Generating human bone marrow organoids for disease modeling and drug discovery
Generating human bone marrow organoids for disease modeling and drug discovery"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The bone marrow supports and regulates hematopoiesis, responding to physiological requirements for blood cell production over ontogeny and during pathological challenges.
Interactions between hematopoietic cells and niche components are challenging to study mechanistically in the human context, but are important to delineate in order to explore the
pathobiology of blood and bone marrow disorders. Organoids are proving transformative in many research settings, but an accurate human bone marrow model incorporating multiple hematopoietic
and stromal elements has been lacking. This protocol describes a method to generate three-dimensional, multilineage bone marrow organoids from human induced pluripotent stem cells (hiPSCs),
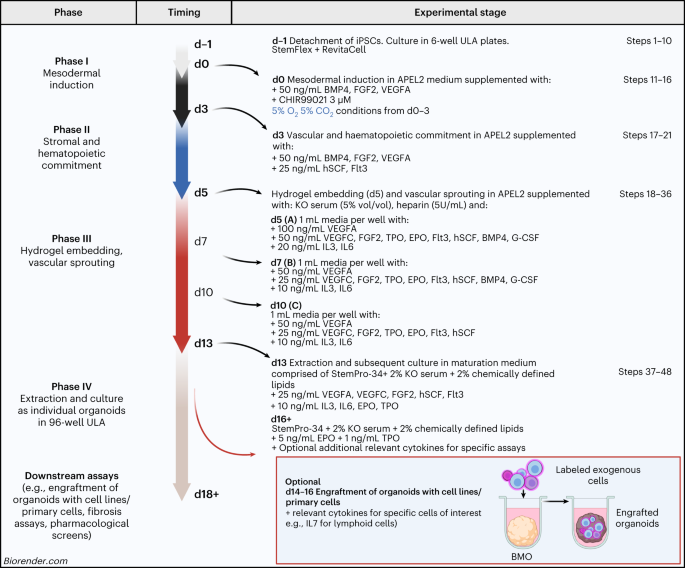
detailing the steps for the directed differentiation of hiPSCs using a series of cytokine cocktails and hydrogel embedding. Over 18 days of differentiation, hiPSCs yield the key lineages
that are present in central myelopoietic bone marrow, organized in a well-vascularized architecture that resembles native hematopoietic tissues. This presents a robust, in vitro system that
can model healthy and perturbed hematopoiesis in a scalable three-dimensional microenvironment. Bone marrow organoids also support the growth of immortalized cell lines and primary cells
from healthy donors and patients with myeloid and lymphoid cancers, including cell types that are poorly viable in standard culture systems. Moreover, we discuss assays for the
characterization of organoids, including interrogation of pathogenic remodeling using recombinant TGF-ß treatment, and methods for organoid engraftment with exogenous cells. This protocol
can be readily adapted to specific experimental requirements, can be easily implemented by users with tissue culture experience and does not require access to specialist equipment. KEY
POINTS * An ex vivo three-dimensional system for modeling human bone marrow is presented. Human induced pluripotent stem cells cultured in a collagen-enriched hydrogel with stimulatory
cytokines generate vascularized bone marrow organoids containing mesenchymal stromal cells, fibroblasts, endothelial and hematopoietic cells. * The bone marrow organoids can be engrafted
with adult donor-derived cells to study the dynamics between these cells and the bone marrow niche, using downstream assays including imaging, genetic characterization and flow cytometry.
Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54
other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and
online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes
which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY
OTHERS GENERATION OF COMPLEX BONE MARROW ORGANOIDS FROM HUMAN INDUCED PLURIPOTENT STEM CELLS Article Open access 19 February 2024 BIOENGINEERED NICHES THAT RECREATE PHYSIOLOGICAL
EXTRACELLULAR MATRIX ORGANISATION TO SUPPORT LONG-TERM HAEMATOPOIETIC STEM CELLS Article Open access 10 July 2024 EXPANSION OF HUMAN MEGAKARYOCYTE-BIASED HEMATOPOIETIC STEM CELLS BY
BIOMIMETIC MICRONICHE Article Open access 18 April 2023 DATA AVAILABILITY The original research relating to this protocol can be accessed in a previous publication22 and via the Github
repository (https://github.com/aokhan/BMorganoidV1/). Single-cell RNA sequencing data relevant to the original publication are available at the Gene Expression Omnibus (accession GSE196684).
Source data are provided with this paper. CODE AVAILABILITY Code used to analyze RNA sequencing data relevant to the original publication is available at
https://github.com/aokhan/BMorganoidV1/ and https://github.com/supatt-lab/SingCellaR. REFERENCES * Tuveson, D. & Clevers, H. Cancer modeling meets human organoid technology. _Science_
364, 952–955 (2019). Article CAS PubMed Google Scholar * Kim, J., Koo, B.-K. & Knoblich, J. A. Human organoids: model systems for human biology and medicine. _Nat. Rev. Mol. Cell
Biol._ 21, 571–584 (2020). Article CAS PubMed PubMed Central Google Scholar * Veninga, V. & Voest, E. E. Tumor organoids: opportunities and challenges to guide precision medicine.
_Cancer Cell_ 39, 1190–1201 (2021). Article CAS PubMed Google Scholar * Li, R. et al. A pro-inflammatory stem cell niche drives myelofibrosis through a targetable galectin 1 axis.
Preprint at _bioRxiv_ https://doi.org/10.1101/2023.08.05.550630 (2023). * Méndez-Ferrer, S. et al. Bone marrow niches in haematological malignancies. _Nat. Rev. Cancer_ 20, 285–298 (2020).
Article PubMed PubMed Central Google Scholar * Lucas, D. Structural organization of the bone marrow and its role in hematopoiesis. _Curr. Opin. Hematol._ 28, 36–42 (2020). Article
Google Scholar * Morrison, S. J. & Scadden, D. T. The bone marrow niche for haematopoietic stem cells. _Nature_ 505, 327–334 (2014). Article CAS PubMed PubMed Central Google Scholar
* Jardine, L. et al. Blood and immune development in human fetal bone marrow and Down syndrome. _Nature_ 598, 327–331 (2021). Article CAS PubMed PubMed Central Google Scholar *
Baryawno, N. et al. A cellular taxonomy of the bone marrow stroma in homeostasis and leukemia. _Cell_ 177, 1915–1932.e16 (2019). Article CAS PubMed PubMed Central Google Scholar *
Tikhonova, A. N. et al. The bone marrow microenvironment at single-cell resolution. _Nature_ 569, 222–228 (2019). Article CAS PubMed PubMed Central Google Scholar * Baccin, C. et al.
Combined single-cell and spatial transcriptomics reveal the molecular, cellular and spatial bone marrow niche organization. _Nat. Cell Biol._ 22, 38–48 (2020). Article CAS PubMed Google
Scholar * Kent, D., Dykstra, B. & Eaves, C. Isolation and assessment of long‐term reconstituting hematopoietic stem cells from adult mouse bone marrow. _Curr. Protoc. Stem Cell Biol._
3, 2A.4.1–2A.4.23 (2007). Google Scholar * Bradley, T. & Metcalf, D. The growth of mouse bone marrow cells in vitro. _Aust. J. Exp. Biol. Med. Sci._ 44, 287–300 (1966). Article CAS
PubMed Google Scholar * de L, M. et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. _N. Engl. J. Med._ 367, 2305–2315 (2012). Article Google Scholar * Khan, A. O. et
al. Post-translational polymodification of β1-tubulin regulates motor protein localisation in platelet production and function. _Haematologica_ 1, 243–260 (2022). Google Scholar * Feng, Q.
et al. Scalable generation of universal platelets from human induced pluripotent stem cells. _Stem Cell Rep._ 3, 817–831 (2014). Article CAS Google Scholar * Ng, E. S. et al.
Differentiation of human embryonic stem cells to HOXA+ hemogenic vasculature that resembles the aorta–gonad–mesonephros. _Nat. Biotechnol._ 34, 1168–1179 (2016). Article CAS PubMed Google
Scholar * Jing, R. et al. EZH1 repression generates mature iPSC-derived CAR T cells with enhanced antitumor activity. _Cell Stem Cell_ 29, 1181–1196.e6 (2022). Article CAS PubMed PubMed
Central Google Scholar * Cao, X. et al. Differentiation and functional comparison of monocytes and macrophages from hiPSCs with peripheral blood derivatives. _Stem Cell Rep._ 12,
1282–1297 (2019). Article CAS Google Scholar * Ebrahimi, M. et al. Differentiation of human induced pluripotent stem cells into erythroid cells. _Stem Cell Res. Ther._ 11, 483 (2020).
Article CAS PubMed PubMed Central Google Scholar * Moreau, T. et al. Large-scale production of megakaryocytes from human pluripotent stem cells by chemically defined forward
programming. _Nat. Commun._ 7, 11208 (2016). Article CAS PubMed PubMed Central Google Scholar * Khan, A. O. et al. Human bone marrow organoids for disease modelling, discovery and
validation of therapeutic targets in hematological malignancies. _Cancer Discov_. https://doi.org/10.1158/2159-8290.cd-22-0199 (2022). * Zhao, Z. et al. Organoids. _Nat. Rev. Methods Prim._
2, 94 (2022). Article CAS Google Scholar * Wimmer, R. A., Leopoldi, A., Aichinger, M., Kerjaschki, D. & Penninger, J. M. Generation of blood vessel organoids from human pluripotent
stem cells. _Nat. Protoc._ 14, 3082–3100 (2019). Article CAS PubMed Google Scholar * Wimmer, R. A. et al. Human blood vessel organoids as a model of diabetic vasculopathy. _Nature_ 565,
505–510 (2019). Article CAS PubMed PubMed Central Google Scholar * Popescu, D.-M. et al. Decoding human fetal liver haematopoiesis. _Nature_ 574, 365–371 (2019). Article CAS PubMed
PubMed Central Google Scholar * Roy, A. et al. Transitions in lineage specification and gene regulatory networks in hematopoietic stem/progenitor cells over human development. _Cell Rep._
36, 109698 (2021). Article CAS PubMed PubMed Central Google Scholar * Raic, A., Naolou, T., Mohra, A., Chatterjee, C. & Lee-Thedieck, C. 3D models of the bone marrow in health and
disease: yesterday, today, and tomorrow. _MRS Commun._ 9, 37–52 (2019). Article CAS PubMed Google Scholar * Sharipol, A., Lesch, M. L., Soto, C. A. & Frisch, B. J. Bone marrow
microenvironment-on-chip for culture of functional hematopoietic stem cells. _Front. Bioeng. Biotechnol._ 10, 855777 (2022). Article PubMed PubMed Central Google Scholar * Bessy, T.,
Itkin, T. & Passaro, D. Bioengineering the bone marrow vascular niche. _Front. Cell Dev. Biol._ 9, 645496 (2021). Article PubMed PubMed Central Google Scholar * Voeltzel, T. et al. A
minimal standardized human bone marrow microphysiological system to assess resident cell behavior during normal and pathological processes. _Biomater. Sci._ 10, 485–498 (2021). Article
Google Scholar * Giger, S. et al. Microarrayed human bone marrow organoids for modeling blood stem cell dynamics. _APL Bioeng._ 6, 036101 (2022). Article CAS PubMed PubMed Central
Google Scholar * Fairfield, H. et al. Development of a 3D bone marrow adipose tissue model. _Bone_ 118, 77–88 (2019). Article PubMed Google Scholar * Glaser, D. E. et al. Organ-on-a-chip
model of vascularized human bone marrow niches. _Biomaterials_ 280, 121245 (2022). Article CAS PubMed Google Scholar * Chou, D. B. et al. On-chip recapitulation of clinical bone-marrow
toxicities and patient-specific pathophysiology. _Nat. Biomed. Eng._ 4, 394–406 (2020). Article PubMed PubMed Central Google Scholar * Zhang, S., Wan, Z. & Kamm, R. D. Vascularized
organoids on a chip: strategies for engineering organoids with functional vasculature. _Lab Chip_ 21, 473–488 (2021). Article CAS PubMed PubMed Central Google Scholar * Aazmi, A. et al.
Engineered vasculature for organ-on-a-chip systems. _Engineering_ 9, 131–147 (2022). Article Google Scholar * Marturano-Kruik, A. et al. Human bone perivascular niche-on-a-chip for
studying metastatic colonization. _Proc. Natl Acad. Sci. USA_ 115, 1256–1261 (2018). Article CAS PubMed PubMed Central Google Scholar * Cuenca, M. V. et al. Engineered 3D vessel-on-chip
using hiPSC-derived endothelial and vascular smooth muscle cells. _Stem Cell Rep._ 16, 2159–2168 (2021). Article Google Scholar * Byambaa, B. et al. Bioprinted osteogenic and vasculogenic
patterns for engineering 3D bone tissue. _Adv. Healthc. Mater._ 6, 1700015 (2017). Article Google Scholar * Simunovic, F. & Finkenzeller, G. Vascularization strategies in bone tissue
engineering. _Cells_ 10, 1749 (2021). Article CAS PubMed PubMed Central Google Scholar * Kopp, H.-G., Avecilla, S. T., Hooper, A. T. & Rafii, S. The bone marrow vascular niche: home
of HSC differentiation and mobilization. _Physiology_ 20, 349–356 (2005). Article CAS PubMed Google Scholar * Demirci, S., Leonard, A. & Tisdale, J. F. Hematopoietic stem cells from
pluripotent stem cells: clinical potential, challenges, and future perspectives. _Stem Cells Transl. Med_ 9, 1549–1557 (2020). Article PubMed PubMed Central Google Scholar * Sugimura,
R. et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. _Nature_ 545, 432–438 (2017). Article CAS PubMed PubMed Central Google Scholar * Sakurai, M. et al.
Chemically defined cytokine-free expansion of human haematopoietic stem cells. _Nature_ 615, 127–133 (2023). Article CAS PubMed Google Scholar * Wilkinson, A. C., Ishida, R., Nakauchi,
H. & Yamazaki, S. Long-term ex vivo expansion of mouse hematopoietic stem cells. _Nat. Protoc._ 15, 628–648 (2020). Article CAS PubMed PubMed Central Google Scholar * Wilkinson, A.
C. et al. Long-term ex vivo haematopoietic-stem-cell expansion allows nonconditioned transplantation. _Nature_ 571, 117–121 (2019). Article CAS PubMed PubMed Central Google Scholar *
Homan, K. A. et al. Flow-enhanced vascularization and maturation of kidney organoids in vitro. _Nat. Methods_ 16, 255–262 (2019). Article CAS PubMed PubMed Central Google Scholar *
Thamodaran, V., Rani, S. & Velayudhan, S. R. Induced pluripotent stem (iPS) cells, methods and protocols. _Methods Mol. Biol._ 2454, 755–773 (2021). Article Google Scholar * Qi, P.,
Zhou, Y., Wang, D., He, Z. & Li, Z. A new collagen solution with high concentration and collagen native structure perfectly preserved. _RSC Adv._ 5, 87180–87186 (2015). Article CAS
Google Scholar * Masselink, W. et al. Broad applicability of a streamlined ethyl cinnamate-based clearing procedure. _Development_ 146, dev166884 (2019). Article PubMed Google Scholar *
Campbell, T. B., Zhang, S. Y., Valencia, A. & Passegue, E. Bone marrow stromal cell remodeling is a common feature of diverse fibrotic myeloproliferative neoplasm models. _Blood_ 128,
25–25 (2016). Article Google Scholar * Heaton, H. et al. Souporcell: robust clustering of single-cell RNA-seq data by genotype without reference genotypes. _Nat. Methods_ 17, 615–620
(2020). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank N. Hayder and S. Reed who helped with sample banking, the University of Birmingham TechHub Imaging
Core Facilities, and the Medical Research Council (MRC) Weatherall Institute of Molecular Medicine Flow Cytometry facility and Imaging core. We thank P. Garcia, G. Murphy, V. Steeples, Y.
Psaras, and C. Toepffer for the generous provision of the hiPSC lines used (BU3-10, BU8C3, KOLF2). A.O.K. is funded by a Sir Henry Wellcome fellowship (218649/Z/19/Z, 218649/A/19/Z). B.P.
receives funding from a Cancer Research UK Advanced Clinician Scientist Fellowship (C67633/A29034), a British Research Council (BRC) Senior Research Fellowship, the Haematology and Stem
Cells Theme of the Oxford BRC, a Kay Kendall Leukemia Fund Project Grant and unit funding from the MRC (awarded to the MRC Molecular Haematology Unit, MRC Weatherall Institute of Molecular
Medicine). A CC BY or equivalent license is applied to the author accepted manuscript arising from this submission, in accordance with the funders’ open access conditions. AUTHOR INFORMATION
Author notes * These authors contributed equally: Antonio Rodriguez-Romera, Zoë C. Wong, Yuqi Shen. AUTHORS AND AFFILIATIONS * MRC Weatherall Institute of Molecular Medicine, Radcliffe
Department of Medicine and National Institute of Health Research, Oxford Biomedical Research Centre, University of Oxford, Oxford, UK Aude-Anais Olijnik, Antonio Rodriguez-Romera, Zoë C.
Wong, Yuqi Shen, Natalie J. Jooss, Bethan Psaila & Abdullah O. Khan * Institute of Cardiovascular Sciences, College of Medical and Dental Sciences, University of Birmingham, Birmingham,
UK Jasmeet S. Reyat, Julie Rayes & Abdullah O. Khan * Department of Physiology, Anatomy and Genetics, Medical Sciences Division, University of Oxford, Oxford, UK Jasmeet S. Reyat Authors
* Aude-Anais Olijnik View author publications You can also search for this author inPubMed Google Scholar * Antonio Rodriguez-Romera View author publications You can also search for this
author inPubMed Google Scholar * Zoë C. Wong View author publications You can also search for this author inPubMed Google Scholar * Yuqi Shen View author publications You can also search for
this author inPubMed Google Scholar * Jasmeet S. Reyat View author publications You can also search for this author inPubMed Google Scholar * Natalie J. Jooss View author publications You
can also search for this author inPubMed Google Scholar * Julie Rayes View author publications You can also search for this author inPubMed Google Scholar * Bethan Psaila View author
publications You can also search for this author inPubMed Google Scholar * Abdullah O. Khan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
A.O.K. devised the differentiation protocol and utilization methods, performed cell culture and imaging experiments, co-authored the manuscript and sourced funding for this project. B.P.
devised experiments and utilization of the organoids for disease modeling, interpreted data, co-wrote the manuscript and sourced funding for this project. A.-A.O., A.R.-R., Z.C.W. and Y.S.
performed flow cytometry experiments, analyzed and interpreted data, and A.-A.O. co-wrote the protocol. J.R. and J.S.R. devised and performed sectioning and related imaging experiments.
N.J.J. critically reviewed and edited the manuscript and curated and interpreted data. CORRESPONDING AUTHORS Correspondence to Bethan Psaila or Abdullah O. Khan. ETHICS DECLARATIONS
COMPETING INTERESTS B.P.: Alethiomics (co-founder, equity, consultancy, research funding), Constellation Therapeutics (consultancy), Blueprint Medicines (advisory board), Galecto (research
funding), Novartis (paid speaking engagements), GSK (advisory board). A.O.K.: Alethiomics (consultancy). The other authors have no conflicts of interest to declare that are relevant to the
content of this article. A patent has been filed by A.O.K. and B.P. relating to work described in this paper (GB2202025.9 and GB221664.47 (WO/2023/156774), PCT/GB2023/050348). PEER REVIEW
PEER REVIEW INFORMATION _Nature Protocols_ thanks Benjamin Frisch, Veronique Maguer-Satta and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RELATED LINKS KEY REFERENCES
USING THIS PROTOCOL Khan, A. O. et al_. Cancer Discov_. 13, 364–385 (2022): https://doi.org/10.1158/2159-8290.cd-22-0199 Li, R. et al. Preprint at _bioRxiv_ (2023):
https://doi.org/10.1101/2023.08.05.550630 SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION Supplementary Figs. 1–8. SOURCE DATA SOURCE DATA FIG. 3 Statistical source data for the
population frequencies in Fig. 3d–i. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing
agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement
and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Olijnik, AA., Rodriguez-Romera, A., Wong, Z.C. _et al._ Generating human bone marrow organoids for disease
modeling and drug discovery. _Nat Protoc_ 19, 2117–2146 (2024). https://doi.org/10.1038/s41596-024-00971-7 Download citation * Received: 28 February 2023 * Accepted: 22 December 2023 *
Published: 26 March 2024 * Issue Date: July 2024 * DOI: https://doi.org/10.1038/s41596-024-00971-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Channelnews : hisense tv’s: dumb.. But cheapHisense has unleashed 10 affordable new TV’s, starting from $299 for a 24″ to $1299 for a 55″ LED TV. The new thin LED ...
Old image of farmer’s death revived amid recent protests_(If you feel suicidal or know someone in distress, please reach out to them with kindness and call these_ _numbers_ _of...
What is the absolute limit for human athletes? Here’s the science …The Olympics have drawn to a close and this year saw the shattering of 19 different world records, some by staggering ma...
October 23, 2009 correspondence to a. Barry randCharles B. Rangel, New York, Chariman Fortney Pete Stark, California Sander M. Levin, Michigan Jim McDermott, Washington...
15-min pedestrian distance life circle and sustainable community governance in chinese metropolitan cities: a diagnosisABSTRACT Urban planning has shifted from “land-oriented” to “human-oriented” and metropolitan cities start to focus on 1...
Latests News
Generating human bone marrow organoids for disease modeling and drug discoveryABSTRACT The bone marrow supports and regulates hematopoiesis, responding to physiological requirements for blood cell p...
[withdrawn] clusters of empty homes fund: allocationsNotice CLUSTERS OF EMPTY HOMES FUND: ALLOCATIONS Funding allocations under the Clusters of Empty Homes fund. Get emails ...
Dave stewart: five days in music cityDave Stewart has been a force in music, as a producer and musician, for more than 30 years. But his name will probably a...
Chevalier | Terra NovaNoteSoutenir les jeunes adultesUne limite d’âge à 25 ans existe en France pour accéder au Revenu de solidarité active (R...
Robert durst has covid, is on ventilatorRobert Durst at his sentencing hearing on October 14. Photo: Myung J. Chun/Getty Images Robert Durst, the 78-year-old re...