Presence-absence of marine macrozoobenthos does not generally predict abundance and biomass
Presence-absence of marine macrozoobenthos does not generally predict abundance and biomass"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Many monitoring programmes of species abundance and biomass increasingly face financial pressures. Occupancy is often easier and cheaper to measure than abundance or biomass. We,
therefore, explored whether measuring occupancy is a viable alternative to measuring abundance and biomass. Abundance- or biomass-occupancy relationships were studied for sixteen
macrozoobenthos species collected across the entire Dutch Wadden Sea in eight consecutive summers. Because the form and strength of these relationships are scale-dependent, the analysis was
completed at different spatiotemporal scales. Large differences in intercept and slope of abundance- or biomass-occupancy relationships were found. Abundance, not biomass, was generally
positively correlated with occupancy. Only at the largest scale, seven species showed reasonably strong abundance-occupancy relationships with large coefficients of determination and small
differences in observed and predicted values (RMSE). Otherwise, and at all the other scales, intraspecific abundance and biomass relationships were poor. Our results showed that there is no
generic relationship between a species’ abundance or biomass and its occupancy. We discuss how ecological differences between species could cause such large variation in these relationships.
Future technologies might allow estimating a species’ abundance or biomass directly from eDNA sampling data, but for now, we need to rely on traditional sampling technology. SIMILAR CONTENT
BEING VIEWED BY OTHERS THE BENBIODEN DATABASE, A GLOBAL DATABASE FOR MEIO-, MACRO- AND MEGABENTHIC BIOMASS AND DENSITIES Article Open access 29 June 2020 SEASONALITY OF PRIMARY PRODUCTION
EXPLAINS THE RICHNESS OF PIONEERING BENTHIC COMMUNITIES Article Open access 27 September 2024 ENVIRONMENTAL DNA REVEALS THE FINE-GRAINED AND HIERARCHICAL SPATIAL STRUCTURE OF KELP FOREST
FISH COMMUNITIES Article Open access 14 July 2021 INTRODUCTION Most conservation efforts depend on monitoring different species to obtain estimates of spatial distributions and population
sizes1. Specimen collection and identification is expensive and labour intensive, and in practice monitoring programmes are constrained by the number of sampling units that can be afforded.
As costs need to be reduced, many long-term monitoring programmes are now under pressure2,3. Cost-reductions could involve new technology that reduces the effort involved in species
identification1, or shifting interest from estimating the abundance of animals to estimating their occupancy, i.e. the proportion of sampling sites in which a species was present4. Because
measures of occupancy are often much easier and cheaper to measure than abundance or biomass5, the question arises whether occupancy is a reliable predictor of a species abundance or
biomass, and whether occupancy sampling could thus reduce the cost of long-term monitoring programmes. Occupancy-abundance relationships are among the most widespread empirical patterns
described in macroecological studies6,7,8,9. The most commonly studied pattern is the interspecific (between species) abundance-occupancy relationship that describes how abundance and
occupancy correlate between species in a particular area. Positive interspecific abundance-occupancy relationships are reported for many different taxa at different spatial scales and in a
wide variety of ecosystems8,9,10,11,12. Even though the underlying mechanisms remain elusive10, possible ecological processes underlying positive abundance-occupancy patterns involve habitat
use6,13,14 and population dynamics, e.g. colonization and extinction rates8,10,15,16,17. Abundance-occupancy relationships have also been studied within species18,19,20,21. Such
intraspecific relationships are divided into temporal and spatial relationships. The intraspecific temporal relationship describes the correlation between abundance and occupancy of a single
species across time. Compared to between-species abundance-occupancy relationships, there have been fewer studies on intraspecific relationships. There is support for positive intraspecific
relationships18,22, but the strength of these relationships depends on the characteristics of each species, e.g., life-history, dispersal, and longevity9,12,23,24. Negative relationships
have, however, also been found8,10 and the generality of a positive relationship remains unresolved25. The intraspecific spatial relationship describes the correlation between abundance and
occupancy of a species at a single point in time across space. Intraspecific spatial relationships are rarely studied and there is no agreement whether the shape of spatial intraspecific
relationships should be positive10. Both types of intraspecific abundance-occupancy relationships have been studied mainly in terrestrial systems26, but rarely in marine systems9,11,23,24.
In this study, we explore whether occupancy can provide accurate estimates of a species’ abundance and thus provide a cost-effective alternative to traditional sampling methods. We analysed
intraspecific abundance-occupancy relationships8, also called distribution-abundance relationships10, for sixteen marine macrozoobenthos species that were collected across the Dutch Wadden
Sea over a period of eight years27,28: the bivalves _Cerastoderma edule_, _Limecola balthica_, _Mya arenaria_, _Abra tenuis, Ensis leei, Mytilus edulis, Scrobicularia plana_, and
_Macomangulus tenuis_, the polychaetes _Scoloplos armiger_, _Heteromastus filiformis_, _Hediste diversicolor_, _Nephtys hombergii_, _Lanice conchilega_, _Marenzelleria viridis_, and
_Arenicola marina_, and the gastropod _Peringia ulvae_. Since the form and strength of abundance-occupancy relationships are scale-dependent9, they were analysed across different
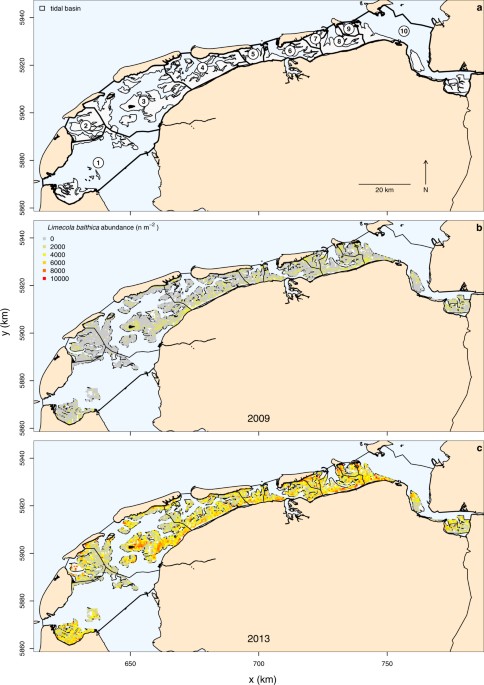
spatiotemporal scales. At the regional scale, yearly variation in abundance and occupancy was analysed across the entire Dutch Wadden Sea. At the local scale, yearly variation in abundance
and occupancy was analysed within the ten tidal basins of the Dutch Wadden Sea (Fig. 1a). We also examined whether abundance-occupancy relationships occurred across geographic space at a
single point in time. For this intraspecific spatial abundance-occupancy relationship, variation in abundance and occupancy between tidal basins within years was analysed. In
abundance-occupancy modelling, abundance is generally measured as the density of the number of individuals8. However, many monitoring programmes are aimed at estimating a species’ biomass
(e.g., as a possible food source for predators29), therefore biomass-occupancy relationships were also considered. RESULTS REGIONAL TEMPORAL RELATIONSHIPS On the regional scale of the entire
Dutch Wadden Sea, the temporal relationships between abundance and occupancy were variable but mainly positive (Table 1, Figs 1b,c, 2a–d, 3a–d, 4, and Supplementary Figs. S1a–d and S2a–d).
The steepest slopes were found for the bivalves _M. arenaria, M. edulis, C. edule_. and _E. leei_, and the polychaete _S. armiger_ (Table 1). Strong relationships, characterised by a large
coefficient of determination (>50%) and small back-transformed RMSE (<50%), were observed in the bivalves _M. edulis_ and _S. plana_, the polychaetes _H. filiformis_ and _S. armiger_,
and the gastropod _P. ulvae_; Weak relationships, characterised by a small coefficient of determination and large RMSEbt, were observed in the bivalves _E. leei_, _M. arenaria_ and _A.
tenuis_, and the polychaete _M. viridis_ (Table 1 and Fig. 5a). Interestingly, the coefficients of determination for the bivalves _M. arenaria_, _L. balthica_ and _C. edule_ were reasonably
large (R2 > 0.5), but the RMSEbt was also large (RMSEbt > 49%, Table 1 and Fig. 5a). On average across the remaining species, small coefficients of variation and large RMSEbt were
found (Table 1 and Fig. 5a). At the regional scale, relationships between biomass and occupancy were highly variable and ranged from positive to negative (Table 2, Figs 2e–h, 3e–h, 4, and
Supplementary Figs S1e–h and S2e–h). The steepest positive slopes were found for the polychaetes _L. conchilega_ and _S. armiger_, and the steepest negative slopes were found for the
bivalves _M. edulis_ and _E. leei_ (Table 2). Strong relationships were observed in the bivalves _L. balthica, M. edulis, E. leei_, and the polychaetes _H. filiformis_ and _S. armiger_; Weak
relationships were found in the bivalves _A. tenuis_, _M. arenaria_, and the polychaete _L. conchilega_ (Table 2 and Fig. 5b). There were several species with small RMSEbt, but they also
had small coefficients of determination (e.g., the polychaetes _A. marina_, _N. hombergii_, and the gastropod _P. ulvae_, Table 2 and Fig. 5b). For these species the overall mean was a
better predictor of biomass than occupancy. Comparing abundance-occupancy and biomass-occupancy relationships showed striking differences for some species (Fig. 4). Although for the bivalves
_M. edulis_, _E. leei_ and _C. edule_ positive relationships were observed between abundance and occupancy, the relationships between biomass and occupancy were negative (Tables 1 and 2,
Figs 2 and 4, and Supplementary Fig. S2). Compared to biomass-occupancy relationships, the RMSEbt were similar but coefficients of determination were larger for abundance-occupancy
relationships (median R2 of abundance and biomass relationships were respectively 0.49 and 0.18). The exceptions were the two bivalves _L. balthica_ and _E. leii_ that showed stronger
occupancy-relationships for biomass than abundance (Tables 1 and 2, and Fig. 5a and b). LOCAL TEMPORAL RELATIONSHIPS On the local scale within tidal basins, the between-year variation in
abundance generally correlated positively with occupancy (Supplementary Fig. S3). However, between tidal basins and between species, we observed large variation in median intercepts (range =
1.85–4.29) and slopes (range = 0.04–0.40) (Supplementary Fig. S3). Additionally, the coefficients of determination were small (median R2 = 0.37) and Root Mean Squared Error large (median
RMSEbt = 51%, Fig. 5c). The weakest relationships were found for the bivalves _M. edulis_ and _A. tenuis_, and the polychaete _L. conchilega_. Compared to the regional scale, relationships
between abundance and occupancy at local scales were more variable (the Inter Quartile Range of slopes were 0.30 and 0.12 for respectively local and regional relationships) as well as weaker
(the median RMSEbt of local and regional relationships were respectively 51 and 34%) with the exception of the bivalves _M. edulis, S. plana_, and the gastropod _P. ulvae_ (Supplementary
Fig. S5a). For biomass, positive, negative or no relationships with occupancy were found within species across tidal basins (Supplementary Fig. S3). Between species, there was also a large
variation in the relationships between biomass and occupancy (median slopes ranged from −0.18 to 0.40, Supplementary Fig. S3), and none of them were strong (Fig. 5d). Compared to the
regional scale relationships, relationships of between-year variation in biomass and occupancy within tidal basins were weaker with similar R2 but larger RMSEbt (median RMSEbt of the local
and regional scale were respectively 63 and 31%, Supplementary Fig. S5b). SPATIAL RELATIONSHIPS In general, positive spatial abundance-occupancy relationships were found, but these
relationships were weak (median slopes ranged from −0.03 to 0.33, Supplementary Fig. S4) and highly variable between years. Only the polychaete _S. armiger_ showed strong spatial
abundance-occupancy relationships (R2 = 0.66 and RMSEbt = 22%, Fig. 5e), but this was still weaker than its temporal relationship at the regional scale (R2 = 0.83 and RMSEbt = 15%, Fig. 5a).
Weakest relationships were found for the bivalves _M. edulis_, _M. arenaria_, the polychaete _L. conchilega_, and the gastropod _P. ulvae_. Comparing spatial relationships with regional
temporal relationships showed that spatial relationships were generally weaker, especially for the bivalves _M. edulis_ (slope of respectively 0.29 and 0.53) and _S. plana_ (slope of
respectively 0.03 and 0.09), and the gastropod _P. ulvae_ (slope of respectively 0.21 and 0.28) (Supplementary Fig. S5c). Only in the cases of the bivalve _E. leei_ and the polychaete _M.
viridis_ did the R2 increase and RMSEbt decrease, but these relationships were still weak (RMSEbt > 68%, Fig. 5e). The spatial relationships were similarly weak as the local temporal
relationships (Supplementary Fig. S4c–f). Only for two polychaete species the spatial relationships were stronger than the local temporal relationships: _S. armiger_ and _A. marina_,
(Supplementary Fig. S5e). However, the regional temporal relationships for these two species were still stronger than both the spatial and local temporal (Fig. 5). For biomass, the spatial
biomass-occupancy relationships revealed large variation in median intercept (range = −0.28–2.57) and slope (range = −0.05–0.56, Supplementary Fig. S4). Moreover, none of the spatial
biomass-occupancy relationships were strong (median R2 = 0.18 and RMSEbt = 82%, Fig. 5f). One of the weakest relationships was observed for the bivalve _M. edulis_ (R2 = 0.19 and RMSEbt =
575%). Compared to the regional temporal biomass-occupancy relationships, the spatial relationships were worse (RMSEbt of respectively 31 and 82%), especially for the bivalves _M. edulis, E.
leei_ and _L. balthica_ (Supplementary Fig. S5d). Also compared to the local temporal abundance-occupancy relationships (median RMSEbt = 63%), the spatial relationships were weaker, e.g.,
for the bivalves _M. edulis_ and _E. leei_ (Supplementary Fig. S5f). When comparing the spatial abundance-occupancy with spatial biomass-occupancy relationships, the biomass-occupancy
relationships were more variable than the abundance-occupancy relationship. That is, the Inter Quartile Ranges of intercepts were 0.65 and 1.45 for the abundance and biomass relationships,
0.18 and 0.32 for the slopes, and 53% and 133% for the RMSEbt respectively (Supplementary Fig. S4). DISCUSSION At the scale of the entire Dutch Wadden Sea, the intraspecific
abundance-occupancy relationships were generally positive. Also, occupancy was usually positively related with biomass, but relationships were more variable than the abundance-occupancy
relationships, and even negative for some species. The local temporal relationships and the spatial relationships were more often negative and weaker, in the cases of both abundance and
biomass, than the regional temporal relationships. These findings suggest that occupancy data at large spatial scales could be informative about the abundance or biomass of selected species
(e.g., the bivalves _L. balthica, S. plana_, _E. leei_ and _M. edulis_, the polychaetes _S. armiger_ and _H. filiformis_, and the gastropod _P. ulvae_.). However, for most species the
predictive power of abundance and/or biomass from occupancy was low, i.e. the coefficients of determination were small and the difference in observed and predicted values large. Moreover,
there were large differences in intercepts and slopes of the relationships between species. Within species, these relationships also varied between years and across geographic space, which
further showed that there was a lack of generality for predicting a species’ abundance or biomass from its occupancy; especially at smaller scales. Several modelling and empirical studies
show that the slope of abundance-occupancy relationships is consistently shallower and weaker for rare species12,20,30. A related factor that could affect abundance-occupancy relationships
is the distribution range (variation) of a species’ measured abundance and occupancy8. In our study, _A. tenuis_ and _M. tenuis_ were relatively rare with little variation in occupancy
(respectively 2–5% and 0–3%), and indeed they showed shallow (slope close to zero) and weak relationships. However, even though _S. plana_ was also rare and had a small occupancy range
(2–5%), its abundance-occupancy relationship was among the strongest, but also with a shallow slope. Likewise, commonness and a large range of occupancies were no guarantee for strong
abundance-occupancy and biomass-occupancy relationships. _M. viridis_ had an occupancy range of 15–54% but weak abundance-occupancy and biomass-occupancy relationships. The variation in
abundance-occupancy patterns observed in this study could be understood by differences in life-histories between species. Theory predicts that abundance-occupancy relationships can be
explained by: niche differentiation in resource and/or environmental use, which result in differences in vital rates and thus abundances along gradients of resources and/or the
environment6,8,10,13,15, or population dynamics mediated by the movement of organisms between sites, which can be driven by competition for resources14,17. Based on the latter mechanism,
weak abundance-occupancy relationships are predicted for species with low dispersal rates16. Thus a species that experiences little dispersion and aggregates locally is expected to have
reduced occupancy compared to a more dispersive species26. A comparison between marine invertebrates showed that dispersal propensity affected abundance-occupancy relationships23. In our
study, _A. tenuis_ has limited dispersal capabilities (it deposits egg masses locally into the sediment31) and indeed showed a shallow slope and weak abundance-occupancy relationship.
Likewise, the species that spawn in the water column and/or have a planktonic juvenile phase, in which the currents can disperse individuals over large distances32,33,34, had the strongest
abundance-occupancy relationships (e.g., _L. balthica_, _M. edulis_, _P. ulvae_). As an example, _M. edulis_ can have a planktonic larval phase of up to two months35 and can potentially
disperse very far. Our findings are, however, not conclusive as some species (e.g., _M. tenuis, L. conchilega, M. viridis_) that have a planktonic phase and should be capable to disperse
over large distances, showed weak abundance- or biomass-occupancy relationships. For many macrozoobenthos species, recruitment dominates population dynamics36,37. Recruits are often
superabundant and disproportionally affect abundance, biomass, and occupancy. That is, small but numerous recruits occupy large areas but with little contribution to biomass, and _vice
versa_ few adults contribute considerably to biomass but survive in restricted areas. Recruitment events could explain the opposing signs of abundance-occupancy versus biomass-occupancy
relationships that were found for _M. edulis_, _E. leei_ and _C. edule_. For instance, in 2011 _C. edule_ had a uniquely strong recruitment with maximum densities of almost 19,000 juveniles
per square meter38. Across the eight years of this study, 2011 had the largest abundance and occupancy, and indeed the smallest biomass as well. Similarly, old and large individuals can also
dominate abundance, biomass and occupancy. Moreover, theory predicts that longevity would cause shallow slopes12,16. _M. arenaria_ can live for 28 years, reach 15 cm in length39, and the
abundance and occupancy of old individuals is small, but biomass is large. Indeed, the weakest relationships for _M. arenaria_ was found. Moreover, longevity introduces strong temporal
autocorrelation, due to cohort effects persisting through time, which might influence abundance-occupancy relationships further. Local conditions can synchronize biomass-variation between
macrozoobenthos species40, e.g., _C. edule, M. edulis, M. arenaria_. Therefore, local temporal relationships (within tidal basins) are predicted to be stronger than regional relationships
(across the entire Dutch Wadden Sea), i.e. spatial variance is reduced. However, the abundance-occupancy relationships on the local scale were generally weaker, particularly for the
above-mentioned species. Perhaps this is caused by smaller sample sizes as we scaled down to tidal basins. Alternatively, it could hint at large-scale processes that synchronise population
dynamics of these species. For instance, the population dynamics of many marine macrozoobenthic species are affected by large-scale weather patterns. Cold winters can cause adult mortality,
and mild winters can cause failed recruitment40,41. Large-scale weather patterns have indeed been found to synchronise population dynamics of different species over large spatial
scales40,42, e.g., _M. edulis_, _M. arenaria_, _L. conchilega_, _C. edule_. Whether these large-scale processes underlie abundance-occupancy relationships, or perhaps influence their shape,
needs to be investigated in further detail. In this study, occupancy was measured in the traditional way by morphological taxonomy. Over the past years there has been an explosion in the use
of environmental DNA (eDNA) metabarcoding as a tool for aiding monitoring programmes1,43,44,45,46,47,48. Studies have shown that eDNA sampling is accurate in collecting presence-absence
data49,50, which could provide a cost-effective alternative to measuring occupancy. One should, however, be careful extrapolating our traditionally measured abundance-occupancy relationships
to occupancies measured with eDNA techniques. There is some evidence that eDNA methods have higher detection rates than traditional field methods, particularly when species occur at low
densities51. Sampling benthic invertebrates with small sediment cores can underestimate a species’ occupancy, i.e. imperfect detection leads to zero-inflated abundances5,52. This might
especially be the case for _M. arenaria_ that live partly below the reach of the sampling core (>30 cm) when they are very large (up to 15 cm)39,53. Indeed, this species showed the
weakest abundance-occupancy relationships. The strength of abundance-occupancy relationships presented in this study could thus be underestimated compared to eDNA sampling techniques.
Occupancy estimates could be improved by modelling detection probabilities5. To fully assess the validity of predicting abundance and biomass from eDNA occupancy data, traditional and eDNA
sampling should be carried out simultaneously at the same locations. In the future, however, new genomic technologies could allow estimating abundance and biomass directly from eDNA sampling
data54,55. In summary, we find support for positive, as well as negative, intraspecific abundance- or biomass-occupancy relationships that could partly and non-conclusively be explained by
ecological differences in life-histories between species. Abundance and biomass of some species could be accurately predicted from occupancy data, but only at the large scale of the entire
Dutch Wadden Sea. At present, there is no generic relationship for predicting a species’ abundance or biomass from its occupancy. For the foreseeable, we therefore need to rely on
traditional sampling technology for estimating a species’ abundance and/or biomass. MATERIAL AND METHODS STUDY SYSTEM The Dutch Wadden Sea (53°16′N, 5°24′E) covers roughly 2500 km2 of which
50% is tidal mud flats56. Due to natural tidal divides, the system is divided into ten physical units of tidal basins separated by watersheds (Fig. 1a). FIELD SAMPLING From 2008 to 2015, the
abundance and biomass of macrozoobenthic invertebrates were sampled across all 1151 km2 of intertidal mudflats in the Dutch Wadden Sea (Fig. 1) from June to September (SIBES Synoptic
Intertidal BEnthic Survey)27,28. Sampling stations were arranged according to a grid sampling design with 0.5 km inter-sample distance and 15 to 20% additional sampling stations randomly
placed onto gridlines27. In total between 3,159 and 4,818 stations were sampled and analysed for the sampling campaigns from 2008 to 2015, with the exception of 2015 where 1,289 samples of
the random sample points have currently been analysed. In 2008, the Ems-Dollard estuary was not sampled. Within tidal basins, the surface area of the intertidal mudflats varies between 25
and 311 km2. Thus on average 75 to 1071 stations were sampled per tidal basin. Sampling stations were located by handheld GPS (Garmin 60 and Dakota 10). At each station, two sediment cores
(1/112 m² each) were taken to a depth of 25–30 cm, washed over a 1-mm square mesh sieve, and then transported to the laboratory. Because of the time between collection and processing, large
bivalves were stored in the freezer and the polychaetes, crustaceans and small bivalves were kept on formalin. In the laboratory, species were identified, individuals counted, and biomass
(g) was determined as ash-free dry mass of the flesh (AFDMflesh)28. The shell and flesh of the gastropod _Peringia ulvae_ were not separated, thus flesh was assumed to contribute 17% of the
AFDM57. For bivalves, shell length (mm) was also measured. ANALYSES Prior to analyses, outliers in AFDMflesh were identified with a non-linear local regression of the log10 of AFDMflesh and
the log10 of shell length58 (R-script available in Supplementary Material Appendix A1). If residuals exceeded twice the Inter Quartile Range (IQR) they were defined as outliers. Because the
lengths of polychaetes could not be accurately determined, they were divided into size-classes (juvenile or adult) and AFDMflesh of outliers was estimated from their size class. _H.
filiformis_ could not be divided into size classes, therefore, outliers were estimated with mean log10 AFDMflesh. If AFDMflesh was not measured, it was estimated from their length (bivalves)
or size class (polychaetes). If both AFDMflesh and length or size class were absent, the measurement was removed from the analyses (0.9% of measurements). Abundance of a single species at a
single sampling location was calculated as the number of individuals divided by the sampled surface area. To obtain the biomass of a single species at a single sampling location, the
AFDMflesh (g) of all individuals was summed. Mean abundance (n m−2) and biomass (g AFDM m−2) were then calculated by averaging abundances and biomasses of a species within occupied patches
only (local mean abundance)59. To calculate presence-absence data, a species was classified as absent (0) or present (1) for each sampling station. Occupancy was calculated as the sum of the
total number of presences divided by the number of stations visited. For the analyses of intraspecific temporal relationships at the regional scale, mean abundance, biomass and occupancy of
a species was calculated across the Dutch Wadden Sea in each year. To evaluate the local temporal relationship, abundances, biomasses and occupancy were averaged for each tidal basins in
each year. The analyses of temporal relationships within tidal basins were restricted to those tidal basins where the species was observed at least six out of eight years. To examine spatial
relationships, we examined the average abundance, biomass and occupancy between tidal basins within a single year, and then for each year separately to assess the yearly variation and
robustness of these relationships across time. Intraspecific relationships were modelled by fitting linear regressions between the log10 of abundance or biomass and the logit of occupancy.
On the scale of tidal basins, abundance, biomass and occupancy data contained zeros. Before taking their logarithm or logit, we therefore added the smallest measured value of abundance or
biomass within tidal basins, and for occupancy half times one over the sample size. To evaluate the form of the abundance- or biomass-occupancy relationships, the intercept and slope were
extracted from linear regression models. Because we were particularly interested in whether occupancy could predict abundance and/or biomass, we also extracted the coefficient of
determination (R2) and the Root Mean Squared Error (RMSE). The coefficient of determination describes the proportion of variance in abundance or biomass explained by occupancy. For
presentation purposes, the Root Mean Squared Error was back-transformed (RMSEbt) by taking the anti-log of RMSE, subtracted by 1, and multiplied by 100. The resulting RMSEbt provided the
percentage difference between observed and predicted values. A strong relationship should be characterised by a large R2 and small RMSEbt, whereas a weak relationship should be characterised
by a small R2 and large RMSEbt. All data was analysed in R v3.2.360. DATA AVAILABILITY All data analysed in this study is available at https://doi.org/10.5281/zenodo.1120347. REFERENCES *
Thomsen, P. F. & Willerslev, E. Environmental DNA – An emerging tool in conservation for monitoring past and present biodiversity. _Biol. Conserv._ 183, 4–18,
https://doi.org/10.1016/j.biocon.2014.11.019 (2015). Article Google Scholar * Biber, E. The challenge of collecting and using environmental monitoring data. _Ecol. Soc._ 18, 68,
https://doi.org/10.5751/ES-06117-180468 (2013). Article Google Scholar * Lindenmayer, D. B. _et al_. Value of long‐term ecological studies. _Austral Ecol._ 37, 745–757 (2012). Article
Google Scholar * Royle, J. A. & Nichols, J. D. Estimating abundance from repeated presence–absence data or point counts. _Ecology_ 84, 777–790 (2003). Article Google Scholar *
MacKenzie, D. I. _et al_ _. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence_. (Elsevier, San Diego, CA., 2006). * Brown, J. H. On the relationship
between abundance and distribution of species. _Am. Nat._ 124, 255–279, https://doi.org/10.1086/284267 (1984). Article Google Scholar * Gaston, K. J., Blackburn, T. M. & Lawton, J. H.
Interspecific abundance-range size relationships: an appraisal of mechanisms. _J. Anim. Ecol._ 66, 579–601 (1997). Article Google Scholar * Gaston, K. J. _et al_. Abundance–occupancy
relationships. _J. Appl. Ecol._ 37, 39–59 (2000). Article Google Scholar * Blackburn, T. M., Cassey, P. & Gaston, K. J. Variations on a theme: sources of heterogeneity in the form of
the interspecific relationship between abundance and distribution. _J. Anim. Ecol._ 75, 1426–1439 (2006). Article PubMed Google Scholar * Borregaard, M. K. & Rahbek, C. Causality of
the relationship between geographic distribution and species abundance. _Q. Rev. Biol._ 85, 3–25 (2010). Article PubMed Google Scholar * Webb, T. J., Barry, J. P. & McClain, C. R.
Abundance–occupancy relationships in deep sea wood fall communities. _Ecography_ (2017). * Buckley, H. L. & Freckleton, R. P. Understanding the role of species dynamics in
abundance–occupancy relationships. _J. Ecol._ 98, 645–658 (2010). Article Google Scholar * Holt, R., Lawton, J., Gaston, K. & Blackburn, T. On the relationship between range size and
local abundance: back to basics. _Oikos_, 183–190 (1997). * Kluyver, H. & Tinbergen, L. Territory and the regulation of density in titmice. _Archives Néerlandaises de Zoologie_ 10,
265–289 (1954). Article Google Scholar * Faulks, L., Svanbäck, R., Ragnarsson-Stabo, H., Eklöv, P. & Östman, Ö. Intraspecific niche variation drives abundance-occupancy relationships
in freshwater fish communities. _Am. Nat._ 186, 272–283 (2015). Article PubMed Google Scholar * Freckleton, R., Gill, J., Noble, D. & Watkinson, A. Large‐scale population dynamics,
abundance–occupancy relationships and the scaling from local to regional population size. _J. Anim. Ecol._ 74, 353–364 (2005). Article Google Scholar * Hanski, I. & Gyllenberg, M.
Uniting two general patterns in the distribution of species. _Science_ 275, 397–400 (1997). Article CAS PubMed Google Scholar * Borregaard, M. K. & Rahbek, C. Prevalence of
intraspecific relationships between range size and abundance in Danish birds. _Divers. Distrib._ 12, 417–422 (2006). Article Google Scholar * Gaston, K. J., Blackburn, T. M. & Gregory,
R. D. Interspecific differences in intraspecific abundance‐range size relationships of British breeding birds. _Ecography_ 21, 149–158 (1998). Article Google Scholar * Webb, T. J., Noble,
D. & Freckleton, R. P. Abundance–occupancy dynamics in a human dominated environment: linking interspecific and intraspecific trends in British farmland and woodland birds. _J. Anim.
Ecol._ 76, 123–134 (2007). Article PubMed Google Scholar * Venier, L. A. & Fahrig, L. Intra-specific abundance-distribution relationships. _Oikos_, 483–490 (1998). * Zuckerberg, B.,
Porter, W. F. & Corwin, K. The consistency and stability of abundance–occupancy relationships in large‐scale population dynamics. _J. Anim. Ecol._ 78, 172–181 (2009). Article PubMed
Google Scholar * Foggo, A., Bilton, D. T. & Rundle, S. D. Do developmental mode and dispersal shape abundance–occupancy relationships in marine macroinvertebrates? _J. Anim. Ecol._ 76,
695–702 (2007). Article PubMed Google Scholar * Webb, T. J., Tyler, E. H. & Somerfield, P. J. Life history mediates large-scale population ecology in marine benthic taxa. _Mar. Ecol.
Prog. Ser._ 396, 293–306 (2009). Article ADS Google Scholar * Gaston, K. J. Implications of interspecific and intraspecific abundance-occupancy relationships. _Oikos_ 86, 195–207,
https://doi.org/10.2307/3546438 (1999). Article Google Scholar * Holt, A. R., Gaston, K. J. & He, F. Occupancy-abundance relationships and spatial distribution: a review. _Basic Appl.
Ecol._ 3, 1–13 (2002). Article Google Scholar * Bijleveld, A. I. _et al_. Designing a benthic monitoring programme with multiple conflicting objectives. _Methods Ecol. Evol._ 3, 526–536,
https://doi.org/10.1111/j.2041-210X.2012.00192.x (2012). Article Google Scholar * Compton, T. J. _et al_. Distinctly variable mudscapes: distribution gradients of intertidal macrofauna
across the Dutch Wadden Sea. _J. Sea Res._ 82, 103–116, https://doi.org/10.1016/j.seares.2013.02.002 (2013). Article ADS Google Scholar * Kraan, C. _et al_. Landscape-scale experiment
demonstrates that Wadden Sea intertidal flats are used to capacity by molluscivore migrant shorebirds. _J. Anim. Ecol._ 78, 1259–1268, https://doi.org/10.1111/j.1365-2656.2009.01564.x
(2009). Article PubMed Google Scholar * Freckleton, R. P., Noble, D. & Webb, T. J. Distributions of habitat suitability and the abundance-occupancy relationship. _Am. Nat._ 167,
260–275 (2006). PubMed Google Scholar * Holmes, S. P., Dekker, R. & Williams, I. D. Population dynamics and genetic differentiation in the bivalve mollusc _Abra tenuis_: aplanic
dispersal. _Mar. Ecol. Prog. Ser._ 268, 131–140 (2004). Article ADS Google Scholar * Pechenik, J. A. On the advantages and disadvantages of larval stages in benthic marine invertebrate
life cycles. _Mar. Ecol. Prog. Ser._ 177, 269–297 (1999). Article ADS Google Scholar * Armonies, W. Migratory rhythms of drifting juvenile mollusks in tidal waters of the Wadden Sea.
_Mar. Ecol. Prog. Ser._ 83, 197–206, https://doi.org/10.3354/meps083197 (1992). Article ADS Google Scholar * Beukema, J. J. & de Vlas, J. Tidal-current transport of thread-drifting
postlarval juveniles of the bivalve _Macoma balthica_ from the Wadden Sea to the North Sea. _Mar. Ecol. Prog. Ser._ 52, 193–200 (1989). Article ADS Google Scholar * Bayne, B. L. Growth
and the delay of metamorphosis of the larvae of _Mytilus edulis_ (L.). _Ophelia_ 2, 1–47, https://doi.org/10.1080/00785326.1965.10409596 (1965). Article Google Scholar * van der Meer, J.,
Beukema, J. J. & Dekker, R. Long-term variability in secondary production of an intertidal bivalve population is primarily a matter of recruitment variability. _J. Anim. Ecol._ 70,
159–169, https://doi.org/10.1046/j.1365-2656.2001.00469.x (2001). Article Google Scholar * Beukema, J. J. Annual variation in reproductive success and biomass of the major macrozoobenthic
species living in a tidal flat area of the Wadden Sea. _Neth. J. Sea Res._ 16, 37–45 (1982). Article Google Scholar * Bijleveld, A. I. _et al_. Understanding spatial distributions:
negative density-dependence in prey causes predators to trade-off prey quantity with quality. _Proc. R. Soc. B_ 283, 20151557, https://doi.org/10.1098/rspb.2015.1557 (2016). Article PubMed
PubMed Central Google Scholar * Cardoso, J. F. M. F., Witte, J. I. & van der Veer, H. W. Differential reproductive strategies of two bivalves in the Dutch Wadden Sea. _Estuar. Coast.
Shelf. S._ 84, 37–44 (2009). Article ADS Google Scholar * Beukema, J. J., Dekker, R., Essink, K. & Michaelis, H. Synchronized reproductive success of the main bivalve species in the
Wadden Sea: causes and consequences. _Mar. Ecol. Prog. Ser._ 211, 143–155, https://doi.org/10.3354/meps211143 (2001). Article ADS Google Scholar * Beukema, J. J. Biomass and species
richness of the macro-benthic animals living on the tidal flats of the Dutch Wadden Sea. _Neth. J. Sea Res._ 10, 236–261 (1976). Article Google Scholar * Beukema, J. J., Essink, K.,
Michaelis, H. & Zwarts, L. Year-to-year variability in the biomass of macrobenthic animals on tidal flats of the Wadden Sea: how predictable is this food source for birds? _Neth. J. Sea
Res._ 31, 319–330, https://doi.org/10.1016/0077-7579(93)90051-S (1993). Article Google Scholar * Thomsen, P. F. _et al_. Monitoring endangered freshwater biodiversity using environmental
DNA. _Mol. Ecol._ 21, 2565–2573 (2012). Article CAS PubMed Google Scholar * Foote, A. D. _et al_. Investigating the potential use of environmental DNA (eDNA) for genetic monitoring of
marine mammals. _PLoS One_ 7, e41781 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Evans, N. T. _et al_. Quantification of mesocosm fish and amphibian species
diversity via environmental DNA metabarcoding. _Mol. Ecol. Resour._ 16, 29–41, https://doi.org/10.1111/1755-0998.12433 (2016). Article CAS PubMed Google Scholar * Valentini, A.,
Pompanon, F. & Taberlet, P. DNA barcoding for ecologists. _Trends Ecol. Evol._ 24, 110–117 (2009). Article PubMed Google Scholar * Dejean, T. _et al_. Improved detection of an alien
invasive species through environmental DNA barcoding: the example of the American bullfrog _Lithobates catesbeianus_. _J. Appl. Ecol._ 49, 953–959,
https://doi.org/10.1111/j.1365-2664.2012.02171.x (2012). Article Google Scholar * Ficetola, G. F., Miaud, C., Pompanon, F. & Taberlet, P. Species detection using environmental DNA from
water samples. _Biol. Lett._ 4, 423–425 (2008). Article PubMed PubMed Central Google Scholar * Pilliod, D. S., Goldberg, C. S., Arkle, R. S. & Waits, L. P. Estimating occupancy and
abundance of stream amphibians using environmental DNA from filtered water samples. _Can. J. Fish. Aquat. Sci._ 70, 1123–1130 (2013). Article CAS Google Scholar * Thomsen, P. F. _et al_.
Detection of a Diverse Marine Fish Fauna Using Environmental DNA from Seawater Samples. _PLoS One_ 7, e41732, https://doi.org/10.1371/journal.pone.0041732 (2012). Article ADS CAS PubMed
PubMed Central Google Scholar * Crampton-Platt, A., Douglas, W. Y., Zhou, X. & Vogler, A. P. Mitochondrial metagenomics: letting the genes out of the bottle. _GigaScience_ 5, 15
(2016). Article PubMed PubMed Central Google Scholar * Lyashevska, O., Brus, D. J. & van der Meer, J. Mapping species abundance by a spatial zero‐inflated Poisson model: a case study
in the Wadden Sea, the Netherlands. _Ecol. Evol._ 6, 532–543 (2016). Article PubMed PubMed Central Google Scholar * Van der Meer, J. A comparison between the SIBES and the
Beukema/Dekker benthos sampling program at Balgzand. (NIOZ Royal Netherlands Institute for Sea Research, Report Version 20150924, 2015). * Zhou, X. _et al_. Ultra-deep sequencing enables
high-fidelity recovery of biodiversity for bulk arthropod samples without PCR amplification. _GigaScience_ 2, 4 (2013). Article CAS PubMed PubMed Central Google Scholar * Liu, S. _et
al_. Mitochondrial capture enriches mito‐DNA 100 fold, enabling PCR‐free mitogenomics biodiversity analysis. _Mol. Ecol. Resour._ 16, 470–479 (2016). Article CAS PubMed Google Scholar *
Wolff, W. J. Causes of extirpations in the Wadden Sea, an estuarine area in the Netherlands. _Conserv. Biol._ 14, 876–885 (2000). Article Google Scholar * Piersma, T. _et al_. Scale and
intensity of intertidal habitat use by knots _Calidris canutus_ in the Western Wadden Sea in relation to food, friends and foes. _Neth. J. Sea Res._ 31, 331–357 (1993). Article Google
Scholar * Bijleveld, A. I., Twietmeyer, S., Piechocki, J., van Gils, J. A. & Piersma, T. Natural selection by pulsed predation: survival of the thickest. _Ecology_ 96, 1943–1956,
https://doi.org/10.1890/14-1845.1 (2015). Article PubMed Google Scholar * Webb, T. J., Freckleton, R. P. & Gaston, K. J. Characterizing abundance–occupancy relationships: there is no
artefact. _Global Ecol. Biogeogr._ 21, 952–957 (2012). Article Google Scholar * R Core Team. _R: a language and environment for statistical computing_. (2015). Download references
ACKNOWLEDGEMENTS We are in very grateful to the large numbers of staff, students and particularly volunteers that collected and analysed the huge amount of data that was used in this study.
We also thank the crew of RV Navicula for the logistical support during field work. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * NIOZ Royal Netherlands Institute for Sea Research,
Department of Coastal Systems, and Utrecht University, P.O. Box 59, 1790 AB, Den Burg, The Netherlands Allert I. Bijleveld, Tanya J. Compton, Lise Klunder, Sander Holthuijsen, Job ten Horn,
Anita Koolhaas, Anne Dekinga, Jaap van der Meer & Henk W. van der Veer Authors * Allert I. Bijleveld View author publications You can also search for this author inPubMed Google Scholar
* Tanya J. Compton View author publications You can also search for this author inPubMed Google Scholar * Lise Klunder View author publications You can also search for this author inPubMed
Google Scholar * Sander Holthuijsen View author publications You can also search for this author inPubMed Google Scholar * Job ten Horn View author publications You can also search for this
author inPubMed Google Scholar * Anita Koolhaas View author publications You can also search for this author inPubMed Google Scholar * Anne Dekinga View author publications You can also
search for this author inPubMed Google Scholar * Jaap van der Meer View author publications You can also search for this author inPubMed Google Scholar * Henk W. van der Veer View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS A.I.B., T.C., L.K., J.v.d.M. and H.v.d.V. conceived the study and designed methodology; S.H., J.t.H.,
A.K., and A.D. collected the data; A.I.B. analysed the data and led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
CORRESPONDING AUTHOR Correspondence to Allert I. Bijleveld. ETHICS DECLARATIONS COMPETING INTERESTS Part of the sampling has been funded by the Nederlandse Aardolie Maatschappij (NAM) and by
The Netherlands Organization for Scientific Research (NWO) via Project 839.08.251 of the National Ocean and Coastal Research Programme (ZKO). ADDITIONAL INFORMATION PUBLISHER'S NOTE:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY MATERIAL RIGHTS AND
PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any
medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Bijleveld, A.I.,
Compton, T.J., Klunder, L. _et al._ Presence-absence of marine macrozoobenthos does not generally predict abundance and biomass. _Sci Rep_ 8, 3039 (2018).
https://doi.org/10.1038/s41598-018-21285-1 Download citation * Received: 29 June 2017 * Accepted: 02 February 2018 * Published: 14 February 2018 * DOI:
https://doi.org/10.1038/s41598-018-21285-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Searching for resources to teach the common standardsIf yearning had a sound, the air would be full of noise right now. That’s because teachers across the country are lookin...
Arsenal hero ian wright urges board to sack unai emery after leicesterArsenal hero Ian Wright has urged the Gunners’ board to make a decision following the defeat to Leicester. Arsenal were ...
Attention Required! | CloudflarePlease enable cookies. Sorry, you have been blocked You are unable to access defatoonline.com.br Why have I been blocked...
Financial Market News, Analysis and Trading Ideas - IG UKSpread bet, trade CFDs or deal shares – decide which of our products is best for you.Discover why so many clients choose...
Taxable benefits and vehicle excise duty: regime for measuring carbon dioxide emissionsPolicy paper TAXABLE BENEFITS AND VEHICLE EXCISE DUTY: REGIME FOR MEASURING CARBON DIOXIDE EMISSIONS This tax informatio...
Latests News
Presence-absence of marine macrozoobenthos does not generally predict abundance and biomassABSTRACT Many monitoring programmes of species abundance and biomass increasingly face financial pressures. Occupancy is...
Chris brown dog bite lawsuit: singer says housekeeper 'only heard' bloody attack on sisterChris Brown says the housekeeper who claims one of his dogs “viciously” mauled her sister at his Los Angeles home has no...
Is edward snowden in danger? Spooky tweet may trace to cryptic bitcoin ‘911’Concerns for the whistleblower subsided late Saturday when journalist Glenn Greenwald responded to a query via Twitter a...
Effects of candesartan and amlodipine on cardiovascular events in hypertensive patients with chronic kidney disease: subanalysis of the case-j studyABSTRACT We examined the effects of candesartan and amlodipine on cardiovascular events in hypertensive patients with ch...
Jordan neely’s father sues daniel penny as nyc jury deliberates verdict for subway chokehold deathJordan Neely’s father is suing Daniel Penny over his son’s chokehold death on a New York City subway car as the jury sti...