Sci. Total Environ._ 368, 585–596 (2006). Article CAS PubMed ADS Google Scholar * Renedo, M. _et al_. Assessment of mercury speciation in feathers using species-specific
isotope dilution analysis. _Talanta_ 174, 100–110 (2017). Article CAS PubMed Google Scholar * Lebreton, J.-D., Burnham, K. P., Clobert, J. & Anderson, D. R. Modeling survival and
testing biological hypotheses using marked animals: a unified approach with case studies. _Ecol. Monogr._ 62, 67–118 (1992). Article Google Scholar * Choquet, R., Lebreton, J.-D., Gimenez,
O., Reboulet, A.-M. & Pradel, R. U-CARE: Utilities for performing goodness of fit tests and manipulating CApture–REcapture data. _Ecography_ 32, 1071–1074 (2009). Article Google
Scholar * Pledger, S., Pollock, K. H. & Norris, J. L. Open capture-recapture models with heterogeneity: I. Cormack-Jolly-Seber model. _Biometrics_ 59, 786–794 (2003). Article
MathSciNet PubMed MATH Google Scholar * Choquet, R., Rouan, L. & Pradel, R. Program E-SURGE: a software application for fitting multievent models. in Modeling demographic processes
in marked populations 845–865 (Springer, 2009). * Akaike, H. Factor analysis and AIC. _Psychometrika_ 52, 317–332 (1987). Article MathSciNet MATH Google Scholar * Amélineau, F. _et al_.
Energyscapes and prey fields shape a North Atlantic seabird wintering hotspot under climate change. _R. Soc. Open Sci._ 5, 171883 (2018). Article PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS This study and long-term monitoring program on little auks have been funded by the French Polar Institute (grant 388 to DG and JF), the National Science
Foundation (NSF Grant 0612504 to Nina Karnovsky & AMAH), the National Geographic Society (GEFNE 41–12 grant to JF), the Belmont Forum (TAMANI project to DG), the European Commission
(Marie Curie IEF and Marie Curie CIG to JF, Projects 273061 and 631203), the French Arctic Initiative – CNRS (PARCS project) and the Mission pour l’Interdisciplinarité – CNRS (Changements en
Sibérie project). We thank all the field assistants for their great work, Delphine Bonnet and Rumsais Blatrix for their help/technical assistance for the zooplankton identification, Francis
Crenner who kindly provided 4 LULs and Jean-Yves Barnagaud for inspiring discussions on the methods. We also thank the platforms ‘Spectrométrie Isotopique’ and ‘Analyses Elémentaires’ at
LIENSs, as well as G. Guillou and M. Brault-Favrou for their help with stable isotope and Hg analyses. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Centre d’Ecologie Fonctionnelle et
Evolutive (CEFE) UMR 5175, CNRS – Université de Montpellier – Université Paul-Valéry Montpellier – EPHE, Montpellier, France Françoise Amélineau, David Grémillet & Rémi Choquet *
Littoral Environnement et Sociétés (LIENSs), UMR 7266 CNRS - Université de La Rochelle, La Rochelle, France Françoise Amélineau & Jérôme Fort * Environmental Science Department, Alaska
Pacific University, Anchorage, AK, USA Ann M. A. Harding * Freshwater Institute, Fisheries and Oceans Canada, 501 University Crescent, Winnipeg, MB, Canada Wojciech Walkusz * Institute of
Oceanology, Polish Academy of Sciences, Sopot, Poland Wojciech Walkusz * Percy FitzPatrick Institute and DST/NRF Excellence Centre at the University of Cape Town, Rondebosch, South Africa
David Grémillet Authors * Françoise Amélineau View author publications You can also search for this author inPubMed Google Scholar * David Grémillet View author publications You can also
search for this author inPubMed Google Scholar * Ann M. A. Harding View author publications You can also search for this author inPubMed Google Scholar * Wojciech Walkusz View author
publications You can also search for this author inPubMed Google Scholar * Rémi Choquet View author publications You can also search for this author inPubMed Google Scholar * Jérôme Fort
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceived and designed the experiments: D.G., A.M.A.H., J.F. Performed the experiments:
D.G., A.M.A.H., J.F., F.A. Analyzed the data: F.A., W.W., R.C. Wrote the paper: F.A., J.F., D.G. All authors reviewed the manuscript. CORRESPONDING AUTHOR Correspondence to Françoise
Amélineau. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Amélineau, F., Grémillet, D., Harding, A.M.A. _et al._ Arctic
climate change and pollution impact little auk foraging and fitness across a decade. _Sci Rep_ 9, 1014 (2019). https://doi.org/10.1038/s41598-018-38042-z Download citation * Received: 13
June 2018 * Accepted: 10 December 2018 * Published: 31 January 2019 * DOI: https://doi.org/10.1038/s41598-018-38042-z SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative
Trending News
What to know about the kids online safety actCongress could potentially pass the first major legislation related to children’s online safety since 1998, as the Kids ...
Trump publicly rebukes putin after deadly strikes in ukrainePresident Donald Trump publicly rebuked Russian President Vladimir Putin on Sunday evening, after Russia bombarded Ukrai...
Real madrid will call tottenham boss mauricio pochettino - balagueZidane resigned as head coach of Real Madrid just days after leading the Spanish club to a third straight Champions Leag...
What to watch after 'the last of us'There was a time when the idea of a video game adaptation sounded alarm bells, with all but guaranteed audience disappoi...
What the last of us season 2 finale's shocking cliffhanger means for the future of the showW_ARNING: THIS POST CONTAINS SPOILERS FOR _THE LAST OF US_ SEASON 2__ FINALE._ The vicious cycle of revenge is cycling o...
Latests News
Arctic climate change and pollution impact little auk foraging and fitness across a decadeABSTRACT Ongoing global changes apply drastic environmental forcing onto Arctic marine ecosystems, particularly through ...
How to improve your posture and preserve joint healthYour mother always nagged you to stand up straight. But even if you followed her advice all these years, sitting for so ...
Bjp-jd(s) alliance wins, congress improves tally: key takeaways from karnatakaThe BJP has been riding on not just the Hindutva sentiment in the communally sensitive areas of coastal Karnataka, but a...
Renewing our commitment to equality on international women’s day — scottish national partyThe SNP Scottish Government is working hard to ensure that future generations of women and girls grow up in a truly equa...
How mastitis detection tech helped reduce antibiotics use - farmers weeklyWrexham dairy farmer Stephen Massey says using technology to sample individual quarters for cell counts has given him th...