Liquid biopsy and minimal residual disease — latest advances and implications for cure
Liquid biopsy and minimal residual disease — latest advances and implications for cure"
- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
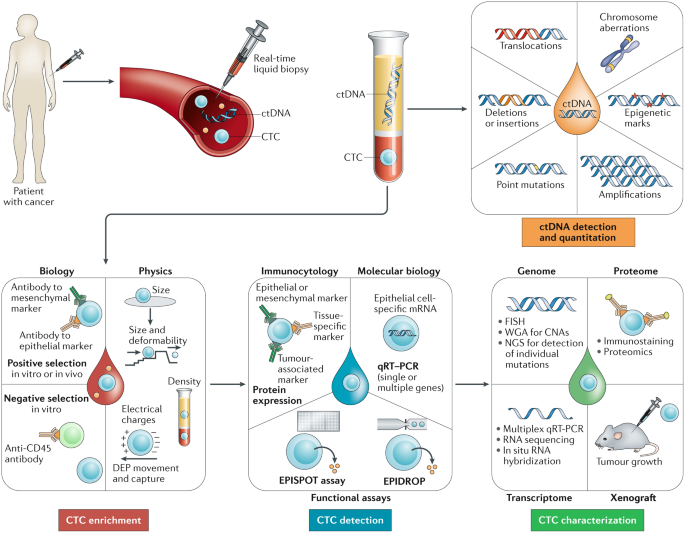
ABSTRACT Liquid biopsy has been introduced as a new diagnostic concept predicated on the analysis of circulating tumour cells (CTCs) or circulating tumour-derived factors, in particular,
cell-free tumour DNA (ctDNA). Highly sensitive liquid biopsy assays have been developed that can now be applied to detect and characterize minimal residual disease (MRD), which reflects the
presence of tumour cells disseminated from the primary lesion to distant organs in patients who lack any clinical or radiological signs of metastasis or residual tumour cells left behind
after local therapy that eventually lead to local recurrence. This application is the new frontier of liquid biopsy analyses, which are challenged by the very low concentrations of CTCs and
ctDNA in blood samples. In this Review, we discuss the key technologies that can be used to detect and characterize CTCs in surveillance of MRD and provide a brief overview of similar roles
of ctDNA analyses. We then focus on the current clinical data on the use of CTCs and ctDNA in the detection and monitoring of MRD and in obtaining information on therapeutic targets and
resistance mechanisms relevant to the management of individual patients with cancer. KEY POINTS * Minimal residual disease (MRD) can be defined as cancer persisting in a patient after
treatment that cannot be detected with current medical imaging modalities and is, therefore, an occult stage of cancer progression. * Liquid biopsy approaches based on the detection of small
numbers of circulating tumour cells (CTCs) or minute amounts of circulating cell-free tumour DNA (ctDNA) now enable MRD detection in patients with various malignancies. * CTC detection at
primary diagnosis of cancer predicts an unfavourable prognosis and is, therefore, applicable to risk stratification strategies beyond the current approaches to tumour staging. * Monitoring
of CTCs and ctDNA during post-surgical follow-up assessments can enable the detection of disease relapse many months earlier than is possible with current radiological imaging procedures. *
Further characterization of CTCs and ctDNA can provide insights into the molecular evolution of MRD during tumour progression, with implications for therapy to delay or even prevent
metastatic relapse. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution
Access Nature and 54 other Nature Portfolio journals Get Nature+, our best-value online-access subscription $29.99 / 30 days cancel any time Learn more Subscribe to this journal Receive 12
print issues and online access $209.00 per year only $17.42 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be
subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR
CONTENT BEING VIEWED BY OTHERS MINIMAL RESIDUAL DISEASE AS A TARGET FOR LIQUID BIOPSY IN PATIENTS WITH SOLID TUMOURS Article 28 November 2024 LIQUID BIOPSY ENTERS THE CLINIC — IMPLEMENTATION
ISSUES AND FUTURE CHALLENGES Article 20 January 2021 THE BREAST IS YET TO COME: CURRENT AND FUTURE UTILITY OF CIRCULATING TUMOUR DNA IN BREAST CANCER Article 26 May 2021 REFERENCES * Pan,
H. et al. 20-year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. _N. Engl. J. Med._ 377, 1836–1846 (2017). Article PubMed PubMed Central Google Scholar *
Merker, J. D. et al. Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. _J. Clin. Oncol._ 36,
1631–1641 (2018). CAS PubMed Google Scholar * Donaldson, J. & Park, B. H. Circulating tumor DNA: measurement and clinical utility. _Annu. Rev. Med._ 69, 223–234 (2018). CAS PubMed
Google Scholar * Heitzer, E., Ulz, P. & Geigl, J. B. Circulating tumor DNA as a liquid biopsy for cancer. _Clin. Chem._ 61, 112–123 (2015). CAS PubMed Google Scholar * Siravegna, G.,
Marsoni, S., Siena, S. & Bardelli, A. Integrating liquid biopsies into the management of cancer. _Nat. Rev. Clin. Oncol._ 14, 531–548 (2017). CAS PubMed Google Scholar *
Alix-Panabieres, C., Mader, S. & Pantel, K. Epithelial-mesenchymal plasticity in circulating tumor cells. _J. Mol. Med._ 95, 133–142 (2017). CAS PubMed Google Scholar * Ohnaga, T.,
Takei, Y., Nagata, T. & Shimada, Y. Highly efficient capture of cancer cells expressing EGFR by microfluidic methods based on antigen-antibody association. _Sci. Rep._ 8, 12005 (2018).
PubMed PubMed Central Google Scholar * Thege, F. I. et al. Microfluidic immunocapture of circulating pancreatic cells using parallel EpCAM and MUC1 capture: characterization, optimization
and downstream analysis. _Lab. Chip_ 14, 1775–1784 (2014). CAS PubMed Google Scholar * El-Heliebi, A. et al. In situ detection and quantification of AR-V7, AR-FL, PSA, and KRAS point
mutations in circulating tumor cells. _Clin. Chem._ 64, 536–546 (2018). CAS PubMed Google Scholar * Santana, S. M., Liu, H., Bander, N. H., Gleghorn, J. P. & Kirby, B. J.
Immunocapture of prostate cancer cells by use of anti-PSMA antibodies in microdevices. _Biomed. Microdevices_ 14, 401–407 (2012). CAS PubMed PubMed Central Google Scholar * Hyun, K. A.,
Lee, T. Y., Lee, S. H. & Jung, H. I. Two-stage microfluidic chip for selective isolation of circulating tumor cells (CTCs). _Biosens. Bioelectron._ 67, 86–92 (2015). CAS PubMed Google
Scholar * Wu, L. L. et al. Chip-assisted single-cell biomarker profiling of heterogeneous circulating tumor cells using multifunctional nanospheres. _Anal. Chem._ 90, 10518–10526 (2018).
CAS PubMed Google Scholar * Paoletti, C. et al. Heterogeneous estrogen receptor expression in circulating tumor cells suggests diverse mechanisms of fulvestrant resistance. _Mol. Oncol._
10, 1078–1085 (2016). CAS PubMed PubMed Central Google Scholar * Coumans, F. A., van Dalum, G., Beck, M. & Terstappen, L. W. Filtration parameters influencing circulating tumor cell
enrichment from whole blood. _PLOS ONE_ 8, e61774 (2013). CAS PubMed PubMed Central Google Scholar * Clawson, G. A. et al. Circulating tumor cells in melanoma patients. _PLOS ONE_ 7,
e41052 (2012). CAS PubMed PubMed Central Google Scholar * Sun, N., Li, X., Wang, Z., Li, Y. & Pei, R. High-purity capture of CTCs based on micro-beads enhanced isolation by size of
epithelial tumor cells (ISET) method. _Biosens. Bioelectron._ 102, 157–163 (2018). CAS PubMed Google Scholar * Kim, T. H., Yoon, H. J., Stella, P. & Nagrath, S. Cascaded spiral
microfluidic device for deterministic and high purity continuous separation of circulating tumor cells. _Biomicrofluidics_ 8, 064117 (2014). PubMed PubMed Central Google Scholar * Huang,
C., Smith, J. P., Saha, T. N., Rhim, A. D. & Kirby, B. J. Characterization of microfluidic shear-dependent epithelial cell adhesion molecule immunocapture and enrichment of pancreatic
cancer cells from blood cells with dielectrophoresis. _Biomicrofluidics_ 8, 044107 (2014). PubMed PubMed Central Google Scholar * Gascoyne, P. R. & Shim, S. Isolation of circulating
tumor cells by dielectrophoresis. _Cancers_ 6, 545–579 (2014). PubMed PubMed Central Google Scholar * Sarioglu, A. F. et al. A microfluidic device for label-free, physical capture of
circulating tumor cell clusters. _Nat. Methods_ 12, 685–691 (2015). CAS PubMed PubMed Central Google Scholar * Gkountela, S. et al. Circulating tumor cell clustering shapes DNA
methylation to enable metastasis seeding. _Cell_ 176, 98–112 (2019). CAS PubMed PubMed Central Google Scholar * Heitzer, E. et al. Complex tumor genomes inferred from single circulating
tumor cells by array-CGH and next-generation sequencing. _Cancer Res._ 73, 2965–2975 (2013). CAS PubMed Google Scholar * Abonnenc, M. et al. Programmable interactions of functionalized
single bioparticles in a dielectrophoresis-based microarray chip. _Anal. Chem._ 85, 8219–8224 (2013). CAS PubMed Google Scholar * Peeters, D. J. et al. Semiautomated isolation and
molecular characterisation of single or highly purified tumour cells from CellSearch enriched blood samples using dielectrophoretic cell sorting. _Br. J. Cancer_ 108, 1358–1367 (2013). CAS
PubMed PubMed Central Google Scholar * Bhagwat, N. et al. An integrated flow cytometry-based platform for isolation and molecular characterization of circulating tumor single cells and
clusters. _Sci. Rep._ 8, 5035 (2018). PubMed PubMed Central Google Scholar * Mastoraki, S. et al. ESR1 methylation: a liquid biopsy-based epigenetic assay for the follow-up of patients
with metastatic breast cancer receiving endocrine treatment. _Clin. Cancer Res._ 24, 1500–1510 (2018). CAS PubMed Google Scholar * Soler, A., Cayrefourcq, L., Mazel, M. &
Alix-Panabieres, C. EpCAM-independent enrichment and detection of viable circulating tumor cells using the EPISPOT assay. _Methods Mol. Biol._ 1634, 263–276 (2017). CAS PubMed Google
Scholar * Ramirez, J. M. et al. Prognostic relevance of viable circulating tumor cells detected by EPISPOT in metastatic breast cancer patients. _Clin. Chem._ 60, 214–221 (2014). CAS
PubMed Google Scholar * Deneve, E. et al. Capture of viable circulating tumor cells in the liver of colorectal cancer patients. _Clin. Chem._ 59, 1384–1392 (2013). CAS PubMed Google
Scholar * Kuske, A. et al. Improved detection of circulating tumor cells in non-metastatic high-risk prostate cancer patients. _Sci. Rep._ 6, 39736 (2016). CAS PubMed PubMed Central
Google Scholar * Eyer, K. et al. Single-cell deep phenotyping of IgG-secreting cells for high-resolution immune monitoring. _Nat. Biotechnol._ 35, 977–982 (2017). CAS PubMed Google
Scholar * Gasch, C. et al. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. _Clin. Chem._
59, 252–260 (2013). CAS PubMed Google Scholar * Carter, L. et al. Molecular analysis of circulating tumor cells identifies distinct copy-number profiles in patients with chemosensitive
and chemorefractory small-cell lung cancer. _Nat. Med._ 23, 114–119 (2017). CAS PubMed Google Scholar * Lohr, J. G. et al. Whole-exome sequencing of circulating tumor cells provides a
window into metastatic prostate cancer. _Nat. Biotechnol._ 32, 479–484 (2014). CAS PubMed PubMed Central Google Scholar * Babayan, A. et al. Comparative study of whole genome
amplification and next generation sequencing performance of single cancer cells. _Oncotarget_ 8, 56066–56080 (2016). PubMed PubMed Central Google Scholar * Muller, C. et al. Hematogenous
dissemination of glioblastoma multiforme. _Sci. Transl Med._ 6, 247ra101 (2014). PubMed Google Scholar * Yates, D. R. et al. Quantitative RT-PCR analysis of PSA and prostate-specific
membrane antigen mRNA to detect circulating tumor cells improves recurrence-free survival nomogram prediction after radical prostatectomy. _Prostate_ 72, 1382–1388 (2012). CAS PubMed
Google Scholar * Strati, A., Kasimir-Bauer, S., Markou, A., Parisi, C. & Lianidou, E. S. Comparison of three molecular assays for the detection and molecular characterization of
circulating tumor cells in breast cancer. _Breast Cancer Res._ 15, R20 (2013). CAS PubMed PubMed Central Google Scholar * Gorges, T. M. et al. Accession of tumor heterogeneity by
multiplex transcriptome profiling of single circulating tumor cells. _Clin. Chem._ 62, 1504–1515 (2016). CAS PubMed Google Scholar * Jordan, N. V. et al. HER2 expression identifies
dynamic functional states within circulating breast cancer cells. _Nature_ 537, 102–106 (2016). CAS PubMed PubMed Central Google Scholar * Gasch, C. et al. Heterogeneity of miR-10b
expression in circulating tumor cells. _Sci. Rep._ 5, 15980 (2015). CAS PubMed PubMed Central Google Scholar * Sinkala, E. et al. Profiling protein expression in circulating tumour cells
using microfluidic western blotting. _Nat. Commun._ 8, 14622 (2017). CAS PubMed PubMed Central Google Scholar * Franzen, B. et al. A fine-needle aspiration-based protein signature
discriminates benign from malignant breast lesions. _Mol. Oncol._ 12, 1415–1428 (2018). CAS PubMed PubMed Central Google Scholar * Baccelli, I. et al. Identification of a population of
blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. _Nat. Biotechnol._ 31, 539–544 (2013). CAS PubMed Google Scholar * Hodgkinson, C.
L. et al. Tumorigenicity and genetic profiling of circulating tumor cells in small-cell lung cancer. _Nat. Med._ 20, 897–903 (2014). CAS PubMed Google Scholar * Zhang, L. et al. The
identification and characterization of breast cancer CTCs competent for brain metastasis. _Sci. Transl Med._ 5, 180ra48 (2013). PubMed Google Scholar * Yu, M. et al. Cancer therapy. Ex
vivo culture of circulating breast tumor cells for individualized testing of drug susceptibility. _Science_ 345, 216–220 (2014). CAS PubMed PubMed Central Google Scholar *
Alix-Panabieres, C. et al. Molecular portrait of metastasis-competent circulating tumor cells in colon cancer reveals the crucial role of genes regulating energy metabolism and DNA repair.
_Clin. Chem._ 63, 700–713 (2017). CAS PubMed Google Scholar * Cayrefourcq, L. et al. Establishment and characterization of a cell line from human circulating colon cancer cells. _Cancer
Res._ 75, 892–901 (2015). CAS PubMed Google Scholar * Fischer, J. C. et al. Diagnostic leukapheresis enables reliable detection of circulating tumor cells of nonmetastatic cancer
patients. _Proc. Natl Acad. Sci. USA_ 110, 16580–16585 (2013). CAS PubMed PubMed Central Google Scholar * Gorges, T. M. et al. Enumeration and molecular characterization of tumor cells
in lung cancer patients using a novel in vivo device for capturing circulating tumor cells. _Clin. Cancer Res._ 22, 2197–2206 (2016). CAS PubMed Google Scholar * Pantel, K. &
Alix-Panabieres, C. Functional studies on viable circulating tumor cells. _Clin. Chem._ 62, 328–334 (2016). CAS PubMed Google Scholar * Alix-Panabieres, C., Bartkowiak, K. & Pantel,
K. Functional studies on circulating and disseminated tumor cells in carcinoma patients. _Mol. Oncol._ 10, 443–449 (2016). PubMed PubMed Central Google Scholar * Soler, A. et al.
Autologous cell lines from circulating colon cancer cells captured from sequential liquid biopsies as model to study therapy-driven tumor changes. _Sci. Rep._ 8, 15931 (2018). PubMed PubMed
Central Google Scholar * Heitzer, E., Haque, I. S., Roberts, C. E. S. & Speicher, M. R. Current and future perspectives of liquid biopsies in genomics-driven oncology. _Nat. Rev.
Genet._ 20, 71–88 (2018). Google Scholar * Abbosh, C., Birkbak, N. J. & Swanton, C. Early stage NSCLC — challenges to implementing ctDNA-based screening and MRD detection. _Nat. Rev.
Clin. Oncol._ 15, 577–586 (2018). CAS PubMed Google Scholar * Riethdorf, S. et al. Prognostic impact of circulating tumor cells for breast cancer patients treated in the neoadjuvant
“Geparquattro” trial. _Clin. Cancer Res._ 23, 5384–5393 (2017). CAS PubMed Google Scholar * Bidard, F. C. et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant
chemotherapy: a meta-analysis. _J. Natl Cancer Inst._ 110, 560–567 (2018). PubMed Google Scholar * Janni, W. J. et al. Pooled analysis of the prognostic relevance of circulating tumor
cells in primary breast cancer. _Clin. Cancer Res._ 22, 2583–2593 (2016). CAS PubMed Google Scholar * Rack, B. et al. Circulating tumor cells predict survival in early average-to-high
risk breast cancer patients. _J. Natl Cancer Inst._ 106, dju066 (2014). PubMed PubMed Central Google Scholar * Yokobori, T. et al. Plastin3 is a novel marker for circulating tumor cells
undergoing the epithelial-mesenchymal transition and is associated with colorectal cancer prognosis. _Cancer Res._ 73, 2059–2069 (2013). CAS PubMed Google Scholar * Effenberger, K. E. et
al. Improved risk stratification by circulating tumor cell counts in pancreatic cancer. _Clin. Cancer Res._ 24, 2844–2850 (2018). CAS PubMed Google Scholar * Garcia-Murillas, I. et al.
Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. _Sci. Transl Med._ 7, 302ra133 (2015). PubMed Google Scholar * Trapp, E. et al. Presence of circulating
tumor cells in high-risk early breast cancer during follow-up and prognosis. _J. Natl Cancer Inst._ https://doi.org/10.1093/jnci/djy152 (2018). Article Google Scholar * Sparano, J. et al.
Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. _JAMA Oncol._ 4, 1700–1706
(2018). PubMed PubMed Central Google Scholar * Goodman, C. R. et al. Association of circulating tumor cell status with benefit of radiotherapy and survival in early-stage breast cancer.
_JAMA Oncol._ 4, e180163 (2018). PubMed PubMed Central Google Scholar * Bidard, F. C. et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a
pooled analysis of individual patient data. _Lancet Oncol._ 15, 406–414 (2014). PubMed Google Scholar * Heller, G. et al. Circulating tumor cell number as a response measure of prolonged
survival for metastatic castration-resistant prostate cancer: a comparison with prostate-specific antigen across five randomized phase III clinical trials. _J. Clin. Oncol._ 36, 572–580
(2018). CAS PubMed Google Scholar * Ignatiadis, M. et al. Trastuzumab versus observation for HER2 nonamplified early breast cancer with circulating tumor cells (EORTC 90091–10093, BIG
1–12, Treat CTC): a randomized phase II trial. _Ann. Oncol._ 29, 1777–1783 (2018). CAS PubMed Google Scholar * Georgoulias, V. et al. Trastuzumab decreases the incidence of clinical
relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. _Ann. Oncol._ 23,
1744–1750 (2012). CAS PubMed Google Scholar * van Dalum, G. et al. Importance of circulating tumor cells in newly diagnosed colorectal cancer. _Int. J. Oncol._ 46, 1361–1368 (2015).
PubMed Google Scholar * Krebs, M. G. et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. _J. Clin. Oncol._ 29, 1556–1563
(2011). PubMed Google Scholar * Hou, J. M. et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with
small-cell lung cancer. _J. Clin. Oncol._ 30, 525–532 (2012). PubMed Google Scholar * Aggarwal, C. et al. Circulating tumor cells as a predictive biomarker in patients with small cell lung
cancer undergoing chemotherapy. _Lung Cancer_ 112, 118–125 (2017). PubMed Google Scholar * Lindsay, C. R. et al. A prospective examination of circulating tumor cell profiles in
non-small-cell lung cancer molecular subgroups. _Ann. Oncol._ 28, 1523–1531 (2017). CAS PubMed Google Scholar * Scher, H. I. et al. Nuclear-specific AR-V7 protein localization is
necessary to guide treatment selection in metastatic castration-resistant prostate cancer. _Eur. Urol._ 71, 874–882 (2017). CAS PubMed Google Scholar * Meng, S. et al. Circulating tumor
cells in patients with breast cancer dormancy. _Clin. Cancer Res._ 10, 8152–8162 (2004). PubMed Google Scholar * Chen, Y. H. et al. Next-generation sequencing of circulating tumor DNA to
predict recurrence in triple-negative breast cancer patients with residual disease after neoadjuvant chemotherapy. _NPJ Breast Cancer_ 3, 24 (2017). PubMed PubMed Central Google Scholar *
Olsson, E. et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. _EMBO Mol. Med._ 7, 1034–1047 (2015). CAS
PubMed PubMed Central Google Scholar * Riva, F. et al. Patient-specific circulating tumor DNA detection during neoadjuvant chemotherapy in triple-negative breast cancer. _Clin. Chem._ 63,
691–699 (2017). CAS PubMed Google Scholar * Tie, J. et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer.
_Sci. Transl Med._ 8, 346ra92 (2016). PubMed PubMed Central Google Scholar * Ng, S. B. et al. Individualised multiplexed circulating tumour DNA assays for monitoring of tumour presence
in patients after colorectal cancer surgery. _Sci. Rep._ 7, 40737 (2017). CAS PubMed PubMed Central Google Scholar * Scholer, L. V. et al. Clinical implications of monitoring circulating
tumor DNA in patients with colorectal cancer. _Clin. Cancer Res._ 23, 5437–5445 (2017). CAS PubMed Google Scholar * Abbosh, C. et al. Phylogenetic ctDNA analysis depicts early-stage lung
cancer evolution. _Nature_ 545, 446–451 (2017). CAS PubMed PubMed Central Google Scholar * Chaudhuri, A. A. et al. Early detection of molecular residual disease in localized lung cancer
by circulating tumor DNA profiling. _Cancer Discov._ 7, 1394–1403 (2017). CAS PubMed PubMed Central Google Scholar * Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next
generation. _Cell_ 144, 646–674 (2011). CAS PubMed Google Scholar * Kuang, Y. et al. Noninvasive detection of EGFR T790M in gefitinib or erlotinib resistant non-small cell lung cancer.
_Clin. Cancer Res._ 15, 2630–2636 (2009). CAS PubMed PubMed Central Google Scholar * Nakamura, T. et al. A noninvasive system for monitoring resistance to epidermal growth factor
receptor tyrosine kinase inhibitors with plasma DNA. _J. Thorac. Oncol._ 6, 1639–1648 (2011). PubMed Google Scholar * Taniguchi, K. et al. Quantitative detection of EGFR mutations in
circulating tumor DNA derived from lung adenocarcinomas. _Clin. Cancer Res._ 17, 7808–7815 (2011). CAS PubMed Google Scholar * Douillard, J. Y. et al. First-line gefitinib in Caucasian
EGFR mutation-positive NSCLC patients: a phase-IV, open-label, single-arm study. _Br. J. Cancer_ 110, 55–62 (2014). CAS PubMed Google Scholar * QIAGEN. therascreen® EGFR RGQ PCR kit
instructions for use (handbook). _FDA_ https://www.accessdata.fda.gov/cdrh_docs/pdf12/P120022c.pdf (2013). * Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer
cells. _N. Engl. J. Med._ 359, 366–377 (2008). CAS PubMed PubMed Central Google Scholar * Mostert, B. et al. KRAS and BRAF mutation status in circulating colorectal tumor cells and their
correlation with primary and metastatic tumor tissue. _Int. J. Cancer_ 133, 130–141 (2013). CAS PubMed Google Scholar * Bettegowda, C. et al. Detection of circulating tumor DNA in early-
and late-stage human malignancies. _Sci. Transl Med._ 6, 224ra24 (2014). PubMed PubMed Central Google Scholar * Siravegna, G. et al. Clonal evolution and resistance to EGFR blockade in
the blood of colorectal cancer patients. _Nat. Med._ 21, 827 (2015). CAS PubMed Google Scholar * Jiang, Y., Palma, J. F., Agus, D. B., Wang, Y. & Gross, M. E. Detection of androgen
receptor mutations in circulating tumor cells in castration-resistant prostate cancer. _Clin. Chem._ 56, 1492–1495 (2010). PubMed Google Scholar * Wyatt, A. W. et al. Genomic alterations
in cell-free DNA and enzalutamide resistance in castration-resistant prostate cancer. _JAMA Oncol._ 2, 1598–1606 (2016). PubMed PubMed Central Google Scholar * Meric-Bernstam, F. et al.
Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. _Clin. Cancer Res._ https://doi.org/10.1158/1078-0432.CCR-18-2275 (2019). Article PubMed
PubMed Central Google Scholar * Schneck, H. et al. Analysing the mutational status of PIK3CA in circulating tumor cells from metastatic breast cancer patients. _Mol. Oncol._ 7, 976–986
(2013). CAS PubMed PubMed Central Google Scholar * Neves, R. P. et al. Genomic high-resolution profiling of single CKpos/CD45neg flow-sorting purified circulating tumor cells from
patients with metastatic breast cancer. _Clin. Chem._ 60, 1290–1297 (2014). CAS PubMed Google Scholar * Pestrin, M. et al. Heterogeneity of PIK3CA mutational status at the single cell
level in circulating tumor cells from metastatic breast cancer patients. _Mol. Oncol._ 9, 749–757 (2015). CAS PubMed Google Scholar * Polzer, B. et al. Molecular profiling of single
circulating tumor cells with diagnostic intention. _EMBO Mol. Med._ 6, 1371–1386 (2014). CAS PubMed PubMed Central Google Scholar * Gasch, C. et al. Frequent detection of PIK3CA
mutations in single circulating tumor cells of patients suffering from HER2-negative metastatic breast cancer. _Mol. Oncol._ 10, 1330–1343 (2016). CAS PubMed PubMed Central Google Scholar
* Schiavon, G. et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. _Sci. Transl Med._ 7, 313ra182 (2015). PubMed
PubMed Central Google Scholar * O’Leary, B. et al. Early circulating tumor DNA dynamics and clonal selection with palbociclib and fulvestrant for breast cancer. _Nat. Commun._ 9, 896
(2018). PubMed PubMed Central Google Scholar * Robinson, D. R. et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. _Nat. Genet._ 45, 1446–1451 (2013). CAS
PubMed PubMed Central Google Scholar * Toy, W. et al. ESR1 ligand-binding domain mutations in hormone-resistant breast cancer. _Nat. Genet._ 45, 1439–1445 (2013). CAS PubMed PubMed
Central Google Scholar * Shaw, J. A. et al. Mutation analysis of cell-free DNA and single circulating tumor cells in metastatic breast cancer patients with high circulating tumor cell
counts. _Clin. Cancer Res._ 23, 88–96 (2017). CAS PubMed Google Scholar * Guttery, D. S. et al. Noninvasive detection of activating estrogen receptor 1 (ESR1) mutations in estrogen
receptor-positive metastatic breast cancer. _Clin. Chem._ 61, 974–982 (2015). CAS PubMed Google Scholar * Chu, D. et al. ESR1 mutations in circulating plasma tumor DNA from metastatic
breast cancer patients. _Clin. Cancer Res._ 22, 993–999 (2016). CAS PubMed Google Scholar * O’Leary, B. et al. The genetic landscape and clonal evolution of breast cancer resistance to
palbociclib plus fulvestrant in the PALOMA-3 trial. _Cancer Discov._ 8, 1390–1403 (2018). PubMed PubMed Central Google Scholar * Villanueva, J., Vultur, A. & Herlyn, M. Resistance to
BRAF inhibitors: unraveling mechanisms and future treatment options. _Cancer Res._ 71, 7137–7140 (2011). CAS PubMed PubMed Central Google Scholar * Long, G. V. et al. Factors predictive
of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. _Lancet
Oncol._ 17, 1743–1754 (2016). CAS PubMed Google Scholar * Sakaizawa, K. et al. Mutation analysis of BRAF and KIT in circulating melanoma cells at the single cell level. _Br. J. Cancer_
106, 939–946 (2012). CAS PubMed PubMed Central Google Scholar * Long, G. V. et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. _N. Engl. J. Med._ 377,
1813–1823 (2017). CAS PubMed Google Scholar * Pailler, E. et al. Method for semi-automated microscopy of filtration-enriched circulating tumor cells. _BMC Cancer_ 16, 477 (2016). PubMed
PubMed Central Google Scholar * Pailler, E. et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. _J. Clin. Oncol._
31, 2273–2281 (2013). PubMed Google Scholar * Pailler, E. et al. High level of chromosomal instability in circulating tumor cells of ROS1-rearranged non-small-cell lung cancer. _Ann.
Oncol._ 26, 1408–1415 (2015). CAS PubMed PubMed Central Google Scholar * Attard, G. et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with
castration-resistant prostate cancer. _Cancer Res._ 69, 2912–2918 (2009). CAS PubMed Google Scholar * Danila, D. C. et al. TMPRSS2-ERG status in circulating tumor cells as a predictive
biomarker of sensitivity in castration-resistant prostate cancer patients treated with abiraterone acetate. _Eur. Urol._ 60, 897–904 (2011). CAS PubMed PubMed Central Google Scholar *
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. _N. Engl. J. Med._ 371, 1028–1038 (2014). PubMed PubMed Central Google Scholar *
Steinestel, J. et al. Detecting predictive androgen receptor modifications in circulating prostate cancer cells. _Oncotarget_ https://doi.org/10.18632/oncotarget.3925 (2015). Article PubMed
PubMed Central Google Scholar * Thadani-Mulero, M. et al. Androgen receptor splice variants determine taxane sensitivity in prostate cancer. _Cancer Res._ 74, 2270–2282 (2014). CAS
PubMed PubMed Central Google Scholar * Antonarakis, E. S. et al. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant
prostate cancer. _JAMA Oncol._ 1, 582–591 (2015). PubMed PubMed Central Google Scholar * Nakazawa, M. et al. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer.
_Ann. Oncol._ 26, 1859–1865 (2015). CAS PubMed PubMed Central Google Scholar * Onstenk, W. et al. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the
presence of AR-V7 in circulating tumor cells. _Eur. Urol._ 68, 939–945 (2015). CAS PubMed Google Scholar * Scher, H. I. et al. Assessment of the validity of nuclear-localized androgen
receptor splice variant 7 in circulating tumor cells as a predictive biomarker for castration-resistant prostate cancer. _JAMA Oncol._ 4, 1179–1186 (2018). PubMed PubMed Central Google
Scholar * Miyamoto, D. T. et al. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. _Science_ 349, 1351–1356 (2015). CAS PubMed PubMed
Central Google Scholar * Maurer, T. et al. PSMA theranostics using PET and subsequent radioguided surgery in recurrent prostate cancer. _Clin. Genitourin. Cancer_ 14, e549–e552 (2016).
PubMed Google Scholar * Ristau, B. T., O’Keefe, D. S. & Bacich, D. J. The prostate-specific membrane antigen: lessons and current clinical implications from 20 years of research.
_Urol. Oncol._ 32, 272–279 (2014). PubMed Google Scholar * Ahmadzadehfar, H. et al. Predictors of overall survival in metastatic castration-resistant prostate cancer patients receiving
[(177)Lu]Lu-PSMA-617 radioligand therapy. _Oncotarget_ 8, 103108–103116 (2017). PubMed PubMed Central Google Scholar * Babayan, A. et al. Heterogeneity of estrogen receptor expression in
circulating tumor cells from metastatic breast cancer patients. _PLOS ONE_ 8, e75038 (2013). CAS PubMed PubMed Central Google Scholar * Paoletti, C. et al. Development of circulating
tumor cell-endocrine therapy index in patients with hormone receptor-positive breast cancer. _Clin. Cancer Res._ 21, 2487–2498 (2015). CAS PubMed Google Scholar * Fehm, T. et al. HER2
status of circulating tumor cells in patients with metastatic breast cancer: a prospective, multicenter trial. _Breast Cancer Res. Treat._ 124, 403–412 (2010). CAS PubMed Google Scholar *
Wang, C. H., Chang, C. J., Yeh, K. Y., Chang, P. H. & Huang, J. S. The prognostic value of HER2-positive circulating tumor cells in breast cancer patients: a systematic review and
meta-analysis. _Clin. Breast Cancer_ 17, 341–349 (2017). CAS PubMed Google Scholar * Philips, G. K. & Atkins, M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. _Int.
Immunol._ 27, 39–46 (2015). CAS PubMed Google Scholar * Mittendorf, E. A. et al. PD-L1 expression in triple-negative breast cancer. _Cancer Immunol. Res._ 2, 361–370 (2014). CAS PubMed
PubMed Central Google Scholar * Mazel, M. et al. Frequent expression of PD-L1 on circulating breast cancer cells. _Mol. Oncol._ 9, 1773–1782 (2015). CAS PubMed PubMed Central Google
Scholar * Nicolazzo, C. et al. Monitoring PD-L1 positive circulating tumor cells in non-small cell lung cancer patients treated with the PD-1 inhibitor nivolumab. _Sci. Rep._ 6, 31726
(2016). CAS PubMed PubMed Central Google Scholar * Strati, A. et al. Prognostic significance of PD-L1 expression on circulating tumor cells in patients with head and neck squamous cell
carcinoma. _Ann. Oncol._ 28, 1923–1933 (2017). CAS PubMed Google Scholar * Cabel, L. et al. Clinical potential of circulating tumour DNA in patients receiving anticancer immunotherapy.
_Nat. Rev. Clin. Oncol._ 15, 639–650 (2018). CAS PubMed Google Scholar * Mohme, M., Riethdorf, S. & Pantel, K. Circulating and disseminated tumour cells — mechanisms of immune
surveillance and escape. _Nat. Rev. Clin. Oncol._ 14, 155–167 (2017). CAS PubMed Google Scholar * Pantel, K. & Hayes, D. F. Disseminated breast tumour cells: biological and clinical
meaning. _Nat. Rev. Clin. Oncol._ 15, 129–131 (2018). PubMed Google Scholar * Ulz, P. et al. Inferring expressed genes by whole-genome sequencing of plasma DNA. _Nat. Genet._ 48, 1273–1278
(2016). CAS PubMed Google Scholar * Guo, N. et al. Circulating tumor DNA detection in lung cancer patients before and after surgery. _Sci. Rep._ 6, 33519 (2016). CAS PubMed PubMed
Central Google Scholar * Alix-Panabieres, C. & Pantel, K. Clinical applications of circulating tumor cells and circulating tumor DNA as liquid biopsy. _Cancer Discov._ 6, 479–491
(2016). CAS PubMed Google Scholar * Newman, A. M. et al. Integrated digital error suppression for improved detection of circulating tumor DNA. _Nat. Biotechnol._ 34, 547–555 (2016). CAS
PubMed PubMed Central Google Scholar * Cohen, J. D. et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. _Science_ 359, 926–930 (2018). CAS
PubMed PubMed Central Google Scholar * Mani, S. A. et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. _Cell_ 133, 704–715 (2008). CAS PubMed
PubMed Central Google Scholar * Fernandez-Cuesta, L. et al. Identification of circulating tumor DNA for the early detection of small-cell lung cancer. _EBioMedicine_ 10, 117–123 (2016).
PubMed PubMed Central Google Scholar * Krimmel, J. D. et al. Ultra-deep sequencing detects ovarian cancer cells in peritoneal fluid and reveals somatic TP53 mutations in noncancerous
tissues. _Proc. Natl Acad. Sci. USA_ 113, 6005–6010 (2016). CAS PubMed PubMed Central Google Scholar * Genovese, G. et al. Clonal hematopoiesis and blood-cancer risk inferred from blood
DNA sequence. _N. Engl. J. Med._ 371, 2477–2487 (2014). PubMed PubMed Central Google Scholar * Kato, S., Lippman, S. M., Flaherty, K. T. & Kurzrock, R. The conundrum of genetic
“drivers” in benign conditions. _J. Natl Cancer Inst._ 108, djw036 (2016). PubMed PubMed Central Google Scholar * Pollock, P. M. et al. High frequency of BRAF mutations in nevi. _Nat.
Genet._ 33, 19–20 (2003). CAS PubMed Google Scholar * Bardelli, A. & Pantel, K. Liquid biopsies, what we do not know (yet). _Cancer Cell_ 31, 172–179 (2017). CAS PubMed Google
Scholar * Gao, X. L., Zhang, M., Tang, Y. L. & Liang, X. H. Cancer cell dormancy: mechanisms and implications of cancer recurrence and metastasis. _Onco Targets Ther._ 10, 5219–5228
(2017). PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS The authors thank N. Reimers for her assistance in designing figure 4. The authors received support from
the German Cancer Aid Fund (Deutsche Krebshilfe); DFG (Deutsche Forschungsgemeinschaft); the French National Institute of Cancer (INCa); CANCER-ID, an Innovative Medicines Initiative Joint
Undertaking under grant agreement no. 115749, resources of which are composed of financial contribution from the European Union’s Seventh Framework Program (FP7/2007-2013) and European
Federation of Pharmaceutical Industries and Associations (EFPIA) companies’ in-kind contributions; and the European Liquid Biopsies Academy (ELBA) Innovative Training Networks (ITN) Horizon
2020 project H2020-MSCA-ITN-2017 (Towards widespread clinical application of blood-based diagnostic tools). COMPETING INTERESTS K.P. and C.A.-P. have ongoing patent applications related to
circulating tumour cells. K.P. has received honoraria from Agena, Novartis, Roche and Sanofi and research funding from European Federation of Pharmaceutical Industries and Associations
(EFPIA) partners (Angle, Menarini and Servier) of the CANCER-ID programme of the European Union–EFPIA Innovative Medicines Initiative. C.A.-P. has received honoraria from Janssen. REVIEWER
INFORMATION _Nature Reviews Clinical Oncology_ thanks J.-Y. Pierga and other anonymous reviewer(s) for their contribution to the peer review of this work. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Tumour Biology, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany Klaus Pantel * Laboratory of Rare Human Circulating Cells (LCCRH), University
Medical Centre and University of Montpellier, Montpellier, France Catherine Alix-Panabières Authors * Klaus Pantel View author publications You can also search for this author inPubMed
Google Scholar * Catherine Alix-Panabières View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Both authors made substantial contributions to
all stages of the preparation of this manuscript. CORRESPONDING AUTHOR Correspondence to Klaus Pantel. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RELATED LINKS BLOODPAC: https://www.bloodpac.org/ CANCER-ID: https://www.cancer-id.eu/ CLINICALTRIALS.GOV DATABASE:
https://clinicaltrials.gov/ ELBA: https://elba.uni-plovdiv.bg/ SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE
THIS ARTICLE Pantel, K., Alix-Panabières, C. Liquid biopsy and minimal residual disease — latest advances and implications for cure. _Nat Rev Clin Oncol_ 16, 409–424 (2019).
https://doi.org/10.1038/s41571-019-0187-3 Download citation * Published: 22 February 2019 * Issue Date: July 2019 * DOI: https://doi.org/10.1038/s41571-019-0187-3 SHARE THIS ARTICLE Anyone
you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the
Springer Nature SharedIt content-sharing initiative
Trending News
Drivers urged to pay car tax ahead of major ved changes next monthThe standard rate will increase by £10 for most cars which were first registered on or after April 1, 2017. For cars reg...
Expressive drawing: a practical guide to freeing the artist withinBOOK DESCRIPTION BUY THIS BOOK! * Amazon * BARNES & NOBLE _Pricing varies by retailer_ The many people who long to d...
France: new president macron is a ‘zombie catholic’Newly-elected President Emmanuel Macron, according to one of his biographers, embodies a new phenomenon in France known ...
Page Not Found很抱歉,你所访问的页面已不存在了。 如有疑问,请电邮[email protected] 你仍然可选择浏览首页或以下栏目内容 : 新闻 生活 娱乐 财经 体育 视频 播客 新报业媒体有限公司版权所有(公司登记号:202120748H)...
Closer look: venison sandwiches; allergies; and moreCloser Look with Rose Scott November 4, 2016 Friday on “Closer Look with Rose Scott and Jim Burress”: * 0:00: Atlanta Jo...
Latests News
Liquid biopsy and minimal residual disease — latest advances and implications for cureABSTRACT Liquid biopsy has been introduced as a new diagnostic concept predicated on the analysis of circulating tumour ...
Don't allow the spin to cloud your view of the backstop | thearticleWhat did politically engaged people in the British Isles talk about, think about and write about before the Irish backst...
AARP Members Edition - Exclusive Articles, Videos, Books and MoreMemorial Day Sale! Join AARP for just $11 per year with a 5-year membership Join now and get a FREE gift. Expires 6/4 G...
Inside Amigoland - The Texas Observer_Oscar Casares’s short story collection _Brownsville_ (Back Bay, 2003) was an instant hit with critics and readers, esta...
The persistent ring of m87* confirms predictionsAccess through your institution Buy or subscribe The first image of a black hole shadow graced the front pages of newspa...