Habenular tcf7l2 links nicotine addiction to diabetes
Habenular tcf7l2 links nicotine addiction to diabetes"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
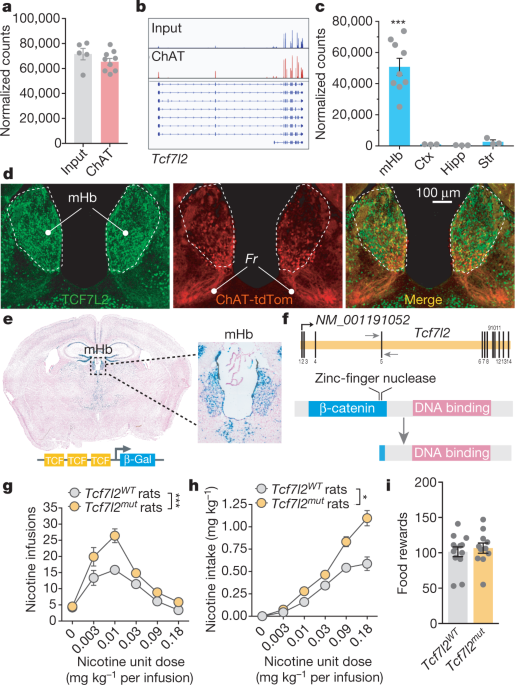
ABSTRACT Diabetes is far more prevalent in smokers than non-smokers, but the underlying mechanisms of vulnerability are unknown. Here we show that the diabetes-associated gene _Tcf7l2_ is
densely expressed in the medial habenula (mHb) region of the rodent brain, where it regulates the function of nicotinic acetylcholine receptors. Inhibition of TCF7L2 signalling in the mHb
increases nicotine intake in mice and rats. Nicotine increases levels of blood glucose by TCF7L2-dependent stimulation of the mHb. Virus-tracing experiments identify a polysynaptic
connection from the mHb to the pancreas, and wild-type rats with a history of nicotine consumption show increased circulating levels of glucagon and insulin, and diabetes-like dysregulation
of blood glucose homeostasis. By contrast, mutant _Tcf7l2_ rats are resistant to these actions of nicotine. Our findings suggest that TCF7L2 regulates the stimulatory actions of nicotine on
a habenula–pancreas axis that links the addictive properties of nicotine to its diabetes-promoting actions. Access through your institution Buy or subscribe This is a preview of subscription
content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access
subscription $32.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 51 print issues and online access $199.00 per year only $3.90 per issue Learn more Buy this article
* Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn
about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS CANNABINOID CB1 RECEPTOR IN DORSAL TELENCEPHALIC GLUTAMATERGIC NEURONS
DRIVES OVERCONSUMPTION OF PALATABLE FOOD AND OBESITY Article Open access 08 February 2021 NEUROTENSIN NEURONS IN THE EXTENDED AMYGDALA CONTROL DIETARY CHOICE AND ENERGY HOMEOSTASIS Article
20 October 2022 THE ARCCRABP1 NEURONS PLAY A CRUCIAL ROLE IN THE REGULATION OF ENERGY HOMEOSTASIS Article Open access 08 March 2025 DATA AVAILABILITY The RNA-seq data generated in this study
are available at the Gene Expression Omnibus (GEO) under the accession code GSE137118. Other data that support the findings of this study are available as Extended Data and Supplementary
Information, including uncropped western blot images. REFERENCES * Stolerman, I. P. & Jarvis, M. J. The scientific case that nicotine is addictive. _Psychopharmacology (Berl.)_ 117,
2–10, discussion 14–20 (1995). Article CAS Google Scholar * Maskos, U. et al. Nicotine reinforcement and cognition restored by targeted expression of nicotinic receptors. _Nature_ 436,
103–107 (2005). Article ADS CAS PubMed Google Scholar * Fowler, C. D., Lu, Q., Johnson, P. M., Marks, M. J. & Kenny, P. J. Habenular α5 nicotinic receptor subunit signalling
controls nicotine intake. _Nature_ 471, 597–601 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Fowler, C. D. & Kenny, P. J. Nicotine aversion: neurobiological
mechanisms and relevance to tobacco dependence vulnerability. _Neuropharmacology_ 76 PT B, 533–544 (2014). Article CAS PubMed Google Scholar * Tuesta, L. M. et al. GLP-1 acts on
habenular avoidance circuits to control nicotine intake. _Nat. Neurosci_. 20, 708–716 (2017). Article CAS PubMed PubMed Central Google Scholar * Liu, Z. & Habener, J. F.
Glucagon-like peptide-1 activation of TCF7L2-dependent Wnt signaling enhances pancreatic beta cell proliferation. _J. Biol. Chem_. 283, 8723–8735 (2008). Article CAS PubMed PubMed Central
Google Scholar * Grant, S. F. et al. Variant of transcription factor 7-like 2 (_TCF7L2_) gene confers risk of type 2 diabetes. _Nat. Genet_. 38, 320–323 (2006). Article CAS PubMed
Google Scholar * Sladek, R. et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. _Nature_ 445, 881–885 (2007). Article ADS CAS PubMed Google Scholar *
Fuchsberger, C. et al. The genetic architecture of type 2 diabetes. _Nature_ 536, 41–47 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Haggard, H. W. & Greenberg,
L. A. The effects of cigarette smoking upon the blood sugar. _Science_ 79, 165–166 (1934). Article ADS CAS PubMed Google Scholar * Sandberg, H., Roman, L., Zavodnick, J. & Kupers,
N. The effect of smoking on serum somatotropin, immunoreactive insulin and blood glucose levels of young adult males. _J. Pharmacol. Exp. Ther_. 184, 787–791 (1973). CAS PubMed Google
Scholar * GBD 2015 Tobacco Collaborators. Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of
Disease Study 2015. _Lancet_ 389, 1885–1906 (2017). Article Google Scholar * Willi, C., Bodenmann, P., Ghali, W. A., Faris, P. D. & Cornuz, J. Active smoking and the risk of type 2
diabetes: a systematic review and meta-analysis. _J. Am. Med. Assoc_. 298, 2654–2664 (2007). Article CAS Google Scholar * Ren, J. et al. Habenula “cholinergic” neurons co-release
glutamate and acetylcholine and activate postsynaptic neurons via distinct transmission modes. _Neuron_ 69, 445–452 (2011). Article CAS PubMed Google Scholar * Görlich, A. et al.
Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons. _Proc. Natl Acad. Sci. USA_ 110, 17077–17082 (2013). Article ADS PubMed CAS
PubMed Central Google Scholar * Dougherty, J. D., Schmidt, E. F., Nakajima, M. & Heintz, N. Analytical approaches to RNA profiling data for the identification of genes enriched in
specific cells. _Nucleic Acids Res_. 38, 4218–4230 (2010). Article CAS PubMed PubMed Central Google Scholar * Ables, J. L. et al. Retrograde inhibition by a specific subset of
interpeduncular α5 nicotinic neurons regulates nicotine preference. _Proc. Natl Acad. Sci. USA_ 114, 13012–13017 (2017). Article CAS PubMed PubMed Central Google Scholar * Boj, S. F. et
al. Diabetes risk gene and Wnt effector _Tcf7l2_/TCF4 controls hepatic response to perinatal and adult metabolic demand. _Cell_ 151, 1595–1607 (2012). Article CAS PubMed Google Scholar
* Korinek, V. et al. Depletion of epithelial stem-cell compartments in the small intestine of mice lacking Tcf-4. _Nat. Genet_. 19, 379–383 (1998). Article CAS PubMed Google Scholar *
Geurts, A. M. et al. Knockout rats via embryo microinjection of zinc-finger nucleases. _Science_ 325, 433 (2009). Article ADS CAS PubMed PubMed Central Google Scholar * Graham, T. A.,
Ferkey, D. M., Mao, F., Kimelman, D. & Xu, W. Tcf4 can specifically recognize β-catenin using alternative conformations. _Nat. Struct. Biol_. 8, 1048–1052 (2001). Article CAS PubMed
Google Scholar * Molenaar, M. et al. XTcf-3 transcription factor mediates beta-catenin-induced axis formation in _Xenopus_ embryos. _Cell_ 86, 391–399 (1996). Article CAS PubMed Google
Scholar * Ip, W., Shao, W., Chiang, Y. T. & Jin, T. The Wnt signaling pathway effector TCF7L2 is upregulated by insulin and represses hepatic gluconeogenesis. _Am. J. Physiol.
Endocrinol. Metab_. 303, E1166–E1176 (2012). Article CAS PubMed PubMed Central Google Scholar * Huang, S. M. et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling.
_Nature_ 461, 614–620 (2009). Article ADS CAS PubMed Google Scholar * McGehee, D. S., Heath, M. J., Gelber, S., Devay, P. & Role, L. W. Nicotine enhancement of fast excitatory
synaptic transmission in CNS by presynaptic receptors. _Science_ 269, 1692–1696 (1995). Article ADS CAS PubMed Google Scholar * Zoli, M., Léna, C., Picciotto, M. R. & Changeux, J.
P. Identification of four classes of brain nicotinic receptors using β2 mutant mice. _J. Neurosci_. 18, 4461–4472 (1998). Article CAS PubMed PubMed Central Google Scholar * Murray, K.
D., Choudary, P. V. & Jones, E. G. Nucleus- and cell-specific gene expression in monkey thalamus. _Proc. Natl Acad. Sci. USA_ 104, 1989–1994 (2007). Article ADS CAS PubMed PubMed
Central Google Scholar * Skoglund, G., Hussain, M. A. & Holz, G. G. Glucagon-like peptide 1 stimulates insulin gene promoter activity by protein kinase A-independent activation of the
rat insulin I gene cAMP response element. _Diabetes_ 49, 1156–1164 (2000). Article CAS PubMed Google Scholar * Giniatullin, R., Nistri, A. & Yakel, J. L. Desensitization of nicotinic
ACh receptors: shaping cholinergic signaling. _Trends Neurosci_. 28, 371–378 (2005). Article CAS PubMed Google Scholar * Paradiso, K. & Brehm, P. Long-term desensitization of
nicotinic acetylcholine receptors is regulated via protein kinase A-mediated phosphorylation. _J. Neurosci_. 18, 9227–9237 (1998). Article CAS PubMed PubMed Central Google Scholar *
Huganir, R. L., Delcour, A. H., Greengard, P. & Hess, G. P. Phosphorylation of the nicotinic acetylcholine receptor regulates its rate of desensitization. _Nature_ 321, 774–776 (1986).
Article ADS CAS PubMed Google Scholar * Li, Y. F. et al. Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP
response element binding protein-mediated neurogenesis in the hippocampus. _Neuropsychopharmacology_ 34, 2404–2419 (2009). Article CAS PubMed Google Scholar * Zhao, T. J. et al. Ghrelin
secretion stimulated by β1-adrenergic receptors in cultured ghrelinoma cells and in fasted mice. _Proc. Natl Acad. Sci. USA_ 107, 15868–15873 (2010). Article ADS CAS PubMed PubMed
Central Google Scholar * Neil-Dwyer, G., Bartlett, J., McAinsh, J. & Cruickshank, J. M. β-adrenoceptor blockers and the blood–brian barrier. _Br. J. Clin. Pharmacol_. 11, 549–553
(1981). Article CAS PubMed PubMed Central Google Scholar * O’Dell, L. E. & Nazarian, A. Enhanced vulnerability to tobacco use in persons with diabetes: a behavioral and
neurobiological framework. _Prog. Neuropsychopharmacol._ _Biol. Psychiatry_ 65, 288–296 (2016). Google Scholar * Perkins, K. A., Epstein, L. H., Sexton, J. E. & Pastor, S. Effects of
smoking cessation on consumption of alcohol and sweet, high-fat foods. _J. Subst. Abuse_ 2, 287–297 (1990). Article CAS PubMed Google Scholar * Yamaguchi, T., Danjo, T., Pastan, I.,
Hikida, T. & Nakanishi, S. Distinct roles of segregated transmission of the septo-habenular pathway in anxiety and fear. _Neuron_ 78, 537–544 (2013). Article CAS PubMed PubMed Central
Google Scholar * Soria-Gómez, E. et al. Habenular CB1 receptors control the expression of aversive memories. _Neuron_ 88, 306–313 (2015). Article PubMed CAS Google Scholar * Zhang, J.
et al. Presynaptic excitation via GABAB receptors in habenula cholinergic neurons regulates fear memory expression. _Cell_ 166, 716–728 (2016). Article CAS PubMed Google Scholar * Chou,
M. Y. et al. Social conflict resolution regulated by two dorsal habenular subregions in zebrafish. _Science_ 352, 87–90 (2016). Article ADS CAS PubMed Google Scholar * Zhao, Z. et al.
A central catecholaminergic circuit controls blood glucose levels during stress. _Neuron_ 95, 138–152.e5 (2017). Article CAS PubMed Google Scholar * Wang, Z. & Ma’ayan, A. An open
RNA-Seq data analysis pipeline tutorial with an example of reprocessing data from a recent Zika virus study _F100Res_. 5, 1574 (2016). Article CAS Google Scholar * Dobin, A. et al. STAR:
ultrafast universal RNA-seq aligner. _Bioinformatics_ 29, 15–21 (2013). Article CAS PubMed Google Scholar * Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general
purpose program for assigning sequence reads to genomic features. _Bioinformatics_ 30, 923–930 (2014). Article CAS PubMed Google Scholar * Clark, N. R. et al. The characteristic
direction: a geometrical approach to identify differentially expressed genes. _BMC Bioinformatics_ 15, 79 (2014). Article PubMed PubMed Central Google Scholar * Chen, E. Y. et al.
Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. _BMC Bioinformatics_ 14, 128 (2013). Article PubMed PubMed Central Google Scholar * Kuleshov, M. V. et
al. Enrichr: a comprehensive gene set enrichment analysis web server 2016 update. _Nucleic Acids Res_. 44 (W1), W90–W97 (2016). Article CAS PubMed PubMed Central Google Scholar Download
references ACKNOWLEDGEMENTS This work was supported by grants from the National Institute on Drug Abuse to P.J.K. (DA020686) and I.I-T. (DA035756). We thank S. Stanley for pRV-GFP virus, J.
Lindstrom for the α4β2α5 nAChR cell line, M. Conkright for the EVX1-CREB-luciferase-GFP reporter, A. Stewart for INS-1 cells and M. Hayes for the AAV1-shGlp1r-GFP virus. The
Lenti-7xTcf-FFluc-SV40-mCherry reporter (7TFC) and dominant-negative _Tcf7l2_ construct (EdTc) were gifts from R. Nusse. Oxycodone and cocaine were supplied by the NIDA Drug Supply Program.
AUTHOR INFORMATION Author notes * Xin-an Liu & Zuxin Chen Present address: Brain Cognition and Brain Disease Institute (BCBDI), Shenzhen Institutes of Advanced Technology, Chinese
Academy of Sciences, Shenzhen-Hong Kong Institute of Brain Science-Shenzhen Fundamental Research Institutions, Shenzhen, China * These authors contributed equally: Alexander Duncan, Mary P.
Heyer, Masago Ishikawa, Stephanie P. B. Caligiuri AUTHORS AND AFFILIATIONS * Nash Family Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, USA Alexander
Duncan, Mary P. Heyer, Masago Ishikawa, Stephanie P. B. Caligiuri, Xin-an Liu, Zuxin Chen, Maria Vittoria Micioni Di Bonaventura, Karim S. Elayouby, Jessica L. Ables, William M. Howe, Purva
Bali, Clementine Fillinger, Maya Williams, Richard M. O’Connor & Paul J. Kenny * Skaggs Graduate School of Chemical and Biological Sciences, Scripps Research, Jupiter, FL, USA Alexander
Duncan * Department of Pharmacological Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, USA Zichen Wang & Avi Ma’ayan * Department of Molecular Medicine, The Scripps
Research Institute, Jupiter, FL, USA Qun Lu & Theodore M. Kamenecka * Institute for Behavioral Genetics, University of Colorado, Boulder, CO, USA Heidi C. O’Neill * The Laboratory of
Molecular Biology, The Rockefeller University, New York, NY, USA Ines Ibanez-Tallon * Department of Physiology, Medical College of Wisconsin, Milwaukee, WI, USA Aron M. Geurts Authors *
Alexander Duncan View author publications You can also search for this author inPubMed Google Scholar * Mary P. Heyer View author publications You can also search for this author inPubMed
Google Scholar * Masago Ishikawa View author publications You can also search for this author inPubMed Google Scholar * Stephanie P. B. Caligiuri View author publications You can also search
for this author inPubMed Google Scholar * Xin-an Liu View author publications You can also search for this author inPubMed Google Scholar * Zuxin Chen View author publications You can also
search for this author inPubMed Google Scholar * Maria Vittoria Micioni Di Bonaventura View author publications You can also search for this author inPubMed Google Scholar * Karim S.
Elayouby View author publications You can also search for this author inPubMed Google Scholar * Jessica L. Ables View author publications You can also search for this author inPubMed Google
Scholar * William M. Howe View author publications You can also search for this author inPubMed Google Scholar * Purva Bali View author publications You can also search for this author
inPubMed Google Scholar * Clementine Fillinger View author publications You can also search for this author inPubMed Google Scholar * Maya Williams View author publications You can also
search for this author inPubMed Google Scholar * Richard M. O’Connor View author publications You can also search for this author inPubMed Google Scholar * Zichen Wang View author
publications You can also search for this author inPubMed Google Scholar * Qun Lu View author publications You can also search for this author inPubMed Google Scholar * Theodore M. Kamenecka
View author publications You can also search for this author inPubMed Google Scholar * Avi Ma’ayan View author publications You can also search for this author inPubMed Google Scholar *
Heidi C. O’Neill View author publications You can also search for this author inPubMed Google Scholar * Ines Ibanez-Tallon View author publications You can also search for this author
inPubMed Google Scholar * Aron M. Geurts View author publications You can also search for this author inPubMed Google Scholar * Paul J. Kenny View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS A.D., M.P.H., M.V.M.DiB., S.P.B.C., W.M.H., P.B., R.M.O.C., M.W., C.F. and K.S.E. performed all behavioural experiments; M.I. performed
electrophysiological recordings; S.P.B.C. designed and validated gRNAs; X.L. and Z.C. performed virus tracing; Q.L. and T.M.K. performed cell culture experiments; J.L.A. and I.I-T. generated
and analysed the TRAP data; A.D. and Q.L. generated the RNA-seq data from wild-type and mutant rats; Z.W. and A.M. analysed RNA-seq data; H.C.O’N. performed the rubidium efflux experiments;
A.M.G. generated the _Tcf7l2_ mutant rats; P.J.K. designed the experiments; A.D. and P.J.K. analysed the data and wrote the manuscript. CORRESPONDING AUTHOR Correspondence to Paul J. Kenny.
ETHICS DECLARATIONS COMPETING INTERESTS P.J.K. is co-founder of Eolas Therapeutics Inc., which has a licensing agreement with AstraZeneca to develop small molecule treatments for drug
dependence. P.J.K. has research support from Eli Lilly and Takeda USA. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. PEER REVIEW INFORMATION _Nature_ thanks Peter Kalivas, Tamas Horvath and the other, anonymous, reviewer(s) for their contribution to the peer
review of this work. EXTENDED DATA FIGURES AND TABLES EXTENDED DATA FIG. 1 GENERATION OF _TCF7L2_ MUTANT RATS. A, Schematic of the _Rattus norvegicus Tcf7l2_ gene. Exons are spliced to
generate _Tcf7l2_ mRNA (NCBI reference sequence: NM_001191052.1). Primers for genotyping and Sanger sequencing are indicated by arrows flanking exon 5. B, Sequencing chromatograph of the
_Tcf7l2_ mutant allele. The site of the 169-bp deletion from exon 5 and the following intron is labelled. C, Illustration of TCF7L2 wild-type protein, containing an N-terminal β-catenin
binding domain (blue) and C-terminal DNA binding domain (red). Predicted open reading frames and truncated proteins generated from the _Tcf7l2_ mutant mRNA. Green regions on predicted
truncated proteins denote ectopic amino acid sequences not found in wild-type TCF7L2 protein. D, Genotyping of wild-type and mutant _Tcf7l2_ rats: wild-type animal (+/+) with single band at
304 bp; heterozygous animal (+/−) with bands at 304 and 144 bp; and mutant animal (−/−) with a single band at 144 bp. Image is representative of genotyping results obtained for wild-type and
mutant _Tcf7l2_ rats used each experiment. E, Graphical representation of mHb in coronal slice of rat brain. Image adapted from the Allen Brain Reference Atlas. F, Nissl staining showed
similar mHb volumes in wild-type and mutant _Tc7l2_ rats. Image is representative of results obtained in three biologically independent animals from each genotype. G, Diffusion tensor
imaging tractography of the fasciculus retroflexus in wild-type (_n_ = 3) and mutant (_n_ = 5) _Tcf7l2_ rats. H, Fractional anisotropy showed similar integrity (left and right sides) of the
fasciculus retroflexus in wild-type (_n_ = 3) and mutant (_n_ = 5) _Tcf7l2_ rats (‘genotype’: _F_1, 6 = 0.000003; _P_ = 0.99; ‘brain side’: _F_1, 6 = 2.562, _P_ = 0.16; ‘genotype × brain
side’: _F_1, 6 = 0.0007, _P_ = 0.98). I, The frequency at different steps of positive current used to calculate the slope of the input–output curve from dorsal mHb neurons. Example traces
showing typical current steps at −20, 0 and 40 pA in dorsal mHb neurons from wild-type and mutant _Tcf7l2_ rats. J, Input–output curve in dorsal mHb neurons from wild-type and mutant
_Tcf7l2_ (_n_ = 16 cells from 5 rats) rats. K, The frequency at different steps of positive current used to calculate the slope of the input-output curve from ventral mHb neurons. L,
Input–output curve in ventral mHb neurons from wild-type and mutant _Tcf7l2_ (_n_ = 16 cells, 5 rats) rats. M, Input resistance from mHb neurons from wild-type (13 cells, 4 rats) and mutant
(16 cells, 5 rats) _Tcf7l2_ rats (_P_ = 0.1036, unpaired two-tailed _t_-test). N, Afterhyperpolarization in mHb neurons from wild-type (13 cells, 4 rats) and mutant (16 cells, 5 rats)
_Tcf7l2_ rats; _P_ = 0.3043, unpaired two-tailed _t_-test. O, Sag current in mHb neurons wild-type (13 cells, 4 rats) and mutant (17 cells, 5 rats) _Tcf7l2_ rats (_P_ = 0.1386, unpaired
two-tailed _t_-test). P, Total distance travelled by drug-naive wild-type (_n_ = 6) and mutant (_n_ = 5) _Tcf7l2_ rats during a 60 min session. Q, Total distance travelled by wild-type (_n_
= 6) and mutant (_n_ = 5) _Tcf7l2_ rats after daily injections of saline or nicotine (0.4 mg kg−1) (15 min pre-treatment time). R, Responses to the training dose of nicotine (0.03 mg kg−1
per infusion) were assessed in a group of wild-type (_n_ = 9) and mutant (_n_ = 11) _Tcf7l2_ rats on days 1 and 35 of access. Nicotine responses were similar between the wild-type and mutant
_Tcf7l2_ rats on day 1 of access, but mutant _Tcf7l2_ rats escalated their intake such that their responses were higher on day 35 compared with wild-type _Tcf7l2_ rats, and compared with
their own intake on day 1 (_F_1, 18 = 30.8, ****_P_ < 0.0001, interaction effect between ‘genotype’ and ‘session’ in two-way ANOVA). Box plots show minimum–maximum range. Data are mean ±
s.e.m. Source data EXTENDED DATA FIG. 2 CRISPR CLEAVAGE OF _TCF7L2_. A, Exon diagram of mouse _Tcf7l2_ with the two pertinent domains highlighted and the sgRNA targeting locus. B,
Bioinformatic comparison of the five different sgRNAs tested against _Tcf7l2_. MM, mismatches. C, Genomic cleavage percentage in mouse N2a cells of the five sgRNAs targeted against _Tcf7l2_.
Data represent _n_ = 3 biologically independent samples. D, T7-endonuclease-based assay illustrating intact PCR and cleaved bands of _Tcf7l2_ via CRISPR gene editing. Observations are from
a single experiment. E, tdTomato expression in N2a cells 48 h after transduction of the AAV carrying sgRNA against _Tcf7l2_ (AAV-sgRNA-Tcf7l2). Data are representative of three biologically
independent samples. F, Relative expression of _Tcf7l2_ transcripts in the N2a cells transfected with AAV-sgRNA-Tcf7l2, AAV-sgRNA-eGFP and AAV-CMV-spCas9 (Vector Biolabs) (***_P_ < 0.001,
unpaired two-tailed _t_-test). Data represent _n_ = 5 biologically independent samples for each gRNA. G, Relative mRNA expression of habenular _Tcf7l2_ 6 weeks after viral stereotaxic
injections of AAV-sgRNA-Tcf7l2, AAV-sgRNA-eGFP or AAV2-hSYN1-iCre into the mHb of _Rosa26__LSL-spCas9-eGFP_ mice. Data represent _n_ = 4 biologically independent samples for each gRNA. H, In
vivo estimation of genomic cleavage of habenular _Tcf7l2_ 6 weeks after viral stereotaxic injection of AAV-sgRNA-Tcf7l2, AAV-sgRNA-eGFP or AAV2-hSYN1-iCre in _Rosa26__LSL-spCas9-eGFP_ mice.
Genomic cleavage efficiency was estimated by average re-annealed mismatches in a T7 endonuclease assay (***_P_ < 0.001, unpaired two-tailed _t_-test). Data represent _n_ = 3 biologically
independent animals for each gRNA. I, Left, representative DAPI-counterstained brain slice showing Cas9-eGFP (green) and _Tcf7l2_ gRNA (red) targeted to the mHb of _Rosa26__LSL-spCas9-eGFP_
mice. Right, whole image of brain slice from which left panels are derived. Representative result from _n_ = 3 mice. J, Medial habenula from _Rosa26__LSL-spCas9-eGFP_ mice injected with
AAV-sgRNA-eGFP (_n_ = 6 independent mice) or AAV-sgRNA-Tcf7l2 (_n_ = 7 independent mice) was dissected and DNA amplicons of targeted region of _Tcf7l2_ sequenced. Percentage of indels
detected in the targeted region of _Tcf7l2_ is shown (****_P_ < 0.0001, unpaired two-tailed _t_-test). Coronal brain image adapted from the Allen Brain Reference Atlas. K, Donut graph
showing Cas9-induced modifications to _Tcf7l2_ in the mHb of _Rosa26__LSL-spCas9-eGFP_ mice treated with AAV-sgRNA-Tcf7l2 (percentage of total amplicons sequenced). A total of 13 amplicons
(_n_ = 6 from AAV-sgRNA-eGFP-treated mice and _n_ = 7 from AAV-sgRNA-Tcf7l2_-_treated mice) were sequenced. Data are mean ± s.e.m. EXTENDED DATA FIG. 3 MECHANISM BY WHICH TCF7L2 REGULATES
NACHR FUNCTION. A, Effects of intra-mHb infusion of vehicle or EX-4 (12.5–100 ng) on nicotine intake in rats (_n_ = 10) (_F_1.696, 15.26 = 38.3, _P_ < 0.0001, one-way repeated measures
ANOVA; **_P_ < 0.01, ***_P_ < 0.001, Bonferroni’s multiple comparisons test). B, Effects of intra-mHb infusion of vehicle or EX-4 (100 nM) on the latency to earn the first and second
nicotine infusion of a self-administration session in rats (_n_ = 10) (_F_1, 29 = 311.4, _P_ < 0.0001, main effect of ‘infusion number’; _F_1, 29 = 125.4, _P_ < 0.0001, main effect of
‘EX-4’ _F_1, 29 = 126.5, ****_P_ < 0.0001, interaction effect; two-way ANOVA). C, Numbers of rats (_n_ = 10 in total) that responded for the first and second nicotine infusion of a
self-administration session after intra-mHb infusion of vehicle or EX-4 (12.5–100 ng). D, Effects of intra-mHb infusion of vehicle or rDKK1 (100 ng per side) on nicotine intake in rats (_n_
= 11) (_P_ = 0.45; unpaired two-tailed _t_-test). E, Effects of intra-mHb infusion of vehicle or XAV939 (12.5 ng per side) on nicotine intake in rats (_n_ = 10) (_P_ = 0.29; unpaired
two-tailed _t_-test). F, Effects of intra-mHb infusion of vehicle or insulin (12.5 ng per side) on nicotine intake in rats (_n_ = 13) (_P_ = 0.29; unpaired two-tailed _t_-test). G–I, LiCl
(G) and constitutively active-β-catenin (CA-β-catenin) (H), but not nicotine (I), increased GFP relative to mCherry expression in PC12 cells transfected with the 7xTcf-eGFP//SV40-mCherry
(7TGC) TCF7L2 reporter. Data reflect results from two independent experiments. J, Levels of β-catenin phosphorylated at serine residue 675 or 552 in rat PC12 cells after treatment with
forskolin, WNT3A or nicotine. Data reflect results from two independent experiments. K, _LacZ_ expression in the mHb of BAT-GAL β-galactosidase reporter mice after nicotine injection. Data
reflect results from two independent animals in each group. L, Expression levels of TCF7L2 (about 69 kDa) in the habenula were measured by western blotting in rats that responded for
intravenous nicotine infusions (0.18 mg kg−1 per infusion; _n_ = 12) or food rewards (_n_ = 12). Each lane contains pooled tissues from _n_ = 3 animals. Experiment was performed on a single
occasion. For uncropped gel image, see Supplementary Fig. 1. M, siRNA-mediated knockdown of TCF7L2 attenuated intracellular calcium transients induced by nicotine (20 nM–320 μM) in HEK293T
cells heterologously expressing α5α4β2 nAChRs (two-way repeated measures ANOVA; ‘siRNA’: _F_1, 4 = 63.38, _P_ < 0.005; ‘nicotine’: _F_15, 60 = 1388, _P_ < 0.0001; ‘siRNA × nicotine’:
_F_15, 60 = 20.89, ***_P_ < 0.0001; Bonferroni post hoc test after interaction effect in two-way ANOVA). Representative result from three experiments. N, 86Rb+ efflux from synaptosomes
generated from IPn tissues derived from wild-type (_n_ = 6) and mutant (_n_ = 6) _Tcf7l2_ rats (_F_1,39 = 4.267, *_P_ = 0.045; extra sum-of-squares _F_ test). Shift in half-maximal effective
concentration (EC50) value between genotypes using comparison of fits in a nonlinear fit model. O, Pharmacologically isolated nAChR currents (normalized) evoked by nicotine (0.1 Hz
application) in mHb neurons from wild-type rats (_n_ = 3 cells from 1 rat) were rapidly and completely blocked by bath application of mecamylamine (10 μM). *_F_1.335, 2.670 = 332.5; _P_ <
0.001; one-way repeated measures ANOVA. P, Baseline nAChR currents in mHb neurons from wild-type (_n_ = 13 cells, 4 rats) and mutant (_n_ = 15 cells, 4 rats) _Tcf7l2_rats. _P_ = 0.8180,
unpaired two-tailed _t_-test. Q, nAChR current decay time after nicotine stimulation (0.1 Hz) before and after nicotine (1 Hz)-induced desensitization in mHb neurons from wild-type (_n_ = 13
cells, 4 rats) and mutant (_n_ = 9 cells, 4 rats) _Tcf7l2_ rats; _P_ = 0.7133, unpaired two-tailed _t_-test. R, Slope of nAChR current decay after nicotine stimulation (0.1 Hz) before and
after nicotine (1 Hz)-induced desensitization in mHb neurons from wild-type (_n_ = 13 cells, 4 rats) and mutant (_n_ = 9 cells, 4 rats) _Tcf7l2_ rats; _P_ = 0.645, unpaired two-tailed
_t_-test. Data are mean ± s.e.m. Source data EXTENDED DATA FIG. 4 GENES REGULATED BY TCF7L2 IN THE MHB. A, qPCR analysis _Akap9_ transcript levels in the mHb of wild-type (_n_ = 8) and
mutant (_n_ = 7) _Tcf7l2_ rats; and _Arhgap5_ transcript levels in the mHb of wild-type (_n_ = 5) and mutant (_n_ = 7) _Tcf7l2_ rats (***_P_ < 0.001, unpaired two-tailed _t_-test). B,
qPCR analysis of α5, α3 and β4 nAChR subunit expression in the mHb of wild-type (_n_ = 5) and mutant (_n_ = 8) _Tcf7l2_ rats. C, Top 15 most abundantly expressed genes in the mHb of
wild-type _Tcf7l2_ rats that show differential downregulation in the mHb of mutant _Tcf7l2_ rats (_n_ = 9 rats per genotype). Genes are organized in descending order according to the
baseline expression levels in wild-type rats. The log2-transformed fold change of downregulated gene expression in the mHb of mutant compared with wild-type _Tcf7l2_ rats is shown. BaseMean,
mean of normalized counts for all samples; lfcSE, s.e.m. of log2-transformed fold change; stat, Wald chi-squared test of normalized counts for gene transcript in mutant versus wild-type
_Tcf7l2_ rats; pval, uncorrected Wald test _P_ value; padj, _P_ value adjusted for multiple testing using Benjamini–Hochberg to estimate the false discovery rate. D–H, Knockdown of
_PAFAHIB1_ (D), _NDFIIP1_ (E), _ARHGAP5_ (F), _HNRNPU_ (G) or _AKAP9_ (H) mRNA transcripts using a pool of validated siRNAs had no effects on nicotine-stimulated increases in [Ca2+]i in
human HEK293T cells stably expressing α5α4β2 nAChRs. Data represent _n_ = 3 biologically independent samples. Data are mean ± s.e.m. EXTENDED DATA FIG 5 TCF7L2 REGULATES CAMP SIGNALLING IN
PC12 CELLS. A, Expression of dominant-negative _Tcf7l2_ (dnTcf7l2) in PC12 cells reduced the activity of a cAMP-responsive luciferase reporter (EVX-1 luciferase). ***_P_ < 0.001, unpaired
two-tailed _t_-test. Data represent biologically independent samples from cells transfected with mCherry (_n_ = 7) or dnTcf7l2 (_n_ = 8). FFL, firefly luciferase; RFU, relative fluorescent
units; RL, _Renilla_ luciferase. B, dnTcf7l2 reduced baseline and EX-4-induced increases in a cAMP-responsive reporter assay in PC12 cells (_F_1, 10 = 19.16, **_P_ < 0.0014, main effect
of ‘dnTcf7l2’; _F_1, 10 = 21.31, _P_ = 0.001, main effect of ‘EX-4’; _F_1, 10 = 0.027, _P_ = 0.87, ‘dnTcf7l2 × EX-4’ interaction; two-way ANOVA). Data represent biologically independent
samples from control cells (_n_ = 3 samples), control cells treated with EX-4 (_n_ = 3 samples), cells transfected dnTcf7l2 (_n_ = 4 samples) and cells transfected dnTcf7l2 and treated with
EX-4 (_n_ = 4 samples). C, dnTcf7l2 reduced baseline and EX-4-evoked increases in EVX-1-luciferase in INS-1 cells, an immortalized rat pancreatic β cell line that constitutively expresses
GLP-1 receptors. Data represent results from a single experiment. D, cAMP content of mHb, IPn and hippocampus were analysed in tissues from wild-type and mutant _Tcf7l2_ rats. Each sample
contained mHb tissue from 3 rats, and data are from 4 independent samples were analysed for a total of 12 rats per genotype. E, Vector map for the pGF-CREB-mCMV-dscGFP-P2A-luciferase (CREB
reporter) lentivirus. F, Brain slices containing the mHb from wild-type and mutant _Tcf7l2_ rats injected with CREB reporter lentivirus into mHb and injected with luciferin just before brain
collection. G, EX-4 increased luciferase activity in the mHb of wild-type (_n_ = 3) but not mutant (_n_ = 4) _Tcf7l2_ rats (_F_1, 11 = 9.398, _P_ = 0.0107, main effect of ‘genotype’; _F_6,
66 = 7.945, ***_P_ < 0.0001, interaction effect between ‘genotype’ and ‘EX-4’; two-way repeated-measures ANOVA). H, Pre-incubation (30 min) of HEK293T cells stably expressing α5α4β2
nAChRs with nicotine (0.1–10 μM) decreased the ability of acetylcholine (0.1 mM) to stimulate increases in [Ca2+]i (_F_3, 12 = 188.1, _P_ < 0.0001, main effect of ‘nicotine’ on one-way
ANOVA). Data represent _n_ = 4 independent experiment. I, 8-Br-cAMP (100–500 μM) attenuated the inhibitory effects of nicotine (0.1 μM) preincubation (30 min) on acetylcholine (0.1 mM)
evoked in increases in [Ca2+]i in α5α4β2 nAChR HEK293T cells (_F_1, 24 = 41.20, _P_ < 0.0001, main effect of ‘cAMP’ in two-way ANOVA). Data represent _n_ = 5 independent experiments. Data
are mean ± s.e.m. EXTENDED DATA FIG. 6 HYPERGLYCAEMIC ACTIONS OF NICOTINE. A, Venn diagram of differentially upregulated genes in the hippocampus, mHb and IPn of mutant _Tcf7l2_ rats
compared with wild-type rats. B, KEGG analysis of differentially upregulated genes identified processes relevant to glucose metabolism as those most likely to be perturbed in the mHb of
mutant _Tcf7l2_ rats (_n_ = 9) compared with wild-type rats (_n_ = 9). _P_ values determined by Fisher exact test. C, Blood glucose was measured before (T0) and 30 min after (T30) rats (_n_
= 7) were injected with saline or nicotine (1 mg kg−1) (_F_1, 13 = 52.3, ***_P_ < 0.0001, interaction effect between ‘nicotine’ and ‘time’; two-way repeated-measures ANOVA). D, Oxycodone
(2.5 mg kg−1) or cocaine (20 mg kg−1) injection had no effects on blood glucose in rats (_n_ = 36). E, _Chrna5-cre_ mice were injected into the IPn with FLEX-GFP (_n_ = 3) or
FLEX-hM3Dq-mCherry (_n_ = 9). Image adapted from the Allen Brain Reference Atlas. F, Blood glucose was measured in both groups of mice before and 30 min after injection of CNO (1 mg kg−1);
**_P_ < 0.0051, unpaired two-tailed _t_-test. G, _Tcf7l2_ mRNA expression was reduced in the mHb of rats after shRNA-mediated knockdown of _Glpr1_ transcript expression. **_P_ <
0.0051, unpaired two-tailed _t_-test. H, Atenolol abolished the hyperglycaemic response to experimenter-administered nicotine injection (1 mg kg−1) in rats (_n_ = 8) (‘atenolol’, _F_3, 25 =
43.54, _P_ < 0.0001; ‘time’, _F_2.406, 60.15 = 48.69, _P_ < 0.0001; ‘atenolol × time’ interaction, _F_9, 75 = 26.88; ***_P_ < 0.0001; two-way ANOVA). I, ICI118,551 abolished the
increases in blood glucose induced by experimenter-administered nicotine injection (1 mg kg−1) in rats (_n_ = 8) (‘nicotine’, _F_1, 7 = 50.83, _P_ = 0.002; ‘ICI118,551’, _F_1, 7 = 13.17, _P_
= 0.0084; ‘ICI118,551 × nicotine’ interaction, _F_1, 7 = 27.75, **_P_ = 0.0012; two-way repeated-measured ANOVA). J, Atenolol abolished the increases in blood glucose induced by CNO (3 mg
kg−1) in rats (_n_ = 8) expressing FLEX-hM3Dq in the mHb–IPn circuit (‘CNO’, _F_1, 8 = 213.0, _P_ < 0.0001; ‘atenolol’, _F_1, 8 = 27.00, _P_ = 0.0008; ‘CNO × atenolol’ interaction, _F_1,
8 = 255.5, ***_P_ < 0.0001; two-way repeated-measures ANOVA). K, Immunostaining for insulin (left), glucagon (middle) and their overlap (right) in mice treated acutely with saline (top;
_n_ = 3) or nicotine (0.5 mg kg−1; bottom; _n_ = 3). L–O, Quantification of insulin intensity (L), insulin relative area (M), glucagon intensity (N) and glucagon relative area (O) in
pancreatic islets from the saline-treated (_n_ = 3) and nicotine-treated (_n_ = 3) mice (**_P_ = 0.0059, ***_P_ < 0.001 _t_-test, unpaired two-tailed _t_-test). Image is representative of
results obtained in from three biologically independent animals in each treatment group. Data are mean ± s.e.m. EXTENDED DATA FIG. 7 CHEMOGENETIC STIMULATION OF THE HABENULA. A, Rats were
injected with AAV-retro-Cre into the IPn and FLEX-GFP or FLEX-hM3Dq-mCherry into the mHb. mCherry-positive cells were detected in the mHb, confirming that virus targeting was effective. B,
CNO (10 μM) had no effects on the relative spike frequency of mCherry-negative cells (_n_ = 4 cells, 2 rats). C, CNO (10 μM) increased the relative spike frequency of mCherry-positive cells
(_n_ = 4 cells, 3 rats) (*_P_ = 0.0124, unpaired two-tailed _t_-test). D, Nicotine (1 μM) increased the relative spike frequency of mHb neurons by a magnitude similar to that seen in
mCherry-positive neurons after CNO treatment (_n_ = 6 cells, 3 rats) (*_P_ = 0.042, unpaired two-tailed _t_-test). Data are mean ± s.e.m. EXTENDED DATA FIG. 8 PRV MAPPING OF POLYSYNAPTIC
PROJECTIONS FROM BRAIN TO PANCREAS AND LIVER. A, C, Images of a pRV-GFP-labelled cells (indicated by white arrows) and fibres in the mHb. B, D, Representative images of pRV-GFP-labelled IPn
neurons (indicated by white arrows). E, Images of GFP-labelled cells in hypothalamus, cortex, substantia nigra and nucleus of the solitary tract (NTS) after pancreas injection of pRV-GFP. R,
Images of a GFP-labelled cells in hypothalamus, ventral tegmental area (VTA) and NTS after liver injection of pRV-GFP. Note the absence of GFP-positive cells in the medial habenula. Images
are representative of results obtained from three separate experiments. Data are mean ± s.e.m. EXTENDED DATA FIG. 9 CONSEQUENCES OF HYPERGLYCAEMIC ACTIONS OF NICOTINE. A, Effects of glucose
(1 mg kg−1, intravenous) on nicotine (0.03 mg kg-1 per infusion) intake in rats (_n_ = 15). B, Effects of glucose (1 mg kg−1, intravenous) on nicotine (0.12 mg kg-1 per infusion) intake in
rats (_n_ = 16). C, Effects of glucose (2 mg kg−1, orally) on nicotine (0.12 mg kg-1 per infusion) intake in rats (_n_ = 16). D, Effects of glucagon (0.2 mg kg−1, intravenous) on nicotine
(0.12 mg kg−1 per infusion) intake in rats (_n_ = 5). E, Atenolol (10 mg kg−1) delivered before the self-administration on three consecutive days did not alter nicotine (0.12 mg kg−1 per
infusion) intake in rats (_n_ = 8). F, Scatter plots of average TRAP immunoprecipitation samples from sucrose-drinking (_y_ axis; _n_ = 28) versus immunoprecipitation samples from
sucrose-naive (_x_ axis; _n_ = 8) _ChAT__DW167_ mice representing increased (>0.5 log2-transformed fold change, magenta) or decreased (< −0.5 log2-transformed fold change, blue) levels
of transcripts undergoing translation (tissues from _n_ = 4 mice were pooled for each sample; 7 samples from sucrose-drinking and 2 samples from sucrose-naive mice were used).
Differentially expressed genes were identified by performing a negative binomial test using DESeq2, with default settings. Significant _P_ values were corrected to control the false
discovery rate of multiple testing according to the Benjamini–Hochberg procedure at 0.05 threshold and minimum threshold of 0.6 log2-transformed fold change. G, Expression levels
(_z_-score-transformed normalized counts) of the top 50 genes affected by sucrose consumption in mHb cholinergic neurons. H, KEGG analysis of differentially upregulated genes in the mHb of
_ChAT__DW167_ mice described in F identified pathways that are likely to be affected in the mHb by sucrose consumption. _P_ values determined by Fisher exact test. I, KEGG analysis of
differentially downregulated genes in the mHb of _ChAT__DW167_ mice described in F identified pathways that are likely to be affected in the mHb by sucrose consumption. _P_ values determined
by Fisher exact test. J, The frequency of action potentials in mHb neurons was unaltered by increasing the glucose concentrations in the extracellular solution from 12.5 to 30 mM (_n_ = 6
cells from 3 rats). K, Maintaining glucose concentration in artificial cerebrospinal fluid (aCSF) in the extracellular solution at 12.5 mM did not alter the magnitude of nicotine-evoked
nAChR currents in mHb neurons (_n_ = 6 cells from 3 rats). L, Increasing the glucose concentration in the extracellular solution from 12.5 to 30 mM decreased the magnitude of nicotine (1 μM)
evoked nAChR currents in mHb neurons (_n_ = 6 cells from 3 rats) (*_P_ < 0.0121, unpaired two-tailed _t_-test). M, Blood glucose levels measured in rats 24 h after their final nicotine
(0.12 mg kg−1 per infusion; _n_ = 7) or saline (_n_ = 8) self-administration session (*_P_ < 0.0223, unpaired two-tailed _t_-test). N, Blood glucose levels measured in rats 6 weeks after
their final nicotine (0.12 mg kg−1 per infusion; _n_ = 7) or saline (_n_ = 8) self-administration session (*_P_ < 0.0371, unpaired two-tailed _t_-test). O, Body weights in post-saline
(_n_ = 8) and post-nicotine rats (_n_ = 6) measured 6 weeks after their final self-administration session. P, Fasting blood glucose levels in wild-type (_n_ = 14 in total) and mutant (_n_ =
14 in total) _Tcf7l2_ rats measured before chronic saline or nicotine injections commenced. Q, Circulating levels of glucagon in nicotine-naive wild-type (_n_ = 7) and mutant (_n_ = 7)
_Tcf7l2_ rats. R, Circulating levels of insulin in nicotine-naive wild-type (_n_ = 7) and mutant (_n_ = 7) _Tcf7l2_ rats. S, Circulating glucagon levels in wild-type (_n_ = 9 in total) and
mutant (_n_ = 10 in total) _Tcf7l2_ rats measured before chronic saline or nicotine injections ended (_F_1, 15 = 4.606, *_P_ < 0.0486, interaction effect of ‘genotype × nicotine’ in
two-way ANOVA). Data are mean ± s.e.m. Source data EXTENDED DATA FIG. 10 PROPOSED MECHANISM BY WHICH TCF7L2 REGULATES THE MOTIVATIONAL PROPERTIES OF NICOTINE AND ITS DISRUPTIVE EFFECTS ON
BLOOD GLUCOSE HOMEOSTASIS. A representation of a mHb neuron projecting monosynaptically to the IPn (both in blue), via the fasciculus retroflexus, and to the pancreas via a polysynaptic
pathway (broken line) is shown. The mHb neurons expresses nAChRs that are activated by nicotine and that undergo nicotine-induced desensitization. In wild-type rats, nAChRs rapidly recover
from desensitization by a process involving cAMP signalling. In mutant _Tcf7l2_ rats, cAMP signalling is compromised, which results in persistently desensitized nAChRs and diminished
sensitivity of mHb neurons to nicotine. When mHb neurons are activated by nicotine, IPn neurons are stimulated by mHb-derived acetylcholine and glutamate. This triggers nicotine avoidance
and a hyperglycaemic response, both of which are attenuated in mutant _Tcf7l2_ rats. After chronic exposure to the hyperglycaemic actions of nicotine, circulating levels of the
pancreas-derived hormones glucagon and insulin are increased, resulting in a diabetes-like disruption of glucose homeostasis. This diabetes-promoting action of nicotine is also attenuated in
mutant _Tcf7l2_ rats. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES This file contains the full-length uncropped Western blot images REPORTING SUMMARY SOURCE DATA SOURCE DATA FIG. 1
SOURCE DATA FIG. 2 SOURCE DATA FIG. 3 SOURCE DATA EXTENDED DATA FIG. 1 SOURCE DATA EXTENDED DATA FIG. 3 SOURCE DATA EXTENDED DATA FIG. 9 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Duncan, A., Heyer, M.P., Ishikawa, M. _et al._ Habenular TCF7L2 links nicotine addiction to diabetes. _Nature_ 574, 372–377 (2019).
https://doi.org/10.1038/s41586-019-1653-x Download citation * Received: 13 November 2018 * Accepted: 12 September 2019 * Published: 16 October 2019 * Issue Date: 17 October 2019 * DOI:
https://doi.org/10.1038/s41586-019-1653-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
Trending News
Myprotein is doing what it takes to turn into a full-blown health & fitness brandThe Hut Group (THG) got into the whey business and launched Myprotein two decades ago. Since originating in the UK, they...
Photo gallery: chinese soccer gets international------------------------- * * X.com * Facebook * E-Mail * * * X.com * Facebook * E-Mail * Messenger * WhatsApp * Dieser ...
Assam encounters: ‘arms smuggler’ gunned down by police in karimganjAn alleged arms smuggler was shot dead by the police in the wee hours of Tuesday in Assam’s Karimganj. The accused who ...
Hospitality graduates celebrate achievementNicola KalmarBroome Advertiser A group of young East Timorese women beamed with pride last week after completing their t...
Cop26 agreed rules on trading carbon emissions – but they’re fatally flawedOne surprise from COP26 – the latest UN climate change conference in Glasgow – was an agreement between world leaders on...
Latests News
Habenular tcf7l2 links nicotine addiction to diabetesABSTRACT Diabetes is far more prevalent in smokers than non-smokers, but the underlying mechanisms of vulnerability are ...
Rachel reeves urged to immediately u-turn on 'cruel farm tax'Jeremy Clarkson warned farmers had been “shafted” in Reeves’s historic Budget (Image: Getty) Labour’s “cruel farm tax” w...
‘effortless’ method to remove yellow pillow stains without bleachDirty pillows and pillowcases can lead to numerous issues such as bad odours and poor sleep hygiene. This can trigger al...
Gp shortage costs kimberley regionA report has revealed people living in the Kimberley have the worst access to general practitioners in the country, with...
Theft brings new heartache for motherJOSH ZIMMERMANSouth Western Times A Bunbury woman recovering from brain surgery is mourning the loss of her daughter for...