Modeling the kinetics of integrin receptor binding to hepatic extracellular matrix proteins
Modeling the kinetics of integrin receptor binding to hepatic extracellular matrix proteins"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The composition of the extracellular matrix (ECM) proteins and the expression of their cognate receptors dictate cell behavior and dynamics. In particular, the interactions of ECM
proteins with integrin receptors are key mediators of these cellular processes, playing a crucial role in the progression of several diseases of the liver, including inflammation,
fibrosis/cirrhosis and cancer. This study establishes a modeling approach combining computation and experiments to evaluate the kinetics of integrin receptor binding to hepatic ECM proteins.
ECM ligand concentration was derived from LC-MS/MS quantification of the hepatic ECM from mice exposed to chronic carbon tetrachloride (CCl4); receptor density was derived from published
literature. Mathematical models for ECM-integrin binding kinetics that were developed incorporate receptor divalence and an aggregation scheme to represent clustering. The computer
simulations reproduced positive cooperativity in the receptor aggregation model when the aggregation equilibrium constant (Ka) was positive and greater than Keq for divalent complex
formation. Importantly, the modeling projected an increase in integrin binding for several receptors for which signaling is known to be increased after CCl4 exposure in the liver. The
proposed modeling approach may be of use to elucidate the kinetics of integrin receptor binding to ECM proteins for homeostatic and diseased livers. SIMILAR CONTENT BEING VIEWED BY OTHERS
COMPUTATIONAL SIMULATION OF LIVER FIBROSIS DYNAMICS Article Open access 18 August 2022 MULTICOMPARTMENT MODELING OF PROTEIN SHEDDING KINETICS DURING VASCULARIZED TUMOR GROWTH Article Open
access 07 October 2020 MODELLING HUMAN LIVER FIBROSIS IN THE CONTEXT OF NON-ALCOHOLIC STEATOHEPATITIS USING A MICROPHYSIOLOGICAL SYSTEM Article Open access 15 September 2021 INTRODUCTION The
extracellular matrix (ECM) consists of a broad range of components that interact bi-directionally with neighboring cells to create a dynamic and responsive microenvironment which regulates
cell signaling, recruitment, and tissue function. The ECM not only provides structure and support for the cells in a tissue, but also acts as a reservoir for growth factors and cytokines and
as a signaling mechanism by which cells can intercommunicate with their environment1. Quantitative and qualitative changes to the ECM structure and superstructure can impact overall health
of the organ and the organism. In particular, the hepatic ECM changes predominantly described in published literature occur in the context of hepatic fibrosis, which is characterized by
robust scarring of the liver with collagen fibrils. However, hepatic ECM is significantly more diverse than collagen ECM. Recent studies further indicate that hepatic ECM content changes
dynamically in response to acute stress and injury2,3,4. Moreover, changes to the hepatic ECM may foster an environment that is conducive to cancer and metastasis. Although the concept that
hepatic ECM changes drive hepatic dysfunction under several conditions is well understood, the mechanisms by which these effects are mediated are not. Integrins comprise a family of
heterodimeric transmembrane glycoprotein receptors that facilitate key interactions between cells and the ECM5,6,7. The binding of ECM ligands to integrins mediates critical processes,
including cell adhesion, migration, proliferation, differentiation, inflammation and apoptosis. Indeed, several of the hallmarks of liver diseases and cancer (e.g., altered proliferation,
angiogenesis and apoptosis) are hypothesized to be mediated via changes in ECM:integrin signaling8. Based on this assumption, integrins have become important therapeutic targets for diseases
of dysregulation, including various cancers, fibrosis, and immune dysfunction. However, few integrin-based therapies have been effective to prevent and/or treat these diseases. This
limitation is partly due, to an incomplete understanding of the complexity of the changes to integrin signaling under dysregulated conditions. The kinetics of ECM:integrin interactions are
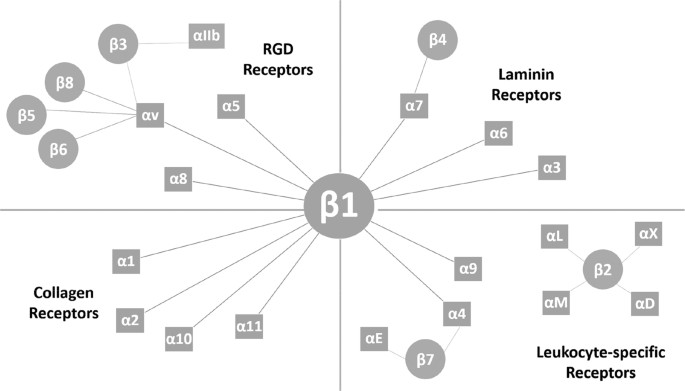
highly intricate. Integrin receptor complexes are structured as non-covalently linked α and β subunits, the various combinations of which contribute to the diversity of receptor types9 (Fig.
1). The overall rate of binding is not driven simply by ligand binding to the receptor, but also by clustering at focal adhesion points and an increase in avidity for binding additional
ligand (i.e., positive cooperativity). Masson-Gandais _et al_. described a two-step model wherein the α subunit binds ligand first, influencing ligand recognition and determinant of
association kinetics10. The β subunit binds second, which creates bond stabilization and determines dissociation kinetics. Ligand binding to the extracellular domain activates the receptor
and initiates its conformational changes to a high-affinity state11,12. This two-step process reflects a divalent kinetics model with the α subunit as the high affinity site, and the β
subunit as the low affinity site13. In addition to binding processivity of individual receptors, ligand binding to distinct integrins favors subsequent binding by other receptors (i.e. focal
adhesion clustering). Furthermore, integrin receptors bind promiscuously to various ECM ligands, creating redundancy, competition and diversity in biofunctionality5,9,14. These complex
interdependent factors affect the kinetics of ECM-integrin interactions in the intact organism. Promiscuity among the repertoire of ECM ligands and integrin receptors, particularly those
with RGD-binding motifs, implies a differential pattern of binding relative to the amounts of substrate available15,16. To explore these interactions as a system, several mathematical
descriptions of integrin binding have been reported with outputs related to spatial clustering and signal transduction, liver fibrosis and haptotaxis17,18,19. Although these models
recapitulate certain aspects of ECM-integrin interactions, they typically focus on one ligand (e.g. collagen or fibronectin) as the ECM substrate. In this study, modeling of integrin
receptor binding kinetics is presented that considers divalent receptor characteristics and employs a simple model of integrin clustering. The kinetic indices of each integrin for each of
its ligands were initially determined to establish a single-species integrin profile. Proteomic data were compiled that assess the liver ECM under homeostatic conditions as well as
experimental fibrosis. These proteomic analyses provided information on relative abundance of hepatic ECM components to calibrate substrate concentrations for the kinetic simulations.
Although data from human fibrotic livers has recently been analyzed20, an animal model was chosen here as it provides a more controlled environment for initial model calibration and testing.
Longer term, by testing homeostatic conditions against the experimental treatment models, how the integrin binding phenotype changes in response to injury could be determined and used to
predict the ECM-integrin binding within the context of transitional tissue remodeling. RESULTS As expected, 4 weeks of CCl4 exposure caused robust fibrotic scarring of the liver in our mouse
model. The resultant phenotype of injury and fibrosis has been previously described to include degradation of basement membrane-like ECM and replacement with fibrillar collagens and other
integrin ligands (Fig. 2)21. The canonical change in ECM content during hepatic fibrosis is an increase in collagen 1 deposition. However, as has been previously described22,23, several
other proteins increase in response to CCl4-induced fibrosis. Analysis of the proteomic data (Table 1) revealed ECM protein expression profiles, and a simple conversion for relating
quantitative exponentially modified protein abundance index (emPAI) values to protein mass was employed as a proteomic ruler to estimate protein concentration under homeostatic and
experimental treatment conditions24,25. Weighting the values with the concentration of extraction fractions, we estimated a relative protein concentration for ECM components. The composition
of the liver ECM as quantitated via proteomic analysis has influence on integrin expression of cells that haptotactically migrate towards ECM protein gradients, and provides the pool of
available ligands for subsequent binding. Qualitatively, the majority of proteins identified were found in both the control and treatment groups; with seven proteins uniquely expressed in
the CCl4 group and only one unique to the control group (Fig. 3). Collagens, glycoproteins and proteoglycans identified via proteomic analysis as ECM substrate were quantified and their
relative concentration was determined (Table 1). Beta-1 and Beta-3 integrins selected for the simulations reflect those involved in hepatic events that relate to CCl4 fibrosis. Integrin-ECM
binding microrates have been determined for various cell types and conditions. Proteomic results were previously validated to confirm relative abundance of identified proteins qualitatively
and quantitatively2. In particular, the amounts and distribution of collagens in the treatment group relative to the control were verified. Here, the presence of trace amounts of Col V in
the CCl4 treatment group was validated to explore whether changes on the nanomolar scale would have pathological consequence. (Fig. 4). Next, to provide for the capability of a system-level
analysis, a computational framework was established using proteomic data for binding species to enable evaluation of integrin receptor binding kinetics (see modeling and experimental details
in Methods). The model was developed taking into consideration sequential binding of subunits. _In silico_ simulations of this model were parameterized using rate constants that correlate
with published literature on binding, or otherwise estimated. The rates are listed in Tables 2 and 3. The simulations were initialized using binding constants from published literature;
where values were not available, parameters were estimated accordingly (see Methods). Collagen fragments for collagen I and IV were plotted together (Fig. 5) and assumed to have the same
rates of binding for the purposes of these experiments. For the other fragmented protein, fibrinogen, only the gamma subunit was considered due to the binding motif located within this
fragment26,27. The binding microrates were set to recapitulate positive cooperativity in divalent receptor saturation and in receptor aggregation pairs, as stipulated in Wanant _et al_.28,
wherein the aggregation equilibrium constant, Ka, drove cooperativity in the aggregate model (Table 3). The simulation graphs in Fig. 5 show left-shifted curves with increased ECM ligand
abundance, indicating increased affinity and avidity for ligand. This is reflected in both the curves for fully occupied divalent receptors (_C_ _d_ ) and for fully occupied aggregate
receptor pairs (_A_ _dd_ ). Steady state values (SS) were recorded for each simulation (Table 4). From these simulation data it appears that upregulated ECMPs reached steady state values in
shorter time, and that aggregation of receptors produced positive cooperativity. Considering the single divalent receptor, _C_ _d_ , the ECM:integrin binding pairs that had the highest
steady state values include both collagen 1 and fibrinogen γ chain in association with the αvβ3 integrin receptor. The combinations with the shortest time to SS were collagen 1 binding αvβ3
or α1β1 receptors. For the aggregated receptor pairs, _A_ _dd_ , the pairs with the highest SS values include von Willebrand factor, fibrinogen γ chain, and collagen 1 binding αvβ3, as well
as fibronectin binding α5β1. The ECM:integrin pairings with the shortest time to SS for _A_ _dd_ pairs were collagen 1 binding αvβ3 and α1β1, and fibronectin binding α5β1. In nearly all
cases, the CCl4 model ECM showed faster rise to SS compared to the control ECM; however, fibrinogen γ chain and Col 4α2 behaved in an opposite manner, owing to the fact that CCl4 actually
downregulated these ECMPs in our dataset. Sensitivity analysis was performed (Table 5) by evaluating the equilibrium constants listed in Table 3 for percent change in steady state after a
ten-fold perturbation to the base values (Table 5). For simple integrin complex formation, i.e. when one ligand binds, increasing the Ki causes a significant increase in aggregated
receptors, while a ten-fold decrease causes an approximate 100% decrease in aggregate pairing. Divalent receptors are moderately decreased when Ki increases, showing that decreased affinity
suppresses the capacity for divalent receptor binding. Filling a single divalent receptor is negatively impacted by an increase in Kc, with aggregate receptor pairs decreasing ~83% for all
three receptor:ligand pairings. A decrease in Kc positively increases the steady state for aggregate pairs, because a lower Keq for the second binding event increases affinity for receptors
with one bound ligand. Kp, the constant for filling empty paired receptors, was nominally affected by perturbation, as were perturbations to filling aggregate pairs. Finally, perturbations
to the aggregation constant, Ka, result in a decrease in steady sate for aggregated pairs when Ka is increased ten-fold, and an increase in steady state when Ka is decreased. This reflects
the condition of a decreased equilibrium constant increasing the affinity for ligand binding, which is expected as the aggregation constant drives ligand affinity in this model. DISCUSSION
Integrin binding to ECM is a vital mechanism for cell migration, invasion, proliferation, and signal transduction between cells and their microenvironment. Diseases of chronic inflammation
and injury, including fibroses and cancer, involve persistent dysregulation of ECM-integrin processes and induce remodeling of the ECM. In addition to their intrinsic utility in cellular
processes, association between immune cells and the ECM is regulated via the β1 & β3 integrin receptor subfamilies29. Elucidating these complex cell-ECM-driven pathological conditions
could lead to improved prognostics and clinical outcomes via more precise therapeutic management of the tissue microenvironment. Several mathematical models of integrin binding have been
reported with outputs relating to spatial clustering and signal transduction, liver fibrosis, and cell migration17,19,30,31. These models recapitulated certain aspects of integrin
interactions; however, these previous studies typically modeled only one ligand, mainly fibronectin or collagen, and utilized generic cognate receptor. In this study, the relative abundance
of ECM components that are canonical substrates of integrin receptors was developed for the proposed modeling framework based on experimentally-obtained liver ECM data. With binding
parameters from published literature, an integrin binding pattern was established for each integrin involved in hepatic processes that are involved in fibrosis. The model from Wanant _et
al_. was adapted to implement the basic model for divalent binding28. Specifically, this model aptly describes initial integrin binding leading to a conformational switch of the receptor
complex from low- to high-affinity. A model of receptor aggregation, which can describe integrin clustering upon attachment to ECM via adhesions5, was also implemented. The simulations
include how each integrin binds with cognate ECM ligands and incorporates the varying affinities that drive this interaction. From these calculations, the kinetic indices of each integrin
for each of its binding partners were determined separately. The impact of changes to the ECM (e.g., in response to CCl4-induced fibrosis) on integrin binding was modeled by calibrating the
substrate concentration based on the proteomic analyses. The extracellular matrix proteome was consistent with the known disease phenotype of the mouse model, with upregulation of specific
ECMPs involved in fulminant fibrosis. The computational results show that in simulations using these ECMPs as substrate for key integrin receptors, interactions involving profibrotic
integrins were predominant. The CCl4 mouse model of liver fibrosis was chosen here due to its robustly characterized pathology and ECM/integrin phenotype (Fig. 2). This model is imperfect in
its recapitulation of human liver fibrosis, but it is the current research standard and therefore has well-defined pathology and changes to the ECM32. Using proteomic data from CCl4-exposed
mouse livers, integrin binding can be explored within the context of fulminant fibrosis. Collagen type Iα1, type III and type IV are excessively deposited due to activated hepatic stellate
cells (HSCs) in response to myofibroblastic transformation induced by activated Kupffer cells and damaged hepatocytes33,34. In agreement with these established phenomena, collagens I, III,
and V were upregulated in the CCl4 cohort in the current study (Table 1). Collagen I is aberrantly produced in this mouse model, and collagen V, a potent nucleating effector for the
co-upregulated fibronectin, exhibited a slight increase from trace levels. In contrast, collagen IV and XVIII levels were decreased relative to the control. Interestingly, collagen XVIII was
identified at relatively minimal levels in the controls, and absent in the CCl4 treated animals (Table 1). This is contrary to an expected increase in collagen XVIII following CCl4
treatment22. Nevertheless, interactions simulated with this ECMP are still based on experimental proteomic analysis. Integrin receptors were not able to be resolved with this particular
method of proteomic analysis, so further proteomic analysis of integrin adhesion complexes in culture is a key component of the future directions for this project. Owing to their involvement
in several critical functions that drive homeostasis and dyshomeostasis, integrins have been identified as key druggable targets in several diseases. For example, integrin inhibitors have
been evaluated to suppress liver fibrogenesis, disrupt attachment and invasion of cancer cells, and to mediate immune response35,36,37,38. Regrettably, many of these drugs fail in early
trials and rarely reach clinical use, perhaps due to an incomplete understanding of integrin binding kinetics, which are traditionally based on single-species models and assumptions; indeed,
even antibodies and small peptide mimetics with specificities for multiple integrins have limited clinical application39,40. Though necessary to target multiple integrins to maximize
efficacy _in vivo_, perhaps the missing link is knowing which targeted doses are most effective for each anti-integrin molecule. In attempting to begin to develop a predictive tool for
effective dosing, the primary goal of this work was to create a framework to simulate simple receptor aggregation and reproduce positive cooperativity induced by aggregate pairing. The
simulations were parameterized to analyze for positive cooperativity of binding in the divalent and aggregation cases. The steady state values and time to steady state for each pairing
correlated to upregulation of key ECMPs in CCl4 liver injury (Fig. 5; Table 4). The integrin receptors that predominated simulations of occupancy were consistent with those known to be at
play in the disease model (Fig. 2). This study offers a first step in which the proposed modeling framework has been initially evaluated using data from a model of fulminant fibrosis and by
which other liver pathologies and how the transitional remodeling of the ECM affects ECM-integrin interactions could be explored. We acknowledge that a more comprehensive test of the model
and its assumptions would require further experiments, which will be pursued in follow-up work. By testing homeostatic conditions against experimental treatment models, this platform could
be broadly employed to predict or confirm changes in integrin binding (and by extension, signaling) caused by remodeling of the hepatic ECM in response to insult or injury. Longer term, a
more complex stochastic model for concurrent integrin binding building upon the results of this study could be developed that considers competitive binding of multiple species. This would
lay the foundation for a more detailed and nuanced analysis of ECM:integrin interactions. METHODS All experiments were performed in accordance with the guidelines and regulations of the
University of Louisville Office of Research Integrity and Institutional Review Board and Biosafety Committee. ANIMALS AND TREATMENTS Male C57BL/6J mice (4–6 w) were purchased from Jackson
Laboratory (Bar Harbor, ME). Mice were housed in a pathogen-free barrier facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and procedures
were approved by the University of Louisville’s Institutional Animal Care and Use Committee. Food and tap water were provided ad libitum. Mice were administered CCl4 (1 ml/kg i.p.; diluted
1:4 in olive oil; Sigma-Aldrich, St. Louis, MO) 2×/wk for 4 wk. Twenty-four h after the last CCl4 administration, mice were anesthetized by injection of a ketamine HCl/xylazine solution
(100/15 mg/kg i.m.; Sigma-Aldrich, St. Louis, MO). Other animals received the same dose of CCl4, but only once, and were sacrificed 12–72 h after intoxication. Blood was collected from the
vena cava just prior to sacrifice by exsanguination and citrated plasma was stored at −80 °C for further analysis. Portions of liver tissue were frozen immediately in liquid nitrogen, while
others were fixed in 10% neutral buffered formalin or embedded in frozen specimen medium (Tissue-Tek OCT compound, Sakura Finetek, Torrance, CA) for subsequent sectioning and mounting on
microscope slides. 3-STEP ECM EXTRACTION SAMPLE PREPARATION AND WASH Snap-frozen liver tissue (75–100 mg) was immediately added to ice-cold phosphate-buffered saline (pH 7.4) wash buffer
containing commercially available protease and phosphatase inhibitors (Sigma Aldrich) and 25 mM EDTA to inhibit proteinase and metalloproteinase activity, respectively. While immersed in
wash buffer, liver tissue was diced into small fragments and washed five times to remove contaminants. Between washes, samples were pelleted by centrifugation at 10,000 × g for 5 min and
wash buffer was decanted. NACL EXTRACTION Diced samples were incubated in 10 volumes of 0.5 M NaCl buffer, containing 10 mM Tris HCl (pH 7.5), proteinase/phosphatase inhibitors, and 25 mM
EDTA. The samples were gently mixed on a plate shaker (800 rpm) overnight at room temperature. The following day, the remaining tissue pieces were pelleted by centrifugation at 10,000 × g
for 10 min. The supernatant was saved and labeled as the NaCl fraction. SDS EXTRACTION The pellet from the NaCl extraction was subsequently incubated in 10 volumes (based on original weight)
of a 1% SDS solution, containing proteinase/phosphatase inhibitors and 25 mM EDTA. The samples were gently mixed on a plate shaker (800 rpm) overnight at room temperature. The following
day, the remaining tissue pieces were pelleted by centrifugation at 10,000 × g for 10 min. The supernatant was saved and labeled as the SDS extract. GUANIDINE HCL EXTRACTION The pellet from
the SDS extraction was incubated with five volumes (based on original weight) of a denaturing guanidine buffer containing 4 M guanidine HCl (pH 5.8), 50 mM sodium acetate, 25 mM EDTA, and
proteinase/phosphatase inhibitors. The samples were vigorously mixed on a plate shaker at 1200 rpm for 48 h at room temperature; vigorous shaking is necessary at this step to aid in the
mechanical disruption of ECM components. The remaining insoluble components were pelleted by centrifugation at 10,000 × g for 10 minutes. This insoluble pellet was retained and solubilized
as described below. The supernatant was saved and labeled as the GnHCl fraction. DEGLYCOSYLATION AND SOLUBILIZATION The supernatants from each extraction were desalted using Zeba Spin
columns (Pierce) according to manufacturer’s instructions. The desalted extracts were then mixed with five volumes of 100% acetone and stored at −20 °C overnight to precipitate proteins. The
precipitated proteins were pelleted by centrifugation at 16,000× g for 45 min. Acetone was evaporated by vacuum drying in a RotoVap for one hour. Dried protein pellets were resuspended in
500 µL deglycosylation buffer (150 mM NaCl, 50 mM sodium acetate, pH 6.8, 10 mM EDTA, and proteinase/phosphatase inhibitors) that contained chondroitinase ABC (_P_. _vulgaris_; 0.025
U/sample), endo-beta-galactosidase (_B_. _fragilis_; 0.01 U/sample) and heparitinase II (_F_. _heparinum_; 0.025 U/sample). Samples were incubated overnight at 37 °C; those containing the
pellet remaining after the guanidine HCl step received 20 µL DMSO for solubilization. Protein concentrations were estimated by absorbance at 280 nm using bovine serum albumin (BSA) in
deglycosylation buffer for reference standards. LC-MS/MS ANALYSIS OF SAMPLES SAMPLE CLEANUP AND PREPARATION FOR LIQUID CHROMATOGRAPHY Pooled samples in deglycosylation buffer were thawed to
room temperature and clarified by centrifugation at 5,000 × g for 5 min at 4 °C. Samples were reduced by adding 1 M DTT to 50 µL (25 µg) of each sample and then incubating at 60 °C for 30
min before addition of 8 M urea in 0.1 M Tris-HCl (pH 8.5) was added to each sample. Each reduced and diluted sample was digested with a modified Filter-Aided Sample Preparation (FASP)
method. Recovered material was dried in a SpeedVac and redissolved in 200 µL of 2% v/v acetonitrile (ACN)/0.4% formic acid (FA). The samples were then trap-cleaned with a C18 PROTOTM 300 Å
Ultra MicroSpin Column (The Nest Group). The sample eluates were incubated at −80 °C for 30 min, dried in a SpeedVac, and stored at −80 °C. Before liquid chromatography, dried samples were
warmed to room temperature and dissolved in 2%v/v ACN/0.1% FA to a final concentration of 0.25 µg/µL. A volume of 16 µL (4 µg) of sample was injected into the Orbitrap Elite. LIQUID
CHROMATOGRAPHY Dionex Acclaim PepMap 100, 75 µM × 2 cm nanoViper (C18, 3 µm, 100 Å) trap and Dionex Acclaim PepMap RSLC, 50 µM × 15 cm nanoViper (C18, 2 µm, 100 Å) separating column were
used. An EASY n-LC (Thermo) UHPLC system was used with mobile phase buffer A (2% v/v acetonitrile/0.1% v/v formic acid), and buffer B (80% v/v acetonitrile/0.1% v/v formic acid). Following
injection of the sample onto the trap, separation was accomplished with a 140 min linear gradient from 0% B to 50% B, followed by a 30 min linear gradient from 50% B to 95% B, and lastly a
10 min wash with 95% B. A 40-mm stainless-steel emitter (Thermo P/N ES542) was coupled to the outlet of the separating column. A Nanospray Flex source (Thermo) was used to position the end
of the emitter near the ion transfer capillary of the mass spectrometer. The ion transfer capillary temperature of the mass spectrometer was set at 225 °C, and the spray voltage was set at
1.6 kV. MASS SPECTROSCOPY An Orbitrap Elite – ETD mass spectrometer (Thermo) was used to collect data from the LC eluate. An Nth Order Double Play with ETD Decision Tree method was created
in Xcalibur v2.2. Scan event one of the method obtained an FTMS MS1 scan for the range 300–2000 m/z. Scan event two obtained ITMS MS2 scans on up to ten peaks that had a minimum signal
threshold of 10,000 counts from scan event one. A decision tree was used to determine whether collision induced dissociation (CID) or electron transfer dissociation (ETD) activation was
used. An ETD scan was triggered if any of the following held: an ion had charge state 3 and m/z less than 650, an ion had charge state 4 and m/z less than 900, an ion had charge state 5 and
m/z less than 950, or an ion had charge state greater than 5; a CID scan was triggered in all other cases. The lock mass option was enabled (0% lock mass abundance) using the 371.101236 m/z
polysiloxane peak as an internal calibrant. PROTEOME DATA ANALYSIS Proteome Discoverer v1.4.0.288 was used to analyze the data collected by the mass spectrometer. The database used in Mascot
v2.4 and SequestHT searches was the 6/2/2014 version of the UniprotKB _Mus musculus_ reference proteome canonical and isoform sequences. In order to estimate the false discovery rate, a
Target Decoy PSM Validator node was included in the Proteome Discoverer workflow. The Proteome Discoverer analysis workflow allows for extraction of MS2 scan data from the Xcalibur RAW file,
separate searches of CID and ETD MS2 scans in Mascot and Sequest, and collection of the results into a single file (.msf extension). The resulting.msf files from Proteome Discoverer were
loaded into Scaffold Q + S v4.3.2. Scaffold was used to calculate the false discovery rate using the Peptide and Protein Prophet algorithms. The results were annotated with mouse gene
ontology information from the Gene Ontology Annotations Database. COMPUTATIONAL MODELING First is considered the divalent receptor model that corresponds to ECM ligand binding of the α
subunit occurring prior to the β subunit7,28,41, where _k_ 1 is the first-order association rate constant and _k_ 2 is the dissociation constant for singly occupied receptors (_C_ _m_ ). _C_
_d_ indicates a fully occupied integrin receptor with two bound ECM ligands, and _k_ 3 and _k_ 4 define the rate constants for association and dissociation, respectively, of the doubly
bound integrin receptor. Differential equations for this model are: $$\frac{dI}{dt}={k}_{2}{C}_{m}-{k}_{1}IE,$$ (1) $$\frac{dE}{dt}={k}_{2}{C}_{m}-{k}_{1}IE+{k}_{4}{C}_{d}-{k}_{3}{C}_{m}E,$$
(2) $$\frac{d{C}_{m}}{dt}={k}_{1}IE-{k}_{2}{C}_{m}+{k}_{4}{C}_{d}-{k}_{3}{C}_{m}E,$$ (3) $$\frac{d{C}_{d}}{dt}={k}_{3}{C}_{m}E-{k}_{4}{C}_{d},$$ (4) The scheme for receptor aggregation and
ligand binding is shown in Fig. 6. In the model of receptor aggregation, we utilized the same scheme as Wanant _et al_.28, wherein receptors pair in a manner such that either singly- or
doubly-bound receptors can aggregate only with an unbound receptor with the aggregation equilibrium constant KA (where KA = _k_ 5 _/k_ 6), and disaggregation equilibrium constant KA’ (KA’ =
_k_ 6 _/k_ 5). Binding constants for an additional ECM ligand binding to the unbound portion of an aggregate pair are the same regardless of whether the bound portion has one or two ligands,
where the equilibrium association constant is KC = _k_ 9 _/k_ 10. The equilibrium constant for adding a second ECM ligand to a singly bound receptor in any pair-configuration is KF = _k_ 7
_/k_ 8. The differential equations describing receptor aggregation are listed below: $$\frac{dI}{dt}={k}_{2}{C}_{m}-{k}_{1}IE+{k}_{6}({A}_{im}+{A}_{id})-{k}_{5}I({C}_{m}+{C}_{d}),$$ (5)
$$\frac{dE}{dt}={k}_{2}{C}_{m}-{k}_{1}IE+{k}_{4}{C}_{d}-{k}_{3}{C}_{m}E+{k}_{10}({A}_{mm}+{A}_{md})-{k}_{9}E({A}_{im}+{A}_{id})+\,{k}_{8}({A}_{id}+{A}_{md}+{A}_{dd})-{k}_{7}E({A}_{im}+{A}_{mm}+{A}_{md}),$$
(6) $$\frac{d{C}_{m}}{dt}={k}_{1}IE-{k}_{2}{C}_{m}+{k}_{4}{C}_{d}-{k}_{3}{C}_{m}E+{k}_{6}{A}_{im}-{k}_{5}I{C}_{m},$$ (7)
$$\frac{d{C}_{d}}{dt}={k}_{3}{C}_{m}E-{k}_{4}{C}_{d}+{k}_{6}{A}_{id}-{k}_{5}I{C}_{d},$$ (8)
$$\frac{d{A}_{im}}{dt}={k}_{5}I{C}_{m}-{k}_{6}{A}_{im}+{k}_{10}{A}_{mm}-{k}_{9}E{A}_{im}+{k}_{8}{A}_{id}-{k}_{7}E{A}_{im},$$ (9)
$$\frac{d{A}_{id}}{dt}={k}_{5}I{C}_{d}-{k}_{6}{A}_{id}+{k}_{10}{A}_{md}-{k}_{9}E{A}_{id}+{k}_{7}E{A}_{im}-{k}_{8}{A}_{id},$$ (10)
$$\frac{d{A}_{mm}}{dt}={k}_{9}E{A}_{im}-{k}_{10}{A}_{mm}+{k}_{8}{A}_{md}-{k}_{7}E{A}_{mm},$$ (11)
$$\frac{d{A}_{md}}{dt}={k}_{9}E{A}_{id}-{k}_{10}{A}_{md}+{k}_{8}E({A}_{dd}-{A}_{md})+{k}_{7}E({A}_{mm}-{A}_{md}),$$ (12) $$\frac{d{A}_{dd}}{dt}={k}_{7}E{A}_{md}-{k}_{8}{A}_{dd},$$ (13) where
_A_ im indicates an aggregate pair comprised of one unbound integrin receptor coupled with a singly-bound receptor; _A_ _id_ is the same combination, except featuring a doubly-bound
receptor. A pair with two singly bound receptors is defined as _A_ _mm_ , with two doubly bound receptors is _A_ _dd_ , and _A_ _md_ indicates a singly bound receptor paired with a doubly
bound one (Fig. 6). The affinity of integrin receptors for ECM proteins fibronectin and laminin are generally in the micromolar range. The Kd measured for ECM:integrin and, in particular,
fibronectin binding, ranges between approximately 10−7–10−6 M42; Takagi _et al_. report nanomolar Kd values for fibronectin binding43. Mallet _et al_. utilized a Kd of 2 × 10−4 M for
tethered RGD peptides in their model of integrin binding17. SIMULATIONS Computer simulations were run using Spyder for Tellurium software version 2.3.5.2; Python version 2.744. Binding
curves were plotted using SigmaPlot 13.0. The model was initialized using ligand concentrations from proteomic analysis (Table 1) and initial integrin concentrations were derived from
published values. Ligand concentration was developed by collapsing the fractionated sample data using MudPIT functionality in Scaffold. Rappsilber _et al_. defined protein abundance index
(PAI) for estimation of absolute protein abundance45, and Ishihama _et al_. report that the emPAI, i.e. exponentially modified PAI, is approximately proportional to protein abundance25.
Using the emPAI quantitative method, proteomic output was normalized by the tissue loading concentration of 0.25 µg/µL; these values for concentration were then divided by the molecular
weight of the protein to convert to molar concentration. Kinetic rates listed in Table 2 were used to calculate microrate parameters relative to the established binding rates from
literature; where exact microrates were unavailable, rates were estimated from various published literature sources. Table 3 relates the equilibrium constants of the system relative to
initial integrin complex formation, such that subsequent binding and clustering steps produce cooperativity when simulated in these proportions. Sensitivity analysis was performed by varying
levels of integrin receptor concentration in 10-fold increments, to explore binding when surface membrane integrin receptor expression is upregulated or downregulated as a consequence of
disease state or in response to microenvironmental fluctuations. DATA AVAILABILITY The datasets generated and/or analysed during the current study are available from the corresponding author
on reasonable request. REFERENCES * Hynes, R. O. The extracellular matrix: not just pretty fibrils. _Science_ 326, 1216–1219 (2009). Article ADS CAS PubMed PubMed Central Google
Scholar * Massey, V. L. _et al_. The hepatic “matrisome” responds dynamically to injury: characterization of transitional changes to the extracellular matrix. _Hepatology_ 65, 969–982
(2017). Article CAS PubMed Google Scholar * Cox, T. R. & Erler, J. T. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. _Dis
Model Mech_ 4, 165–178 (2011). Article CAS PubMed PubMed Central Google Scholar * Poole, L. G. & Arteel, G. E. Transitional remodeling of the hepatic extracellular matrix in
alcohol-induced liver injury. _BioMed Res Intl_ 2016 (2016). * Humphries, J. D., Byron, A. & Humphries, M. J. Integrin ligands at a glance. _J Cell Sci_ 119, 3901–3903 (2006). Article
CAS PubMed PubMed Central Google Scholar * Hynes, R. O. Integrins: a family of cell surface receptors. _Cell_ 48, 549–554 (1987). Article CAS PubMed Google Scholar * Hynes, R. O.
Integrins. _Cell_ 110, p673–p687 (2002). Article Google Scholar * Patsenker, E. & Stickel, F. Role of integrins in fibrosing liver diseases. _Am J Physiol Gastrointest Liver Physiol_
301, G425–G434 (2011). Article CAS PubMed Google Scholar * Campbell, I. D. & Humphries, M. J. Integrin structure, activation, and interactions. _Cold Spring Harb Perspect Biol_ 3,
a004994 (2011). Article PubMed PubMed Central Google Scholar * Masson-Gadais, B., Pierres, A., Benoliel, A. M., Bongrand, P. & Lissitzky, J. C. Integrin (alpha) and beta subunit
contribution to the kinetic properties of (alpha)2beta1 collagen receptors on human keratinocytes analyzed under hydrodynamic conditions. _J Cell Sci_ 112, 2335–2345 (1999). CAS PubMed
Google Scholar * Legate, K. R., Wickstrom, S. A. & Fassler, R. Genetic and cell biological analysis of integrin outside-in signaling. _Genes & Dev_ 23, 397–418 (2009). Article CAS
Google Scholar * Pouwels, J., Nevo, J., Pellinen, T., Ylänne, J. & Ivaska, J. Negative regulators of integrin activity. _J Cell Sci_ 125, 3271–3280 (2012). Article CAS PubMed
Google Scholar * Zhao, T., Li, Y. & Dinner, A. R. How focal adhesion size depends on integrin affinity. _Langmuir_ 25, 1540–1546 (2009). Article CAS PubMed Google Scholar * Plow, E.
F., Haas, T. A., Zhang, L., Loftus, J. & Smith, J. W. Ligand binding to integrins. _J Biological Chem_ 275, 21785–21788 (2000). Article CAS Google Scholar * Sánchez-Cortés, J. &
Mrksich, M. The platelet integrin aIIbb3 binds to the RGD and AGD motifs in fibrinogen. _Chem Biol_ 16, 990–1000 (2009). Article PubMed PubMed Central Google Scholar * Garratt, A. N.
& Humphries, M. J. Recent insights into ligand binding, activation and signalling by integrin adhesion receptors. _Acta Anatomica_ 154, 34–45 (1995). Article CAS PubMed Google Scholar
* Mallet, D. G. & Pettet, G. J. A mathematical model of integrin-mediated haptotactic cell migration. _. Bull Math Biol_ 68, 231–253 (2006). Article MathSciNet CAS PubMed MATH
Google Scholar * Dutta-Moscato, J. _et al_. A multiscale agent-based in silico model of liver fibrosis progression. _Front Bioeng Biotechnol_ 2, 18 (2014). Article PubMed PubMed Central
Google Scholar * Welf, E. S., Naik, U. P. & Ogunnaike, B. A. A spatial model for integrin clustering as a result of feedback between integrin activation and integrin binding. _Biophys
J_ 103, 1379–1389 (2012). Article CAS PubMed PubMed Central Google Scholar * Baiocchini, A. _et al_. _E_xtracellular matrix molecular remodeling in human liver fibrosis evolution. _PLoS
One_ 11, e0151736 (2016). Article PubMed PubMed Central Google Scholar * Iredale, J. P., Thompson, A. & Henderson, N. C. Extracellular matrix degradationin liver fibrosis:
Biochemistry and regulation. _Biochimica et Biophysica Acta Mol Basis Disease_ 1832, 876–883 (2013). Article CAS Google Scholar * Duncan, M. B. _et al_. _T_ype XVIII collagen is essential
for survival during acute liver injury in mice. _Dis Model Mech_ 6, 942–951 (2013). Article CAS PubMed PubMed Central Google Scholar * Jiang, Y., Liu, J., Waalkes, M. & Kang, Y. J.
Changes in the gene expression associated with carbon tetrachloride-induced liver fibrosis persist after cessation of dosing in mice. _Toxicological Sci_ 79, 404–410 (2004). Article CAS
Google Scholar * Wisniewski, J. R., Hein, M. Y., Cox, J. & Mann, M. A “proteomic ruler” for protein copy number and concentration estimation without spike-in standards. _Mol Cell
Proteomics_ 13, 3497–3506 (2014). Article CAS PubMed PubMed Central Google Scholar * Ishihama, Y. _et al_. Exponentially modified protein abundance index (emPAI) for estimation of
absolute protein amount in proteomics by th enumber of sequeced peptides per protein. _Mol & Cellular Proteomics_ 4, 1265–1272 (2005). Article CAS Google Scholar * Peerschke, E. I.,
Francis, C. W. & Marder, V. J. Fibrinogen binding to human blood platelets: effect of gamma chain carboxyterminal structure and length. _Blood_ 67, 385–390 (1986). CAS PubMed Google
Scholar * Ware, S., Donahue, J. P., Hawiger, J. & Anderson, W. F. Structure of the fibrinogen gamma-chain integrin binding and factor XIIIa cross-linking sites obtained through carrier
protein driven crystallization. _Protein Sci_ 8, 2663–2671 (1999). Article CAS PubMed PubMed Central Google Scholar * Wanant, S. & Quon, M. J. Insulin receptor binding kinetics:
Modeling and simulation studies. _J Theor Biol_ 205, 355–364 (2000). Article CAS PubMed Google Scholar * Bruck, R. _et al_. _T_he use of synthetic analogues of Arg-Gly-Asp (RGD) and
soluble receptor of tumor necrosis factor to prevent acute and chronic experimental liver injury. _Yale J Biol Med_ 70, 391–402 (1997). CAS PubMed PubMed Central Google Scholar * Caré,
B. R. & Soula, H. A. Impact of receptor clustering on ligand binding. _BMC Systems Biol_ 5, 48 (2011). Article Google Scholar * Nieto, N. & Lutolf, M. P. Extracellular matrix
bioengineering and systems biology approaches in liver disease. _Systems Synthetic Biol_ 5, 11 (2011). Article Google Scholar * Delire, B., Stärkel, P. & Leclercq, I. Animal models for
fibrotic liver diseases: What we have, what we need, and what is under development. _J Clin Translational Hepatol_ 3, 53–66 (2015). Article Google Scholar * Friedman, S. L. Molecular
Regulation of hepatic fibrosis, an integrated cellular response to tissue injury. _J Biological Chem_ 275, 2247–2250 (2000). Article CAS Google Scholar * Liedtke, C. _et al_.
_E_xperimental liver fibrosis research: update on animal models, legal issues and translational aspects. _Fibrogenesis Tissue Repair_ 6, 19 (2013). Article PubMed PubMed Central Google
Scholar * Rosenow, F. _et al_. _I_ntegrins as antimetastatic targets of RGD-independent snake venom components in liver metastasis. _Neoplasia_ 10, 168–176 (2008). Article CAS PubMed
PubMed Central Google Scholar * Shimaoka, M. & Springer, T. A. Therapeutic antagonists and conformational regulation of integrin function. _Nature Rev Drug Discovery_ 2, 703–716
(2003). Article CAS Google Scholar * Henderson, N. C. _et al_. _T_argeting of av integrin identifies a core molecular pathway that regulates fibrosis in several organs. _Nature Med_ 19,
1617–1624 (2013). Article CAS PubMed Google Scholar * Agarwal, S. K. Integrins and cadherins as therapeutic targets in fibrosis. _Front Pharmacol_ 5, 131 (2014). Article PubMed PubMed
Central Google Scholar * Millard, M., Odde, S. & Neamati, N. Integrin targeted therapeutics. _Theranostics_ 1, 154–188 (2011). Article CAS PubMed PubMed Central Google Scholar *
Goodman, S. L. & Picard, M. Integrins as therapeutic targets. _Trends Pharmacol Sci_ 33, 405–412 (2012). Article CAS PubMed Google Scholar * Sauro, H. M. _Enzyme Kinetics for Systems
Biology_, _2nd Ed_.(Ambrosius Publishing and Future Skill Software, Seattle, 2012). * Buck, C. A. & Horwitz, A. F. Cell surface receptors for extracellular matrix molecules. _Ann Rev
Cell Biol_ 3, 179–205 (1987). Article CAS PubMed Google Scholar * Takagi, J., Strokovich, K., Springer, T. A. & Walz, T. Structure of integrin a5b1 in complex with fibronectin. _EMBO
J_ 22, 4607–4615 (2003). Article CAS PubMed PubMed Central Google Scholar * Choi, K., Medley, J. K., König, M., Stocking, K. & Cannistra, C. Tellurium, version 2.3.5.2. Co_mputer
Program_ (2014). * Rappsilber, J., Ryder, U., Lamond, A. I. & Mann, M. Large-scale proteomic analysis of the human spliceosome. _Mol & Cellular Proteomics_ 4, 1265–1272 (2002).
Google Scholar * Kislinger, T., Gramolini, A. O., MacLennan, D. H. & Emili, A. Multidimensional protein identification technology (MudPIT): Technical overview of a profiling method
optimized for the comprehensive proteomic investigation of normal and diseased heart tissue. _Proteomics and Disease_ 16, 1207 (2005). CAS Google Scholar * Schirmer, E. C., Yates, I. J. R.
& Gerace, L. MudPIT: A powerful proteomics tool for discovery. _Discov Med_ 3, 38–39 (2003). PubMed Google Scholar * Kim, J. K. _et al_. A novel binding site in collagen type III for
integrins a1b1 and a2b1. _J Biological Chem_ 280, 32512–32520 (2005). Article CAS Google Scholar * Kern, A., Eble, J., Golbik, R. & Kühn, K. Interaction of type IV collagen with the
isolated integrins a1b1. _Eur J Biochem_ 215, 151–159 (1993). Article CAS PubMed Google Scholar * Denis, C., Williams, J. A., Lu, X., Meyer, D. & Baruch, D. Solid-phase von
Willebrand factor contains a conformationally active RGD motif that mediates endothelial cell adhesion through the alpha v beta 3 receptor. _Blood_ 82, 3622–3630 (1993). CAS PubMed Google
Scholar * Xu, Y. _et al_. Multiple binding sites in collagen type I for the integrins a1b1 and a2b1. _J Biological Chem_ 275, 38981–38989 (2000). Article CAS Google Scholar Download
references ACKNOWLEDGEMENTS We are grateful to William L. Dean (Biochemistry & Molecular Biology, University of Louisville) for useful discussions and advice. AUTHOR INFORMATION Author
notes * Hermann B. Frieboes and Gavin E. Arteel jointly supervised this work. AUTHORS AND AFFILIATIONS * Department of Pharmacology and Toxicology, University of Louisville, Louisville, KY,
40202, USA Shanice V. Hudson, Christine E. Dolin, Lauren G. Poole, Veronica L. Massey, Daniel Wilkey, Juliane I. Beier, Michael L. Merchant, Hermann B. Frieboes & Gavin E. Arteel *
Department of Bioengineering, University of Louisville, Louisville, KY, 40208, USA Shanice V. Hudson & Hermann B. Frieboes * James Graham Brown Cancer Center, University of Louisville,
Louisville, KY, 40202, USA Hermann B. Frieboes * University of Louisville Alcohol Research Center, University of Louisville, Louisville, 40202, KY, USA Gavin E. Arteel Authors * Shanice V.
Hudson View author publications You can also search for this author inPubMed Google Scholar * Christine E. Dolin View author publications You can also search for this author inPubMed Google
Scholar * Lauren G. Poole View author publications You can also search for this author inPubMed Google Scholar * Veronica L. Massey View author publications You can also search for this
author inPubMed Google Scholar * Daniel Wilkey View author publications You can also search for this author inPubMed Google Scholar * Juliane I. Beier View author publications You can also
search for this author inPubMed Google Scholar * Michael L. Merchant View author publications You can also search for this author inPubMed Google Scholar * Hermann B. Frieboes View author
publications You can also search for this author inPubMed Google Scholar * Gavin E. Arteel View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
Conceived and organized the study (S.V.H., H.B.F., G.E.A.); directed proteomics analysis (M.L.M.); directed animal study for proteomics samples (V.L.M., J.I.B.); prepared proteomic samples
(L.G.P.); performed proteomic analysis (C.E.D., D.W.); implemented modeling and simulations (S.V.H.); analyzed the results (S.V.H., H.B.F.); wrote initial manuscript (S.V.H.); revised and
approved final manuscript (S.V.H., G.E.A., H.B.F.). CORRESPONDING AUTHOR Correspondence to Gavin E. Arteel. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no
competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY TABLE S1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which
permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Hudson, S.V., Dolin, C.E., Poole, L.G. _et al._ Modeling the Kinetics of Integrin Receptor Binding to Hepatic Extracellular Matrix
Proteins. _Sci Rep_ 7, 12444 (2017). https://doi.org/10.1038/s41598-017-12691-y Download citation * Received: 13 June 2017 * Accepted: 14 September 2017 * Published: 29 September 2017 * DOI:
https://doi.org/10.1038/s41598-017-12691-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Submerged valleys and barrier reefsABSTRACT As I have never visited the Pacific Islands, I do not attempt to bring their valleys under the same category as...
Statement: rfu put their full backing behind allianz premier 15’s - ruckBY STELLA MILLS THE RFU HAVE TODAY ANNOUNCED A TEN-YEAR STRATEGY PLAN TO GROW THE ALLIANZ PREMIER 15’S, WHICH WILL SEE A...
Lipid storage disorders block lysosomal trafficking by inhibiting a trp channel and lysosomal calcium releaseABSTRACT Lysosomal lipid accumulation, defects in membrane trafficking and altered Ca2+ homoeostasis are common features...
Martin Kimber | TheArticleFirst {{register.errors.names}} Last Gender What's this for? Age bracket What's this for? This is to help us s...
What to watch after 'the last of us'There was a time when the idea of a video game adaptation sounded alarm bells, with all but guaranteed audience disappoi...
Latests News
Modeling the kinetics of integrin receptor binding to hepatic extracellular matrix proteinsABSTRACT The composition of the extracellular matrix (ECM) proteins and the expression of their cognate receptors dictat...
‘crashed ufo’ found in russia housing 'extra-terrestrial remains’It was hailed as a major coup in the search for evidence of extra-terrestrial life, when the team from the Kuzbassrazrez...
Pest warning: toxic caterpillars and superslugs to invade britainThe slimy creatures have arrived on home soil and gardeners are on high alert as the pests are highly poisonous and dest...
Selena gomez shares yard photos on instagram promoting puma sneakersBy PAUL CHAVEZ FOR DAILYMAIL.COM Published: 01:23 EDT, 13 July 2020 | Updated: 08:26 EDT, 13 July 2020 Selena Gomez took...
Separate 'garoland' to be main poll plank of garo national councilSeparate ‘Garoland’ is the main agenda for the Garo National Council (GNC ) and the party will make this its main poll p...