Functionalized gold nanorod nanocomposite system to modulate differentiation of human mesenchymal stem cells into neural-like progenitors
Functionalized gold nanorod nanocomposite system to modulate differentiation of human mesenchymal stem cells into neural-like progenitors"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A 2D multifunctional nanocomposite system of gold nanorods (AuNRs) was developed. Gold nanorods were functionalized via polyethylene glycol with a terminal amine, and, were
characterized using transmission and scanning electron microscopy, ultra violet-visible and X-ray photoelectron spectroscopy, and Zeta-potential. The system was cytocompatible to and
maintained the integrity of Schwann cells. The neurogenic potential of adipose tissue – derived human mesenchymal stem cells (hMSCs) was evaluated _in vitro_. The expression pattern and
localization of Vimentin confirmed the mesenchymal origin of cells and tracked morphological changes during differentiation. The expression patterns of S100β and glial fibrillary acidic
protein (GFAP), were used as indicator for neural differentiation. Results suggested that this process was enhanced when the cells were seeded on the AuNRs compared to the tissue-culture
surface. The present study indicates that the design and the surface properties of the AuNRs enhances neural differentiation of hMSCs and hence, would be beneficial for neural tissue
engineering scaffolds. SIMILAR CONTENT BEING VIEWED BY OTHERS MAGNETIC NANOWIRES SUBSTRATE INCREASES ADIPOSE-DERIVED MESENCHYMAL CELLS OSTEOGENESIS Article Open access 06 October 2022 4D
PRINTING OF STRETCHABLE NANOCOOKIE@CONDUIT MATERIAL HOSTING BIOCUES AND MAGNETOELECTRIC STIMULATION FOR NEURITE SPROUTING Article Open access 11 September 2020 NANOCOMPOSITE MAGNETIC
HYDROGEL WITH DUAL ANISOTROPIC PROPERTIES INDUCES OSTEOGENESIS THROUGH THE NOTCH-DEPENDENT PATHWAYS Article Open access 22 March 2024 INTRODUCTION Bionanotechnology presents a revolutionary
new approach to regenerative medicine and tissue engineering. Nanotechnology offers the possibility of creating tunable surface chemistry and adjustable size and surface area on a
biocompatible substrate—features that can be extremely useful for complex biomedical applications. The interaction between a biocompatible substrate and a cell membrane has been cited as an
essential control factor in cellular fate1. The therapeutic action of regenerative devices is a function of their chemistry profile, which factors into their control and enhancement of the
tissue regeneration process2,3. In turn, this essential chemistry profile facilitates the selection process of loading the necessary components within the biocompatible substrate4.
Therefore, the behavior of nanomaterials should be thoroughly understood when considering them for use as therapeutic aides for biological responses. Previously, significant work has been
conducted to explore the use of nanoparticles (NPs) in regenerative medicine, especially in the application of the NPs’ electrical, chemical, and other unique properties for neural tissue
regeneration. For example, Ciofani and his team have established electrical stimulation treatment of cells that relies on the piezoelectric behavior of boron nitride nanotubes. Using this
method, they saw a 30% increase in axonal outgrowth of the PC12 cells absorbed after 9 days of treatment compared to the control5. Gold NPs have also been used to modify the surface of a
bioactive substrate—a chitosan-gold NP system was grafted onto poly(D,L-lactide) to design nerve conduits, and the resulting system improved the regeneration process of an _in vivo_
transection model of the rat sciatic nerve6. Gold nanorods (AuNRs) are extremely unique in having an optically active surface, caused by localized surface plasmon resonance7. It has been
reported that activation of this feature of AuNRs leads to enhanced axonal extension of the NG108-15 neuronal cells8; axonal enhancement is connected with a moderate increase in
intracellular calcium (Ca2+) concentration9. It is believed that the excitation of the active plasmonic surface could produce transient heating, which in turn would change the membrane
capacitance and activate specific sensitive ion channels located in the cell membrane10. Several studies have explored the role of AuNRs in enhancing cellular activity8,9,10,11,12,13,14. For
example, Paviolo _et al_.8 observed a noticeable increase in neurite length when NG108-15 neuronal cells were posed to cell culture medium containing 5% (v/v) AuNRs solution. The study
compared differences when cells were exposed to pure AuNR versus poly (4-styrenesulfonic acid) - and silica-coated AuNRs. The incubated cells were exposed to 1.2–7.5 W/cm2 laser at 780 nm to
stimulate neuronal cells. In another study, Yong _et al_.11 claimed that the laser-induced heating of AuNRs can stimulate electrical activity in auditory neurons. They demonstrated this by
incubating rat primary auditory neurons with a medium culture containing silica-coated AuNRs and silica-coated gold nanospheres. The incubated cells were exposed to a near-infrared laser at
780 nm to stimulate the primary neurons. Even though these procedures demonstrate the potential of AuNRs in generating a neural response, the methods are not adequate for preparing a 2D
substrate and ultimately a 3D scaffold. Furthermore, it is technologically challenging to incorporate AuNR solution that has been added to the cells in previous studies and hence, cannot be
used to form a physical device to treat nerve injuries. Additionally, the studies described above, do not provide real data about the interaction between the cell membrane and the substrate,
since this interaction is based on cellular encapsulation of the AuNRs rather than cellular adherence. It has been demonstrated in recent years that adult-tissue derived mesenchymal stem
cells (MSCs) are valuable for cell-based therapies in tissue engineering and regeneration. This is primarily due to their inherent multipotent potential to undergo differentiation into
osteocytes, adipoctyes, chondrocytes, myocytes, and hepatocytes. Human MSCs (hMSCs) can be isolated from any adult tissue, including, bone marrow, peripheral blood, dental pulp, synovial
fluid, synovial membrane and adipose tissue. Human adipose-tissue is a relatively easy, and noninvasive source of MSCs, and is the preferred source in human medicine15,16,17,18. In the last
decade or so, it has been established that hMSCs also have the potential to differentiate into electrically functional neural cells, which can easily translate into novel strategies for the
treatment of neurological disorders and/or peripheral nerve injuries19,20,21 Since, the adhesion, proliferation, and differentiation potentials of MSCs can be significantly affected by
biomaterials22,23, it is imperative that these properties are tested before the cell/biomaterial constructs are implanted in an animal. Using _in vitro_ cell proliferation and
differentiation assays, these properties can be tested reliably prior to clinical applications. Although there are several reports suggesting the potential to use AuNRs in regenerative
medicine, all studies thus far, are focused on the _in vitro_ based analyses of immortalized cell lines, like NG108-15 neuronal cell8, or Schwann cells14. Similar assays assessing the
differentiation potentials of hMSCs in presence of neuromimetic AuNRs are lacking. It has been suggested that the physical and chemical properties of biomaterials can significantly affect
the adhesion, proliferation and differentiation abilities of hMSCs, and hence, it is imperative that these properties are tested _in vitro_ prior to their application _in vivo_. In this
study, we established a novel 2D nanocomposite system that incorporates AuNRs as an active layer for cell adhesion, proliferation, and cell-substrate interaction. Based on previous reports,
AuNRs with an aspect ratio 3 were chosen as the building blocks to form a 2D substrate. A novel and relatively simple procedure was used to produce a 2D system of where cell-substrate
interactions could be easily investigated. By utilizing polyethylene glycol (PEG), the surface chemistry was controlled in such a way that a coating of AuNRs was successfully deposited on
the substrate. By using a negatively charged substrate and positively charged AuNRs, strong adhesion was achieved. Moreover we expect that the positive nature of the free terminal amine
(opposite the substrate) on the AuNRs will enhance the cellular adhesion and thus, their interactions. The ability to modify surface chemistry of AuNRs, by changing the terminal groups of
PEG, opens a new route to combine a layer of AuNRs with immortalized and primary cultures of cells, such as progenitor cells, mesenchymal stem cells and Schwann cells, which could lead to
new cellular therapeutic neural devices. In the present report, the 2D system of AuNRs was prepared and used to study the effect of neural differentiation of hMSCs. The cytocompatibility of
the AuNRs was first tested using RT4-D6P2T, a commercially available immortalized Schwann cell line. Subsequently, the neural differentiation of hMSCs was evaluated _in vitro_, by assessing
the expression patterns of Vimentin, glial fibrillary acidic protein (GFAP) and a calcium binding protein B (S100β), specific to the mesenchymal and the neural progenitor cells. Since, one
of the first steps in neural development includes differentiation of neurons from neural stem cells or progenitor cells, this study is the first step before the AuNRs can be implemented _in
vivo_. METHODS MATERIALS All biochemicals, cell culture supplements, and disposable tissue culture supplies were purchased from Thermo Fisher Scientific unless otherwise noted. In all
preparation steps, deionized (DI) water from a Siemens Labostar unit with a resistance of 18 M/cm was used. Sodium borohydride (99%), gold (III) chloride trihydrate (99%), L-ascorbic acid
(98%), _N_-(3-Dimethylaminopropyl)-_N_′-ethylcarbodiimide hydrochloride (EDC), and N-hydroxysuccinimide (NHS) were purchased from Sigma-Aldrich. Cetyltrimethylammonium bromide (CTAB 99%) was
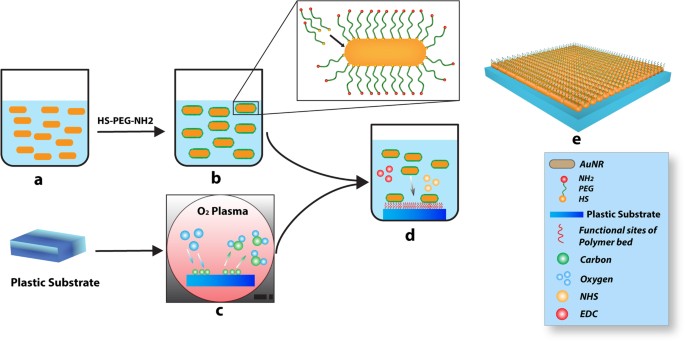
purchased from MP Biomedicals. HS-PEG and HS-PEG-NH2 were purchased from Nanocs Inc. PREPARATION OF GOLD NANOROD (AUNR) SUBSTRATE AuNRs with dimensions of ~12 nm diameter and ~36 nm length
were prepared according to the seed-mediated method described earlier24. The purified AuNRs (Fig. 1a) were then functionalized with HS–PEG–NH2 (Fig. 1b) to modify their surface chemistry and
has been previously reported7,25. Commercially available 15 mm diameter Thermanox coverslips were used as the substrate to coat functionalized AuNRs. Prior to coating, the substrate was
treated with oxygen plasma (100 mTorr O2 with 65 W) for 30 minutes (Fig. 1c). This step produced a more hydrophilic surface and induced reactive oxygen functional groups26. Subsequent to the
plasma treatment, NHS/EDC conjugation assay27 was performed to cover the substrate with a coating of AuNRs-HS-PEG-NH2 (Fig. 1d). Briefly, 10 mg/mL stock solutions of EDC and NHS each were
prepared in 1xPBS. 2 ml of 2 mg/ml functionalized AuNRs were added to each of the O2-treated Thermanox cover slips, followed by the addition of 228 μl of EDC solution and 112 μl of NHS
solution. Using an orbital shaker, the mixture was gently shaken at 100 rpm for 4 hours in order to achieve full conjugation. The excess liquid above the substrate was removed, and the
substrate was washed extensively with DI water to remove any unbound AuNRs-SH-PEG-NH2 molecules. A virtual design depicting the final product is shown in Fig. 1e. CHARACTERIZATION OF AUNRS
The size, structure and morphology of AuNRs were observed using a JEOL-2100F transmission electron microscope with an accelerating voltage of 80 kV, and a JEOL JSM7000F scanning electron
microscope. For TEM, a few drops of AuNRs and AuNR-SH-PEG-NH2 suspended in ethanol, were deposited on carbon coated copper grids, and dried at room temperature for 30 minutes. For SEM,
substrates were coated with a thin carbon layer for visualization. UV-Vis-NIR spectra was recorded on a UV 3600 Shimadzu spectrophotometer to determine the transverse and longitudinal peaks
for AuNRs, both in solution and in the substrate form. A 500 µg/ml solution of AuNRs was used to procure the measurement in solution, while the thin film measurement method was used for the
substrate. All measurements were taken between 400–1000 nm. Electrophoretic mobility (zeta-potential) of the nanoparticles was determined using a Zeta Reader (MARK 21). Briefly, in deionized
water, 500 µg/ml of pure and functionalized AuNRs were loaded in 5 ml syringes, and the data was collected at an applied potential of 20 eV. X-ray photoelectron spectroscopy analysis was
conducted with a Thermo Scientific Model K-Alpha XPS instrument using monochromatic Al Kα radiation (1486.7 eV) with an output power of 36 W. Data were collected and processed using the
Thermo Scientific Avantage XPS software package. Spectra were charge-corrected using the main C1s peak due to adventitious hydrocarbon set to 284.80 eV. Peak fitting was performed using
mixed Gaussian/Lorentzian peak shapes and a Shirley/Smart type background. IMMORTALIZED SCHWANN CELLS An immortalized Schwann cell line, RT4-D6P2T was obtained from the American Type Culture
Collection. A second passage of RT4-D6P2T cells, were incubated for 7 days over the AuNRs substrates, and used in the experiments described below. Tissue culture treated Thermanox cover
slips were used as positive controls. Cells were incubated at 37 °C (5% CO2, 95% air) in DMEM medium supplemented with 10% fetal bovine serum (ATCC, 63310972) and 1% penicillin/
streptomycin. The medium was changed every two days. RT4-D6P2T cells seeded on the AuNRs were evaluated for their viability, adherence, morphology and the expression of Schwann cell protein
markers using the WST-1 and immunofluorescence assays, respectively. WST-1 ASSAY WST-1 Cell Proliferation Assay Kit was used to determine the mitochondrial metabolic activity of RT4-D6P2T
cells, and thus, was used as a measure of cell viability as per the manufacturer’s instructions. The reconstituted WST-1 mixture was added to each well in a 1:10 dilution, and incubated for
2 hours in the dark at 37 °C. A microplate reader was used to measure the absorbance at 450 nm. Wells containing the AuNRs substrate, media, and WST-1 reagent alone were measured as a
background controls. All assays were performed in triplicate and data was analyzed using Students’ TTest analysis. P value of <0.05 was considered statistically significant.
IMMUNOFLUORESCENCE For immunofluorescence, all cells at specified time points were fixed with 4% paraformaldehyde at room temperature for 10 minutes; rinsed twice with HBSS (Hanks’ Balanced
Salt Solution), and then permeabilized with 0.1% Triton X-100. Cells then blocked with a Universal Blocking Reagent for 30 minutes at room temperature. Cells were subsequently incubated with
2µgs of primary antibodies for S100β, GFAP and Vimentin (BD Pharmingen) at 4 °C for 24 hours. Subsequently, cells were washed twice with HBSS and incubated with appropriate Alexa Fluor
secondary antibodies at room temperature for 30 minutes in dark. The cells were then mounted with Prolong Gold antifade reagent with 4′, 6-diamidino-2-phenylindole (DAPI). The cells were
imaged and captured with a laser scanning spectral confocal microscope (Leica TCS SP2) at 20x magnification. ISOLATION, AND _EX VIVO_ EXPANSION OF HUMAN MSCS Human adipose tissue was
obtained from patients undergoing panniculectomies in accordance to a protocol approved by the IRB at the University of Tennessee Medical Center. Informed client consent was obtained prior
to the harvest. After resection, the adipose tissue was immediately processed as previously described28 The cells were grown to 80–90% confluence and then harvested with 0.05% trypsin/EDTA,
for cryopreservation (80% FBS, 10% DMEM/F12, 10% DMSO) or split and seeded into new flasks for expansion. All experiments were performed using cells from passage 2–6 in complete growth media
(CGM) (DMEM/F12, 1% penicillin-streptomycin/amphotericin B, 10% FBS). CHARACTERIZATION OF HUMAN MSCS Isolated human MSCs were characterized by a combination of specific
cluster-of-differentiation (CD) markers expressed on their cell surface. Approximately 1 × 106 human MSCs were used to stain cells with: anti-human CD29-PE/CD44-APC, CD73-PE/Cy7,
CD90-Alexa-fluor 647/CD105-PE, CD34-Alexa-fluor 647/CD45-PE, CD106-PE/HLA-DR-APC or their corresponding isotype matched controls (Biolegend). Antibodies were used at the manufacturer’s
recommended concentrations. Cells were harvested, counted, blocked in 1% goat serum in PBS for 20 minutes at room temperature then stained with each antibody for 20 minutes at room
temperature in darkness. Cells were washed with PBS, collected by centrifugation and fixed with 4% paraformaldehyde/PBS for 10 minutes at room temperature in darkness. Cells were resuspended
in PBS and 20,000 events were measured using a BD FACS Calibur. The raw data was analyzed by FlowJo software. Human MSCs were also evaluated for their ability to undergo tri-lineage
differentiation, _in vitro_, using the following differentiation cocktails in CGM: Osteogenic (100 nM dexamethasone, 10 mM β-glycerol phosphate and 155μM ascorbic acid), Adipogenic (1μM
dexamethasone, 500μM 3-isobutyl-1-methylxanthine (IBMX), 5μg/ml recombinant human insulin, 621μM ascorbate-2-phosphate and 60μM indomethacin), Chondrogenic (100 nM dexamethasone, 155 nM
ascorbate-2-phosphate, 20ng/ml TGF-β1, 1X ITS-G, 50μg/ml proline and 1 mM sodium pyruvate). In all differentiation experiments, non-induced (i.e. cells grown in the absence of any
differentiation media) were used as controls. At 21 days, cells were fixed in 4% paraformaldehyde/PBS for 10 minutes at room temperature. Alizarin red, oil-red-o and alcian blue were used to
detect calcium, lipid and glycosaminoglycan deposition, respectively, in cells undergoing osteogenesis, adipogenesis and chondrogenesis, respectively. All images were obtained using a Zeiss
Axiovert 40 C microscope (Carl Zeiss) equipped with Nikon Digital Sight DS-Qi1Mc camera (Nikon Instruments). DIFFERENTIATION OF HUMAN MSCS INTO NEURAL-LIKE CELLS Human MSCs isolated above
were induced to undergo differentiation into neural-like cells as described earlier29. Neural differentiation was induced in CGM supplemented with 0.5 mM 3-isobutyl-1-methylxanthine (IBMX)/1
mM dibutyryl cAMP (dbcAMP) for 24 h, and 6 days. Media was replenished every 3 days, and differentiation was evaluated by the changes in cell morphology during the induction process. The
expression of GFAP and S100β was evaluated in the undifferentiated and neural-like differentiated hMSCs in presence of AuNRs using immunofluorescence. Imaging of Vimentin was used to assess
morphological changes in mesenchymal cells morphology during differentiation. All cells were fixed at 24hrs and 6 days post differentiation. AuNRs alone without any cells served as negative
controls, whereas, cells seeded on the glass coverslips alone were included as the positive controls. RESULTS AND DISCUSSION PREPARATION AND CHARACTERIZATION OF AUNRS A relatively simple
layer technique was used in the preparation of AuNRs 2D nanocomposite system. Gold nanorods were functionalized with PEG and containing free amine groups were layered on a plastic plasma
treated surface. The nanocomposite thus generated was used in cell adhesion and neural differentiation assays. Figure 1 illustrates the strategy used in the preparation of AuNRs. A plastic
substrate (Thermanox) was treated with oxygen plasma to increase the hydrophilicity of the substrate, and to “activate” the surface with oxygen groups (such as those present in carboxylic
acid) that can form covalent bonds with the surface groups of the AuNRs. There are two forces which might hold the functionalized AuNRs with the polymer substrate: (1) hydrophilic
interactions between PEG polymeric chains and the hydrophilic polymer substrate and (2) covalent bonds between functionalized AuNRs and the substrate surface. The AuNRs (with aspect ratio of
3, average diameter of 12 nm & average length 36 nm) are functionalized with PEG that have terminal amine functional groups which form amide bonds with the plastic substrate through
EDC/NHS reaction. AuNRs were characterized using a combination of different physicochemical methods. As shown in Fig. 2a, native AuNRs with ~12 nm diameter, ~36 nm length, were confirmed by
electron microscopy. In order to control the fabrication of AuNRs layer on the plastic substrate, the surface chemistry of AuNRs should be modified. Generally, the common way to modify the
Au nanoparticles is to attach thiol molecules over Au surface by strong semi-covalent Au–S bonds7. TEM and zeta potential were used to investigate the presence of Thiol-PEG-Amine
(SH-PEG-NH2) layer on the surface of the AuNRs. After functionalization approximately a 1 nm shield can be observed on the AuNR in Fig. 2b thus, confirming the assembly of AuNR with the
HS–PEG–NH2 group. As a result of water absorbed by the PEG chains, the effective thickness of the AuNR particle is expected to be larger than 1 nm. Although the TEM indicated a 1 nm shield
of SH-PEG-NH2 of the AuNRs, this is observed only under vacuum conditions since, the PEG chains collapse under dry or semidry conditions30. On the other hand, PEG chains tend to expand in
non-dry condition as a result of water absorbed. The UV-Vis spectrum (Fig. 2c) of the functionalized AuNRs shows the expected surface plasmon profile (λmax at 520 nm and 780 nm) as described
in previous published work31. Moreover, the zeta-potential of pure AuNRs was −15mV and after functionalization with HS–PEG–NH2 it shifted to +24 mV, confirming an exterior positive surface
change due to terminal amine groups. The assembly and attachment of AuNR-SH-PEG-NH2 to the plastic Thermanox substrate and the presence of the NH2 terminal over the AuNR layer was confirmed
using SEM, XPS, and UV-Vis spectroscopy. The SEM images in Fig. 2d show a coating of AuNR-SH-PEG-NH2 over the plastic substrate even though the AuNR-SH-PEG-NH2 nanorods do not align
uniaxially as proposed in the virtual design Fig. 1e. The UV-Vis spectrum for the layer of AuNRs-SH-PEG-NH2 on the plastic substrate shown in Fig. 2e, indicates that this structure has both
transverse and longitudinal plasmon resonance peaks, located at 520 and 830 nm, respectively. Since the longitudinal peak is more prominent than when the functionalized AuNRs are in solution
it is evident that the AuNRs are laying on their long axis. Furthermore, the longitudinal surface plasmon is significantly shifted toward the IR region compare to control (the AuNRs
dispersed in DI water, where UV-Vis shows transverse and longitudinal plasmon resonance peaks located at 520 and 790 nm). The shift in longitudinal plasmon resonance peak is attributed to
aggregation of AuNRs on the substrate; this change in plasmonic resonance peak has been explained previously in detail32. The XPS spectra of Au4f and N1s from the constructed layer of
AuNR-SH-PEG-NH2 are given in Fig. 2f and g. The sampling depth of our XPS system for metals, which is defined as three times the mean free path of electrons, is approximately 3–3.5 nm. The
organic SH-PEG-NH2 shell is ~1 nm thick, as shown by TEM. The Au4f spectra clearly demonstrates the existence of Au atoms in the nanorods33. The collected spectra also indicates an Au4f5/2
binding energy of 83.42 eV, as shown in Fig. 2f. The N1s binding energy value of ~400 eV attributed to NH2 bonds34 is used as an evidence of a functionalized organic layer as shown in Fig.
2g. The results presented here reflect the successful preparation of the AuNRs 2D nanocomposite system and the attachment of functionalized AuNRs to the plastic substrate. There are many
advantages of combining the AuNRs with SH-PEG-NH2. The AuNR-SH-PEG-NH2 system can be easily used to form coatings over various plastic surfaces, either by interaction between the NH2
terminal of SH-PEG-NH2 with functional sites of the O2 plasma treated plastic substrate, or by hydrophilic interactions between the hydrophilic polymer substrate with AuNR-SH-PEG-NH2.
Surface hydrophilicity, which has been achieved by the incorporation of PEG35 within this configuration, is an important feature or enhanced cell interaction36,37. The free terminal amine of
the functionalized AuNRs also provides a positively charged surface, an environment favorable for cell interaction, and improved neuronal growth36. THE AUNRS ARE CYTOCOMPATIBLE WITH
RT4-D6P2T CELLS The cytotoxicity of the AuNRs was assessed by evaluating cell viability and the fluorescent staining of the cell cytoskeleton using WST-1 assay (Fig. 3A) and phalloidin Alexa
fluor 488 staining of F-actin (Fig. 3B), respectively. The RT4-D6P2T cells were seeded on the AuNRs and assayed after 7 days. As shown in Fig. 3A, there was a 14% significant increase in
cell viability on AuNRs compared to the Thermanox tissue culture treated surface (P = 0.005451393). Immunofluorescence results showed a high actin cytoskeleton organization in the cells at
the same time point. Z stacking showed that the cells adhered and spread uniformly onto the surface of AuNRs while still maintaining their structural integrity. The WST1 assay and the actin
staining results confirm that AuNRs are biocompatible, and do not release any molecules toxic to the cells within the one week study period and thus, can be used as a substrate to support
cell adhesion. In addition to the above, immunofluorescence was also used to evaluate that the Schwann cells maintained the expression of their protein markers, S100β and GFAP, thus,
maintaining their integrity on AuNRs (Fig. 3C). CHARACTERIZATION OF HMSCS In order to maintain uniformity in the classification of progenitor cells, cells are classified as MSCs if they
satisfy certain criteria15 One is to adhere and proliferate on polystyrene treated surface with a spindle-like fibroblastic morphology, and second is to express a combination of specific
cluster-of-differentiation (CD) markers. Subjective evaluation demonstrated fibroblastic morphology of cells during _in vitro_ culturing and serial passaging from passage 1 through 6. Flow
cytometric analyses revealed that >99% cells isolated and expanded in culture from the human adipose tissue were positive for CD29, CD44, CD73, CD90 and CD105 (Fig. 4). The endothelial
surface marker, CD106, showed minimal antigenic reactivity (4.68%) in passage 2 cells, while the hematopoietic marker, CD34, showed 61.4% expression. In passage 6 cells, the expression of
CD34 and CD106 was reduced to 11% and 1.75% respectively. Similarly, the passage 2 cells were <3% positive for the monocyte-macrophage marker, CD45, and the human MHC Class II marker,
HLA-DR, the levels of which were nearly undetectable in passage 6. The third criteria to identify cells as MSCs is to demonstrate their potential to undergo tri-lineage differentiation _in
vitro_. As shown in Fig. 5, when the isolated MSCs were incubated in a lineage-specific inducing cocktail they did undergo differentiation into the expected cell types compared to the
uninduced control cells. Alizarin red, oil-red-o and alcian blue staining confirmed the presence of calcium, lipids and glycosaminoglycans, respectively, when the MSCs revealed osteogenesis,
adipogenesis and chondrogenesis, respectively. Thus the isolated MSCs met the criteria for having the multipotential to undergo tri-lineage differentiation. In order to evaluate the
potential of hMSCs to undergo differentiation into cells of neural lineage and subsequently to assess their behavior on AuNRs, hMSCs were first differentiated into a neural cell-like
phenotype on a polystyrene-coated tissue culture dish, and the expressions of Vimentin, S100β, and GFAP were confirmed using immunofluorescence after 24 hrs and 6 days of differentiation.
The _in vitro_ patterns of expression were as expected (Fig. 6). Vimentin was expressed at 24h and 6days post differentiation (Fig. 6A,B) indicating the maintenance of cell shape, integrity
of the cytoplasm, and stable cytoskeletal interactions in hMSCs during differentiation. Noteworthy is the bipolar morphology of the cells indicating that the hMSCs were becoming neural-like
as early as 24 hours in presence of cAMP and IBMX. This is further confirmed by the relatively higher expression of S100β in the 24 hr sample (Fig. 6C), and of GFAP after 6 days (Fig. 6F).
The hMSCs were next seeded on the AuNRs and differentiated into neural lineage under conditions described above, and analyzed after 24 hrs and 6 days for biomarkers as described above (Fig.
7). As expected, Vimentin was expressed at 24hrs and 6 days displaying the bipolar morphology, integrity of the cytoskeleton and the cytoplasm of cells as early as 24 hrs. and maintaining it
until 6 days (Fig. 7A,B) in the differentiation medium. Importantly, an increase in cell density as visualized in the undifferentiated control cells (Fig. 7A,B insets), further confirmed
and supported the WST-1 assay data, that the AuNRs are not cytotoxic to the hMSCs. The expression patterns of S100β and GFAP on AuNRs were interesting. Unlike, the expression pattern on the
polystyrene surfaces, the S100β was expressed after 24hrs of differentiation and its expression was maintained even after 6 days (Fig. 7C,D). GFAP was expressed in hMSCs on AuNRs as early as
24hrs and persisted even after 6 days, suggesting that the differentiation process was accelerated in presence of AuNRs. Neurogenesis is the process by which neurons are generated from
neural stem cells and progenitor cells. It plays a central role in neural development. Neurogenesis is a complex process that is controlled by a variety of key proteins and their targets38.
The S100 protein family is the largest subgroup of the calcium –binding EF hand protein group39. S100β, is one member of this family, considered to be an important neuro-biomarker and which
regulates the cytoskeletal structure, cell proliferation and neural differentiation. These actions of S100β may be modulated via the MAPK or the NF-kβ signaling pathways. GFAP, a class-III
intermediate filament, is a cell-specific marker, solely expressed in astrocytes, and used to distinguish astrocytes from other glial cells during development40. In summary, the results
presented in this study, demonstrate that the early expression of GFAP when hMSCs differentiate on AuNRs in presence of the inducers like cAMP and IBMX, indicates that their differentiation
was accelerated. The morphological changes in MSCs coincided with the increase in expression of both GFAP and S100β. The cells developed neural-like characteristic morphologic features in
presence of AuNRs within 24 hrs of seeding, and the expression of GFAP was detected as early as 24hrs indicated that the naïve, undifferentiated hMSCs were differentiating into astrocytes as
early as 24hrs rather than 6 days as observed on polystyrene tissue culture surfaces. Researchers have demonstrated that cells exhibit many of the characteristics of neuroendocrine cells
_in vitro_ when the intracellular cAMP levels are elevated by adding inducers like, epinephrine, isoproterenol, forskolin, and IBMX to the culture medium41,42,43. Specifically, neuron-like
cells differentiated from MSCs exhibited increased cytoplasmic Ca2+ levels44. Hence, it is reasonable to speculate that the surface properties of the AuNRs in conjunction with the chemical
cues that are triggered in the hMSCs, may cause the cells to progress towards the neural lineage. The signaling mechanisms resulting in these changes are unknown. Future investigations are
required to study the mechanism(s) of these cellular changes in the AuNR + hMSC nanocomposite system. CONCLUSION Figuress study, a novel protocol was developed to form a layer of 2D
AuNRs-SH-PEG-NH2 nanocomposite on a plastic surface (Fig. 1). Such a structure will allow us to investigate the role AuNRs as a bioactive substrate, by evaluating cell-substrate interaction.
The AuNRs were of 12 nm diameter, functionalized with thiolated PEG and contained –NH2 groups for enhanced cell adhesion (Fig. 2). Immortalized, commercially available Schwann cells adhered
to and were viable on AuNRs and maintained their cellular integrity for 7 days (Fig. 3), confirming the lack of cytotoxicity of the materials. Finally, the AuNRs were evaluated as a
suitable substrate for neural differentiation of human MSCs. Human MSCs were isolated from the human adipose tissue and were characterized using standard cellular assays (Figs 4 and 5).
Finally, the differentiation of hMSCs into neural cells was compared in presence and absence of AuNRs using the expression of three target proteins, Vimentin, S100β and GFAP (Figs 6 and 7).
The expression pattern of Vimentin confirmed that the cells maintained their fibroblastic morphology, and depicted changes when neural differentiation was induced. The expression patterns of
S100β and GFAP suggested that the process of neural differentiation was accelerated in presence of AuNRs. This observation is important and necessary for the development and design of the
3D scaffold of AuNRs. A major finding of this study is that the surface plasmon of the AuNRs nanocomposite system can be activated by an IR laser source. This activated surface can thus,
potentially promote cell growth and neural differentiation by opening Ca + 2 ion channels, thereby, modulating the neurogenesis process. Hence, future experiments to use the 3D AuNRs
scaffold with improved potential for neural differentiation in an _in vivo_ animal model and to evaluate the signaling mechanism(s) of neural differentiation of human MSCs in presence of
AuNRs, should be carried out. REFERENCES * Dubiel, E. A., Martin, Y. & Vermette, P. Bridging the Gap Between Physicochemistry and Interpretation Prevalent in Cell−Surface Interactions.
_Chemical Reviews_ 111, 2900–2936, https://doi.org/10.1021/cr9002598 (2011). Article CAS PubMed Google Scholar * Castro, C., Evora, C., Baro, M., Soriano, I. & Sanchez, E. Two-month
ciprofloxacin implants for multibacterial bone infections. _European journal of pharmaceutics and biopharmaceutics: official journal of Arbeitsgemeinschaft fur Pharmazeutische
Verfahrenstechnik e.V_ 60, 401–406, https://doi.org/10.1016/j.ejpb.2005.02.005 (2005). Article CAS Google Scholar * Rousseau, M. _et al_. _In vivo_ assessment of a multicomponent and
nanostructural polymeric matrix as a delivery system for antimicrobials and bone morphogenetic protein-2 in a unicortical tibial defect in goats. _American journal of veterinary research_
75, 240–250, https://doi.org/10.2460/ajvr.75.3.240 (2014). Article CAS PubMed Google Scholar * Alghazali, K. M. _et al_. Bone-tissue engineering: complex tunable structural and
biological responses to injury, drug delivery, and cell-based therapies. _Drug Metabolism Reviews_ 47, 431–454, https://doi.org/10.3109/03602532.2015.1115871 (2015). Article CAS PubMed
Google Scholar * Ciofani, G. _et al_. Enhancement of Neurite Outgrowth in Neuronal-Like Cells following Boron Nitride Nanotube-Mediated Stimulation. _ACS Nano_ 4, 6267–6277,
https://doi.org/10.1021/nn101985a (2010). Article CAS PubMed Google Scholar * Ni, H. C., Lin, Z. Y., Hsu, S. H. & Chiu, I. M. The use of air plasma in surface modification of
peripheral nerve conduits. _Acta biomaterialia_ 6, 2066–2076, https://doi.org/10.1016/j.actbio.2009.12.038 (2010). Article CAS PubMed Google Scholar * Chen, H., Shao, L., Li, Q. &
Wang, J. Gold nanorods and their plasmonic properties. _Chemical Society Reviews_ 42, 2679–2724, https://doi.org/10.1039/C2CS35367A (2013). Article CAS PubMed Google Scholar * Paviolo,
C. _et al_. Laser exposure of gold nanorods can increase neuronal cell outgrowth. _Biotechnology and bioengineering_ 110, 2277–2291, https://doi.org/10.1002/bit.24889 (2013). Article CAS
PubMed Google Scholar * Henley, J. & Poo, M.-m Guiding Neuronal Growth Cones by Ca(2+) Signals: During axon pathfinding in the developing nervous system, spatiotemporal patterns of
Ca(2+) signals can govern growth cone extension and steering—by symmetric versus asymmetric regulation of cytoskeletal and membrane dynamics. _Trends in cell biology_ 14, 320–330,
https://doi.org/10.1016/j.tcb.2004.04.006 (2004). Article CAS PubMed PubMed Central Google Scholar * Paviolo, C., Haycock, J. W., Cadusch, P. J., McArthur, S. L. & Stoddart, P. R.
Laser exposure of gold nanorods can induce intracellular calcium transients. _J Biophotonics_ 7, 761–765, https://doi.org/10.1002/jbio.201300043 (2014). Article CAS PubMed Google Scholar
* Yong, J. _et al_. Gold-Nanorod-Assisted Near-Infrared Stimulation of Primary Auditory Neurons. _Advanced Healthcare Materials_ 3, 1862–1868, https://doi.org/10.1002/adhm.201400027
(2014). Article CAS PubMed Google Scholar * Paviolo, C., McArthur, S. L. & Stoddart, P. R. Gold Nanorod-assisted Optical Stimulation of Neuronal Cells. e52566,
doi:https://doi.org/10.3791/52566 (2015). * Paviolo, C. & Stoddart, P. R. Metallic nanoparticles for peripheral nerve regeneration: is it a feasible approach? _Neural Regeneration
Research_ 10, 1065–1066, https://doi.org/10.4103/1673-5374.160083 (2015). Article PubMed PubMed Central Google Scholar * Paviolo, C.; Haycock, J. W.; Stoddart, P. R.; McArthur, S. L. In
Effects of laser-exposed gold nanorods on biochemical pathways of neuronal cells, 2013; pp 89231A-89231A-9. * Dominici, M. _et al_. Minimal criteria for defining multipotent mesenchymal
stromal cells. The International Society for Cellular Therapy position statement. _Cytotherapy_ 8, 315–317, https://doi.org/10.1080/14653240600855905 (2006). Article CAS PubMed Google
Scholar * Gronthos, S., Mankani, M., Brahim, J., Robey, P. G. & Shi, S. Postnatal human dental pulp stem cells (DPSCs) _in vitro_ and _in vivo_. _Proc Natl Acad Sci USA_ 97,
13625–13630, https://doi.org/10.1073/pnas.240309797 (2000). Article ADS CAS PubMed PubMed Central Google Scholar * Pittenger, M. F. _et al_. Multilineage potential of adult human
mesenchymal stem cells. _Science_ 284, 143–147 (1999). Article ADS CAS PubMed Google Scholar * Zuk, P. A. _et al_. Human adipose tissue is a source of multipotent stem cells. _Molecular
biology of the cell_ 13, 4279–4295, https://doi.org/10.1091/mbc.E02-02-0105 (2002). Article CAS PubMed PubMed Central Google Scholar * Woodbury, D., Schwarz, E. J., Prockop, D. J.
& Black, I. B. Adult rat and human bone marrow stromal cells differentiate into neurons. _Journal of neuroscience research_ 61, 364–370,
https://doi.org/10.1002/1097-4547(20000815)61:4<364::aid-jnr2>3.0.co;2-c (2000). Article CAS PubMed Google Scholar * Capkin, M. _et al_. Random/aligned electrospun PCL/PCL-collagen
nanofibrous membranes: comparison of neural differentiation of rat AdMSCs and BMSCs. _Biomedical materials (Bristol, England)_ 7, 045013, https://doi.org/10.1088/1748-6041/7/4/045013
(2012). Article ADS Google Scholar * Ladak, A., Olson, J., Tredget, E. E. & Gordon, T. Differentiation of mesenchymal stem cells to support peripheral nerve regeneration in a rat
model. _Experimental Neurology_ 228, 242–252, https://doi.org/10.1016/j.expneurol.2011.01.013 (2011). Article CAS PubMed Google Scholar * Chai, C. & Leong, K. W. Biomaterials
Approach to Expand and Direct Differentiation of Stem Cells. _Molecular therapy: the journal of the American Society of Gene Therapy_ 15, 467–480, https://doi.org/10.1038/sj.mt.6300084
(2007). Article CAS Google Scholar * Tam, R. Y., Fuehrmann, T., Mitrousis, N. & Shoichet, M. S. Regenerative Therapies for Central Nervous System Diseases: a Biomaterials Approach.
_Neuropsychopharmacology_ 39, 169–188, https://doi.org/10.1038/npp.2013.237 (2014). Article CAS PubMed Google Scholar * Nikoobakht, B. & El-Sayed, M. A. Preparation and Growth
Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. _Chemistry of Materials_ 15, 1957–1962, https://doi.org/10.1021/cm020732l (2003). Article CAS Google Scholar * Nima, Z.
A. _et al_. Circulating tumor cell identification by functionalized silver-gold nanorods with multicolor, super-enhanced SERS and photothermal resonances. _Scientific reports_ 4, 4752
(2014). Article CAS PubMed PubMed Central Google Scholar * Vesel, A. & Mozetic, M. Surface modification and ageing of PMMA polymer by oxygen plasma treatment. _Vacuum_ 86, 634–637,
https://doi.org/10.1016/j.vacuum.2011.07.005 (2012). Article ADS CAS Google Scholar * Kulin, S., Kishore, R., Hubbard, J. B. & Helmerson, K. Real-Time Measurement of Spontaneous
Antigen-Antibody Dissociation. _Biophysical Journal_ 83, 1965–1973, https://doi.org/10.1016/S0006-3495(02)73958-1 (2002). Article ADS CAS PubMed PubMed Central Google Scholar * Yu, G.
_et al_. Adipogenic differentiation of adipose-derived stem cells. _Methods Mol Biol_ 702, 193–200, https://doi.org/10.1007/978-1-61737-960-4_14 (2011). Article CAS PubMed Google Scholar
* Deng, W., Obrocka, M., Fischer, I. & Prockop, D. J. _In vitro_ differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase
intracellular cyclic AMP. _Biochemical and biophysical research communications_ 282, 148–152, https://doi.org/10.1006/bbrc.2001.4570 (2001). Article CAS PubMed Google Scholar * Mönch, W.
_et al_. In _Biophotonics_ 405–476 (Wiley-VCH Verlag GmbH & Co. KGaA, 2006). * Nima, Z. A. _et al_. Circulating tumor cell identification by functionalized silver-gold nanorods with
multicolor, super-enhanced SERS and photothermal resonances. _Sci Rep_ 4, 4752, https://doi.org/10.1038/srep04752 (2014). Article CAS PubMed PubMed Central Google Scholar * Bao, Y.,
Vigderman, L., Zubarev, E. R. & Jiang, C. Robust Multilayer Thin Films Containing Cationic Thiol-Functionalized Gold Nanorods for Tunable Plasmonic Properties. _Langmuir_ 28, 923–930,
https://doi.org/10.1021/la203993m (2012). Article CAS PubMed Google Scholar * Fumiya, W. _et al_. X-ray photoelectron spectroscopy and transmission electron microscopy analysis of
silver-coated gold nanorods designed for bionanotechnology applications. _Nanotechnology_ 28, 025704 (2017). Article Google Scholar * Dementjev, A. P. _et al_. X-Ray photoelectron
spectroscopy reference data for identification of the C3N4 phase in carbon–nitrogen films. _Diamond and Related Materials_ 9, 1904–1907, https://doi.org/10.1016/S0925-9635(00)00345-9 (2000).
Article ADS CAS Google Scholar * Israelachvili, J. The different faces of poly(ethylene glycol). _Proceedings of the National Academy of Sciences of the United States of America_ 94,
8378–8379 (1997). Article ADS CAS PubMed PubMed Central Google Scholar * Guo, H., Su J Fau - Wei, J., Wei J Fau - Kong, H., Kong H Fau - Liu, C. & Liu, C. Biocompatibility and
osteogenicity of degradable Ca-deficient hydroxyapatite scaffolds from calcium phosphate cement for bone tissue engineering. _Acta biomaterialia_ 5, 268–278 (2009). Article CAS PubMed
Google Scholar * Webb, K., Hlady, V. & Tresco, P. A. Relative importance of surface wettability and charged functional groups on NIH 3T3 fibroblast attachment, spreading, and
cytoskeletal organization. _Journal of biomedical materials research_ 41, 422–430 (1998). Article CAS PubMed PubMed Central Google Scholar * Yao, B. & Jin, P. Unlocking epigenetic
codes in neurogenesis. _Genes and Development_ 28, 1253–1271, https://doi.org/10.1101/gad.241547.114 (2014). Article CAS PubMed PubMed Central Google Scholar * Sedaghat, F. &
Notopoulos, A. S100 protein family and its application in clinical practice. _Hippokratia_ 12, 198–204 (2008). CAS PubMed PubMed Central Google Scholar * Gomes, F. C., Paulin, D. &
Moura Neto, V. Glial fibrillary acidic protein (GFAP): modulation by growth factors and its implication in astrocyte differentiation. _Brazilian journal of medical and biological research =
Revista brasileira de pesquisas medicas e biologicas_ 32, 619–631 (1999). CAS PubMed Google Scholar * Cox, M. E., Deeble, P. D., Lakhani, S. & Parsons, S. J. Acquisition of
neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. _Cancer research_ 59, 3821–3830 (1999). CAS PubMed Google Scholar *
Sharma, S. K. & Raj, A. B. Transient increase in intracellular concentration of adenosine 3′:5′-cyclic monophosphate results in morphological and biochemical differentiation of C6 glioma
cells in culture. _Journal of neuroscience research_ 17, 135–141, https://doi.org/10.1002/jnr.490170207 (1987). Article CAS PubMed Google Scholar * Ghosh, P. & Singh, U. N.
Intercellular communication in rapidly proliferating and differentiated C6 glioma cells in culture. _Cell biology international_ 21, 551–557, https://doi.org/10.1006/cbir.1997.0193 (1997).
Article CAS PubMed Google Scholar * Gong, M. _et al_. _Retinoic acid receptor beta mediates all-trans retinoic aci_d facilitation of mesenchymal stem cells neuronal differentiation. _The
International Journal of Biochemistry & Cell Biology_ 45, 866–875, https://doi.org/10.1016/j.biocel.2013.01.002 (2013). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS The authors from the University of Tennessee acknowledge the partial support of the University of Tennessee, Center of Excellence in Livestock Diseases and Human Health. The
Center for Nanotechnology Services acknowledge partial support by the Center for Advanced Surface Engineering, under the National Science Foundation Grant No. IIA-1457888, the Arkansas
EPSCoR Program, ASSET III. The Higher Committee For Education Development in Iraq (HCED-IRAQ) is highly appreciated for providing scholarship to KK. The editorial assistance of Emily Davis
is acknowledged. AUTHOR INFORMATION Author notes * Karrer M. Alghazali and Steven D. Newby contributed equally to this work. AUTHORS AND AFFILIATIONS * Center of Integrative Nanotechnology
Sciences, University of Arkansas at Little Rock, Little Rock, AR, 72204, USA Karrer M. Alghazali, Zeid A. Nima, Rabab N. Hamzah, Fumiya Watanabe, Shawn E. Bourdo & Alexandru S. Biris *
College of Veterinary Medicine, University of Tennessee, Knoxville, TN, 37996, USA Steven D. Newby, David E. Anderson & Madhu s. Dhar * University of Tennessee Graduate School of
Medicine, Knoxville, TN, 37996, USA Thomas J. Masi & Stacy M. Stephenson Authors * Karrer M. Alghazali View author publications You can also search for this author inPubMed Google
Scholar * Steven D. Newby View author publications You can also search for this author inPubMed Google Scholar * Zeid A. Nima View author publications You can also search for this author
inPubMed Google Scholar * Rabab N. Hamzah View author publications You can also search for this author inPubMed Google Scholar * Fumiya Watanabe View author publications You can also search
for this author inPubMed Google Scholar * Shawn E. Bourdo View author publications You can also search for this author inPubMed Google Scholar * Thomas J. Masi View author publications You
can also search for this author inPubMed Google Scholar * Stacy M. Stephenson View author publications You can also search for this author inPubMed Google Scholar * David E. Anderson View
author publications You can also search for this author inPubMed Google Scholar * Madhu s. Dhar View author publications You can also search for this author inPubMed Google Scholar *
Alexandru S. Biris View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All authors were involved in the various aspects of the study design,
data analysis, interpretations and drafting the manuscript; K.M.A., Z.A.N., R.N.H., F.W., S.E.B., and A.S.B. planned and carried out all the experiments related to the generation of the gold
nanorods; S.N., T.M., S.S., D.E.A. and M.D. planned and carried out all the mesenchymal stem cell experiments. All authors read and approved the final manuscript. CORRESPONDING AUTHORS
Correspondence to Madhu s. Dhar or Alexandru S. Biris. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no competing interests. ADDITIONAL INFORMATION
PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article
is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Alghazali, K.M., Newby, S., Nima, Z.A. _et al._
Functionalized gold nanorod nanocomposite system to modulate differentiation of human mesenchymal stem cells into neural-like progenitors. _Sci Rep_ 7, 16654 (2017).
https://doi.org/10.1038/s41598-017-16800-9 Download citation * Received: 23 August 2017 * Accepted: 13 November 2017 * Published: 30 November 2017 * DOI:
https://doi.org/10.1038/s41598-017-16800-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
When making thanksgiving dressing, grandma monnette had one simple ruleUPDATED NOVEMBER 21, 2023 AT 5:24 AM ET _All Things We're Cooking is an NPR series that features family recipes fro...
World war 3: conflict between china and us-backed taiwan brewingTaiwan's President Tsai Ing-wen said on Wednesday the island would not accept a "one country, two systems"...
How hiv’s evasion tactics could help fight the fluOne vaccine. Lifetime immunity. This is the goal for thousands of researchers tackling one of the world’s most evasive p...
50 years ago, chimeras gave a glimpse of gene editing’s futureAbby Wallace is the digital engagement producer at _Science News_. She has an undergraduate degree in biology from Georg...
Meet mama's boy, a pandemic-born family bandMICHEL MARTIN, HOST: And finally today, what does a family do when they have to quarantine together? (SOUNDBITE OF SONG,...
Latests News
Functionalized gold nanorod nanocomposite system to modulate differentiation of human mesenchymal stem cells into neural-like progenitorsABSTRACT A 2D multifunctional nanocomposite system of gold nanorods (AuNRs) was developed. Gold nanorods were functional...
James bond star would 'love' to return to 007 franchise despite deathDuring this, the star was asked about her character, Frost, and if she would make her way back to the franchise. "I...
Rheumatoid arthritis: could gluten be triggering your flare-ups?* Rheumatoid arthritis patients may have inflammatory reactions to gluten * Having an autoimmune condition could increas...
High cholesterol: best cooking oil to keep cholesterol levels downInstead, people are better off using oils high in unsaturated fats, such as vegetable and seed oils. Two great examples ...
‘prashant kishor travelled to kolkata amid lockdown,’ alleges bjpIn a statement, Bihar BJP spokesperson Nikhil Anand demanded to know by whose permission Kishor, who is not a government...