Altered ISGylation drives aberrant macrophage-dependent immune responses during SARS-CoV-2 infection
Altered ISGylation drives aberrant macrophage-dependent immune responses during SARS-CoV-2 infection"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Ubiquitin-like protein ISG15 (interferon-stimulated gene 15) (ISG15) is a ubiquitin-like modifier induced during infections and involved in host defense mechanisms. Not surprisingly, many
viruses encode deISGylating activities to antagonize its effect. Here we show that infection by Zika, SARS-CoV-2 and influenza viruses induce ISG15-modifying enzymes. While influenza and
Zika viruses induce ISGylation, SARS-CoV-2 triggers deISGylation instead to generate free ISG15. The ratio of free versus conjugated ISG15 driven by the papain-like protease (PLpro) enzyme
of SARS-CoV-2 correlates with macrophage polarization toward a pro-inflammatory phenotype and attenuated antigen presentation. In vitro characterization of purified wild-type and mutant
PLpro revealed its strong deISGylating over deubiquitylating activity. Quantitative proteomic analyses of PLpro substrates and secretome from SARS-CoV-2-infected macrophages revealed several
glycolytic enzymes previously implicated in the expression of inflammatory genes and pro-inflammatory cytokines, respectively. Collectively, our results indicate that altered free versus
conjugated ISG15 dysregulates macrophage responses and probably contributes to the cytokine storms triggered by SARS-CoV-2.
Interferons (IFNs) are the first line of defense against virus infections1 and are critical drivers of the innate immune response. Although hosts deficient in type I IFN are more susceptible
to virus infections2, an excess of type I IFN or aberrant cytokine response may lead to extensive tissue damage as is commonly observed in highly pathogenic cases of influenza3 and
coronaviruses4. Mice lacking the IFN-α receptor (IFNAR) have a higher survival rate to influenza and coronavirus infections than wild-type (WT) animals5,6, again pointing to a dysregulation
of IFN signaling underpinning the immunopathology of severe cases.
Macrophages are cells of the innate immune system that play key roles in modulating disease severity during virus infections. They can be infected by a range of viruses and are the major
producers of pro-inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), IFN-β, IP-10 (C-X-C motif chemokine 10) and monocyte chemoattractant protein 1 (MCP-1), which have an impact
on the pathogenesis and clinical outcomes in the host7,8,9,10. Regulation of cytokines in macrophages is essential. Overproduction of cytokines, commonly referred to as ‘cytokine storms’,
aggravates lung damage with uncontrolled extravasation of immune cells into infection sites4,11, although the exact sequence of events is not yet completely understood.
ISG15 is a ubiquitin-like modifier with broad-spectrum antiviral activity that is part of the first line of defense against pathogens. Post-translational modifications by ubiquitin and ISG15
are frequently targeted by viruses to perturb host immune responses12. ISG15 can be conjugated to proteins in a process termed ISGylation or be secreted in its free form. Among the hundreds
of modifiable substrates, many have immune-related functions13,14,15, and ISG15 (free or conjugated) has been shown to protect the host against infections16.
The fate of ISGylation in virus-infected macrophages has not been reported so far. The impact of ISG15 has only been investigated in influenza virus-infected epithelial cells where
ISG15-modified influenza NS1 inhibited virus replication. In addition, ISGylated TSG101, a member of the endosomal sorting complexes required for transport, inhibited transport of influenza
virus proteins15,17. However, much of the immune response at the site of infection emanates from monocyte-derived and tissue-resident macrophages. In this study, we investigated how viruses
interact with the immune activation pathways in infected macrophages using Zika and SARS-CoV-2 as two (+)RNA viruses from distinct families. Zika is a member of the Flaviviridae family while
SARS-CoV-2 is a coronavirus. Both Zika and SARS-CoV-2 are single-stranded, positive-sense RNA viruses that replicate in the cytoplasm within membrane-delineated replication organelles.
Macrophages are permissive to infection by both viruses; however, they are the primary target cells of Zika virus and support replication to high titers. We also compared these responses to
those infected with influenza virus, which is an orthomyxovirus with a vastly different genome organization (negative-sense segmented RNA) and unlike the former, replicates in the nucleus.
Influenza and Zika viruses promoted cellular ISGylation while SARS-CoV-2 triggered secretion of ISG15. Expression of the WT but not the catalytically inactive SARS-CoV-2 PLpro alone was
sufficient to drive deISGylation and aberrant macrophage responses. Proteomic analyses revealed that glycolytic enzymes that regulate inflammatory responses are the primary substrates of
PLpro deISGylase activity. The secretome from SARS-CoV-2-infected macrophages also revealed enrichment of nonclassical secretory components and pro-inflammatory cytokines. Collectively, our
data underscore the critical impact of altered free versus conjugated ISG15 on macrophage function, potentially underpinning the onset of lymphopenia and cytokine storms during infections by
SARS-CoV-2.
ISG15 is produced during virus infections downstream of type I IFNs18,19. Upregulation of ISG15 and modifying enzymes, including the E1 activating enzyme ubiquitin-like modifier-activating
enzyme 7 (UBE1L), E2 conjugation enzyme ubiquitin/ISG15-conjugating enzyme E2 L6 (UBE2L6/UBCH8), E3 ISG15–protein ligase HERC5 (HERC5) and deISGylase Ubl carboxyl-terminal hydrolase 18
(USP18) have been reported, albeit only in virus-infected epithelial cells20,21. To determine the magnitude of expression of ISG15 and its modifying enzymes in virus-infected macrophages, we
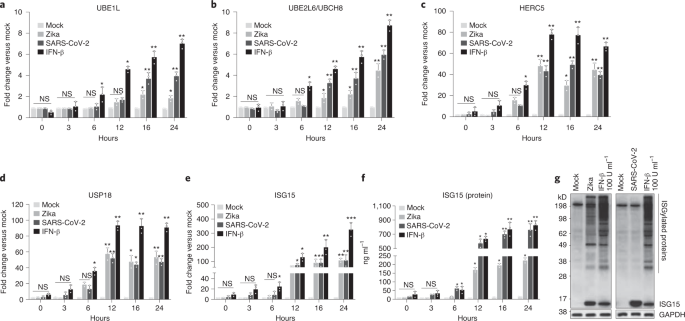
performed quantitative PCR with reverse transcription (RT–qPCR) to quantify the messenger RNA levels of ISG15, UBE1L (E1), UBE2L6/UBCH8 (E2), HERC5 (E3) and USP18 on Zika and SARS-CoV-2
infection. We treated macrophages with IFN-β as positive control, where ISG15 and its conjugating enzymes were all induced as anticipated (Fig. 1a–d). In those infected by either Zika or
SARS-CoV-2, all enzymes of the ISGylation pathway were substantially induced (Fig. 1a–e). We also measured their expression in influenza A-infected cells. Like +RNA viruses, in cells
infected by human influenza A (H1N1) or avian influenza A (H9N2) virus, expression of all mRNAs, with the exception of UBE1L, was induced to similar levels (Supplementary Fig. 1a–e).
Intracellular ISG15 protein, measured by ELISA, was equivalently upregulated after infection with influenza (Supplementary Fig. 1f), Zika virus and most significantly SARS-CoV-2 (Fig. 1f).
These results indicate that ISG15 and modifying enzymes are expressed in macrophages and markedly induced after virus infection.
a–e, Macrophages were infected with Zika or SARS-CoV-2 at an MOI of 2. At the indicated time intervals, changes in mRNA expression levels of the ISG15-modifying enzymes UBE1L (a),
UBE2L6/UBCH8 (b), HERC5 (c) and USP18 (d), and ISG15 (e) against mock infection were quantified by qPCR. NS, not significant. f, Intracellular ISG15 protein levels in Zika- or
SARS-CoV-2-infected macrophages were quantified by ELISA. a–f, Data are displayed as the means ± s.e.m. of three biologically independent experiments. *P
Trending News
VIC News - 9News - Latest updates and breaking headlines VictoriaThis is a collection page for Victoria news. Check this page for breaking headlines from Melbourne plus surrounding regi...
'banks too big to care, all about profits'When people take up part-time or seasonal work, their Centrelink payment is clawed back. After paying income tax, this a...
Factors controlling the interconversion of enzyme–substrate compounds of pig heart lactate dehydrogenaseABSTRACT Stopped flow and temperature jump experiments make possible a description of the individual catalytic steps. De...
Liverpool handed marcelo brozovic transfer boost after inter talksThe defensive midfielder has gone on to make 186 appearances for Inter during which time he has scored 23 goals and prov...
Pogba could stay at man utd as juventus identity transfer alternativeHis last 90 minute performance for United came back at the end of September, when the Red Devils drew 1-1 with Arsenal. ...
Latests News
Altered ISGylation drives aberrant macrophage-dependent immune responses during SARS-CoV-2 infectionUbiquitin-like protein ISG15 (interferon-stimulated gene 15) (ISG15) is a ubiquitin-like modifier induced during infecti...
All we know about new Madeleine McCann search as cops scan underground 10-mins from hotel - Daily StarAll we know about new Madeleine McCann search as cops scan underground 10-mins from hotelGerman authorities have linked ...
Book Review | NatureAccess through your institution Buy or subscribe This is a preview of subscription content, access via your institution ...
SVAHCS Postpones Non-Essential ProceduresThe .gov means it’s official.Federal government websites often end in .gov or .mil. Before sharing sensitive information...
Case Histories of Innovations | NatureABSTRACT Technologically slanted case histories of the development of industrial products are valuable supplements to mo...