Within-host evolution of a gut pathobiont facilitates liver translocation
Within-host evolution of a gut pathobiont facilitates liver translocation"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Gut commensal bacteria with the ability to translocate across the intestinal barrier can drive the development of diverse immune-mediated diseases1,2,3,4. However, the key factors
that dictate bacterial translocation remain unclear. Recent studies have revealed that gut microbiota strains can adapt and evolve throughout the lifetime of the host5,6,7,8,9, raising the
possibility that changes in individual commensal bacteria themselves over time may affect their propensity to elicit inflammatory disease. Here we show that within-host evolution of the
model gut pathobiont _Enterococcus gallinarum_ facilitates bacterial translocation and initiation of inflammation. Using a combination of in vivo experimental evolution and comparative
genomics, we found that _E. gallinarum_ diverges into independent lineages adapted to colonize either luminal or mucosal niches in the gut. Compared with ancestral and luminal _E.
gallinarum_, mucosally adapted strains evade detection and clearance by the immune system, exhibit increased translocation to and survival within the mesenteric lymph nodes and liver, and
induce increased intestinal and hepatic inflammation. Mechanistically, these changes in bacterial behaviour are associated with non-synonymous mutations or insertion–deletions in defined
regulatory genes in _E. gallinarum_, altered microbial gene expression programs and remodelled cell wall structures. _Lactobacillus reuteri_ also exhibited broadly similar patterns of
divergent evolution and enhanced immune evasion in a monocolonization-based model of within-host evolution. Overall, these studies define within-host evolution as a critical regulator of
commensal pathogenicity that provides a unique source of stochasticity in the development and progression of microbiota-driven disease. Access through your institution Buy or subscribe This
is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our
best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue
Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL
ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS QUANTIFYING THE ADAPTIVE LANDSCAPE OF
COMMENSAL GUT BACTERIA USING HIGH-RESOLUTION LINEAGE TRACKING Article Open access 21 February 2024 INTRAHOST EVOLUTION OF THE GUT MICROBIOTA Article 17 April 2023 TRANSCRIPTIONAL
DIVERSIFICATION IN A HUMAN-ADAPTING ZOONOTIC PATHOGEN DRIVES NICHE-SPECIFIC EVOLUTION Article Open access 28 February 2025 DATA AVAILABILITY The sequencing data generated in this study are
available at the NCBI Sequence Read Archive (SRA) database. Genomic sequences are under the accession PRJNA743649 and RNA-seq data are under the accession PRJNA743979. Source data are
provided with this paper. REFERENCES * Manfredo Vieira, S. et al. Translocation of a gut pathobiont drives autoimmunity in mice and humans. _Science_ 359, 1156–1161 (2018). Article ADS CAS
PubMed Google Scholar * Nakamoto, N. et al. Gut pathobionts underlie intestinal barrier dysfunction and liver T helper 17 cell immune response in primary sclerosing cholangitis. _Nat.
Microbiol._ 4, 492–503 (2019). Article CAS PubMed Google Scholar * Chow, J., Tang, H. & Mazmanian, S. K. Pathobionts of the gastrointestinal microbiota and inflammatory disease.
_Curr. Opin. Immunol._ 23, 473–480 (2011). Article CAS PubMed PubMed Central Google Scholar * Ruff, W. E., Greiling, T. M. & Kriegel, M. A. Host-microbiota interactions in
immune-mediated diseases. _Nat. Rev. Microbiol._ 18, 521–538 (2020). Article CAS PubMed Google Scholar * Schloissnig, S. et al. Genomic variation landscape of the human gut microbiome.
_Nature_ 493, 45–50 (2013). Article ADS PubMed CAS Google Scholar * Zhao, S. et al. Adaptive evolution within gut microbiomes of healthy people. _Cell Host Microbe_ 25, 656–667 (2019).
Article CAS PubMed PubMed Central Google Scholar * Elhenawy, W., Tsai, C. N. & Coombes, B. K. Host-specific adaptive diversification of Crohn's disease-associated
adherent-invasive _Escherichia coli_. _Cell Host Microbe_ 25, 301–312 (2019). Article CAS PubMed Google Scholar * Barroso-Batista, J. et al. Specific eco-evolutionary contexts in the
mouse gut reveal _Escherichia coli_ metabolic versatility. _Curr. Biol._ 30, 1049–1062 (2020). Article CAS PubMed Google Scholar * Yilmaz, B. et al. Long-term evolution and short-term
adaptation of microbiota strains and sub-strains in mice. _Cell Host Microbe_ 29, 650–663 (2021). Article CAS PubMed Google Scholar * Group, N. H. W. et al. The NIH Human Microbiome
Project. _Genome Res._ 19, 2317–2323 (2009). Article CAS Google Scholar * The Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. _Nature_ 569,
641–648 (2019). * Plumbridge, J. Control of the expression of the _manXYZ_ operon in _Escherichia coli_: Mlc is a negative regulator of the mannose PTS. _Mol. Microbiol._ 27, 369–380 (1998).
Article CAS PubMed Google Scholar * Honeyman, A. L. & Curtiss, R. 3rd Isolation, characterization and nucleotide sequence of the _Streptococcus_ mutans lactose-specific enzyme II
(_lacE_) gene of the PTS and the phospho-beta-galactosidase (_lacG_) gene. _J. Gen. Microbiol._ 139, 2685–2694 (1993). Article CAS PubMed Google Scholar * Dubrac, S. & Msadek, T.
Tearing down the wall: peptidoglycan metabolism and the WalK/WalR (YycG/YycF) essential two-component system. _Adv. Exp. Med. Biol._ 631, 214–228 (2008). Article CAS PubMed Google Scholar
* Fried, L., Behr, S. & Jung, K. Identification of a target gene and activating stimulus for the YpdA/YpdB histidine kinase/response regulator system in _Escherichia coli_. _J.
Bacteriol._ 195, 807–815 (2013). Article CAS PubMed PubMed Central Google Scholar * Dobihal, G. S., Brunet, Y. R., Flores-Kim, J. & Rudner, D. Z. Homeostatic control of cell wall
hydrolysis by the WalRK two-component signaling pathway in _Bacillus subtilis_. _eLife_ 8, e52088 (2019). Article CAS PubMed PubMed Central Google Scholar * Auchtung, J. M., Lee, C. A.,
Garrison, K. L. & Grossman, A. D. Identification and characterization of the immunity repressor (ImmR) that controls the mobile genetic element ICE_Bs1_ of _Bacillus subtilis_. _Mol.
Microbiol._ 64, 1515–1528 (2007). Article CAS PubMed PubMed Central Google Scholar * Donaldson, G. P., Lee, S. M. & Mazmanian, S. K. Gut biogeography of the bacterial microbiota.
_Nat. Rev. Microbiol._ 14, 20–32 (2016). Article CAS PubMed Google Scholar * Boneca, I. G. et al. A critical role for peptidoglycan _N_-deacetylation in _Listeria_ evasion from the host
innate immune system. _Proc. Natl Acad. Sci. USA_ 104, 997–1002 (2007). Article ADS CAS PubMed PubMed Central Google Scholar * Benachour, A. et al. The lysozyme-induced peptidoglycan
N-acetylglucosamine deacetylase PgdA (EF1843) is required for _Enterococcus faecalis_ virulence. _J. Bacteriol._ 194, 6066–6073 (2012). Article CAS PubMed PubMed Central Google Scholar
* Thurlow, L. R., Thomas, V. C., Fleming, S. D. & Hancock, L. E. _Enterococcus faecalis_ capsular polysaccharide serotypes C and D and their contributions to host innate immune evasion.
_Infect. Immun._ 77, 5551–5557 (2009). Article CAS PubMed PubMed Central Google Scholar * Dalton, J. E. et al. Intraepithelial γδ+ lymphocytes maintain the integrity of intestinal
epithelial tight junctions in response to infection. _Gastroenterology_ 131, 818–829 (2006). Article CAS PubMed Google Scholar * Hoytema van Konijnenburg, D. P. et al. Intestinal
epithelial and intraepithelial T cell crosstalk mediates a dynamic response to infection. _Cell_ 171, 783–794 (2017). Article CAS PubMed PubMed Central Google Scholar *
Olivares-Villagomez, D. & Van Kaer, L. Intestinal intraepithelial lymphocytes: sentinels of the mucosal barrier. _Trends Immunol._ 39, 264–275 (2018). Article CAS PubMed Google
Scholar * McPherson, A. C., Pandey, S. P., Bender, M. J. & Meisel, M. Systemic immunoregulatory consequences of gut commensal translocation. _Trends Immunol._ 42, 137–150 (2021).
Article CAS PubMed Google Scholar * Zegarra-Ruiz, D. F. et al. A diet-sensitive commensal _Lactobacillus_ strain mediates TLR7-dependent systemic autoimmunity. _Cell Host Microbe_ 25,
113–127 (2019). Article CAS PubMed Google Scholar * Lee, S. M. et al. Bacterial colonization factors control specificity and stability of the gut microbiota. _Nature_ 501, 426–429
(2013). Article ADS CAS PubMed PubMed Central Google Scholar * Brown, S. P., Cornforth, D. M. & Mideo, N. Evolution of virulence in opportunistic pathogens: generalism, plasticity,
and control. _Trends Microbiol._ 20, 336–342 (2012). Article CAS PubMed PubMed Central Google Scholar * Culyba, M. J. & Van Tyne, D. Bacterial evolution during human infection:
adapt and live or adapt and die. _PLoS Pathog._ 17, e1009872 (2021). Article CAS PubMed PubMed Central Google Scholar * Ha, C. W. Y. et al. Translocation of viable gut microbiota to
mesenteric adipose drives formation of creeping fat in humans. _Cell_ 183, 666–683(2020). Article CAS PubMed PubMed Central Google Scholar * Van Tyne, D. et al. Impact of antibiotic
treatment and host innate immune pressure on enterococcal adaptation in the human bloodstream. _Sci. Transl. Med._ 11, eaat8418 (2019). Article PubMed PubMed Central CAS Google Scholar
* Young, B. C. et al. Severe infections emerge from commensal bacteria by adaptive evolution. _eLife_ 6, e30637 (2017). Article PubMed PubMed Central Google Scholar * Palm, N. W. et al.
Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. _Cell_ 158, 1000–1010 (2014). Article CAS PubMed PubMed Central Google Scholar * Wick, R. R.,
Judd, L. M., Gorrie, C. L. & Holt, K. E. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. _PLoS Comput. Biol._ 13, e1005595 (2017). Article ADS
PubMed PubMed Central CAS Google Scholar * Andrews, S. FastQC: a quality control tool for high throughput sequence data (2010); http://www.bioinformatics.babraham.ac.uk/projects/fastqc/
* Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. _Bioinformatics_ 30, 2114–2120 (2014). Article CAS PubMed PubMed Central Google
Scholar * De Coster, W., D'Hert, S., Schultz, D. T., Cruts, M. & Van Broeckhoven, C. NanoPack: visualizing and processing long-read sequencing data. _Bioinformatics_ 34, 2666–2669
(2018). Article PubMed PubMed Central CAS Google Scholar * Aziz, R. K. et al. The RAST Server: rapid annotations using subsystems technology. _BMC Genomics_ 9, 75 (2008). Article
PubMed PubMed Central CAS Google Scholar * Overbeek, R. et al. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). _Nucleic Acids Res._ 42,
D206–D214 (2014). Article CAS PubMed Google Scholar * Seemann, T. Prokka: rapid prokaryotic genome annotation. _Bioinformatics_ 30, 2068–2069 (2014). Article CAS PubMed Google Scholar
* Seemann, T. snippy: fast bacterial variant calling from NGS reads (2015); https://github.com/tseemann/snippy * Deatherage, D. E. & Barrick, J. E. Identification of mutations in
laboratory-evolved microbes from next-generation sequencing data using breseq. _Methods Mol. Biol._ 1151, 165–188 (2014). Article CAS PubMed PubMed Central Google Scholar * Treangen, T.
J., Ondov, B. D., Koren, S. & Phillippy, A. M. The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. _Genome Biol._ 15,
524 (2014). Article PubMed PubMed Central CAS Google Scholar * Bankevich, A. et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. _J. Comput.
Biol._ 19, 455–477 (2012). Article MathSciNet CAS PubMed PubMed Central Google Scholar * Darling, A. C., Mau, B., Blattner, F. R. & Perna, N. T. Mauve: multiple alignment of
conserved genomic sequence with rearrangements. _Genome Res._ 14, 1394–1403 (2004). Article CAS PubMed PubMed Central Google Scholar * Okonechnikov, K., Golosova, O. & Fursov, M.,
team, U. Unipro UGENE: a unified bioinformatics toolkit. _Bioinformatics_ 28, 1166–1167 (2012). Article CAS PubMed Google Scholar * Letunic, I. & Bork, P. Interactive Tree Of Life
(iTOL) v5: an online tool for phylogenetic tree display and annotation. _Nucleic Acids Res._ 49, W293–W296 (2021). Article CAS PubMed PubMed Central Google Scholar * Page, A. J. et al.
Roary: rapid large-scale prokaryote pan genome analysis. _Bioinformatics_ 31, 3691–3693 (2015). Article CAS PubMed PubMed Central Google Scholar * Eren, A. M. et al. Community-led,
integrated, reproducible multi-omics with anvi’o. _Nat. Microbiol._ 6, 3–6 (2021). Article CAS PubMed PubMed Central Google Scholar * Martin, M. Cutadapt removes adapter sequences from
high-throughput sequencing reads. _EMBnet J._ 17, 10–12 (2011). Article Google Scholar * Howe, K. L. et al. Ensembl 2021. _Nucleic Acids Res._ 49, D884–D891 (2021). Article CAS PubMed
Google Scholar * Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. _Bioinformatics_ 29, 15–21 (2013). Article CAS PubMed Google Scholar * Anders, S., Pyl, P. T. & Huber,
W. HTSeq—a Python framework to work with high-throughput sequencing data. _Bioinformatics_ 31, 166–169 (2015). Article CAS PubMed Google Scholar * Love, M. I., Huber, W. & Anders, S.
Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. _Genome Biol._ 15, 550 (2014). PubMed PubMed Central Google Scholar * Subramanian, A. et al. Gene set
enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. _Proc. Natl Acad. Sci. USA_ 102, 15545–15550 (2005). Article ADS CAS PubMed PubMed
Central Google Scholar * Langmead, B. & Salzberg, S. L. Fast gapped-read alignment with Bowtie 2. _Nat. Methods_ 9, 357–359 (2012). Article CAS PubMed PubMed Central Google Scholar
* Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. _Bioinformatics_ 26, 841–842 (2010). Article CAS PubMed PubMed Central Google
Scholar * Cullen, T. W. et al. Antimicrobial peptide resistance mediates resilience of prominent gut commensals during inflammation. _Science_ 347, 170–175 (2015). Article ADS CAS
PubMed PubMed Central Google Scholar * Leenhouts, K. et al. A general system for generating unlabelled gene replacements in bacterial chromosomes. _Mol. Gen. Genet._ 253, 217–224 (1996).
Article CAS PubMed Google Scholar * Laute-Caly, D. L. et al. The flagellin of candidate live biotherapeutic _Enterococcus gallinarum_ MRx0518 is a potent immunostimulant. _Sci. Rep._ 9,
801 (2019). Article ADS PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank R. Medzhitov, A. Wang and the members of the Palm laboratory for
discussions and comments; Y. Cao, T. A. Rice and S. R. Leopold for experimental assistance; the staff at the Centre for Cellular and Molecular Imaging (CCMI) Electron Microscopy Facility at
Yale University for electron micrograph acquisition; members of the Yale Research Histology Core for histological staining; and staff at the Yale Centre for Genome Analysis for
next-generation sequencing. This work was supported by the National Institute on Aging of the National Institutes of Health under award number R01AG068863 (to N.W.P.). Approximately 50% of
the funding for this research project (around US$300,000) was financed with NIH funds; the remainder was financed by non-governmental sources. N.W.P. also acknowledges support from the
Common Fund of the NIH (DP2DK125119), the Leona M. and Henry B. Helmsley Charitable Trust (3083), the Chan Zuckerberg Initiative, Aligning Science Across Parkinson's through the Michael
J. Fox Foundation for Parkinson’s Research, the Ludwig Family, the Mathers Foundation, the Pew Charitable Trust, the NIGMS (RM1GM141649) and F. Hoffmann-La Roche. M.A.K. acknowledges
support from the Lupus Research Alliance. The funders of this work had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The
content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Schematic figures were created using
BioRender.com. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Immunobiology, Yale University School of Medicine, New Haven, CT, USA Yi Yang, Mytien Nguyen, Varnica Khetrapal,
Nicole D. Sonnert, Anjelica L. Martin, Haiwei Chen, Martin A. Kriegel & Noah W. Palm * Department of Microbial Pathogenesis, Yale University School of Medicine, New Haven, CT, USA Nicole
D. Sonnert * Department of Translational Rheumatology and Immunology, Institute of Musculoskeletal Medicine, University of Münster, Münster, Germany Martin A. Kriegel * Section of
Rheumatology and Clinical Immunology, Department of Medicine, University Hospital Münster, Münster, Germany Martin A. Kriegel Authors * Yi Yang View author publications You can also search
for this author inPubMed Google Scholar * Mytien Nguyen View author publications You can also search for this author inPubMed Google Scholar * Varnica Khetrapal View author publications You
can also search for this author inPubMed Google Scholar * Nicole D. Sonnert View author publications You can also search for this author inPubMed Google Scholar * Anjelica L. Martin View
author publications You can also search for this author inPubMed Google Scholar * Haiwei Chen View author publications You can also search for this author inPubMed Google Scholar * Martin A.
Kriegel View author publications You can also search for this author inPubMed Google Scholar * Noah W. Palm View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS N.W.P. and Y.Y. conceived the project, designed the experiments and wrote the manuscript. Y.Y., M.N., V.K., N.D.S. and H.C. designed and performed experiments. M.N.
isolated _E. gallinarum_ isolates from SPF NZW × BXSB F1 mice. V.K. generated the _pgdA_ mutant strain. N.D.S. and H.C. assessed potential _E. gallinarum_ phenotypes. Y.Y. performed all of
the other experiments and all data analyses. A.L.M. assisted with gnotobiotic mouse experiments. M.A.K. participated in the conceptualization of the project and provided NZW × BXSB F1 mice,
the original liver isolate of _E. gallinarum_ from autoimmune-prone SPF mice and critical intellectual input. All of the authors edited the manuscript. CORRESPONDING AUTHOR Correspondence to
Noah W. Palm. ETHICS DECLARATIONS COMPETING INTERESTS N.W.P. is a co-founder of Artizan Biosciences and Design Pharmaceuticals and has received research funding from Artizan Biosciences and
F. Hoffmann-La Roche. M.A.K. holds a patent on the use of microbiota manipulations to treat immune-mediated diseases (US patent no. PCT/US18/14368) and consults for Eligo Biosciences. The
other authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature_ thanks Kenya Honda and the other, anonymous, reviewer(s) for their contribution to the peer review
of this work. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. EXTENDED DATA
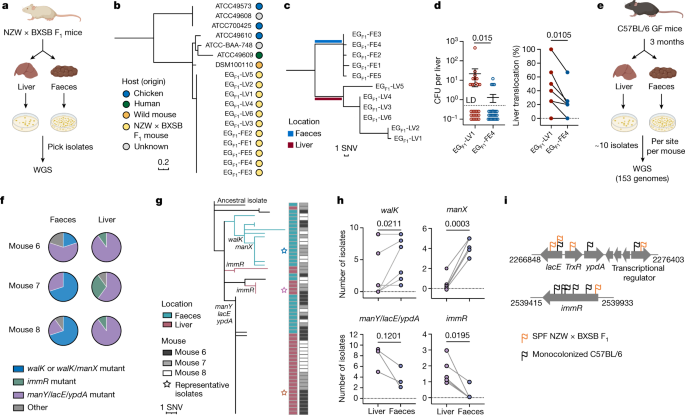
FIGURES AND TABLES EXTENDED DATA FIG. 1 MUTATIONS DETECTED IN _E. GALLINARUM_ ISOLATES FROM SPF NZW × BXSB F1 MICE. A, Pangenome of eighteen _E. gallinarum_ strains. B, Schematic of the
complete genome of _E. gallinarum_ strain EGF1-FE1. C, Number of singleton or shared variants detected in _E. gallinarum_ faecal or liver isolates. D, Functional classes of genes with
nonsynonymous mutations or indels. n = 13 genes. E, Relative abundance of liver and faecal _E. gallinarum_ isolates in the faeces of co-colonized SPF C57BL/6 mice. Mice were gavaged with
equal doses of EGF1-LV1 and EGF1-FE4 and faecal samples were analysed one week after colonization. n = 6 mice across two independent experiments. Data represent mean ± SEM. Unpaired
two-tailed t-test (e). NSY, nonsynonymous SNVs; SYN, synonymous SNVs; Indel, small insertion/deletion variant; Inter, intergenic variant. EG, _E. gallinarum_. Source Data EXTENDED DATA FIG.
2 MUTATIONS DETECTED IN _E. GALLINARUM_ ISOLATES FROM MONOCOLONIZED C57BL/6 MICE. A, Schematic of cage setup of the experimental evolution in monocolonized mice. B, Distribution of detected
mutations across the _E. gallinarum_ genome. n = 159 unique mutations detected in 153 isolates. C, Number of singleton or shared variants. D, Functional classes of genes with nonsynonymous
mutations or indels. n = 30 genes. NSY, nonsynonymous SNVs; SYN, synonymous SNVs; Indel, small insertion/deletion variant; Inter, intergenic variant. The schematic in A was created using
BioRender. EXTENDED DATA FIG. 3 WITHIN-HOST EVOLUTION OF _E. GALLINARUM_ IN MONOCOLONIZED MICE. A–C, Phylogenetic tree of liver and faecal _E. gallinarum_ isolates sampled from mouse 1 (A),
mouse 2 (B) or mice 3-5 (C). Reference-based alignments. D, Position, effects, and frequency of mutations at _walK_, _manX_, _manY_, _lacE_, _ypdA_, and _immR_ in liver or faecal isolates
sampled from each individual mouse. Rows represent mutations and columns represent individual mice. E, Relative abundance of _E. gallinarum_ isolates in the faeces of bi-colonized mice.
EGF1-FE4, EGmono6-LV1, or EGmono6-LV10 was co-gavaged with an equal dose of EGmono7-FE2 and samples were collected 4 weeks after co-colonization. See Supplementary Table 7 for a summary of
all mutants. n = 3 mice. Representative of two independent experiments. Data represent mean ± SEM. Two-way ANOVA with Sidak’s post hoc test. F, Colony morphologies of EGmono6-LV1,
EGmono6-LV10, and EGmono7-FE2 after 48-hour aerobic culture on GAM agar plates. Source Data EXTENDED DATA FIG. 4 RECONSTRUCTED EVOLUTIONARY PATHS OF _E. GALLINARUM_ WITHIN INDIVIDUAL HOSTS.
Reconstructed phylogenetic histories of _E. gallinarum_ isolates from mouse 1 (A), mouse 2 (B), mice 3–5 (C), and mice 6–8 (D). The mouse 7 is also presented in Fig. 2a. Bar plots show
bacterial load recovered from the liver of each monocolonized mouse. n = 1 mouse. Circles represent sequenced isolates and squares represent hypothetical intermediate genotypes. Different
colours indicate distinct genotypes. Arrows connect related genotypes and dashed lines connect genetically identical isolates. Liver and faecal populations are separated by a blue dashed
line. The schematics in A-D were created using BioRender. Source Data EXTENDED DATA FIG. 5 TRANSCRIPTOMIC COMPARISONS OF LIVER AND FAECAL _E. GALLINARUM_ ISOLATES, AND SUSCEPTIBILITY OF _E.
GALLINARUM PGDA_ MUTANT TO LIVER CLEARANCE. A, The minimum inhibitory concentrations (MIC) of mCRAMP against _E. gallinarum_ isolates. B, Gating strategy used to select live macrophages and
quantify CFSE signal in Fig. 3c. C–G, Volcano plots showing adjusted p-value versus fold change for 3575 genes expressed in EGmono6-LV1 (C), EGmono6-LV10 (D) as compared to EGmono7-FE2, or
in EGmono6-LV1 (E), EGmono6-LV10 (F) and EGmono7-FE2 (G) isolate as compared to the ancestral strain EGF1-FE4. Horizontal lines show the p-value cut-off for significance after correction for
multiple testing (adjust p-value < 0.05). Differentially expressed genes are coloured as red. n = 3 independent cultures. H, Overrepresented Pfam functional classes (EGmono6-LV10 _vs_.
EGmono7-FE2). I, Expressions of _pgdA_ gene in cultured _E. gallinarum_ isolates. n = 3 independent cultures. J, Liver clearance of intravenously injected wild-type or _pgdA_ mutant strains
of _E. gallinarum_. Liver bacterial load 5 days post-injection. n = 4 mice. Representative of two independent experiments. Data in (I, J) represent mean ± SEM. Two-tailed Wald test with
Benjamini-Hochberg correction (FDR = 0.05) (C–G), one-way ANOVA with Benjamini-Hochberg correction (FDR = 0.05) (I), unpaired two-tailed t-test (J). Source Data EXTENDED DATA FIG. 6 LIVER
AND FAECAL _E. GALLINARUM_ ISOLATES ELICIT DISTINCT IMMUNE RESPONSES IN THE INTESTINE AND LIVER. A–E, Transcriptomics of ileal epithelia and ileal tissues. A, B, Eight-hour monocolonization.
Enriched pathways (A) and differentially expressed genes (B) in IECs between mice colonized by EGmono6-LV10 or EGmono7-FE2. n = 4 mice. C–E, Two-week monocolonization. C, Pathways enriched
in IECs or ileal tissues induced by EGmono6-LV10 or EGmono7-FE2 colonization. D, E, Enriched pathways (D) and differentially expressed genes (E) in IECs or ileal tissues between mice
colonized by EGmono6-LV1 or EGmono7-FE2. IECs (n = 4), ileal tissues (n = 3). Left and right heatmaps display distinct gene sets. F, Density of _E. gallinarum_ in distal ileum after 8-hour
or 2-week monocolonization. 8 h: EGmono6-LV10 (n = 6), EGmono7-FE2 (n = 8). 2 weeks: n = 4 mice. G, H, PAS staining (G) or CD3 immunohistochemistry staining (H) showing goblet cells (G, n =
40 villi) or IELs (H, n = 90 villi) in distal ileum of monocolonized mice 2 weeks (G) or 18 h (H) post-gavage. Scale bars, 50 μm. I, Gut permeability of monocolonized mice.
_MyD88_-/-_Trif_-/-: EGmono6-LV1 (n = 6), EGmono6-LV10 and EGmono7-FE2 (n = 7). _Rag1_-/-: EGmono6-LV1 (n = 7), EGmono6-LV10 and EGmono7-FE2 (n = 6). J, Percentage of monocolonized mice
showing liver translocation. K, Pathways enriched in livers induced by EGmono6-LV10 or EGmono7-FE2 monocolonization. Results are representative of n =2 (A-I) or 5 (J) independent
experiments. Data represent mean ± SEM (F, I), violin plots (G, H) showing median (red) and quartiles (blue). Two-tailed Wald test with Benjamini-Hochberg correction (FDR = 0.05) (B, E),
two-tailed Wilcoxon test (F, left panel), Kruskal-Wallis test with Benjamini-Hochberg correction (FDR = 0.05) (F, right panel; H, I), one-way ANOVA with Tukey’s post hoc test (G), paired
two-tailed t-test (J). NES, normalized enrichment score. Source Data EXTENDED DATA FIG. 7 LIVER _E. GALLINARUM_ ISOLATES FROM SPF NZW × BXSB F1 MICE EXHIBIT SIMILAR FEATURES AS
EXPERIMENTALLY EVOLVED LIVER ISOLATES FROM MONOCOLONIZATIONS. A, TEM images of cultured _E. gallinarum_ isolates from NZW × BXSB F1 mice. Two bacterial cells of each isolate were shown. B,
Minimum inhibitory concentration for mCRAMP against NZW × BXSB F1 _E. gallinarum_ isolates. C, Liver clearance of intravenously injected NZW × BXSB F1 _E. gallinarum_ isolates. Liver
bacterial load 5 days post-injection. n = 9 mice. D, Differentially expressed genes in IECs (EGF1-LV1: n = 4, EGF1-FE4: n = 3) or ileal tissues (n = 4) between mice monocolonized with
EGF1-LV or EGF1-FE4 for 2 weeks. Left and right heatmaps display distinct gene sets. E, Gut permeability of mice monocolonized with EGF1-LV1 (n = 11) or EGF1-FE4 (n = 9) for 2 weeks. F–I,
Imiquimod-induced autoimmune phenotypes in mice monocolonized with EGF1-LV1 or EGF1-FE4. F, Schematic of imiquimod-induced autoimmunity. _E. gallinarum_ translocation to the liver (G), mLNs
(H) and spleen (I). n = 9 mice. Results in (A–I) are representative of two independent experiments. Data in (C, E, G–I) represent mean ± SEM. Two-tailed Mann-Whitney test (C, G–I), unpaired
two-tailed t-test (E), two-tailed Wald test with Benjamini-Hochberg correction (FDR = 0.05) (D). The schematic in F was created using BioRender. Source Data EXTENDED DATA FIG. 8 DIVERGENT
EVOLUTION OF _B. FRAGILIS_ IN MONOCOLONIZED MICE. A, Schematic of experimental evolution of _B. fragilis_ in monocolonized mice. Pie charts showing proportions of mice with liver
translocation. n = 5 mice. B, Reconstructed phylogenetic histories of _B. fragilis_ isolates sampled from two co-housed mice (BF1 and BF2). Circles represent sequenced isolates and squares
represent hypothetical intermediate genotypes. Arrows connect related genotypes and dashed lines connect genetically identical isolates. SIM and faecal populations are separated by a blue
dashed line. C, Liver clearance of intravenously injected _B. fragilis_. Representative SIM (BFmono1-LV5, BFmono2-LV8) and faecal (BFmono1-LV6) isolates. Liver bacterial load 1 or 3 dpi. n =
4 mice. Representative of two independent experiments. Data represent mean ± SEM. One-way ANOVA with Tukey’s post hoc test (C). SIM, small intestinal mucosa. dpi, days post-injection. The
schematic in A was created using BioRender. Source Data EXTENDED DATA FIG. 9 IMPACTS OF WITHIN-HOST EVOLUTION OF _E. GALLINARUM_ ON BACTERIAL TRANSLOCATION AND INITIATION OF INFLAMMATION IN
THE INTESTINE AND LIVER. Within-host evolution of _E. gallinarum_ facilitates divergence into independent lineages with distinct niche preferences and capacities for translocation. Faecal
lineages preferentially colonize the intestinal lumen and are highly susceptible to immune recognition and clearance. By contrast, mucosa-associated lineages are adapted to colonize mucosal
niches and exhibit enhanced resistance to immune recognition and clearance via remodelling of the bacterial cell wall. Functionally, the faecal lineage elicits robust immune responses at the
intestinal epithelium, including enhanced mucus production and increased IEL recruitment; these responses reinforce the integrity of the gut barrier and limit bacterial translocation. By
contrast, mucosally adapted _E. gallinarum_ lineages evade initial detection at the epithelial surface, translocate across the ‘leaky’ gut barrier, exhibit increased survival after
translocation, and trigger inflammation in the intestinal lamina propria and liver. The schematic was created using BioRender. SUPPLEMENTARY INFORMATION REPORTING SUMMARY SUPPLEMENTARY TABLE
1 Mutations detected in NZW × BXSB F1 mice-derived _E. gallinarum_ isolates. This table lists mutations detected in 11 _E. gallinarum_ isolates sampled from the livers or faeces of
18-week-old NZW × BXSB F1 hybrid mice. The EGF1-FE1 strain was used as the reference genome. SUPPLEMENTARY TABLE 2 Mutations detected in _E. gallinarum_ isolates evolved in monocolonized
C57BL/6 mice. This table lists mutations detected in 153 _E. gallinarum_ isolates sampled from the livers or faeces of eight monocolonized C57BL/6 mice. _E. gallinarum_ isolates were
collected after colonization for 3 months. EGF1-FE4 was the ancestral strain and was used as the reference genome. SUPPLEMENTARY TABLE 3 Mutations detected in _E. gallinarum_ isolates
evolved in a simplified mock microbial community. This table lists mutations detected in 20 _E. gallinarum_ isolates sampled from the livers or faeces of a gnotobiotic C57BL/6 mouse
colonized by _E. gallinarum_ and a nine-species mock community. _E. gallinarum_ isolates were collected after colonization for 7 weeks. EGF1-FE4 was the ancestral strain and was used as the
reference genome. SUPPLEMENTARY TABLE 4 Mutations detected in _E. gallinarum_ isolates evolved in SPF C57BL/6 mice. This table lists mutations detected in 39 _E. gallinarum_ isolates sampled
from the SIM or faeces of two cohoused SPF C57BL/6 mice. _E. gallinarum_-free C57BL/6 mice were purchased from Jackson Laboratory and were inoculated with EGF1-FE4 strain. _E. gallinarum_
isolates were collected after colonization for 1.5 years. EGF1-FE4 was the ancestral strain and was used as the reference genome. SUPPLEMENTARY TABLE 5 Mutations detected in _L. reuteri_
isolates evolved in monocolonized C57BL/6 mice. This table lists mutations detected in 20 _L. reuteri_ isolates sampled from the livers or faeces of a monocolonized C57BL/6 mouse. _L.
reuteri_ isolates were collected after colonization for 3 months. The draft genome of the ancestral strain was used as the reference genome. SUPPLEMENTARY TABLE 6 Mutations detected in _B.
fragilis_ isolates evolved in monocolonized C57BL/6 mice. This table lists mutations detected in 48 _B. fragilis_ isolates sampled from the SIM or faeces of two cohoused monocolonized
C57BL/6 mouse. _B. fragilis_ isolates were collected after colonization for 3 months. The draft genome of the ancestral strain was used as the reference genome. SUPPLEMENTARY TABLE 7
Metadata of selected bacterial strains used in this study. This table lists the identifier, species, host of origin, duration of colonization, source location and bacterial genotype of
bacterial strains assessed in this study. SUPPLEMENTARY TABLE 8 Annotation of select mutated genes in this study. This table lists annotations of select mutated genes by RAST server v.2.0
(https://rast.nmpdr.org/rast.cgi) or Prokka v.1.12. SOURCE DATA SOURCE DATA FIG. 1 SOURCE DATA FIG. 2 SOURCE DATA FIG. 3 SOURCE DATA FIG. 4 SOURCE DATA FIG. 5 SOURCE DATA EXTENDED DATA FIG.
1 SOURCE DATA EXTENDED DATA FIG. 3 SOURCE DATA EXTENDED DATA FIG. 4 SOURCE DATA EXTENDED DATA FIG. 5 SOURCE DATA EXTENDED DATA FIG. 6 SOURCE DATA EXTENDED DATA FIG. 7 SOURCE DATA EXTENDED
DATA FIG. 8 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, Y., Nguyen, M., Khetrapal, V. _et al._ Within-host evolution of a gut pathobiont
facilitates liver translocation. _Nature_ 607, 563–570 (2022). https://doi.org/10.1038/s41586-022-04949-x Download citation * Received: 26 September 2021 * Accepted: 08 June 2022 *
Published: 13 July 2022 * Issue Date: 21 July 2022 * DOI: https://doi.org/10.1038/s41586-022-04949-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Review: a reimagined 'twilight: los angeles, 1992' revival arrives at a time of protracted crisisThe most powerful moments in the revised version of “Twilight: Los Angeles, 1992,” Anna Deavere Smith’s landmark drama a...
Sheikh hasina's india stay will not affect indo-bangladesh ties, says key interim government adviserMohammed Touhid Husain, Foreign Affairs Adviser to Bangladesh's interim government, has said that former Bangladesh...
Not our story: mumbai-based ngo chirag steps up to change the way we look at rural indiaChirag Rural Development Foundation, a Mumbai based NGO has been working in villages across the country for 13 years now...
Can the next generation of russians modernize their country?Can the Next Generation of Russians Modernize Their Country? | Carnegie Endowment for International Peace A generational...
Chicago’s vietnam war stories | jim mcnerney – bob hopeChicago’s Vietnam War Stories Clip | 58sVideo has Closed Captions | CC Vietnam veteran Jim McNerney remembers getting to...
Latests News
Within-host evolution of a gut pathobiont facilitates liver translocationABSTRACT Gut commensal bacteria with the ability to translocate across the intestinal barrier can drive the development ...
Clipper race: gybing along africa(September 20, 2019; Race 2, Day 5) – The Clipper 2019-20 Round the World Yacht Race slides along the east of Western Sa...
Up minister succumbs to covid-19; state mourning announcedThe Yogi Adityanath government has announced a state mourning after the demise of Uttar Pradesh Minister Kamal Rani Varu...
China’s strong-arming won’t work in marcos’ philippines – analysisBy Jenny Balboa and Shinji Takenaka Despite careful words from Philippine officials, the latest US–Philippines Balikatan...
New royal enfield classic 350 revealed: launching september 1 with major updatesRoyal Enfield has unveiled the latest version of its iconic Classic 350, a motorcycle that has remained a top seller sin...