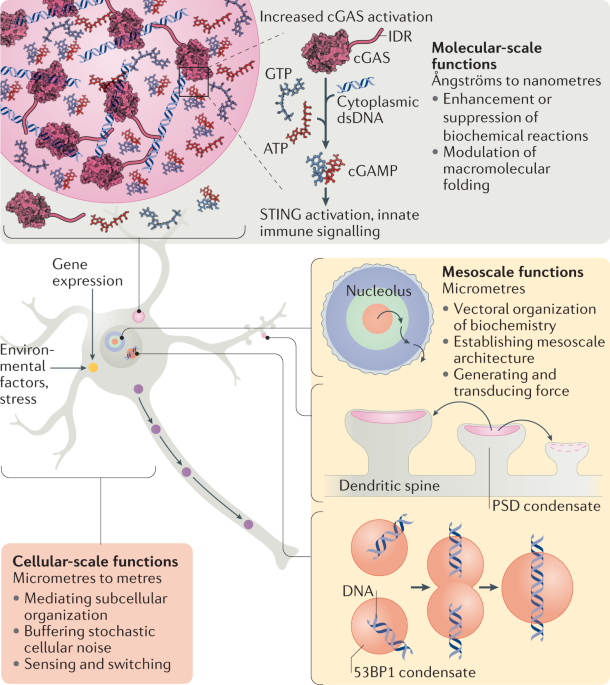
A framework for understanding the functions of biomolecular condensates across scales
A framework for understanding the functions of biomolecular condensates across scales"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Biomolecular condensates are found throughout eukaryotic cells, including in the nucleus, in the cytoplasm and on membranes. They are also implicated in a wide range of cellular
functions, organizing molecules that act in processes ranging from RNA metabolism to signalling to gene regulation. Early work in the field focused on identifying condensates and
understanding how their physical properties and regulation arise from molecular constituents. Recent years have brought a focus on understanding condensate functions. Studies have revealed
functions that span different length scales: from molecular (modulating the rates of chemical reactions) to mesoscale (organizing large structures within cells) to cellular (facilitating
localization of cellular materials and homeostatic responses). In this Roadmap, we discuss representative examples of biochemical and cellular functions of biomolecular condensates from the
recent literature and organize these functions into a series of non-exclusive classes across the different length scales. We conclude with a discussion of areas of current interest and
challenges in the field, and thoughts about how progress may be made to further our understanding of the widespread roles of condensates in cell biology. Access through your institution Buy
or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Access Nature and 54 other Nature Portfolio journals Get
Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12 print issues and online access $209.00 per year only
$17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout
ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS FUNCTIONAL SPECIFICITY IN
BIOMOLECULAR CONDENSATES REVEALED BY GENETIC COMPLEMENTATION Article 21 October 2024 BIOMOLECULAR CONDENSATES – EXTANT RELICS OR EVOLVING MICROCOMPARTMENTS? Article Open access 21 June 2023
RNA CONTRIBUTIONS TO THE FORM AND FUNCTION OF BIOMOLECULAR CONDENSATES Article 06 July 2020 REFERENCES * Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates:
organizers of cellular biochemistry. _Nat. Rev. Mol. Cell Biol._ 18, 285–298 (2017). CAS PubMed PubMed Central Google Scholar * Shin, Y. & Brangwynne, C. P. Liquid phase condensation
in cell physiology and disease. _Science_ 357, eaaf4382 (2017). PubMed Google Scholar * Banjade, S. & Rosen, M. K. Phase transitions of multivalent proteins can promote clustering of
membrane receptors. _eLife_ 3, e04123 (2014). PubMed Central Google Scholar * Li, P. et al. Phase transitions in the assembly of multivalent signalling proteins. _Nature_ 483, 336–340
(2012). CAS PubMed PubMed Central Google Scholar * Kato, M. et al. Cell-free formation of RNA granules: low complexity sequence domains form dynamic fibers within hydrogels. _Cell_ 149,
753–767 (2012). CAS PubMed PubMed Central Google Scholar * Gibson, B. A. et al. Organization of chromatin by intrinsic and regulated phase separation. _Cell_ 179, 470–484.e21 (2019). CAS
PubMed PubMed Central Google Scholar * Wang, J. et al. A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. _Cell_ 174, 688–699.e16
(2018). CAS PubMed PubMed Central Google Scholar * Martin, E. W. et al. Valence and patterning of aromatic residues determine the phase behavior of prion-like domains. _Science_ 367,
694–699 (2020). CAS PubMed PubMed Central Google Scholar * Lin, Y. H., Forman-Kay, J. D. & Chan, H. S. Theories for sequence-dependent phase behaviors of biomolecular condensates.
_Biochemistry_ 57, 2499–2508 (2018). CAS PubMed Google Scholar * Vernon, R. M. et al. Pi–Pi contacts are an overlooked protein feature relevant to phase separation. _eLife_ 7, e31486
(2018). PubMed PubMed Central Google Scholar * Jain, A. & Vale, R. D. RNA phase transitions in repeat expansion disorders. _Nature_ 546, 243–247 (2017). CAS PubMed PubMed Central
Google Scholar * Langdon, E. M. et al. mRNA structure determines specificity of a polyQ-driven phase separation. _Science_ 360, 922–927 (2018). CAS PubMed PubMed Central Google Scholar
* Nott, T. J. et al. Phase transition of a disordered nuage protein generates environmentally responsive membraneless organelles. _Mol. Cell_ 57, 936–947 (2015). CAS PubMed PubMed Central
Google Scholar * Banani, S. F. et al. Compositional control of phase-separated cell. bodies. _Cell_ 166, 651–663 (2016). CAS PubMed PubMed Central Google Scholar * Yoshizawa, T. et
al. Nuclear import receptor inhibits phase separation of FUS through binding to multiple sites. _Cell_ 173, 693–705.e22 (2018). CAS PubMed PubMed Central Google Scholar * Qamar, S. et
al. FUS phase separation is modulated by a molecular chaperone and methylation of arginine cation–pi interactions. _Cell_ 173, 720–734.e15 (2018). CAS PubMed PubMed Central Google Scholar
* Guo, L. et al. Nuclear-import receptors reverse aberrant phase transitions of RNA-binding proteins with prion-like domains. _Cell_ 173, 677–692.e20 (2018). CAS PubMed PubMed Central
Google Scholar * Hofweber, M. et al. Phase separation of FUS is suppressed by its nuclear import receptor and arginine methylation. _Cell_ 173, 706–719.e13 (2018). CAS PubMed Google
Scholar * Riback, J. A. et al. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. _Cell_ 168, 1028–1040.e19 (2017). THIS PAPER SHOWS THAT THE EXQUISITE
TEMPERATURE SENSITIVITY OF PAB1 CONDENSATION SERVES AS A CELLULAR-SCALE SENSOR FOR HEAT STRESS, COUPLED WITH MOLECULAR-SCALE CHANGES IN MRNA BINDING ACTIVITY THAT REGULATE THE HEAT SHOCK
RESPONSE. CAS PubMed PubMed Central Google Scholar * Franzmann, T. M. et al. Phase separation of a yeast prion protein promotes cellular fitness. _Science_ 359, eaao5654 (2018). PubMed
Google Scholar * Kato, M. et al. Redox state controls phase separation of the yeast ataxin-2 protein via reversible oxidation of its methionine-rich low-complexity domain. _Cell_ 177,
711–721.e8 (2019). CAS PubMed PubMed Central Google Scholar * Nedelsky, N. B. & Taylor, J. P. Bridging biophysics and neurology: aberrant phase transitions in neurodegenerative
disease. _Nat. Rev. Neurol._ 15, 272–286 (2019). PubMed Google Scholar * Alberti, S. & Dormann, D. Liquid–liquid phase separation in disease. _Annu. Rev. Genet._ 53, 171–194 (2019).
CAS PubMed Google Scholar * Berry, J., Brangwynne, C. P. & Haataja, M. Physical principles of intracellular organization via active and passive phase transitions. _Rep. Prog. Phys._
81, 046601 (2018). PubMed Google Scholar * Brangwynne, C. P., Tompa, P. & Pappu, R. V. Polymer physics of intracellular phase transitions. _Nat. Phys._ 11, 899–904 (2015). CAS Google
Scholar * Andersson, I. & Backlund, A. Structure and function of Rubisco. _Plant Physiol. Biochem._ 46, 275–291 (2008). CAS PubMed Google Scholar * Wunder, T., Oh, Z. G. &
Mueller-Cajar, O. CO2-fixing liquid droplets: towards a dissection of the microalgal pyrenoid. _Traffic_ 20, 380–389 (2019). CAS PubMed Google Scholar * Wang, H. et al. Rubisco condensate
formation by CcmM in β-carboxysome biogenesis. _Nature_ 566, 131–135 (2019). CAS PubMed Google Scholar * Oltrogge, L. M. et al. Multivalent interactions between CsoS2 and Rubisco mediate
α-carboxysome formation. _Nat. Struct. Mol. Biol._ 27, 281–287 (2020). CAS PubMed PubMed Central Google Scholar * Wunder, T., Cheng, S. L. H., Lai, S.-K., Li, H.-Y. & Mueller-Cajar,
O. The phase separation underlying the pyrenoid-based microalgal Rubisco supercharger. _Nat. Commun._ 9, 5076 (2018). PubMed PubMed Central Google Scholar * Freeman Rosenzweig, E. S. et
al. The eukaryotic CO2-concentrating organelle is liquid-like and exhibits dynamic reorganization. _Cell_ 171, 148–162.e19 (2017). TOGETHER WITH WANG ET AL. (_NATURE_, 2019), OLTROGGE ET AL.
(2020) AND WUNDER ET AL. (2018), THIS PAPER DEMONSTRATES HOW RUBISCO CONDENSATES CO-CONCENTRATE ENZYME AND SUBSTRATE TO ENHANCE REACTION RATES. CAS PubMed PubMed Central Google Scholar
* Giordano, M., Beardall, J. & Raven, J. A. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. _Annu. Rev. Plant. Biol._ 56, 99–131 (2005). CAS
PubMed Google Scholar * Kaplan, A. & Reinhold, L. CO2 concentrating mechanisms in photosynthetic microorganisms. _Annu. Rev. Plant. Physiol. Plant Mol. Biol._ 50, 539–570 (1999). CAS
PubMed Google Scholar * Nishimura, T., Yamaguchi, O., Takatani, N., Maeda, S. & Omata, T. In vitro and in vivo analyses of the role of the carboxysomal β-type carbonic anhydrase of the
cyanobacterium _Synechococcus elongatus_ in carboxylation of ribulose-1,5-bisphosphate. _Photosynth. Res._ 121, 151–157 (2014). CAS PubMed Google Scholar * Dou, Z. et al. CO2 fixation
kinetics of _Halothiobacillus neapolitanus_ mutant carboxysomes lacking carbonic anhydrase suggest the shell acts as a diffusional barrier for CO2. _J. Biol. Chem._ 283, 10377–10384 (2008).
CAS PubMed Google Scholar * Varesio, L. M., Willett, J. W., Fiebig, A. & Crosson, S. A carbonic anhydrase pseudogene sensitizes select brucella lineages to low CO2 tension. _J.
Bacteriol._ 201, e00509-19 (2019). CAS PubMed PubMed Central Google Scholar * Du, M. & Chen, Z. J. DNA-induced liquid phase condensation of cGAS activates innate immune signaling.
_Science_ 361, 704–709 (2018). THIS PAPER DEMONSTRATES THE FORMATION OF PHASE-SEPARATED CGAS CONDENSATES UPON INTRODUCTION OF DOUBLE-STRANDED DNA, LEADING TO ENHANCED CGAS PRODUCTION. CAS
PubMed Google Scholar * Zhang, X. et al. The cytosolic DNA sensor cGAS forms an oligomeric complex with DNA and undergoes switch-like conformational changes in the activation loop. _Cell
Rep._ 6, 421–430 (2014). CAS PubMed PubMed Central Google Scholar * Wu, J. et al. Cyclic GMP–AMP is an endogenous second messenger in innate immune signaling by cytosolic DNA. _Science_
339, 826–830 (2013). CAS PubMed Google Scholar * Sheu-Gruttadauria, J. & MacRae, I. J. Phase transitions in the assembly and function of human miRISC. _Cell_ 173, 946–957 e916 (2018).
THIS PAPER SHOWS THAT CONDENSATES FORMED BY COMPONENTS OF THE RNA-INDUCED SILENCING COMPLEX ENHANCE RATES OF MRNA DEADENYLATION. CAS PubMed PubMed Central Google Scholar * Chu, C. Y.
& Rana, T. M. Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. _PLoS Biol._ 4, e210 (2006). PubMed PubMed Central Google Scholar * Eulalio,
A., Behm-Ansmant, I., Schweizer, D. & Izaurralde, E. P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. _Mol. Cell Biol._ 27, 3970–3981 (2007). CAS PubMed
PubMed Central Google Scholar * Brouhard, G. J. & Rice, L. M. Microtubule dynamics: an interplay of biochemistry and mechanics. _Nat. Rev. Mol. Cell Biol._ 19, 451–463 (2018). CAS
PubMed PubMed Central Google Scholar * Woodruff, J. B. et al. The centrosome is a selective condensate that nucleates microtubules by concentrating tubulin. _Cell_ 169, 1066–1077.e10
(2017). THIS PAPER SHOWS THAT THE CENTROSOME IS A LIQUID-LIKE COMPARTMENT THAT NUCLEATES MICROTUBULES THROUGH CONCENTRATION OF TUBULIN AND MICROTUBULE REGULATORY PROTEINS. CAS PubMed
Google Scholar * King, M. R. & Petry, S. Phase separation of TPX2 enhances and spatially coordinates microtubule nucleation. _Nat. Commun._ 11, 270 (2020). CAS PubMed PubMed Central
Google Scholar * Jiang, H. et al. Phase transition of spindle-associated protein regulate spindle apparatus assembly. _Cell_ 163, 108–122 (2015). CAS PubMed PubMed Central Google Scholar
* Huang, Y. et al. Aurora A activation in mitosis promoted by BuGZ. _J. Cell Biol._ 217, 107–116 (2018). CAS PubMed PubMed Central Google Scholar * Schwayer, C. et al. Mechanosensation
of tight junctions depends on ZO-1 phase separation and flow. _Cell_ 179, 937–952.e18 (2019). CAS PubMed Google Scholar * Beutel, O., Maraspini, R., Pombo-García, K., Martin-Lemaitre, C.
& Honigmann, A. Phase separation of zonula occludens proteins drives formation of tight junctions. _Cell_ 179, 923–936.e11 (2019). TOGETHER WITH SCHWAYER ET AL. (2019), THIS PAPER
DEMONSTRATES THAT PHASE SEPARATION CONTRIBUTES TO THE FORMATION OF CELL ADHESIVE TIGHT JUNCTIONS, WITH CONDENSATES CONTRIBUTING TO MECHANOSENSATION. CAS PubMed Google Scholar * Peeples,
W. & Rosen, M. K. Phase separation can increase enzyme activity by concentration and molecular organization. Preprint at _bioRxiv_
https://www.biorxiv.org/content/10.1101/2020.09.15.299115v1 (2020). * Hirose, T. et al. NEAT1 long noncoding RNA regulates transcription via protein sequestration within subnuclear bodies.
_Mol. Biol. Cell_ 25, 169–183 (2014). PubMed PubMed Central Google Scholar * Powers, S. K. et al. Nucleo-cytoplasmic partitioning of ARF proteins controls auxin responses in _Arabidopsis
thaliana_. _Mol. Cell_ 76, 177–190.e5 (2019). CAS PubMed PubMed Central Google Scholar * Chakraborty, A. K. & Weiss, A. Insights into the initiation of TCR signaling. _Nat. Immunol._
15, 798–807 (2014). CAS PubMed PubMed Central Google Scholar * Su, X. et al. Phase separation of signaling molecules promotes T cell receptor signal transduction. _Science_ 352, 595–599
(2016). THIS PAPER DEMONSTRATES THAT THE CORE TCR SIGNALLING COMPONENTS PHASE-SEPARATE IN VITRO AND THE CLUSTERING INDUCES LOCALIZED ACTIN POLYMERIZATION. CAS PubMed PubMed Central
Google Scholar * Douglass, A. D. & Vale, R. D. Single-molecule microscopy reveals plasma membrane microdomains created by protein–protein networks that exclude or trap signaling
molecules in T cells. _Cell_ 121, 937–950 (2005). CAS PubMed PubMed Central Google Scholar * Zeng, M. et al. Reconstituted postsynaptic density as a molecular platform for understanding
synapse formation and plasticity. _Cell_ 174, 1172–1187.e16 (2018). CAS PubMed Google Scholar * Zhu, J., Shang, Y. & Zhang, M. Mechanistic basis of MAGUK-organized complexes in
synaptic development and signalling. _Nat. Rev. Neurosci._ 17, 209–223 (2016). CAS PubMed Google Scholar * Wei, M.-T. et al. Phase behaviour of disordered proteins underlying low density
and high permeability of liquid organelles. _Nat. Chem._ 9, 1118–1125 (2017). CAS PubMed Google Scholar * Huang, W. Y. C. et al. Phosphotyrosine-mediated LAT assembly on membranes drives
kinetic bifurcation in recruitment dynamics of the Ras activator SOS. _Proc. Natl Acad. Sci. USA_ 113, 8218–8223 (2016). CAS PubMed PubMed Central Google Scholar * Case, L. B., Zhang,
X., Ditlev, J. A. & Rosen, M. K. Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. _Science_ 363, 1093–1097 (2019). CAS PubMed PubMed Central
Google Scholar * Huang, W. Y. C. et al. A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. _Science_ 363, 1098–1103 (2019). TOGETHER WITH CASE ET
AL. (2019), THIS PAPER DEMONSTRATES HOW THE DWELL TIME OF MOLECULES WITHIN MEMBRANE-ASSOCIATED CONDENSATES CAN LEAD TO INCREASED ACTIVITY THROUGH A KINETIC PROOFREADING MECHANISM. CAS
PubMed PubMed Central Google Scholar * Lafontaine, D. L. J., Riback, J. A., Bascetin, R. & Brangwynne, C. P. The nucleolus as a multiphase liquid condensate. _Nat. Rev. Mol. Cell
Biol._ https://doi.org/10.1038/s41580-020-0272-6 (2020). Article PubMed Google Scholar * Kim, T. H. et al. Phospho-dependent phase separation of FMRP and CAPRIN1 recapitulates regulation
of translation and deadenylation. _Science_ 365, 825–829 (2019). THIS PAPER SHOWS A COUNTER-INTUITIVE ACCELERATION OF A REACTION IN A MULTIPHASE CONDENSATE WHERE THE ENZYME AND SUBSTRATE ARE
SUBSTANTIALLY ENRICHED IN THE DIFFERENT PHASES. CAS PubMed Google Scholar * Bouchard, J. J. et al. Cancer mutations of the tumor suppressor SPOP disrupt the formation of active,
phase-separated compartments. _Mol. Cell_ 72, 19–36.e8 (2018). CAS PubMed PubMed Central Google Scholar * Ma, W. & Mayr, C. A membraneless organelle associated with the endoplasmic
reticulum enables 3′ UTR-mediated protein–protein interactions. _Cell_ 175, 1492–1506.e19 (2018). CAS PubMed PubMed Central Google Scholar * Frottin, F. et al. The nucleolus functions as
a phase-separated protein quality control compartment. _Science_ 365, 342–347 (2019). THIS PAPER IDENTIFIES A NOVEL, MOLECULAR-SCALE ACTIVITY OF THE NUCLEOLUS IN REGULATING THE FOLDING
STATE OF NUCLEAR PROTEINS UNDER HEAT STRESS. CAS PubMed Google Scholar * Kedersha, N. & Anderson, P. in _Progress in Molecular Biology and Translational Science_ Vol. 90 155–185
(Academic, 2009). * Kedersha, N. et al. G3BP–Caprin1–USP10 complexes mediate stress granule condensation and associate with 40S subunits. _J. Cell Biol._ 212, e201508028 (2016). Google
Scholar * Matsuki, H. et al. Both G3BP1 and G3BP2 contribute to stress granule formation. _Genes Cell_ 18, 135–146 (2013). CAS Google Scholar * Yang, P. et al. G3BP1 is a tunable switch
that triggers phase separation to assemble stress granules. _Cell_ 181, 325–345.e28 (2020). CAS PubMed PubMed Central Google Scholar * Sanders, D. W. et al. Competing protein–RNA
interaction networks control multiphase intracellular organization. _Cell_ 181, 306–324.e28 (2020). CAS PubMed PubMed Central Google Scholar * Guillen-Boixet, J. et al. RNA-induced
conformational switching and clustering of G3BP drive stress granule assembly by condensation. _Cell_ 181, 346–361.e17 (2020). TOGETHER WITH YANG ET AL. (2020) AND SANDERS ET AL. (2020),
THIS PAPER DEMONSTRATES THE IMPORTANCE OF G3BP1 IN FORMING STRESS GRANULES, WHICH CAN PREVENT RNA ENTANGLEMENT AND AGGREGATION. CAS PubMed PubMed Central Google Scholar * Hondele, M. et
al. DEAD-box ATPases are global regulators of phase-separated organelles. _Nature_ 573, 144–148 (2019). CAS PubMed Google Scholar * Nott, T. J., Craggs, T. D. & Baldwin, A. J.
Membraneless organelles can melt nucleic acid duplexes and act as biomolecular filters. _Nat. Chem._ 8, 569–575 (2016). CAS PubMed Google Scholar * Küffner, A. M. et al. Acceleration of
an enzymatic reaction in liquid phase separated compartments based on intrinsically disordered protein domains. _ChemSystemsChem_ 2, e2000001 (2020). Google Scholar * Feric, M. et al.
Coexisting liquid phases underlie nucleolar subcompartments. _Cell_ 165, 1686–1697 (2016). THIS PAPER SHOWS THAT THE MATERIAL PROPERTIES OF CONDENSATES FORMED BY NUCLEOLAR PROTEINS RESULTS
IN A MULTICOMPARTMENT STRUCTURE THAT CONTRIBUTES TO VECTORAL ORGANIZATION OF RIBOSOME ASSEMBLY REACTIONS. CAS PubMed PubMed Central Google Scholar * Yao, R.-W. et al. Nascent pre-rRNA
sorting via phase separation drives the assembly of dense fibrillar components in the human nucleolus. _Mol. Cell_ 76, 767–783.e11 (2019). CAS PubMed Google Scholar * Zhu, L. et al.
Controlling the material properties and rRNA processing function of the nucleolus using light. _Proc. Natl Acad. Sci. USA_ 116, 17330–17335 (2019). CAS PubMed PubMed Central Google
Scholar * Mitrea, D. M. et al. Self-interaction of NPM1 modulates multiple mechanisms of liquid–liquid phase separation. _Nat. Commun._ 9, 842 (2018). PubMed PubMed Central Google Scholar
* Wan, G. et al. Spatiotemporal regulation of liquid-like condensates in epigenetic inheritance. _Nature_ 557, 679–683 (2018). THIS PAPER IDENTIFIES A NOVEL CONDENSATE COMPOSED OF THREE
SEPARATE, COEXISTING PHASES INVOLVED IN TRANSGENERATIONAL EPIGENETIC INHERITANCE OF RNAI, POTENTIALLY FUNCTIONING VIA VECTORAL ORGANIZATION OF BIOCHEMISTRY SIMILAR TO THE NUCLEOLUS. CAS
PubMed PubMed Central Google Scholar * Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. _Science_ 324, 1729–1732
(2009). CAS PubMed Google Scholar * Phillips, C. M., Montgomery, T. A., Breen, P. C. & Ruvkun, G. MUT-16 promotes formation of perinuclear Mutator foci required for RNA silencing in
the _C. elegans_ germline. _Genes. Dev._ 26, 1433–1444 (2012). CAS PubMed PubMed Central Google Scholar * Tibble, R. W., Depaix, A., Kowalska, J., Jemielity, J. & Gross, J. D.
Biomolecular condensates amplify mRNA decapping by coupling protein interactions with conformational changes in Dcp1/Dcp2. Preprint at _bioRxiv_
https://www.biorxiv.org/content/10.1101/2020.07.09.195057v1 (2020). * Wu, X. et al. RIM and RIM-BP form presynaptic active-zone-like condensates via phase separation. _Mol. Cell_ 73,
971–984.e5 (2019). CAS PubMed Google Scholar * Zeng, M. et al. Phase transition in postsynaptic densities underlies formation of synaptic complexes and synaptic plasticity. _Cell_ 166,
1163–1175.e12 (2016). TOGETHER WITH ZENG ET AL. (2018) AND WU ET AL. (2019), THIS PAPER DEMONSTRATES THE ROLES OF CONDENSATE FORMATION IN REGULATING THE MESOSCALE ARCHITECTURE OF THE PSD AND
PRESYNAPTIC ACTIVE ZONES. CAS PubMed PubMed Central Google Scholar * Südhof, T. C. The presynaptic active zone. _Neuron_ 75, 11–25 (2012). PubMed PubMed Central Google Scholar *
Boke, E. et al. Amyloid-like self-assembly of a cellular compartment. _Cell_ 166, 637–650 (2016). CAS PubMed PubMed Central Google Scholar * Rebane, A. A. et al. Liquid–liquid phase
separation of the Golgi matrix protein GM130. _FEBS Lett._ 594, 1132–1144 (2020). CAS PubMed Google Scholar * Maser, R. S., Monsen, K. J., Nelms, B. E. & Petrini, J. H. hMre11 and
hRad50 nuclear foci are induced during the normal cellular response to DNA double-strand breaks. _Mol. Cell Biol._ 17, 6087–6096 (1997). CAS PubMed PubMed Central Google Scholar * Haaf,
T., Golub, E. I., Reddy, G., Radding, C. M. & Ward, D. C. Nuclear foci of mammalian Rad51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal
complexes. _Proc. Natl Acad. Sci. USA_ 92, 2298–2302 (1995). CAS PubMed PubMed Central Google Scholar * Singatulina, A. S. et al. PARP-1 activation directs FUS to DNA damage sites to
form PARG-reversible compartments enriched in damaged DNA. _Cell Rep._ 27, 1809–1821.e5 (2019). THIS PAPER SHOWS THAT CO-PHASE SEPARATION OF FUS AND PAR AT SITES OF DNA DAMAGE ORGANIZES A
MESOSCALE DNA REPAIR COMPARTMENT. CAS PubMed Google Scholar * Patel, A. et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. _Cell_ 162,
1066–1077 (2015). CAS PubMed Google Scholar * Altmeyer, M. et al. Liquid demixing of intrinsically disordered proteins is seeded by poly(ADP-ribose). _Nat. Commun._ 6, 8088 (2015). CAS
PubMed Google Scholar * Bunting, S. F. et al. 53BP1 inhibits homologous recombination in Brca1-deficient cells by blocking resection of DNA breaks. _Cell_ 141, 243–254 (2010). CAS PubMed
PubMed Central Google Scholar * Kilic, S. et al. Phase separation of 53BP1 determines liquid-like behavior of DNA repair compartments. _Embo J._ 38, e101379 (2019). PubMed PubMed
Central Google Scholar * Pessina, F. et al. Functional transcription promoters at DNA double-strand breaks mediate RNA-driven phase separation of damage-response factors. _Nat. Cell Biol._
21, 1286–1299 (2019). THIS PAPER SHOWS THAT PHASE SEPARATION APPEARS TO BE INVOLVED IN THE TURNOVER OF DNA REPAIR FOCI AS LIQUID, REVERSIBLE PROPERTIES ALLOW DISPERSAL UPON RESOLUTION OF
DAMAGE. CAS PubMed PubMed Central Google Scholar * Shin, Y. et al. Liquid nuclear condensates mechanically sense and restructure the genome. _Cell_ 175, 1481–1491.e13 (2018). CAS PubMed
PubMed Central Google Scholar * Oshidari, R. et al. DNA repair by Rad52 liquid droplets. _Nat. Commun._ 11, 695 (2020). CAS PubMed PubMed Central Google Scholar * Parker, M. W. et
al. A new class of disordered elements controls DNA replication through initiator self-assembly. _eLife_ 8, e48562 (2019). CAS PubMed PubMed Central Google Scholar * Xiang, W. et al.
Correlative live and super-resolution imaging reveals the dynamic structure of replication domains. _J. Cell Biol._ 217, 1973–1984 (2018). CAS PubMed PubMed Central Google Scholar *
Cook, P. R. The organization of replication and transcription. _Science_ 284, 1790–1795 (1999). CAS PubMed Google Scholar * Wang, Z. & Zhang, H. Phase separation, transition, and
autophagic degradation of proteins in development and pathogenesis. _Trends Cell Biol._ 29, 417–427 (2019). CAS PubMed Google Scholar * Sun, D., Wu, R., Zheng, J., Li, P. & Yu, L.
Polyubiquitin chain-induced p62 phase separation drives autophagic cargo segregation. _Cell Res._ 28, 405–415 (2018). CAS PubMed PubMed Central Google Scholar * Yamasaki, A. et al.
Liquidity is a critical determinant for selective autophagy of protein condensates. _Mol. Cell_ 77, 1163–1175.e9 (2020). CAS PubMed Google Scholar * Fujioka, Y. et al. Phase separation
organizes the site of autophagosome formation. _Nature_ 578, 301–305 (2020). TOGETHER WITH SUN ET AL. (2018) AND YAMASAKI ET AL. (2020), THIS PAPER DEMONSTRATE HOW PHASE SEPARATION CREATES
MESOSCALE, DISCRETE STRUCTURES THAT CAN BE ENGULFED BY AUTOPHAGOSOMES. CAS PubMed Google Scholar * Bergeron-Sandoval, L.-P. et al. Endocytosis caused by liquid–liquid phase separation of
proteins. Preprint at _bioRxiv_ https://www.biorxiv.org/content/10.1101/145664v3 (2018). * Weatheritt, R. J., Gibson, T. J. & Babu, M. M. Asymmetric mRNA localization contributes to
fidelity and sensitivity of spatially localized systems. _Nat. Struct. Mol. Biol._ 21, 833–839 (2014). CAS PubMed PubMed Central Google Scholar * Maday, S., Twelvetrees, A. E.,
Moughamian, A. J. & Holzbaur, E. L. Axonal transport: cargo-specific mechanisms of motility and regulation. _Neuron_ 84, 292–309 (2014). CAS PubMed PubMed Central Google Scholar *
Ishizuka, N., Cowan, W. M. & Amaral, D. G. A quantitative analysis of the dendritic organization of pyramidal cells in the rat hippocampus. _J. Comp. Neurol._ 362, 17–45 (1995). CAS
PubMed Google Scholar * Donnelly, C. J., Fainzilber, M. & Twiss, J. L. Subcellular communication through RNA transport and localized protein synthesis. _Traffic_ 11, 1498–1505 (2010).
CAS PubMed PubMed Central Google Scholar * Gopal, P. P., Nirschl, J. J., Klinman, E. & Holzbaur, E. L. Amyotrophic lateral sclerosis-linked mutations increase the viscosity of
liquid-like TDP-43 RNP granules in neurons. _Proc. Natl Acad. Sci. USA_ 114, E2466–E2475 (2017). CAS PubMed PubMed Central Google Scholar * Holt, C. E. & Schuman, E. M. The central
dogma decentralized: new perspectives on RNA function and local translation in neurons. _Neuron_ 80, 648–657 (2013). CAS PubMed PubMed Central Google Scholar * Liao, Y. C. et al. RNA
granules hitchhike on lysosomes for long-distance transport, using annexin A11 as a molecular tether. _Cell_ 179, 147–164.e20 (2019). THIS PAPER DEMONSTRATES HOW RIBONUCLEOPROTEIN
CONDENSATES ‘HITCHHIKE’ ON MEMBRANOUS ORGANELLES FOR LONG-DISTANCE TRANSPORT IN NEURONS. CAS PubMed PubMed Central Google Scholar * Meaburn, K. J. & Misteli, T. Cell biology:
chromosome territories. _Nature_ 445, 379–781 (2007). CAS PubMed Google Scholar * Bintu, B. et al. Super-resolution chromatin tracing reveals domains and cooperative interactions in
single cells. _Science_ 362, eaau1783 (2018). PubMed PubMed Central Google Scholar * Lieberman-Aiden, E. et al. Comprehensive mapping of long-range interactions reveals folding principles
of the human genome. _Science_ 326, 289–293 (2009). CAS PubMed PubMed Central Google Scholar * Rao, S. S. P. et al. Cohesin loss eliminates all loop domains. _Cell_ 171, 305–320.e24
(2017). CAS PubMed PubMed Central Google Scholar * Larson, A. G. et al. Liquid droplet formation by HP1α suggests a role for phase separation in heterochromatin. _Nature_ 547, 236–240
(2017). CAS PubMed PubMed Central Google Scholar * Sanulli, S. et al. HP1 reshapes nucleosome core to promote phase separation of heterochromatin. _Nature_ 575, 390–394 (2019). CAS
PubMed PubMed Central Google Scholar * Strom, A. R. et al. Phase separation drives heterochromatin domain formation. _Nature_ 547, 241–245 (2017). THIS PAPER SHOWS THAT PHASE SEPARATION
OF HP1Α APPEARS TO PLAY A ROLE IN HETEROCHROMATIN FORMATION (BUT SEE ALSO ERDEL ET AL. (2020)). CAS PubMed PubMed Central Google Scholar * Zaidi, S. K. et al. Mitotic bookmarking of
genes: a novel dimension to epigenetic control. _Nat. Rev. Genet._ 11, 583–589 (2010). CAS PubMed PubMed Central Google Scholar * Palozola, K. C., Lerner, J. & Zaret, K. S. A
changing paradigm of transcriptional memory propagation through mitosis. _Nat. Rev. Mol. Cell Biol._ 20, 55–64 (2019). CAS PubMed PubMed Central Google Scholar * Liu, X. et al. Mitotic
implantation of the transcription factor prospero via phase separation drives terminal neuronal differentiation. _Dev. Cell_ 52, 277–293.e8 (2020). THIS PAPER DEMONSTRATES HOW A CONDENSATE
COMPOSED OF THE PROSPERO TRANSCRIPTION FACTOR LOCALIZES TO SPECIFIC REGIONS OF THE GENOME EVEN THROUGH MITOSIS TO FACILITATE RAPID FORMATION OF HETEROCHROMATIN FOLLOWING MITOSIS IN NEURAL
PROGENITOR CELLS, DRIVING TERMINAL NEURONAL DIFFERENTIATION. CAS PubMed Google Scholar * Eldar, A. & Elowitz, M. B. Functional roles for noise in genetic circuits. _Nature_ 467,
167–173 (2010). CAS PubMed PubMed Central Google Scholar * Stoeger, T., Battich, N. & Pelkmans, L. Passive noise filtering by cellular compartmentalization. _Cell_ 164, 1151–1161
(2016). CAS PubMed Google Scholar * Dill, K. A. & Bromberg, S. _Molecular Driving Forces. Statistical Thermodynamics in Biology, Chemistry, Physics, and Nanoscience_ 2nd edn (Garland
Science, 2010). * Klosin, A. et al. Phase separation provides a mechanism to reduce noise in cells. _Science_ 367, 464–468 (2020). THIS PAPER PROVIDES THEORETICAL AND EXPERIMENTAL EVIDENCE
THAT PHASE SEPARATION MAY PROVIDE A MEANS OF REDUCING FLUCTUATIONS IN PROTEIN CONCENTRATION, THEREBY INCREASING ROBUSTNESS OF CELLULAR PROCESSES. CAS PubMed Google Scholar * Riback, J. A.
et al. Composition-dependent thermodynamics of intracellular phase separation. _Nature_ 581, 209–214 (2020). THIS PAPER DEMONSTRATES THE COMPLEXITY OF MULTICOMPONENT PHASE-SEPARATED
CONDENSATES, WHERE SATURATION CONCENTRATIONS ARE NOT FIXED BUT VARY BASED ON CONCENTRATIONS OF CONDENSATE COMPONENTS. CAS PubMed PubMed Central Google Scholar * Ruff, K. M., Roberts, S.,
Chilkoti, A. & Pappu, R. V. Advances in understanding stimulus-responsive phase behavior of intrinsically disordered protein polymers. _J. Mol. Biol._ 430, 4619–4635 (2018). CAS PubMed
Google Scholar * Hyman, A. A., Weber, C. A. & Julicher, F. Liquid–liquid phase separation in biology. _Annu. Rev. Cell Dev. Biol._ 30, 39–58 (2014). CAS PubMed Google Scholar *
Yoo, H., Triandafillou, C. & Drummond, D. A. Cellular sensing by phase separation: using the process, not just the products. _J. Biol. Chem._ 294, 7151–7159 (2019). CAS PubMed PubMed
Central Google Scholar * Sengupta, P. & Garrity, P. Sensing temperature. _Curr. Biol._ 23, R304–R307 (2013). CAS PubMed PubMed Central Google Scholar * Lindquist, S. &
Petersen, R. Selective translation and degradation of heat-shock messenger RNAs in _Drosophila_. _Enzyme_ 44, 147–166 (1990). CAS PubMed Google Scholar * Iserman, C. et al. Condensation
of Ded1p promotes a translational switch from housekeeping to stress protein production. _Cell_ 181, 818–831.e19 (2020). THIS PAPER SHOWS THAT STRESS-INDUCED CONDENSATE FORMATION SWITCHES
THE CELLULAR TRANSLATIONAL REGULATORY REGIME TO MOUNT A HOMEOSTATIC RESPONSE, AND PROVIDES EVIDENCE THAT EVOLUTIONARY PROCESSES HAVE TUNED CONDENSATE FORMATION AT TEMPERATURES SUITING
ORGANISMS’ ENVIRONMENTS. CAS PubMed PubMed Central Google Scholar * Delarue, M. et al. mTORC1 controls phase separation and the biophysical properties of the cytoplasm by tuning
crowding. _Cell_ 173, 338–349.e20 (2018). THIS PAPER SHOWS THAT MTORC INHIBITION LEADS TO REDUCED RIBOSOME ABUNDANCE AND DECREASED CYTOPLASMIC CROWDING, IMPACTING THE SATURATION
CONCENTRATION FOR CONDENSATE FORMATION. Google Scholar * Xing, W., Muhlrad, D., Parker, R. & Rosen, M. K. A quantitative inventory of yeast P body proteins reveals principles of
composition and specificity. _eLife_ 9, e56525 (2020). CAS PubMed PubMed Central Google Scholar * Jain, S. et al. ATPase-modulated stress granules contain a diverse proteome and
substructure. _Cell_ 164, 487–498 (2016). CAS PubMed PubMed Central Google Scholar * Markmiller, S. et al. Context-dependent and disease-specific diversity in protein interactions within
stress granules. _Cell_ 172, 590–604.e13 (2018). CAS PubMed PubMed Central Google Scholar * Youn, J. Y. et al. High-density proximity mapping reveals the subcellular organization of
mRNA-associated granules and bodies. _Mol. Cell_ 69, 517–532.e11 (2018). CAS PubMed Google Scholar * Hubstenberger, A. et al. P-body purification reveals the condensation of repressed
mRNA regulons. _Mol. Cell_ 68, 144–157.e5 (2017). TOGETHER WITH XING ET AL. (2020), JAIN ET AL. (2016), MARKMILLER ET AL. (2018) AND YOUN ET AL. (2018), THIS PAPER CHARACTERIZES THE
COMPLEXITY OF RNA GRANULES AND BODIES, SETTING THE STAGE FOR FUTURE WORK IN UNDERSTANDING HOW INTERACTIONS BETWEEN NUMEROUS COMPONENTS GOVERN THE FUNCTION OF HIGHLY COMPLEX NATIVE
CONDENSATES. CAS PubMed Google Scholar * McSwiggen, D. T., Mir, M., Darzacq, X. & Tjian, R. Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences.
_Genes Dev._ 33, 1619–1634 (2019). CAS PubMed PubMed Central Google Scholar * Teixeira, D., Sheth, U., Valencia-Sanchez, M. A., Brengues, M. & Parker, R. Processing bodies require
RNA for assembly and contain nontranslating mRNAs. _RNA_ 11, 371–382 (2005). CAS PubMed PubMed Central Google Scholar * Wheeler, R. J. & Hyman, A. A. Controlling compartmentalization
by non-membrane-bound organelles. _Philos. Trans. R. Soc. B_ 373, 20170193 (2018). Google Scholar * Alberti, S., Gladfelter, A. & Mittag, T. Considerations and challenges in studying
liquid–liquid phase separation and biomolecular condensates. _Cell_ 176, 419–434 (2019). CAS PubMed PubMed Central Google Scholar * Soding, J., Zwicker, D., Sohrabi-Jahromi, S.,
Boehning, M. & Kirschbaum, J. Mechanisms for active regulation of biomolecular condensates. _Trends Cell Biol._ 30, 4–14 (2020). PubMed Google Scholar * Bratek-Skicki, A., Pancsa, R.,
Meszaros, B., Van Lindt, J. & Tompa, P. A guide to regulation of the formation of biomolecular condensates. _FEBS J._ 287, 1924–1935 (2020). CAS PubMed Google Scholar * Choi, J. M.,
Dar, F. & Pappu, R. V. LASSI: a lattice model for simulating phase transitions of multivalent proteins. _PLoS Comput. Biol._ 15, e1007028 (2019). CAS PubMed PubMed Central Google
Scholar * Cai, L. H., Panyukov, S. & Rubinstein, M. Mobility of nonsticky nanoparticles in polymer liquids. _Macromolecules_ 44, 7853–7863 (2011). CAS PubMed PubMed Central Google
Scholar * Cho, W. K. et al. Mediator and RNA polymerase II clusters associate in transcription-dependent condensates. _Science_ 361, 412–415 (2018). CAS PubMed PubMed Central Google
Scholar * Sabari, B. R. et al. Coactivator condensation at super-enhancers links phase separation and gene control. _Science_ 361, eaar3958 (2018). PubMed PubMed Central Google Scholar *
Mir, M. et al. Dynamic multifactor hubs interact transiently with sites of active transcription in _Drosophila_ embryos. _eLife_ 7, e40497 (2018). TOGETHER WITH CHO ET AL. (2018) AND SABARI
ET AL. (2018), THIS KEY PAPER DISCUSSES THE ROLE OF CONDENSATES IN TRANSCRIPTION, A HIGHLY ACTIVE AREA OF RESEARCH WHERE MANY QUESTIONS REMAIN UNSETTLED. PubMed PubMed Central Google
Scholar * Williamson, D. J. et al. Pre-existing clusters of the adaptor Lat do not participate in early T cell signaling events. _Nat. Immunol._ 12, 655–662 (2011). CAS PubMed Google
Scholar * Rao, B. S. & Parker, R. Numerous interactions act redundantly to assemble a tunable size of P bodies in _Saccharomyces cerevisiae_. _Proc. Natl Acad. Sci. USA_ 114, E9569
(2017). CAS PubMed PubMed Central Google Scholar * Hill, T. L. _Thermodynamics of Small Systems, Parts 1 & 2_ (Dover, 2013). * Puglisi, A., Sarracino, A. & Vulpiani, A.
_Thermodynamics and Statistical Mechanics of Small Systems_ (published by the authors, 2018). * Elowitz, M. B., Levine, A. J., Siggia, E. D. & Swain, P. S. Stochastic gene expression in
a single cell. _Science_ 297, 1183–1186 (2002). CAS PubMed Google Scholar * Ozbudak, E. M., Thattai, M., Kurtser, I., Grossman, A. D. & van Oudenaarden, A. Regulation of noise in the
expression of a single gene. _Nat. Genet._ 31, 69–73 (2002). CAS PubMed Google Scholar * Boija, A. et al. Transcription factors activate genes through the phase-separation capacity of
their activation domains. _Cell_ 175, 1842–1855.e16 (2018). CAS PubMed Google Scholar * Lu, H. et al. Phase-separation mechanism for C-terminal hyperphosphorylation of RNA polymerase II.
_Nature_ 558, 318–323 (2018). CAS PubMed PubMed Central Google Scholar * Chong, S. et al. Imaging dynamic and selective low-complexity domain interactions that control gene
transcription. _Science_ 361, eaar2555 (2018). PubMed PubMed Central Google Scholar * Mir, M. et al. Dense Bicoid hubs accentuate binding along the morphogen gradient. _Genes Dev._ 31,
1784–1794 (2017). CAS PubMed PubMed Central Google Scholar * Boehning, M. et al. RNA polymerase II clustering through carboxy-terminal domain phase separation. _Nat. Struct. Mol. Biol._
25, 833–840 (2018). TOGETHER WITH BOIJA ET AL. (2018), LU ET AL. (2018), CHONG ET AL. (2018) AND MIR ET AL. (2017), THIS IMPORTANT PAPER DISCUSSES PHASE SEPARATION, CONDENSATES AND
TRANSCRIPTION. CAS PubMed Google Scholar * Julin, J., Napari, I., Merikanto, J. & Vehkamaki, H. A thermodynamically consistent determination of surface tension of small Lennard-Jones
clusters from simulation and theory. _J. Chem. Phys._ 133, 044704 (2010). PubMed Google Scholar * Lau, G. V., Hunt, P. A., Muller, E. A., Jackson, G. & Ford, I. J. Water droplet excess
free energy determined by cluster mitosis using guided molecular dynamics. _J. Chem. Phys._ 143, 244709 (2015). PubMed Google Scholar * Nguyen, V. D., Schoemaker, F. C., Blokhuis, E. M.
& Schall, P. Measurement of the curvature-dependent surface tension in nucleating colloidal liquids. _Phys. Rev. Lett._ 121, 246102 (2018). CAS PubMed Google Scholar * Gsponer, J.
& Babu, M. M. Cellular strategies for regulating functional and nonfunctional protein aggregation. _Cell Rep._ 2, 1425–1437 (2012). CAS PubMed PubMed Central Google Scholar *
Chavali, S. et al. Constraints and consequences of the emergence of amino acid repeats in eukaryotic proteins. _Nat. Struct. Mol. Biol._ 24, 765–777 (2017). CAS PubMed PubMed Central
Google Scholar * Kuriyan, J. & Eisenberg, D. The origin of protein interactions and allostery in colocalization. _Nature_ 450, 983–990 (2007). CAS PubMed Google Scholar * Kawecki, T.
J. et al. Experimental evolution. _Trends Ecol. Evol._ 27, 547–560 (2012). PubMed Google Scholar * Sanchez de Groot, N. et al. The fitness cost and benefit of phase-separated protein
deposits. _Mol. Syst. Biol._ 15, e8075 (2019). PubMed PubMed Central Google Scholar * So, C. et al. A liquid-like spindle domain promotes acentrosomal spindle assembly in mammalian
oocytes. _Science_ 364, eaat9557 (2019). CAS PubMed PubMed Central Google Scholar * Al-Husini, N., Tomares, D. T., Bitar, O., Childers, W. S. & Schrader, J. M. α-Proteobacterial RNA
degradosomes assemble liquid–liquid phase-separated RNP bodies. _Mol. Cell_ 71, 1027–1039.e14 (2018). CAS PubMed Google Scholar * Ying, Y. et al. Splicing activation by Rbfox requires
self-aggregation through its tyrosine-rich domain. _Cell_ 170, 312–323.e10 (2017). CAS PubMed PubMed Central Google Scholar * Tatomer, D. C. et al. Concentrating pre-mRNA processing
factors in the histone locus body facilitates efficient histone mRNA biogenesis. _J. Cell Biol._ 213, 557–570 (2016). CAS PubMed PubMed Central Google Scholar * Novotný, I., Blažíková,
M., Staneˇk, D., Herman, P. & Malinsky, J. In vivo kinetics of U4/U6·U5 tri-snRNP formation in Cajal bodies. _Mol. Biol. Cell_ 22, 513–523 (2011). PubMed PubMed Central Google Scholar
* Samir, P. et al. DDX3X acts as a live-or-die checkpoint in stressed cells by regulating NLRP3 inflammasome. _Nature_ 573, 590–594 (2019). CAS PubMed PubMed Central Google Scholar *
Lasker, K. et al. Selective sequestration of signalling proteins in a membraneless organelle reinforces the spatial regulation of asymmetry in _Caulobacter crescentus_. _Nat. Microbiol._ 5,
418–429 (2020). CAS PubMed PubMed Central Google Scholar * Milovanovic, D., Wu, Y., Bian, X. & De Camilli, P. A liquid phase of synapsin and lipid vesicles. _Science_ 361, 604–607
(2018). CAS PubMed PubMed Central Google Scholar * Zaffagnini, G. et al. p62 filaments capture and present ubiquitinated cargos for autophagy. _EMBO J._ 37, e98308 (2018). PubMed PubMed
Central Google Scholar * Park, J.-E. et al. Phase separation of Polo-like kinase 4 by autoactivation and clustering drives centriole biogenesis. _Nat. Commun._ 10, 4959 (2019). PubMed
PubMed Central Google Scholar * Saha, S. et al. Polar positioning of phase-separated liquid compartments in cells regulated by an mRNA competition mechanism. _Cell_ 166, 1572–1584.e16
(2016). CAS PubMed PubMed Central Google Scholar * Chakravarty, A. K., Smejkal, T., Itakura, A. K., Garcia, D. M. & Jarosz, D. F. A non-amyloid prion particle that activates a
heritable gene expression program. _Mol. Cell_ 77, 251–265.e9 (2020). CAS PubMed Google Scholar * Itakura, A. K., Chakravarty, A. K., Jakobson, C. M. & Jarosz, D. F. Widespread
prion-based control of growth and differentiation strategies in _Saccharomyces cerevisiae_. _Mol. Cell_ 77, 266–278.e6 (2020). CAS PubMed Google Scholar * Gaglia, G. et al. HSF1 phase
transition mediates stress adaptation and cell fate decisions. _Nat. Cell Biol._ 22, 151–158 (2020). CAS PubMed PubMed Central Google Scholar * Guo, Y. E. et al. Pol II phosphorylation
regulates a switch between transcriptional and splicing condensates. _Nature_ 572, 543–548 (2019). CAS PubMed PubMed Central Google Scholar * Zamudio, A. V. et al. Mediator condensates
localize signaling factors to key cell identity genes. _Mol. Cell_ 76, 753–766.e6 (2019). CAS PubMed PubMed Central Google Scholar * Erdel, F. et al. Mouse heterochromatin adopts digital
compaction states without showing hallmarks of HP1-driven liquid–liquid phase separation. _Mol. Cell_ 78, 236–249.e7 (2020). CAS PubMed PubMed Central Google Scholar * Wang, L. et al.
Histone modifications regulate chromatin compartmentalization by contributing to a phase separation mechanism. _Mol. Cell_ 76, 646–659.e6 (2019). CAS PubMed Google Scholar * Davis, D. et
al. Human antiviral protein MxA forms novel metastable membraneless cytoplasmic condensates exhibiting rapid reversible tonicity-driven phase transitions. _J. Virol._ 93, e01014–e01019
(2019). CAS PubMed PubMed Central Google Scholar * Heinrich, B. S., Maliga, Z., Stein, D. A., Hyman, A. A. & Whelan, S. P. J. Phase transitions drive the formation of vesicular
stomatitis virus replication compartments. _mBio_ 9, e02290-17 (2018). PubMed PubMed Central Google Scholar * Zhou, Y., Su, J. M., Samuel, C. E. & Ma, D. Measles virus forms inclusion
bodies with properties of liquid organelles. _J. Virol._ 93, e00948-19 (2019). PubMed PubMed Central Google Scholar * Yasuda, S. et al. Stress- and ubiquitylation-dependent phase
separation of the proteasome. _Nature_ 578, 296–300 (2020). CAS PubMed Google Scholar * Sala, K. et al. The ERC1 scaffold protein implicated in cell motility drives the assembly of a
liquid phase. _Sci. Rep._ 9, 13530 (2019). PubMed PubMed Central Google Scholar * Celetti, G. et al. The liquid state of FG-nucleoporins mimics permeability barrier properties of nuclear
pore complexes. _J. Cell Biol._ 219, e201907157 (2019). PubMed Central Google Scholar * A, P. & Weber, S. C. Evidence for and against liquid–liquid phase separation in the nucleus.
_NonCoding RNA_ 5, 50 (2019). PubMed Central Google Scholar * Falahati, H. & Wieschaus, E. Independent active and thermodynamic processes govern the nucleolus assembly in vivo. _Proc.
Natl Acad. Sci. USA_ 114, 1335–1340 (2017). THIS PAPER DEMONSTRATES THAT BOTH THERMODYNAMIC PROCESSES, SUCH AS PHASE SEPARATION, AND ACTIVE PROCESSES CONTRIBUTE TO NUCLEOLUS ASSEMBLY. CAS
PubMed PubMed Central Google Scholar * Pareek, V., Tian, H., Winograd, N. & Benkovic, S. J. Metabolomics and mass spectrometry imaging reveal channeled de novo purine synthesis in
cells. _Science_ 368, 283–290 (2020). CAS PubMed PubMed Central Google Scholar * Itia, A. F.-B., Alexander, B. S., Ethan, K. S. & Halina, R.-D. Optical trapping in vivo: theory,
practice, and applications. _Nanophotonics_ 8, 1023–1040 (2019). Google Scholar * Mittasch, M. et al. Non-invasive perturbations of intracellular flow reveal physical principles of cell
organization. _Nat. Cell Biol._ 20, 344–351 (2018). CAS PubMed Google Scholar * Taylor, J. P., Brown, R. H. Jr. & Cleveland, D. W. Decoding ALS: from genes to mechanism. _Nature_ 539,
197–206 (2016). PubMed PubMed Central Google Scholar * Boulay, G. et al. Cancer-specific retargeting of BAF complexes by a prion-like domain. _Cell_ 171, 163–178.e19 (2017). CAS PubMed
PubMed Central Google Scholar * Tulpule, A. et al. Kinase-mediated RAS signaling via membraneless cytoplasmic protein granules. Preprint at _bioRxiv_
https://www.biorxiv.org/content/10.1101/704312v3 (2020). THIS PAPER REPORTS THAT ABERRANT CONDENSATES FORMED BY ONCOGENIC RECEPTOR TYROSINE KINASE FUSION PROTEINS DRIVE RAS SIGNALLING. *
Takeuchi, K. Discovery stories of RET fusions in lung cancer: a mini-review. _Front. Physiol._ 10, 216 (2019). PubMed PubMed Central Google Scholar * Grunewald, T. G. P. et al. Ewing
sarcoma. _Nat. Rev. Dis. Prim._ 4, 5 (2018). PubMed Google Scholar * Wheeler, R. J. et al. Small molecules for modulating protein driven liquid–liquid phase separation in treating
neurodegenerative disease. Preprint at _bioRxiv_ https://www.biorxiv.org/content/10.1101/721001v2 (2020). * Berchtold, D., Battich, N. & Pelkmans, L. A systems-level study reveals
regulators of membrane-less organelles in human cells. _Mol. Cell_ 72, 1035–1049.e5 (2018). CAS PubMed Google Scholar * Rai, A. K., Chen, J. X., Selbach, M. & Pelkmans, L.
Kinase-controlled phase transition of membraneless organelles in mitosis. _Nature_ 559, 211–216 (2018). CAS PubMed Google Scholar * Monahan, Z. et al. Phosphorylation of the FUS
low-complexity domain disrupts phase separation, aggregation, and toxicity. _Embo J._ 36, 2951–2967 (2017). CAS PubMed PubMed Central Google Scholar * Wang, J. T. et al. Regulation of
RNA granule dynamics by phosphorylation of serine-rich, intrinsically disordered proteins in _C. elegans_. _eLife_ 3, e04591 (2014). PubMed PubMed Central Google Scholar * Zhang, G.,
Wang, Z., Du, Z. & Zhang, H. mTOR regulates phase separation of PGL granules to modulate their autophagic degradation. _Cell_ 174, 1492–1506.e22 (2018). CAS PubMed Google Scholar *
Guccione, E. & Richard, S. The regulation, functions and clinical relevance of arginine methylation. _Nat. Rev. Mol. Cell Biol._ 20, 642–657 (2019). CAS PubMed Google Scholar * Dao,
T. P. et al. Ubiquitin modulates liquid–liquid phase separation of UBQLN2 via disruption of multivalent interactions. _Mol. Cell_ 69, 965–978.e6 (2018). CAS PubMed PubMed Central Google
Scholar * Saito, M. et al. Acetylation of intrinsically disordered regions regulates phase separation. _Nat. Chem. Biol._ 15, 51–61 (2019). CAS PubMed Google Scholar * Buchan, J. R.,
Kolaitis, R. M., Taylor, J. P. & Parker, R. Eukaryotic stress granules are cleared by autophagy and Cdc48/VCP function. _Cell_ 153, 1461–1474 (2013). CAS PubMed PubMed Central Google
Scholar * James, J. R. Tuning ITAM multiplicity on T cell receptors can control potency and selectivity to ligand density. _Sci. Signal._ 11, eaan1088 (2018). PubMed PubMed Central Google
Scholar * Cao, Q., Boyer, D. R., Sawaya, M. R., Ge, P. & Eisenberg, D. S. Cryo-EM structure and inhibitor design of human IAPP (amylin) fibrils. _Nat. Struct. Mol. Biol._ 27, 653–659
(2020). CAS PubMed PubMed Central Google Scholar * Griner, S. L. et al. Structure-based inhibitors of amyloid β core suggest a common interface with tau. _eLife_ 8, e46924 (2019). PubMed
PubMed Central Google Scholar * Lu, J. et al. Structure-based peptide inhibitor design of amyloid-β aggregation. _Front. Mol. Neurosci._ 12, 54 (2019). CAS PubMed PubMed Central
Google Scholar * Sangwan, S. et al. Inhibition of synucleinopathic seeding by rationally designed inhibitors. _eLife_ 9, e46775 (2020). CAS PubMed PubMed Central Google Scholar * Klein,
I. A. et al. Partitioning of cancer therapeutics in nuclear condensates. _Science_ 368, 1386 (2020). CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS
Research on biomolecular condensates is supported in the Rosen laboratory by the Howard Hughes Medical Institute, a Paul G. Allen Frontiers Distinguished Investigator Award to M.K.R. and
grants from the Welch Foundation (I-1544 to M.K.R.) and the National Institutes of Health (NIH) (F32 GM136058 to A.S.L.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of
Biophysics and Howard Hughes Medical Institute, University of Texas Southwestern Medical Center, Dallas, TX, USA Andrew S. Lyon, William B. Peeples & Michael K. Rosen Authors * Andrew S.
Lyon View author publications You can also search for this author inPubMed Google Scholar * William B. Peeples View author publications You can also search for this author inPubMed Google
Scholar * Michael K. Rosen View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS The authors contributed equally to all aspects of the article.
CORRESPONDING AUTHOR Correspondence to Michael K. Rosen. ETHICS DECLARATIONS COMPETING INTERESTS M.K.R. is a co-founder of the biotechnology company Faze Medicines. A.S.L and W.B.P. declare
no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Reviews Molecular Cell Biology_ thanks M. Babu, S. Michnick and the other, anonymous, reviewer(s) for their
contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. GLOSSARY
* Material properties In the context of biomolecular condensates, physical properties of the assembly of constituent macromolecules including viscosity, surface tension and porosity. *
Interfacial tension (Also known as surface tension). For separate liquid phases in contact with each other, the work required to increase the surface area of contact between the two phases.
In the absence of external forces, interfacial/surface tension causes phase-separated liquids to form spherical droplets as spheres have minimal surface area for a given volume. *
Multivalent interactions Interactions occurring between macromolecules with multiple sites of interaction, such that each molecule can interact with multiple binding partners. *
Intrinsically disordered regions Protein regions that do not adopt any stable ordered three-dimensional structure. * Law of mass action In the context of enzymology, the principle that the
chemical reaction rate is proportional to the concentration of enzymes and substrates. * Ribulose bisphosphate carboxylase/oxygenase (Rubisco). An enzyme acting in carbon fixation in
photosynthetic organisms, catalysing the reaction between ribulose bisphosphate and atmospheric carbon dioxide. * Cyclic guanosine monophosphate–adenosine monophosphate (cGAMP) synthase
(cGAS). An innate immune signalling enzyme that senses cytosolic DNA, a pathogen-associated molecular pattern, and produces cGAMP, which activates the stimulator of interferon genes (STING)
protein to induce pro-inflammatory transcriptional responses. * Allostery Regulation of enzyme activity via binding by a second molecule at a site other than the enzyme’s active site, often
by inducing a conformational change. * Scaffold In simple molecular systems, a macromolecule that is required for condensate formation. The other, general group of condensate components are
client molecules, which bind to and selectively partition into condensates without affecting condensate formation. In many natural condensates, this distinction is not absolute, and whereas
some macromolecules act as pure scaffolds and some as pure clients, others can have varying impacts on the formation (threshold concentration) and composition of the compartment. * _K_ M A
parameter of the Michaelis–Menten model of enzyme kinetics, describing the concentration of a substrate molecule at which the rate of product formation reaches half of the maximum possible
rate under a given set of conditions. If the rate of enzyme–substrate binding is rapid relative to catalysis, the _K_M value approximates the dissociation constant for the enzyme–substrate
complex. * Paraspeckles Nuclear condensates implicated in RNA base editing as well as transcriptional regulation. Paraspeckles are formed from the long non-coding RNA NEAT1 and the DBHS
family of proteins (NONO, SFPQ and PSPC1). * Kinetic proofreading A biochemical error-correction mechanism favouring reaction pathways that lead to correct over incorrect products, wherein
an irreversible step that leads to exit of reaction intermediates from the pathway is more likely to occur for incorrect intermediates. * Processing bodies Cytoplasmic condensates found in
yeast and humans that contains mRNA, RNA decapping and RNA degradation machinery. P bodies are thought to either store or degrade mRNA during stress. * Argonaute A protein component of the
RNA-induced silencing complex that binds several classes of small non-coding RNAs, which direct the complex to mRNA targets via sequence complementarity to downregulate expression through
endonucleolytic mRNA cleavage or translational inhibition. * Transgenerational epigenetic inheritance Biological processes that allow transmission of epigenetic regulatory molecules or
modifications, such as RNAi factors or DNA methylation, from parent to offspring without altering DNA sequences. * P granules Biomolecular condensates formed by liquid–liquid phase
separation in _Caenorhabditis elegans_ composed of RNA and proteins involved in the maintenance of germ cell fate via post-transcriptional regulation and small RNA biogenesis. * Z granules
Biomolecular condensates in _Caenorhabditis elegans_ containing the proteins ZNFX1 and WAGO4 required for transgenerational epigenetic inheritance of RNAi. Associates with both P granules
and Mutator foci, forming a bridge between the two condensates. * Mutator foci A type of biomolecular condensate in _Caenorhabditis elegans_ consisting of proteins encoded by _mutator_ class
genes, originally discovered in genetic screens for activation of transposons in the germline. Functions in siRNA amplification and RNA silencing. * Voltage-gated calcium channels Membrane
protein channels that allow ingress of calcium into the cell at presynaptic terminals of neurons when activated by membrane depolarization. Calcium activates exocytosis of neurotransmitter
vesicles. * _N_-Methyl-d-aspartate (NMDA) receptor A postsynaptic membrane protein channel activated by the excitatory neurotransmitter glutamate, allowing ingress of cations to depolarize
the postsynaptic neuron. * Dendritic spines Small protrusions on postsynaptic dendrites that are sites of excitatory signalling by glutamate neurotransmitter receptors. * Balbiani body A
condensate specifically found during oocyte development that includes nuage, mitochondria and rough endoplasmic reticulum. Although the function is not fully understood, it is thought to
preserve eggs in a dormant state prior to ovulation. * Optical trapping The use of highly focused laser beams to apply force to (‘trap’) very small objects. * Chemogenetic approaches A class
of experimental techniques that introduce proteins or protein domain fusion constructs that have engineered small molecule-dependent activities into cells or in vitro biochemical reactions
to achieve control over cellular or biochemical activities. * Optogenetics A class of experimental techniques using light-responsive proteins or engineered protein domain fusions to acutely
modulate cellular or protein activities by illuminating cells or in vitro biochemical reactions. * Partition coefficient The ratio of molecular concentration within a biomolecular condensate
relative to the concentration in the surrounding solution. * Michaelis-like recruitment For the binding of a molecule to some structure, a non-linear, saturable relationship between the
molecular concentration and the fraction bound described by the rectangular hyperbola of the Michaelis–Menten model of enzyme kinetics. * Promyelocytic leukaemia nuclear bodies Nuclear
condensates formed by the promyelocytic leukaemia protein (PML). Fusion of PML to the retinoic acid receptor causes acute promyelocytic leukaemia. PML bodies are implicated in various
processes, including transcription regulation, viral immunity, post-translational modification and apoptosis. * CAR T cells In cancer immunotherapy, T cells that express engineered T cell
receptors (TCRs) where the native extracellular domains have been replaced by a heterologous binding domain targeted to a tumour-specific cell-surface protein in order to direct increased
cytotoxic activity towards tumour cells. * Kinetic trapping A phenomenon in which a thermodynamically less stable state is maintained due to the high energy barrier, and thus long time
period, required to move to the more stable state. * Split enzyme system The use of an enzyme that has been expressed as two separate polypeptide chains and is only active when the fragments
are brought together to reconstitute the full enzyme. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Lyon, A.S., Peeples, W.B. & Rosen, M.K. A
framework for understanding the functions of biomolecular condensates across scales. _Nat Rev Mol Cell Biol_ 22, 215–235 (2021). https://doi.org/10.1038/s41580-020-00303-z Download citation
* Accepted: 01 October 2020 * Published: 09 November 2020 * Issue Date: March 2021 * DOI: https://doi.org/10.1038/s41580-020-00303-z SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative
Trending News
Nature chemical biology - volume 13 issue 8, august 2017The cover depicts conidiophores of the fungus _Aspergillus nidulans_ carrying a fungal artificial chromosome (FAC), imag...
Households warned over common mistake that will damage hanging basket bloomsTHE DAYS ARE WARMING UP, THE NIGHTS ARE BALMIER BUT DON'T GET COMPLACENT, EVEN A COOL NIGHT COULD DAMAGE AN UNCOVER...
Dietary nutrient composition affects progression of liver diseaseAccess through your institution Buy or subscribe Ioannou, G. N. _ et al_. Association between dietary nutrient compositi...
David beckham weighs into gary neville vs nottingham forest row with commentGARY NEVILLE HAS BEEN BANNED FROM WORKING AT THE CITY GROUND TO COVER NOTTINGHAM FOREST VS CHELSEA ON SUNDAY, PROMPTING ...
Ruben amorim details how bruno fernandes could stay at man utd amid exit fearsMANCHESTER UNITED'S 1-0 DEFEAT TO TOTTENHAM IN THE EUROPA LEAGUE FINAL HAS ROUNDED OFF A HORROR SEASON AND NOW THER...
Latests News
A framework for understanding the functions of biomolecular condensates across scalesABSTRACT Biomolecular condensates are found throughout eukaryotic cells, including in the nucleus, in the cytoplasm and ...
At this experimental culinary event, the cutlery is high artWelcome to the real-life Mad Hatter's tea party: Guests eat out of spiraling ceramics from spoons as long as their ...
Understanding the problem of pain and its treatmentMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
8 affordable ways to get training for a new careerFind a mentor Sometimes there’s nothing like a little one-on-one handholding to give you the skills you need next. There...
Microrna target predictions in animalsABSTRACT In recent years, microRNAs (miRNAs) have emerged as a major class of regulatory genes, present in most metazoan...