Elovanoids are novel cell-specific lipid mediators necessary for neuroprotective signaling for photoreceptor cell integrity
Elovanoids are novel cell-specific lipid mediators necessary for neuroprotective signaling for photoreceptor cell integrity"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Docosahexaenoic acid (DHA, 22:6 n-3) is abundant in the retina and is enzymatically converted into pro-homeostatic docosanoids. The DHA- or eicosapentaenoic acid (EPA)-derived 26
carbon fatty acid is a substrate of elongase ELOVL4, which is expressed in photoreceptor cells and generates very long chain (≥C28) polyunsaturated fatty acids including n-3 (VLC-PUFAs,n-3).
While ELOVL4 mutations are linked to vision loss and neuronal dysfunctions, the roles of VLC-PUFAs remain unknown. Here we report a novel class of lipid mediators biosynthesized in human
retinal pigment epithelial (RPE) cells that are oxygenated derivatives of VLC-PUFAs,n-3; we termed these mediators elovanoids (ELV). ELVs have structures reminiscent of docosanoids but with
different physicochemical properties and alternatively-regulated biosynthetic pathways. The structures, stereochemistry, and bioactivity of ELVs were determined using synthetic materials
produced by stereo-controlled chemical synthesis. ELVs enhance expression of pro-survival proteins in cells undergoing uncompensated oxidative stress. Our findings unveil a novel
autocrine/paracrine pro-homeostatic RPE cell signaling that aims to sustain photoreceptor cell integrity and reveal potential therapeutic targets for retinal degenerations. SIMILAR CONTENT
BEING VIEWED BY OTHERS ACYL-COA SYNTHETASE 6 CONTROLS ROD PHOTORECEPTOR FUNCTION AND SURVIVAL BY SHAPING THE PHOSPHOLIPID COMPOSITION OF RETINAL MEMBRANES Article Open access 21 August 2024
PRETREATMENT OF HUMAN RETINAL PIGMENT EPITHELIAL CELLS WITH STERCULIC ACID FORESTALLS FENRETINIDE-INDUCED APOPTOSIS Article Open access 23 December 2022 PNPLA6 REGULATES RETINAL HOMEOSTASIS
BY CHOLINE THROUGH PHOSPHOLIPID TURNOVER Article Open access 13 March 2025 INTRODUCTION Disease onset and progression trigger a complex cellular response that disrupts homeostasis1, 2.
Referred to as inflammation, this is a defensive mechanism that includes the generation of protective mediators, including bioactive lipids3,4,5,6,7, and engages immune cells, blood vessels,
neurons, astrocytes, retinal pigment epithelial (RPE) cells and other cells, aiming to sustain homeostasis, remove triggering factors and cell debris, and set in motion cellular and tissue
restoration. Pro-homeostatic signaling is set in motion in RPE cells, photoreceptor cells (PRCs) and, likely, in other retinal cells at the beginning of cellular disruptions such as
uncompensated oxidative stress (UOS), as well as at the onset of retinal degenerations8,9,10 or other neurodegenerative diseases. The omega-3 fatty acid docosahexaenoic acid (DHA) is
abundant in the central nervous system (CNS), which includes the retina5, 6, 9, 11, and serves as the precursor for 22-carbon chain length docosanoids, which have neuroprotective and
pro-homeostatic bioactivities9, 10, 12, 13. DHA also can be the target of excessive oxidative damage that evolves into retinal pathology14. Photoreceptor cells express the elongase enzyme
ELOVL4 (ELOngation of Very Long chain fatty acids-4), which is evolutionarily conserved in the retina15 and catalyzes the biosynthesis of very long chain polyunsaturated fatty acids (≥C28)
including n-3 (VLC-PUFAs,n-3) from 26:6 fatty acids derived from DHA or eicosapentaenoic acid (EPA)16, 17; EPA has been shown to be the preferred substrate16. Even though the levels of EPA
are quite low in the retina compared to DHA, retroconversion of DHA to EPA in peroxisomes takes place, and it is possible that EPA produced by this reaction will generate the 26:6 substrate
for ELOVL416. These fatty acids become acyl chains of phosphatidylcholines and sphingolipids and are enriched in the inner segment of PRCs. ELOVL4 synthesizes VLC-PUFAs in the retina18,19,20
and testes21, and it synthesizes VLC saturated fatty acids (VLC-SFAs) in the skin and brain22, 23. Mutant ELOVL4 causes juvenile macular degeneration in autosomal dominant Stargardt’s
disease (STGD3), with loss of central vision, progressive degeneration of the macula and peripheral retina18,19,20, 22,23,24,25,26,27,28, and early functional defects in RPE cells and
PRCs29. Also, recent studies have linked spinocerebellar ataxia to ELOVL4 mutations30,31,32. Moreover, recessive mutations in ELOVL4 result in impaired neural development, neuronal
dysfunction, hyper-excitability and seizures28, 33, and neuroichthyotic disorders34. In addition, ELOVL4 is necessary in the skin-permeability barrier and neonatal survival23. One of the
proposed mechanisms for PRC degeneration is that mutations in ELOVL4 that cause dominant Stargardt’s disease are due to the loss of its C-terminal endoplasmic reticulum (ER) retention
signal, leading to protein mislocalization and aggregation18, 19, 28, 35,36,37. Thus, mislocalization of the truncated ELOVL4 protein causes cellular stress that leads to PRC death.
Alternatively, mislocalization of an enzymatically-active truncated ELOVL4 protein from the ER leads to accumulation of toxic products (_i.e_., 3-keto intermediates) because the truncated
protein still contains the putative active site. Production and accumulation of these toxic keto intermediates by the truncated ELOVL4 could be an additive insult to the overall reduction in
the ELOVL4-derived products (_i.e_., VLC-PUFAs). Furthermore, ELOVL4 knockout (KO) mice have VLC-PUFA-deficient PRC terminals with reduced rod terminal vesicles and a disorganized outer
plexiform layer38, 39. The ELOVL4 protein is targeted via its C-terminal di-lysine motif KXKXX to the ER for elongation by a four-step cyclical process of condensation, reduction,
dehydration and reduction, yielding a fatty acid elongated by two carbons. The initial condensation reaction and rate-limiting step is catalyzed by an elongase and mediated by
iron-coordinating histidines in the active site, which condenses malonyl CoA (the two-carbon donor) and a fatty acyl-CoA to yield a 3-keto-acyl-CoA intermediate. The 3-keto compound is then
reduced to the 3-hydroxy product, dehydrated to a trans-2,3-enoyl fatty acyl-CoA, which is further reduced to form the final product, a fatty acid that is two carbons longer than the
precursor. The initial and final reduction steps are catalyzed by 3-keto-acyl-CoA reductase (KAR), trans-2,3-enoyl-CoA reductase (TER) enzymes, respectively, both of which require NADPH as a
cofactor. The dehydration step is carried out by one of four different 3-hydroxyacyl-CoA dehydratases (HACD1, HACD2, HACD3, or HACD4), and the chain length of the final product is
determined by the particular elongase that catalyzes the reaction. After VLC-PUFAs are generated via ELOVL4, they are incorporated into phospholipids in the PRC inner segment, where they
become part of the PRC outer membrane biogenesis20 and tightly interact with rhodopsin40. Additionally, VLC-PUFAs are assumed to be important in the overall functions of PRC, including
longevity, synaptic function, and neuronal connectivity. However, the molecular mechanisms by which VLC-PUFAs exert these important functions and its protective role remain unknown. Herein,
we have explored an alternative mechanistic rationale for the significance of ELOVL4 in PRC survival. The genetic ablation of adiponectin receptor 1 (AdipoR1) leads to the depletion of the
phosphatidylcholine molecular species (PCMS) that contain 32:6n3, 34:6n3, and DHA (22:6n3), which in turn leads to photoreceptor degeneration that resembles various forms of human retinal
degenerative diseases41. Thus, a shortage in the protective bioactive mediators derived from VLC-PUFAs may be a fundamental factor in the onset and early progression of these diseases.
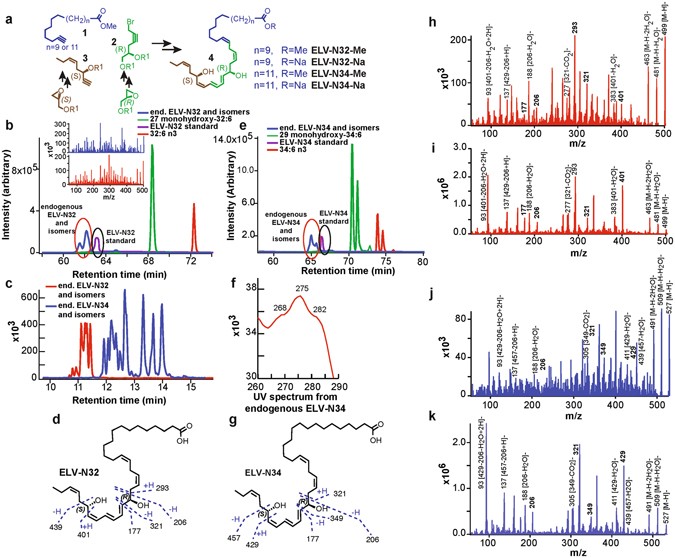
ELV-N32 AND ELV-N34 FORMATION, STRUCTURE AND STEREOCHEMISTRY IN PRIMARY HUMAN RPE CELLS The complete structures and stereochemistry of the novel 32- and 34-carbon elovanoids ELV-N32 and
ELV-N34 were established through a direct comparison with compounds prepared via stereo-controlled total organic synthesis by adapting our previously reported methodologies for the total
synthesis of the DHA-derived lipid mediator, neuroprotectin D1 (NPD1; 10_R_,17_S_-dihydroxydocosa- (4_Z_,7_Z_,11_E_,13_E_,15_Z_,19_Z_)-hexaenoic acid)42, 43. Further validation of these
structural assignments was established by synthesizing deuterium-labelled derivatives (ELV-N32-d2 and ELV-N34-d2) for liquid chromatography tandem mass spectrometry (LC-MS/MS) analysis.
ELV-N32 and ELV-N34 were prepared by stereo-controlled total chemical synthesis (Fig. 1a). The availability of synthetic materials with fully-defined structures and stereochemistry allowed
us to determine the complete R/S configuration as well as the Z/E geometry of the double bonds in these human primary RPE cell-derived elovanoids (ELVs). Confocal images of immunostained
primary human RPE cells (using the specific markers ZO-1 (Zona occludens-1), RPE65 (retinal pigment epithelim-specific 65 kDa protein), MITF (Micro-ophtalmia-associated Transcription Factor)
and β-catenin) are depicted in Fig. 2, as well as light microscopy morphology at different passages in culture. In brief, these cells were cultured for 24 to 48 hours followed by a 24-hour
incubation with 10 μM free 32:6n6 plus 34:6n6. Then cells were incubated with 1 mM H2O2 for 24 hours after a 24-hour serum deprivation. The incubation media were collected, and lipids were
extracted and loaded onto a liquid chromatography tandem mass spectrometer for analysis. We also generated synthetic stereochemically-pure deuterium-labeled ELVs, and by matching them with
endogenously-produced molecules by LC-MS/MS, we further confirmed their structure and stereochemistry. Following matching with human primary RPE cell culture media-derived elovanoids, the
complete structures of ELV-N32 (from a 32 carbon omega-3 polyunsaturated fatty acid) and ELV-N34 (from a 34 carbon omega-3 polyunsaturated fatty acid) were confirmed to be as follows:
ELV-N32: (14_Z_,17_Z_,20_R_,21_E_,23_E_,25_Z_,27_S_,29_Z_)-20,27-dihydroxydo-triaconta-14,17,21,23, 25,29-hexaenoic acid; ELV-N34:
(16_Z_,19_Z_,22_R_,23_E_,25_E_,27_Z_,29_S_,31_Z_)-22,29-dihydroxytetra-triaconta-16,19,23, 25,27,31-hexaenoic acid. Both of these elovanoids and their precursor VLC-PUFAs were detected in
RPE cells under uncompensated oxidative stress (UOS) (Fig. 1b–k). We used m/z 499 → 93 and 499 → 401 MRM transitions for ELV-N32, and m/z 527 → 93 and 527 → 429 transitions for ELV-N34 for
detection. For the corresponding precursors, we used m/z 483 → 385 for 27-hydroxy-32:6n3, and m/z 511 → 413 for 29–hydroxyl-34:6n3. For further identification, we performed full
fragmentation on ELVs and found good matches to the standards. In summary, here we show the identification and structural characterization of a novel class of oxygenated lipid mediators
derived from 32:6n3 or 34:6n3 in primary human RPE cells; we have named these mediators “elovanoids.” The structure and stereochemistry of the novel elovanoids ELV-N32 and ELV-N34, having
structures reminiscent of NPD1, were established using synthetic materials produced by stereo-controlled chemical synthesis. We characterized these ELVs in the incubation media from primary
human RPE cells exposed to UOS, including deuterium-labeled ELVs for matching experiments. Elovanoids from longer fatty acid chains also are likely to occur. 32:6N3 OR 34:6N3 ELICITS POTENT
CYTOPROTECTION 32:6n3 or 34:6n3 are precursors of ELVs, and in fact they are converted into the novel ELVs under our experimental conditions (Fig. 1). Therefore, we asked whether free 32:6n3
or 34:6n3 elicit protection against UOS in RPE cells. To test the efficacy of 32:6n3 and 34:6n3 VLC-PUFAs in modulating human RPE cell homeostasis and survival rates, we incubated human
ARPE-19 cells undergoing UOS with both 32:6n3 or 34:6n3 (3 μM each) for 16 hours. The addition of H2O2 (800 μM) plus tumor necrosis factor alpha (TNFα) (10 ng/ml) induced apoptosis (50% cell
death). Both 32:6n3 and 34:6n3 successfully prevented cell death in a concentration-dependent fashion (Fig. 3a). A similar protective effect was observed in primary human RPE cells (Fig.
4). Oxidative stress stimulation initiates the enzymatic oxygenation of DHA through the activation of 15-lipoxygenase-1 (15-LOX-1)44, leading to the biosynthesis of NPD112. NPD1 is a
stress-response lipid mediator derived from DHA5, 11, 12, and it enhances survival signaling in RPE cells confronted with oxidative stress by promoting modulation of the activity and content
of proteins directly involved in deciding cell fate9, 10, 44,45,46,47. Primary human RPE cells, formerly serum-deprived for 12 hours, were incubated with the 15-LOX-1 inhibitor (PD146176)
(10 μM for 1 hour), then bathed with 600 μM H2O2/TNFα in conjunction with a mixture of 32:6n3 plus 34:6n3 (3 μM each) for 16 hours (Fig. 5). The 15-LOX-1 inhibitor sensitizes cells;
therefore, a lower concentration of H2O2 than in the cytoprotection experiment was used. As mentioned above, adding H2O2 and TNFα induced RPE cell apoptosis, but treatment with a mixture of
32:6n3 and 34:6n3 successfully prevented cell death (Fig. 5), indicating that 15-LOX-1 is not involved in this free fatty acid cell protection mechanism using primary human RPE cells; this
issue remains to be defined in the future. Thus, newly-identified ELVs are different from other endogenous cytoprotective mediators because, among other reasons, they involve a phospholipid
molecular species endowed with acyl chains that are precursors of neuroprotective lipids. DHA, the precursor of the bioactive “docosanoids,” is anchored at position C2 of the glycerol
backbone, while 32:6n3 or 34:6n3 are located at position C1 and serve as the reservoir of the precursors of the novel ELVs described herein. 32:6N3 AND 34:6N3 ENHANCE ANTI-APOPTOTIC AND
PRO-SURVIVAL PROTEIN EXPRESSION We observed that 32:6n3 and 34:6n3 upregulated the expression of pro-survival Bcl-2 and Bcl-xL (Fig. 3e,f; Supplementary Fig. 1) and down regulated the
pro-apoptotic proteins Bax, Bim, and Bid (Fig. 3g–i; Supplementary Fig. 2). Moreover, the pro-homeostatic effects of 32:6n3 and 34:6n3 was concentration-dependent (Fig. 3j; Supplementary
Fig. 2), and sirtuin-1 (SIRT1) and Iduna abundance were augmented (Fig. 3c,d; Supplementary Fig. 1). So since 32:6n3 or 34:6n3 are the precursors of ELV-N32 and ELV-N34, respectively, under
the present experimental conditions we interpreted this to mean that these results are mediated by ELVs (Fig. 1). Moreover, in Fig. 6 (Supplementary Figs 3 and 4), we tested similar targets
using ELVs and found similar results. Since sirtuins have been shown to be involved in retinal disease48 and play a role in aging49, 50, mitochondrial function51, and overall homeostasis52,
this protein is important in eliciting the biological activities of the novel lipid mediators. The other protein targeted is Iduna (ring finger protein 146 (RNF146). Iduna is a
PARsylation-directed ring finger E3 ubiquitin ligase engaged in protein quality control and DNA repair, and it facilitates protection against parthanatos53, 54, which is a form of cell death
dependent on poly(ADP-ribose) polymerase-1 (PARP-1)53, 55,56,57. PARPs catalyze the transfer of ADP-ribose from nicotinamide adenine dinucleotide (NAD) to target proteins and are
indispensable for genomic integrity, the cell cycle, and gene expression58. Recently, it was found that NPD1 augments the abundance of Iduna in RPE cells when confronted with UOS59. ELVS
UPREGULATE PRO-HOMEOSTATIC AND ANTI-APOPTOTIC PROTEIN ABUNDANCE WITH ATTENUATION OF APOPTOSIS IN RPE VLC-PUFAs play a vital role in PRC structure and function, which are compromised when DHA
levels are reduced due to genetic ablation of AdipoR141. A consequence of this is that retinal degeneration ensues. To counter neurodegeneration, cells activate neuroprotective pathways
that sustain a balance between pro- and anti-apoptotic signaling. The genetic and cellular mechanisms that govern the expression of these pro-survival mechanisms, in addition to the
mediators that signal to proteins that carry protective actions, are not known. Therefore, we next explored whether ELVs enhance the expression of pro-survival and pro-homeostatic proteins
in RPE cells undergoing UOS. Figure 6 shows that either the sodium salt or the methyl ester form of ELVs potently regulate the abundance of several key proteins. ELV-N32-Na and ELV-N34-Na
upregulated SIRT1 abundance in UOS RPE cells in a dose-dependent manner (100–200 nM) (Fig. 6a; Supplementary Fig. 3), and they also enhanced Iduna expression in RPE cells under UOS at
concentrations of 200 nM (Fig. 6b; Supplementary Fig. 3). ELV-N32-Na or ELV-N34-Na also enhanced the abundance of the anti-apoptotic proteins Bcl-2 and Bcl-xL (Fig. 6c; Supplementary Fig.
3). On the other hand, pro-apoptotic Bax (Fig. 6d; Supplementary Fig. 4), Bid (Fig. 6e; Supplementary Fig. 4), and Bim (Fig. 6f; Supplementary Fig. 4) were decreased by ELV-N32 or ELV-N34
(with the sodium salts or methyl esters). It is interesting to note that while Bcl-2 and Bcl-xL were upregulated (Fig. 6c; Supplementary Fig. 3), Bax, Bim, and Bid were downregulated by
either the sodium salts or methyl esters (Fig. 6d–f; Supplementary Fig. 4). Prohibitin (type-1), a cell-survival protein47, 60,61,62, was upregulated by both ELV-N32 and ELV-N34 (sodium
salts and methyl ester forms) in a concentration-dependent manner (100–200 nM) in RPE cells undergoing UOS (Fig. 6g; Supplementary Fig. 4). We have shown here that ELV-N32 or ELV-N34 (sodium
salts or methyl esters) upregulate SIRT1 and Iduna proteins, while inhibition of apoptosis takes place in RPE under UOS. These observations suggest that ELVs may be playing an important
role in cell signaling5, 13. Moreover, the 32:6n3 and 34:6n3 cytoprotective response was not affected by the presence of the 15-LOX-1 inhibitor in the primary human RPE cells. ELV
upregulation of the expression of Bcl-2 and Bcl-xL, and ELV downregulation of Bax, Bim and Bid in RPE cells undergoing UOS, indicates that ELVs are involved in modulating cell apoptotic
pathways18, 23. Moreover, Fig. 6h shows that ELV-N32-Na and ELV-N34-Me attenuate apoptosis in RPE cells in a concentration-dependent fashion (50–500 nM). The highest inhibition was at 500 nM
(for both the sodium salt and methyl ester forms) and the lowest was at 50 nM (Fig. 6h). Furthermore, the increased SIRT1 abundance induced by 32:6n3 and 34:6n3 (Fig. 3c; Supplementary Fig.
1) and by ELVs (Fig. 6a; Supplementary Fig. 3) highlights an additional target of these novel mediators on pro-homeostatic bioactivity. The observation that ELVs upregulated prohibitin
(type-1) in these RPE cells undergoing UOS is of interest in the biology of senescence and cell survival. Prohibitins are ubiquitous, evolutionarily-conserved proteins that form a ring-like,
high-molecular-mass complex at the inner membrane of mitochondria and other cellular compartments60,61,62,63. In addition, they also are involved in energy metabolism, proliferation,
apoptosis, and senescence30. Prohibitin regulates signaling of membrane transport, control of transcription activation, and the cell cycle, while the mitochondrial prohibitin complex
stabilizes the mitochondrial genome and modulates mitochondrial dynamics, morphology, biogenesis, and the intrinsic apoptotic pathway63. Therefore, we suggest that by manipulating the
intracellular abundance of prohibitin (type-1), ELVs could provide a possible way to control aging and other pro-homeostatic functions in mammalian cells. ADIPOR1 REGULATES DHA UPTAKE AND
ELV FORMATION RPE cells sustain PRC functional integrity, and their demise is involved in the onset of several forms of retinal degenerations (Fig. 7d). One of the functions of the RPE cell
is to retrieve DHA during PRC renewal and return it through the interphotoreceptor matrix to the PRC inner segment for new outer segment disc membrane biogenesis64. Recently, adiponectin
receptor 1 (AdipoR1) was found to be necessary for DHA availability to photoreceptor cells41, and a single amino acid mutation in this receptor is causative of autosomal dominant retinitis
pigmentosa65. Genetic ablation of this receptor leads to PRC degeneration and to shutting off VLC-PUFA,n-3 synthesis in the retina. Here we show in Fig. 7 that the pool size of free 32:6n3
and of 34:6n3 in retinas of AdipoR1 knockout (KO) mice (red) is drastically decreased as compared with that in WT (blue). Moreover, ELV-N32 and ELV-N34 in KO (red) were undetectable.
Mono-hydroxy 32:6n3 and 34:6n3, the stable derivatives of the hydroperoxy precursors of ELV-N32 and of ELV-N34 respectively, lack a detectable signal in the KO (red), unlike the wild type
(blue) (Fig. 7b,c). ELV-N32 and ELV-N34 were found to be secreted from RPE cells when confronted with UOS, suggesting paracrine or autocrine bioactivity. We also show that these ELVs target
and enhance the expression of pro-survival and pro-homeostatic proteins in the RPE cells undergoing UOS. ELVS PROTECT RPE CELLS, WHICH SUSTAIN PRC INTEGRITY PUFA elongation in the inner
segment of photoreceptors by ELOVL4 leads to the biosynthesis of VLC-PUFAs,n-3 and their insertion at the C1 position of phosphatidylcholine within PRC disk membranes. However, under
conditions of stress, these VLC-PUFAs are cleaved by phospholipase A1 (PLA1) for the synthesis of mono- and di-hydroxy VLC-PUFAs (ELVs) (Fig. 7a). Light-induced oxidative stress in mouse
retinas triggers the production of free 32:6n3 and 34:6n3, as well as their mono- and di-hydroxy derivatives (Fig. 7a). In AdipoR1 KO mice, no detectable amounts of these molecules were
found (Fig. 7b,c, red curves). Therefore, the lack of the VLC-PUFA,n-3 precursor DHA results in retinal degeneration (Fig. 7d)41, preceded by a remarkable downregulation of the free
VLC-PUFA,n-3 molecular species and ELV biosynthesis. These observations support our present hypothesis that VLC-PUFA,n-3 are precursors of novel bioactive mediators that elicit
pro-homeostatic protective bioactivity. CONCLUDING REMARKS Here we report the discovery of elovanoids (ELVs), the first bioactive lipid mediators derived from VLC-PUFA,n-3, which are the
biosynthetic products of elongase ELOVL4. We established the structure and stereochemistry of ELVs with 32 and 34 carbons (ELV-N32, ELV-N34) using synthetic materials obtained by
stereocontrolled total synthesis. ELV availability is abolished in the retinas of mice with genetically-ablated AdipoR1 (Fig. 7). Dietary DHA (or derived from dietary 18:3n3) is supplied to
tissues by the liver and captured by AdipoR1, followed by elongation in the inner segment of photoreceptor cells by ELOVL4 to VLC-PUFA,n-3 and incorporation into phosphatidylcholine
molecular species, which are also endowed with DHA. ELOVL4 uses EPA as a preferred substrate16, in spite of the fact that the abundance of EPA is very low in the retina compared to DHA.
Retroconversion of DHA to EPA in peroxisomes has been demonstrated, and EPA generated by this reaction might generate the 26 carbon PUFA that is the substrate for ELOVL416. During daily PRC
outer segment renewal, these phosphatidylcholine molecular species interact with rhodopsin40 and, after shedding and phagocytosis, become part of the RPE cells. UOS or other disruptors of
homeostasis triggers the release of VLC-PUFAs. Figure 7a shows that 32:6n3 and 34:6n3 generate a hydroperoxy molecule and then an ELV-N32 or ELV-N34, respectively. ELVs are biosynthesized in
human RPE cells and have protective functions in RPE cells undergoing UOS (Figs 4, 5 and 7). The bioactivity targets of ELVs in RPE cells reveal strong pro-homeostatic functions. The
discovery of ELVs points to previously unknown pathways for preserving PRC integrity. ELV formation likely involves an alternative activation pathway since VLC-PUFA,n-3 are incorporated at
position C1 of phospholipids, while DHA is located at position C2. ELV biosynthesis, in combination with alternative regulation by PRC-specific phospholipases A1 and A2, point to a novel
neuroprotective mechanism in the retina. We did not detect VLC-PUFAs n-3 in sphingomyelin or in other phospholipids, such as phosphatidylethanolamine and phosphatidylserine. Our data show
that the availability of free VLC-PUFA n-3 plus UOS leads to the activation of pro-homeostatic events and RPE cytoprotection. We characterized the synthesis of the novel lipids
biosynthesized under these conditions, including the S-precursor and the stable analogs of hydroxyl-derivatives66. Many new questions emerge from our observations: Are phospholipases A1 and
A2 modulated in a coordinated fashion? Are neurotrophins a modulator of the pathways that these enzymes create, as in the case of NPD1 synthesis47, 67? If so, how are neurotrophins
instructed to cleave C1 or C2? Are there synergies between NPD1 (or other docosanoids) and elovanoids? The novel ELV bioactive lipids disclosed here (ELV-N32 and ELV-N34) involve the prior
release of either 32:6n3 or 34:6n3 from the C1 position of the phosphatidyl choline. Since this phospholipid molecular species also has DHA in the C2 position, we suggest that NPD1 also can
be made from the same precursor. Therefore, we reveal here a different signal bifurcation mechanism that aims to sustain PRC and RPE cell integrity. This is supported by our observation that
genetic ablation of AdipoR1, which results in depletion of molecular species of PRC that contains 32:6n3 or 34:6n3 and DHA in the mouse, leads to photoreceptor degeneration resembling
various human forms of retinal degenerative diseases41. We anticipate that other ELVs also might be made to regulate cell function in other cells. Mutant ELOVL4 causes juvenile macular
degeneration and other neurological conditions. Among the proposed mechanisms for photoreceptor cell degeneration caused by mutant ELOVL4 is the loss of its C-terminal ER retention signal,
leading to protein mislocalization of the truncated ELOVL4 protein that, in turn, causes cellular stress that leads to photoreceptor cell death. The data presented here suggest an
alternative mechanism for the deleterious effects of mutant ELOVL4, which would limit the occurrence of VLC-PUFA,n-3 in the C1 position of phosphatidylcholines and sphingolipids. Thus,
VLC-PUFAs,n-3 are converted to the corresponding ELVs, which are protective in cell survival under UOS conditions. The RPE and retina, under continuous stress, might need ELVs to sustain the
functional integrity of RPE cells and the overall function of photoreceptor cells: vision. The bioactivities of ELV-N32 and ELV-N34 include some unusual and unique features. In addition to
their potent neuroprotective actions, these lipid mediators: (a) are cell selective; (b) involve a relationship between PRC and RPE cells that is necessary for vision; (c) are derived from
VLC-PUFA,n-3, the biosynthesis of which is regulated by a PRC-specific enzyme, ELOVL4; and (d) have precursor fatty acids (VLC-PUFA,n-3) that are positioned as acyl chains at position C1 of
the phosphatidylcholine, unlike DHA (the precursor of NPD1), which is incorporated at position C2. Since they are derived from an alternative fatty acid precursor regulated by ELOVL4 and
stored at an alternative phospholipid position, the ELVs are likely to involve an alternative activation pathway for exerting their neuroprotective bioactivity in the retina. Another
significant question raised by our novel findings is as follows: Which signaling mechanism targets the novel phosphatidylcholine molecular species that, after shedding and phagocytosis,
appears in RPE cells? The phosphatidylcholine molecular species in the RPE cell stores precursors of two lipid mediators, DHA in the C2 position, and VLC-PUFAs 32:6n3 or 34:6n3 (the
precursors of ELV-N32 and ELV-N34) in the C1 position. The phosphatidylcholine molecular species is targeted for release of the acyl chains at C1 and C2 when confronted with UOS as in the
onset of retinal degenerations. The new ELVs reported here provide a novel autocrine/paracrine pro-homeostatic RPE signaling that aims to sustain PRC and RPE cell integrity, thus revealing
the potential for developing novel therapeutic approaches for retinal degenerations. METHODS EXPERIMENTAL APPROVAL All animal experiments conducted were approved by the Institutional Animal
Care and Use Committee of Louisiana State University Health New Orleans (LSUHNO), and all experiments involving primary human retinal pigment epithelia (RPE) cells were approved by the
Institutional Review Board of LSUHNO; all experiments were conducted in accordance with National Institutes of Health guidelines. Cells were collected from anonymous human donors provided by
eye banks, thus the identity of the donors was unknown. ANTIBODIES The following antibodies were used: β-catenin (catalog# sc-7963, lot# K0812) Santa Cruz Biotechnology: (concentration used
1:50); ZO-1 (catalog# 187430, lot# 1633993 A) Life Technologies: (concentration used 1:100); MITF (catalog# ab59232, lot# GR52475-3) ABCAM: (concentration used 1:250); RPE65 (catalog#
ab78036, lot 3GR254004-1), ABCAM: (concentration used 1:250). HUMAN RPE CELL CULTURES Globes of a 19-year-old Caucasian male without eye pathology were obtained from NDRI within 24 hours
after death (head trauma). Globes were opened, and then RPE cells were harvested and cultured68, 69. Cells from passage 4 were placed in medium containing 10% DMSO and frozen in liquid N2.
When needed, cells were unfrozen, placed in T75 flasks, and used after passage 8. Cells were cultured in T75 flasks in MEM medium containing 10% FBS, 5% NCS, MEM-NEAA (ThermoFisher
Scientific, Waltham, MA), 1x Penicillin/Streptomycin and 10 ng/ml FGF at 37 °C 5% CO2, 99% relative humidityfor 24–48 hours followed by a 24-hour incubation with 10 μM free 32:6n3 and 34:6n3
fatty acid mixture. Figure 2 depicts immunostaining of primary human RPE cells using specific markers ZO-1 (Zona occludens-1), RPE65, MITF (Micro-ophtalmia-associated Transcription Factor)
and β-catenin, as well as light microscopy depicting primary human RPE cell morphology at different passages in culture. ARPE-19 cells were grown and maintained in T75 flasks in DMEM F12
medium containing 10% FBS and incubated at 37 °C with a constant supply of 5% CO2. Cells at 75–80% confluence (72 hours growth in DMEM/F12 + 10% FBS) in 6-well plates were serum-starved for
8 hours before exposure. EXPOSURE OF RPE CELLS TO UOS AND 32:6N3, 34:6N3 OR ELVS ARPE-19 cells at 75–80% confluence (72 hours growth in DMEM/F12 + 10% FBS) in 6-well plates were
serum-starved for 8 hours. Then cells were treated with TNFα (Sigma–Aldrich, St. Louis, MO) (10 ng/ml) and H2O2 (600 μM) to induce uncompensated oxidative stress either for 6 hours (for
Western-blot analysis of selected proteins) or 16 hours (for apoptosis assessment) while the cells were treated with increasing concentrations (50–500 nM) of 32:6n3 and 34:6n3. These fatty
acids were applied as follows: the stocks of the free fatty acid forms of 32:6n3, 34:6n3 or ELVs (sodium salt or methyl ester) were dried under N2 and resuspended in ethanol. No precipitates
were formed. In order to add them to the cell cultures, fatty acids or ELVs were dissolved in medium containing 0.5% serum and incubated with cells. All control samples received appropriate
amounts of ethanol; no cell toxicity was observed. For primary human RPE cell viability assay experiments, cells were treated with 32:6n3 plus 34:6n3 (3 μM each) or separately for the
entire duration of the experiment (Figs 5 and 6). For the inhibition studies, the 15-LOX-1 inhibitor (PD146176) (10 μm) was added to the cells 1 hour before oxidative stress induction and
kept throughout the incubation period. ANALYSIS OF PROTEINS Bcl-2 family proteins, SIRT1 and Prohibitin (type-1), and Iduna proteins were analyze by Western blot analysis. In brief, 20–25 μg
equivalents of each cell extracts were subjected to electrophoresis on a 4–12% gels (Promega) at 125 V for 2 hours. The proteins were transferred to a nitrocellulose membrane by an I-blot
transfer apparatus. The membranes were subjected to treatment with primary antibodies of Bcl-2, Bcl-xL, Bax, Bid, Bim, SIRT1 and prohibitin (type-1) (Santa Cruz Biotechnology) and Iduna
(Neuro-Mab Lab, UCLA, Los Angeles, CA) overnight at 4 °C and probed for 45 minutes with secondary antibody, goat anti-mouse Ig:horseradish peroxidase, and horseradish peroxidase-conjugated
anti-biotin antibody, and then proteins were evaluated by using an ECL kit (Amersham). IMMUNOCYTOCHEMISTRY AND CELL APOPTOSIS ASSESSMENT Immunocytochemistry assays were performed in 8-well
slide chambers. Briefly, cells were fixed in 4% paraformaldehyde (PFA) for 20 minutes, permeabilized with Triton X-100 0.1% in PBS, and non-specific epitopes were blocked in 10% bovine serum
albumin (BSA) in 1 × PBS for 1 hour at room temperature. Immunostaining was accomplished by incubating primary antibodies overnight at 4 °C. Samples were incubated for 2 hours at room
temperature with Alexa Fluor 555 conjugated secondary antibodies diluted at 1 in 250 (MeridianLife Science Inc., Memphis, TN), and nuclei were stained with Hoechst (2 μM Hoechst33258).
Pictures were taken with a Zeiss LSM 510 confocal microscope and a Zeiss Axioplan-2 deconvolution microscope. To assess cell death, primary human RPE cells and ARPE-19 cells were fixed with
methanol for 15 minutes, washed with 1 × PBS, then loaded with 2 μM Hoechst dissolved in a Locke’s solution (Promega) and incubated for another 15 minutes before imaging. Cells were then
viewed by using a Zeiss LSM 510 confocal microscope under UV fluorescence. Images were recorded, and cell apoptosis was assessed by using an automated unbiased method70. LC-MS/MS OF ELV-N32
AND ELV-N34 IN RPE CELLS Human RPE cells (at passage 19) were cultured in T75 flasks for 24–48 hours followed by a 24-hour incubation with 10 μM free 32:6n3 and 34:6n3 fatty acid mixture.
Cells were incubated with 1 mM H2O2 for 24 hours promptly after a 24-hour serum deprivation. Fatty acids were extracted using a liquid-liquid lipid extraction method from the collected cell
culture medium. Extracts were loaded onto a liquid chromatography tandem mass spectrometer for analysis. We analyzed fatty acids, monohydroxy fatty acid derivatives (27-hydroxy-fatty acid
32:6n3 and 29–hydroxyl-fatty acid 34:6n3), ELV-N32 (20,27-dihydroxy-fatty acid 32:6n3), and ELV-N34 (22,29-dihydroxy-fatty acid 34:6n3). ELV-N32 and ELV-N34 and their deuterium-labeled
derivatives, ELV-N32-d2 and ELV-N34-d2, were prepared by stereo-controlled chemical synthesis and used for matching with cell-generated derivatives. PHOTO-OXIDATIVE STRESS C57BL/6 wild type
and AdipoR1 knockout mice were housed in a temperature-controlled room at 21–23 °C with a 12-hour:12-hour light-dark cycle. For light-induced oxidative stress, mice were exposed for 1 hour
to bright light (using an 8-light array of 10-inch circular fluorescent 22 W bulbs; Cool White, FTC8T9/CW; General Electric, Fairfield, CT; 18 klux; 270 µE m-2 s). After light exposure,
animals were sacrificed by cervical dislocation, and eyes were enucleated. The cornea, iris and lens were discarded and the retina was separated from the rest of the eyecup. These tissues
were then flash-frozen. Retinas from animals of the same genotype were pooled together. Samples were processed for lipid extraction and LC-MS/MS-based lipidomic analysis. REFERENCES * Korn,
T. & Kallies, A. T cell responses in the central nervous system. _Nat. Rev. Immunol_. doi:10.1038/nri.2016.144. (2017). * Becher, B., Spath, S. & Goverman, J. Cytokine networks in
neuroinflammation. _Nat. Rev. Immunol._ 17, 49–59 (2017). Article CAS PubMed Google Scholar * Serhan, C. N. Treating inflammation and infection in the 21st century: new hints from
decoding resolution mediators and mechanisms. _FASEB J_., doi:10.1096/fj.201601222R (2017). * Serhan, C. N., Dalli, J., Colas, R. A., Winkler, J. W. & Chiang, N. Protectins and maresins:
New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. _Biochim. Biophys. Acta._ 1851, 397–413 (2015). Article CAS PubMed Google Scholar *
Bazan, N. G. Homeostatic regulation of photoreceptor cell integrity: significance of the potent mediator neuroprotectin D1 biosynthesized from docosahexaenoic acid: the Proctor Lecture.
_Invest. Ophthalmol. Vis. Sci._ 48, 4866–4881 (2007). Article PubMed Google Scholar * Bazan, N. G. Neuroprotectin D1-mediated anti-inflammatory and survival signaling in stroke, retinal
degenerations, and Alzheimer’s disease. _J. Lipid Res._ 50(Suppl), S400–S405 (2009). Article PubMed PubMed Central Google Scholar * Serhan, C. N. & Petasis, N. A. Resolvins and
protectins in inflammation resolution. _Chem. Rev._ 111, 5922–5943 (2011). Article CAS PubMed PubMed Central Google Scholar * Wang, H. & Hartnett, M. E. Regulation of signaling
events involved in the pathophysiology of neovascular AMD. _Mol. Vis._ 22, 189–202 (2016). CAS PubMed PubMed Central Google Scholar * Bazan, N. G. Cell survival matters: docosahexaenoic
acid signaling, neuroprotection and photoreceptors. _Trends Neurosci._ 29, 263–271 (2006). Article CAS PubMed Google Scholar * Bazan, N. G., Calandria, J. M. & Serhan, C. N. Rescue
and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. _J. Lipid Res._ 51, 2018–2031 (2010). Article CAS PubMed PubMed Central Google
Scholar * Gordon, W. C. & Bazan, N. G. Docosahexaenoic acid utilization during rod photoreceptor cell renewal. _J. Neurosci._ 10, 2190–2202 (1990). CAS PubMed Google Scholar *
Mukherjee, P. K., Marcheselli, V. L., Serhan, C. N. & Bazan, N. G. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from
oxidative stress. _Proc. Natl. Acad. Sci. USA_ 101, 8491–8496 (2004). Article ADS CAS PubMed PubMed Central Google Scholar * Bazan, N. G. Cellular and molecular events mediated by
docosahexaenoic acid-derived neuroprotectin D1 signaling in photoreceptor cell survival and brain protection. _Prostaglandins Leukot. Essent. Fatty Acids._ 81, 205–211 (2009). Article CAS
PubMed PubMed Central Google Scholar * Hollyfield, J. G. _et al_. Oxidative damage-induced inflammation initiates age-related macular degeneration. _Nat. Med_ 14, 194–198 (2008). Article
CAS PubMed PubMed Central Google Scholar * Lagali, P. S. _et al_. Evolutionarily conserved ELOVL4 gene expression in the vertebrate retina. _Invest. Ophthalmol. Vis. Sci._ 44, 2841–50
(2003). Article PubMed Google Scholar * Yu, M. _et al_. ELOVL4 protein preferentially elongates 20:5n3 to very long chain PUFAs over 20:4n6 and 22:6n3. _J. Lipid Res._ 53, 494–504 (2012).
Article CAS PubMed PubMed Central Google Scholar * Suh, M. & Clandinin, M. T. 20:5n-3 but not 22:6n-3 is a preferred substrate for synthesis of n-3 very-long-chain fatty acids
(C24-C36) in retina. _Curr. Eye Res._ 30, 959–968 (2005). Article CAS PubMed Google Scholar * Agabaga, M. P. _et al_. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the
biosynthesis of very long chain fatty acids. _Proc. Natl. Acad. Sci. USA_ 105, 12843–12848 (2008). Article ADS Google Scholar * Agabaga, M. P. _et al_. Retinal very long-chain PUFAs: new
insights from studies on ELOVL4 protein. _J. Lipid Res._ 51, 1624–1642 (2010). Article Google Scholar * Aveldano, M. I. A novel group of very long chain polyenoic fatty acids in
dipolyunsaturated phosphatidylcholines from vertebrate retina. _J. Biol. Chem._ 262, 1172–1179 (1987). CAS PubMed Google Scholar * Oresti, G. M. _et al_. Sequential depletion of rat
testicular lipids with long-chain and very long-chain polyenoic fatty acids after X-ray-induced interruption of spermatogenesis. _J. Lipid Res._ 51, 2600–2610 (2010). Article CAS PubMed
PubMed Central Google Scholar * Monroig, O. _et al_. Expression and role of Elovl4 elongases in biosynthesis of very long-chain fatty acids during zebrafish Danio rerio early development.
_Biochemica et Biophysica Acta_ 1801, 1145–1154 (2010). Article CAS Google Scholar * Cameron, D. J. _et al_. Essential role of Elovl4 in very long chain fatty acid synthesis, skin
permeability barrier function, and neonatal survival. _Int. J. Biol. Sci._ 3, 111–119 (2007). Article CAS PubMed PubMed Central Google Scholar * Zhang, K. _et al_. A 5-bp deletion in
ELOVL4 is associated with two related forms of autosomal dominant macular dystrophy. _Nat. Genet._ 27, 89–93 (2001). CAS PubMed Google Scholar * Edwards, A. O., Donoso, L. A. &
Ritter, R. 3rd A novel gene for autosomal dominant Stargardt-like macular dystrophy with homology to the SUR4 protein family. _Invest. Ophthalmol. Vis. Sci._ 42, 2652–2663 (2001). CAS
PubMed Google Scholar * Bernstein, P. S. _et al_. Diverse macular dystrophy phenotype caused by a novel complex mutation in the ELOVL4 gene. _Invest. Ophthalmol. Vis. Sci._ 42, 3331–3336
(2001). CAS PubMed Google Scholar * Maugeri, A. _et al_. A novel mutation in the ELOVL4 gene causes autosomal dominant Stargardt-like macular dystrophy. _Invest Ophthalmol Vis Sci_ 45,
4263–4267 (2004). Article PubMed Google Scholar * Agbaga, M. P. Different mutations in ELOVL4 affect very long chain fatty acid biosynthesis to cause variable neurological disorders in
humans. _Adv. Exp. Med. Biol._ 854, 129–135 (2016). Article PubMed Google Scholar * Kuny, S., Cho, W. J., Dimopoulos, I. S. & Sauvé, Y. Early onset ultrastructural and functional
defects in RPE and photoreceptors of a Stargardt-like macular dystrophy (STGD3) transgenic mouse model. _Invest. Ophthalmol. Vis. Sci._ 56, 7109–7121 (2015). Article CAS PubMed Google
Scholar * Bourassa, C. V. _et al_. A new ELOVL4 mutation in a case of spinocerebellar ataxia with erythrokeratodermia. _JAMA Neurol._ 72, 942–943 (2015). Article PubMed Google Scholar *
Ozaki, K. _et al_. A novel mutation in ELOVL4 leading to spinocerebellar ataxia (SCA) with the hot cross bun sign but lacking erythrokeratodermia: A broadened spectrum of SCA34. _JAMA
Neurol._ 72, 797–805 (2015). Article PubMed Google Scholar * Cadieux-Dion, M. _et al_. Expanding the clinical phenotype associated with ELOVL4 mutation: study of a large French-Canadian
family with autosomal dominant spinocerebellar ataxia and erythrokeratodermia. _JAMA Neurol_ 71, 470–475 (2014). Article PubMed Google Scholar * Aldahmesh, M. A. _et al_. Recessive
mutations in ELOVL4 cause ichthyosis, intellectual disability, and spastic quadriplegia. _Am. J. Hum. Genet._ 89, 745–750 (2011). Article CAS PubMed PubMed Central Google Scholar * Mir,
H. _et al_. A novel recessive mutation in the gene ELOVL4 causes a neuro-ichthyotic disorder with variable expressivity. _BMC Med. Genet._ 15, 25 (2014). Article PubMed PubMed Central
Google Scholar * Ambasudhan, R. _et al_. Atrophic macular degeneration mutations in ELOVL4 result in the intracellular misrouting of the protein. _Genomics_ 83, 615–625 (2004). Article CAS
PubMed Google Scholar * Karan, G. _et al_. Loss of ER retention and sequestration of the wild-type ELOVL4 by Stargardt disease dominant negative mutants. _Mol. Vis._ 11, 657–664 (2005).
CAS PubMed Google Scholar * Vasireddy, V. _et al_. Stargardt-like macular dystrophy protein ELOVL4 exerts a dominant negative effect by recruiting wild-type protein into aggresomes. _Mol.
Vis._ 11, 665–676 (2005). CAS PubMed Google Scholar * Agbaga, M. P. Mutant ELOVL4 that causes autosomal dominant stargardt-3 macular dystrophy is misrouted to rod outer segment disks.
_Invest. Ophthalmol. Vis. Sci._ 55, 3669–3680 (2014). Article CAS PubMed PubMed Central Google Scholar * Bennett, L. D. _et al_. Examination of VLC-PUFA-deficient photoreceptor
terminals. _Invest. Ophthalmol. Vis. Sci._ 55, 4063–4072 (2014). Article PubMed PubMed Central Google Scholar * Aveldano, M. I. Phospholipid species containing long and very long
polyenoic fatty acids remain with rhodopsin after hexane extraction of photoreceptor membranes. _Biochemistry._ 27, 1229–1239 (1988). Article CAS PubMed Google Scholar * Rice, D. _et
al_. Adiponectin receptor 1 conserves docosahexaenoic acid and promotes photoreceptor cell survival. _Nat. Commun_. 6, 6228 (2015). * Serhan, C. N. _et al_. Anti-inflammatory actions of
neuroprotectin D1/protectin D1 and its natural stereoisomers: Assignments of dihydroxy-containing docosatrienes. _J. Immunol._ 176, 1848–1859 (2006). Article CAS PubMed Google Scholar *
Petasis, N. A. _et al_. Stereocontrolled total synthesis of neuroprotectin D1/protectin D1 and its aspirin-triggered stereoisomer. _Tetrahedron Lett._ 53, 1695–1698 (2012). Article CAS
PubMed PubMed Central Google Scholar * Calandria, J. M. _et al_. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic
acid-derived mediator, neuroprotectin D1. _J. Biol. Chem._ 284, 17877–17882 (2009). Article CAS PubMed PubMed Central Google Scholar * Antony, R., Lukiw, W. J. & Bazan, N. G.
Neuroprotectin D1 induces dephosphorylation of Bcl-xL in a PP2A-dependent manner during oxidative stress and promotes retinal pigment epithelial cell survival. _J. Biol. Chem._ 285,
18301–18308 (2010). Article CAS PubMed PubMed Central Google Scholar * Bazan, N. G. The docosanoid neuroprotectin D1 induces homeostatic regulation of neuroinflammation. _Prostaglandins
Leukot. Essent. Fatty Acids._ 88, 127–129 (2013). Article CAS PubMed Google Scholar * Mukherjee, P. K., Chawla, A., Loayza, M. S. & Bazan, N. G. Docosanoids are multifunctional
regulators of neural cell integrity and fate: significance in aging and disease. _Prostaglandins Leukot. Essent. Fatty Acids._ 77, 233–238 (2007). Article CAS PubMed PubMed Central
Google Scholar * Balaiya, S., Abu-Amero, K. K., Kondkar, A. A. & Chalam, K. V. Sirtuins expression and their role in retinal diseases. _Oxid. Med. Cell Longev_ 2017, 3187594 (2017).
Article PubMed PubMed Central Google Scholar * van de Ven, R. A., Santos, D. & Haigis, M. C. Mitochondrial sirtuins and molecular mechanisms of aging. _Trends Mol. Med_ S1471–4914,
30024–2 (2017). Google Scholar * Grabowska, W., Sikora, E., & Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. _Biogerontology_.
doi:10.1007/s10522-017-9685-9 (2017). * Hershberger, K. A., Martin, A. S. & Hirschey, M. D. Role of NAD+ and mitochondrial sirtuins in cardiac and renal diseases. _Nat. Rev. Nephrol_ 13,
213–225 (2017). Article CAS PubMed Google Scholar * Jokinen, R., Pirnes-Karhu, S., Pietiläinen, K. H. & Pirinen, E. Adipose tissue NAD+ -homeostasis, sirtuins and poly(ADP-ribose)
polymerases - important players in mitochondrial metabolism and metabolic health. _Redox. Biol_ 2, 246–263 (2017). Article Google Scholar * Kang, H. C. _et al_. Iduna is a poly(ADP-ribose)
(PAR)-dependent E3 ubiquitin ligase that regulates DNA damage. _Proc. Natl. Acad. Sci. USA_ 108, 14103–14108 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Andrabi, S.
A. _et al_. Iduna protects the brain from glutamate excitotoxicity and stroke by interfering with poly(ADP-ribose) polymer-induced cell death. _Nat. Med_ 17, 692–699 (2011). Article CAS
PubMed PubMed Central Google Scholar * Zhang, J. _et al_. Augmentation of poly(ADP-ribose) Q5 polymerase-dependent neuronal cell death by acidosis. _J. Cereb. Blood Flow Metab_.
pii:0271678X16658491 (2016). * Lee, Y. _et al_. Parthanatos mediates AIMP2-activated age-dependent dopaminergic neuronal loss. _Nat. Neurosci._ 16, 1392–1400 (2013). Article CAS PubMed
PubMed Central Google Scholar * Andrabi, S. A. _et al_. Poly(ADP-ribose) (PAR) polymer is a death signal. _Proc. Natl. Acad. Sci. USA_ 103, 18308–18313 (2006). Article ADS CAS PubMed
PubMed Central Google Scholar * Krietsch, J. _et al_. Reprogramming cellular events by poly(ADP-ribose)-binding proteins. _Mol. Aspects Med._ 34, 1066–1087 (2013). Article CAS PubMed
Google Scholar * Belayev, L. _et al_. Neuroprotectin D1 upregulates Iduna expression and provides protection in cellular uncompensated oxidative stress and in experimental ischemic stroke.
_Cell Death Differ_, doi:10.1038/cdd.2017.55 (2017). * Li, L. _et al_. Prohibitin 1 gene delivery promotes functional recovery in rats with spinal cord injury. _Neuroscience._ 286, 27–36
(2015). Article CAS PubMed Google Scholar * Sripathi, S. R. _et al_. Prohibitin as the molecular binding switch in the retinal pigment epithelium. _Protein J._ 35, 1–16 (2016). Article
CAS PubMed PubMed Central Google Scholar * Sripathi, S. R. _et al_. Altered cytoskeleton as a mitochondrial decay signature in the retinal pigment epithelium. _Protein J._ 35, 179–192
(2016). Article CAS PubMed PubMed Central Google Scholar * Nijtmans, L. G. _et al_. Prohibitins act as a membrane-bound chaperone for the stabilization of mitochondrial proteins. _EMBO
J_ 19, 2444–2451 (2000). Article CAS PubMed PubMed Central Google Scholar * Mukherjee, P. K. _et al_. Photoreceptor outer segment phagocytosis attenuates oxidative stress-induced
apoptosis with concomitant neuroprotectin D1 synthesis. _Proc. Natl. Acad. Sci. USA_ 104, 13158–13163 (2007). Article ADS CAS PubMed PubMed Central Google Scholar * Zhang, J. _et al_.
A mutation in ADIPOR1 causes nonsyndromic autosomal dominant retinitis pigmentosa. _Hum. Genet._ 135, 1375–1387 (2016). Article CAS PubMed Google Scholar * Bazan, N. G., Molina, M. F.
& Gordon, W. C. Docosahexaenoic acid signalolipidomics in nutrition: significance in aging, neuroinflammation, macular degeneration, Alzheimer’s, and other neurodegenerative diseases.
_Annu. Rev. Nutr._ 31, 321–351 (2011). Article CAS PubMed PubMed Central Google Scholar * Mukherjee, P. K. _et al_. Neurotrophins enhance retinal pigment epithelial cell survival
through neuroprotectin D1 signaling. _Proc. Natl. Acad. Sci. USA_ 104, 13152–13157 (2007). Article ADS CAS PubMed PubMed Central Google Scholar * Ishida, M., Lui, G. M., Yamani, A.,
Sugino, I. K. & Zarbin, M. A. Culture of human retinal pigment epithelial cells from peripheral scleral flap biopsies. _Curr. Eye Res._ 17, 392–402 (1998). Article CAS PubMed Google
Scholar * Calandria, J. M. _et al_. Ataxin-1 poly(Q)-induced proteotoxic stress and apoptosis are attenuated in neural cells by docosahexaenoic acid-derived neuroprotectin D1. _J. Biol.
Chem._ 287, 23726–23739 (2012). Article CAS PubMed PubMed Central Google Scholar * Stark, D. T. & Bazan, N. G. Synaptic and extrasynaptic NMDA receptors differentially modulate
neuronal cyclooxygenase-2 function, lipid peroxidation, and neuroprotection. _J. Neurosci._ 31, 13710–13721 (2011). Article CAS PubMed PubMed Central Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by National Eye Institute grant EY005121 (NGB), National Institute of General Medical Sciences grant GM103340 (NGB), the Eye, Ear, Nose & Throat
Foundation, and in part by an unrestricted departmental grant from Research to Prevent Blindness, Inc., New York, NY. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Neuroscience Center of
Excellence, School of Medicine, Louisiana State University Health New Orleans, New Orleans, LA, USA Bokkyoo Jun, Pranab K. Mukherjee, Aram Asatryan, Marie-Audrey Kautzmann, Jessica Heap,
William C. Gordon, Surjyadipta Bhattacharjee & Nicolas G. Bazan * Department of Chemistry and Loker Hydrocarbon Research Institute, University of Southern California, Los Angeles, CA,
USA Rong Yang & Nicos A. Petasis Authors * Bokkyoo Jun View author publications You can also search for this author inPubMed Google Scholar * Pranab K. Mukherjee View author publications
You can also search for this author inPubMed Google Scholar * Aram Asatryan View author publications You can also search for this author inPubMed Google Scholar * Marie-Audrey Kautzmann
View author publications You can also search for this author inPubMed Google Scholar * Jessica Heap View author publications You can also search for this author inPubMed Google Scholar *
William C. Gordon View author publications You can also search for this author inPubMed Google Scholar * Surjyadipta Bhattacharjee View author publications You can also search for this
author inPubMed Google Scholar * Rong Yang View author publications You can also search for this author inPubMed Google Scholar * Nicos A. Petasis View author publications You can also
search for this author inPubMed Google Scholar * Nicolas G. Bazan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS N.G.B. and N.A.P.
conceived this project and wrote the paper; all authors reviewed and edited the manuscript; J.B.K. performed the lipidomic/MS studies; P.M. performed the ARPE-19 cell culture studies; A.S.
and S.B. performed the experiments with primary RPE cells in culture; R.Y. and N.A.P. performed the stereo-controlled total chemical synthesis; M.-A.K., J.H. and W.C.G. performed the
experiments with wild type and the AdipoR1 KO animals. CORRESPONDING AUTHOR Correspondence to Nicolas G. Bazan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare that they have no
competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which
permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to
the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless
indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or
exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jun, B., Mukherjee, P.K., Asatryan, A. _et al._ Elovanoids are novel cell-specific lipid mediators necessary for neuroprotective
signaling for photoreceptor cell integrity. _Sci Rep_ 7, 5279 (2017). https://doi.org/10.1038/s41598-017-05433-7 Download citation * Received: 02 March 2017 * Accepted: 09 May 2017 *
Published: 13 July 2017 * DOI: https://doi.org/10.1038/s41598-017-05433-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link
Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Fruits masks Latest News in Hindi, Photos, Videos on Fruits masks InextLive Jagraninextlive के साथ रहिए खबरों की दुनिया से जुड़े। यहां पढ़िए Fruits Masks से जुड़ी हिन्दी न्यूज़ Fruits Masks Hindi News औ...
Sharon stone stays true to herselfMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
Science and the Nation | NatureABSTRACT IT is the fate of many symposia to fail as a whole by the very excellence of the parts; relationship and proxim...
The aarp minute: april 29, 2020Memorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
Trump blasts judge for making us ‘look weak’ after travel ban blockedFollowing news a federal judge in Hawaii issued a temporary restraining order, barring the executive order from taking e...
Latests News
Elovanoids are novel cell-specific lipid mediators necessary for neuroprotective signaling for photoreceptor cell integrityABSTRACT Docosahexaenoic acid (DHA, 22:6 n-3) is abundant in the retina and is enzymatically converted into pro-homeosta...
All-round records | Combined Test, ODI and T20I records | Cricinfo Statsguru | ESPNcricinfo.comESPN CricinfoLive ScoresLive Scores HomeScheduleResultsMonth viewSeason viewInternational calendarDesktop ScoreboardSeri...
On the design and analysis of gene expression studies in human populationsAccess through your institution Buy or subscribe To the Editor: In a recent _Nature Genetics_ Letter entitled “Common ge...
Cruise crew member shares his ‘biggest tip’ for guestsSimon Egerton is an entertainment manager for Fred. Olsen and he spoke to Express.co.uk from onboard the line’s _Boreali...
Secret life of scientists and engineers | eran egozy: game developer | season 2009 | episode 22- [Announcer] Funding for the Secret Life of Scientists is provided by the Alfred P. Sloan Foundation. - At Harmonics, w...